Abstract
Objective
To systemically review the effects of low-intensity pulsed ultrasound (LIPUS) on pain relief and functional recovery in patients with knee osteoarthritis (KOA).
Data sources
PubMed, Web of Science, Cochrane Library, Physiotherapy Evidence Database (PEDro), and China National Knowledge Infrastructure (CNKI) were used from inception to 18 March 2022.
Review Methods
Meta-analysis was performed to evaluate pain and function recovery between control and LIPUS groups. Standardized mean difference (SMD) or mean difference (MD) and 95% confidence interval (CI) were calculated, and data were combined using the fixed or random-effect model.
Results
Thirteen studies involving 807 patients with KOA were included. Patients’ outcomes treated by LIPUS were improved significantly, including Visual analog scale (VAS) score (MD = −0.95, 95% CI: −1.43 to −0.48,P < 0.001), Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) score (MD = −4.35, 95% CI: −8.30 to −0.40, P = 0.0309), Lysholm score (SMD = 1.59, 95% CI: 1.29 to 1.90, P < 0.001), Lequesne index (MD = −1.33, 95% CI: −1.69 to −0.96, P < 0.001), Range of motion (ROM) (MD = 2.43, 95% CI: 0.39 to 4.46, P = 0.0197) and 50 meter walking time (SMD = 1.48, 95% CI: 0.46 to 2.49, P = 0.0044). Subgroup analyses showed monotherapy of LIPUS produced a better effect on reducing VAS score (P = 0.0213), and the shorter therapeutic period (≤4 weeks) produced a more significant effect on raising the WOMAC score (P = 0.0083).
Conclusion
LIPUS was beneficial for pain relief and functional knee recovery and maybe as an alternative therapy in KOA rehabilitation.
Keywords: Knee osteoarthritis, low-intensity pulsed ultrasound, physical therapy, a systematic review, meta-analysis
Introduction
Knee osteoarthritis (KOA) is a common degenerative knee joint disease, causing joint pain, stiffness, swelling, muscle weakness, loss of physical function, and even disability. 1 The number of KOA patients has increased with population aging, seriously affecting patients’ quality of life and significantly burdens society.
Until now, medical therapy for KOA includes surgical therapies and nonsurgical therapies. Total knee arthroplasty is the definitive therapy for KOA. Before surgery, some nonsurgical therapies could relieve pain and promote functional recovery. Nonsurgical therapies include pharmacological and non-pharmacological approaches. Pharmacological approaches refer to analgesic drugs and supplements for promoting cartilage repair.2–4 However, these drugs often have lower curative effects and some serious side effects. Exercise, weight loss, and physical factors are mainly non-pharmacological treatments, but exercise and weight loss have poor compliance.5, 6 In recent years, physicians have paid increasing attention to physical factors therapies, including ultrasound, laser therapy, and electroanalgesia, to manage osteoarthritis.7–12low-intensity pulsed ultrasound (LIPUS), one kind of therapeutic ultrasound, has been approved to treat fresh fracture and nonunion for more than 20 years in the United States. In recent years, the effect of LIPUS on osteoarthritis has been discussed by various studies. 13 A systematic review published in 2018 and five randomized controlled trials showed that LIPUS played a beneficial role in pain relief and functional recovery and had no adverse effects on KOA patients. However, the number of trials is limited, with heterogeneity but no subgroup analysis. 14 Another systematic review published in 2019 only assessed the effect of LIPUS therapeutic ultrasound on the pain in patients with KOA. 15
Fortunately, in recent years, high-quality randomized controlled trials have evaluated the impact of LIPUS on the treatment effect of KOA. Therefore, based on relevant studies, we designed and updated a meta-analysis to comprehensively analyze and determine the efficacy of LIPUS on KOA.
Methods
This meta-analysis met the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). 16 The International Open Science Framework Registry Link is https://doi.org/10.17605/OSF.IO/FHSD9. The scientific research funding project from the Department of Education of Liaoning Province (LJKZ1048) supported this work.
Electronic literature searches were conducted on PubMed, Web of Science, Cochrane Library, Physiotherapy Evidence Database (PEDro), and China National Knowledge Infrastructure (CNKI) from their inception to 18 March 2022. We searched the keywords “Low-Intensity Pulsed Ultrasound,” “Knee Osteoarthritis,” and corresponding retrieved terms of Medical Subject Headings (MeSH). Each keyword and corresponding Medical Subject Headings term were combined with OR operator and two keywords with AND operator. The language was restricted to English and Chinese. We also screened the reference lists of the papers identified in database searches. The search strategy for each database was shown in Appendix 1.
The inclusion criteria based on the PICOS framework 17 (population, intervention, comparison, outcome, and study) are as follows: (1) Randomized controlled trials; (2) At least one group in the study using LIPUS as an intervention; (3) Outcomes reflecting efficacy (including symptoms, outcome measures); (4) Papers in English and Chinese; (5) Randomized controlled trials studies at a high methodological quality (Jadad score > 3) 18 ; (6) Studies with follow-up.
The following were the exclusion criteria: (1) Experimental studies (for example, animal studies); (2) A cohort study, case-control, or cross-sectional study; review articles, or conference abstracts; (3) Studies whose full text is not available or studies with no data available; and (4) randomized controlled trials studies at a low methodological quality (Jadad score ≤ 3). 18
Following a systematic search, the retrieved articles were screened by two independent reviewers (HQC and ZW) for eligibility based on the inclusion criteria described below to determine whether the article title and abstract met the criteria. After excluding articles that did not meet the criteria, those likely to meet the criteria were read in detail and assessed in detail for final inclusion.
Two reviewers (HQC and ZW) independently extracted the data. The following data were extracted from the included articles: First author and year of publication; Characteristics of the subject (country or region, age, and gender of the participants); Intervention and Comparison group; LIPUS treatment parameters; Grading criteria for knee osteoarthritis; Outcome measurements; and Follow-up.
According to the Cochrane Collaboration, two reviewers (HQC and ZW) independently assessed the risk of the bias of the included studies. 19 The assessment involved six items (selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias). The associated risks were divided into unclear, low, and high. Any differences or discrepancies that exist were discussed or resolved by a third reviewer (MLS). Moreover, these have been agreed upon by researchers before.
Data from the included studies were analyzed using The R Programming Language × 64 4.0.5 software (R Development Core Team, Vienna, Austria).20,21 The package (University of Freiburg, Germany) commands of “meta” and “ metacont” called under R software were used to calculate the overall effect size, the forest plot is represented by “forest,” and the publication bias is represented by “funnel” and “metabias”.22–24 For continuous outcomes with different scoring units or a big difference in the mean between different studies, each outcome metric's estimated effect size was pooled using a standardized mean difference (SMD) with a 95% CI. Continuous results had the same score, and the unit estimated effect size used the mean difference (MD) with a 95% CI to summarize each outcome indicator. I2 test was used to evaluate the statistical heterogeneity, and the low heterogeneity I2 ≤ 25%; Moderate heterogeneity 25% < I2 < 50%; significant heterogeneity 50% < I2 < 75%; High heterogeneity I2 ≥75%. 25 When I2 < 50%, P > 0.1, the fixed-effect model was used for the summary mean difference evaluation of 95% CI, on the contrary, the random effect model was used.26,27
In addition, subgroup analysis and sensitivity analysis were performed to identify potential determinants of efficacy and heterogeneity,respectively. Furthermore, the potential publication bias was tested using Egger's test. 28 P < 0.05 was considered statistically significant.
Result
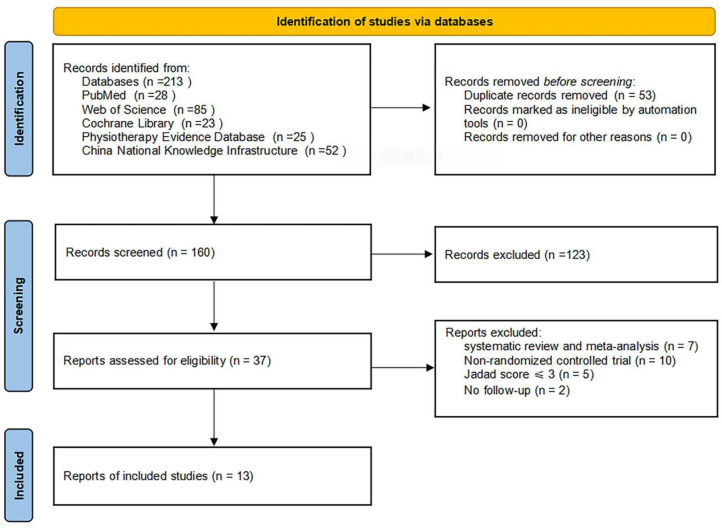
The flow diagram of the article screening process is shown in Figure 1. The search strategy proposed retrieved 213 documents. After the whole selection process, 37 articles were included in the systematic review, of which 13 studies with 807 subjects were included in the meta-analysis for statistical comparison.29–41 The characteristics of the included studies are shown in Table 1.
Figure 1.
Flowchart for the selection of included trials.
Table 1.
Characteristics of eligible randomized controlled trials included in the meta-analysis.
| First author-Year | Characteristics of subject | Intervention group (I) Comparison group (C) |
LIPUS parameter | Treatment duration | Joint Kellgren and Lawrence class rating | Outcome measurements | Follow-up |
|---|---|---|---|---|---|---|---|
| Huang
29
-2005 |
Taiwan, China Age: 40-77 years M/F: 113/27 |
(I) LIPUS + Isokinetic exercises n = 35 (C) Isokinetic exercises n = 35 |
1MHZ,2.5W/cm2 | 5 min/ 3 times a week 8 weeks |
Knee (Altman grade II) |
①②⑩⑬⑯ | 1 year |
| Huang
30
-2005 |
Taiwan, China Age: 42-72 years M/F: 20/100 |
(I) LIPUS + Isokinetic exercises n = 30 (C) Isokinetic exercises n = 30 |
1MHZ, 2.5W/cm2 | 5 min/ 3 times a week 8 weeks |
Knee (Altman grade II) |
①②⑩⑬⑯ | 1 year |
| Tascioglu
31
-2010 |
Turkey Age: 54-70 years M/F: 25/56 |
(I) LIPUS n = 28 (C) Sham LIPUS + Placebo n = 27 |
1MHZ, 2W/cm2 | 5 min/ 5 times a week 2 weeks |
Knee, 2-3 | ①④⑮ | 2 weeks |
| Loyola
32
-2012 |
Canada Age:≥45 years M/F: 21/27 |
(I) LIPUS n = 13 (C) Sham LIPUS n = 14 |
1MHZ, 0.2W/cm2 112.5J/cm2 |
3 times a week, 8 weeks |
Knee (OARSI-Medical JSN, grade 1 or 2) |
④⑭ | 2 months |
| Li
33
-2013 |
China Age: 39-72 years M/F: 32/76 |
(I) LIPUS + Sodium hyaluronate n = 30 (C) Sodium hyaluronate n = 30 |
1MHZ | 35 min/ the first week 20 min/the next 4 weeks 3 times a week 8 weeks |
Knee, 1-3 | ①② | 5 weeks |
| Cakir
34
-2014 |
Turkey Age: 40-80 years M/F: 13/47 |
(I) LIPUS + Self-discipline n = 20 (C) Sham LIPUS + Self-discipline n = 20 |
1MHZ, 1W/cm2 | 12 min/ 5 times a week 2 weeks |
Knee, 2-3 | ①④⑮ | 6 months |
| Yildiz
35
-2015 |
Turkey Age: 40-65 years M/F: 15/75 |
(I) LIPUS n = 30 (C) Sham LIPUS + Placebo n = 30 |
1MHZ, 1.5W/cm2 | 5 min/ 5 times a week 2 weeks |
Knee, 2-3 | ①②⑩ | 2 months |
| Gao
36
-2016 |
China Age: 25-45 years M/F: 32/76 |
(I) LIPUS + NSAID (a) n = 20 (C) NSAID (a) n = 20 |
3MHZ, 40mW/cm2 | 20 min/ 6 times a week 6 weeks |
Knee (ICRS, grades I-II) | ①③④⑪ | 6 weeks |
| Jia
37
-2016 |
China Age:≥40 years M/F: 30/76 |
(I) LIPUS + NSAID (b) n = 53 (C) NSAID (b) n = 53 |
0.6 MHZ, 120mW/cm2 a pulse repetition frequency 300HZ | 20 min/ 1 time a day 10 times in 2 weeks |
Knee, 2-3 | ①②④⑤⑩⑬ | 12 weeks |
| Cheng
38
-2019 |
China Age: 43-71 years M/F: 45/56 |
LIPUS + NSAID (b) n = 52 (C) NSAID (b) n = 52 |
3MHZ, 40mW/cm2 0.6MHZ,30mW/cm2 |
40 min, 4 weeks | unclear | ①③⑥⑦⑧ | 4 weeks |
| Luo
39
-2019 |
China Age:≥40 years M/F: 25/50 |
(I) LIPUS + NSAID (b) n = 36 (C) NSAID (b) n = 39 |
3MHZ,40mW/cm2 0.6MHZ,30mW/cm2 |
40 min, 8 weeks | Knee, 1-3 | ①②③ | 8 weeks |
| Kim
40
-2019 |
Korea Age: 25-45 years M/F: 32/76 |
(I) LIPUS + TENs n = 19 (C) TENs n = 19 |
1MHZ, 0.1W/cm2 | 20 min,≤3 times a day, >10 times a week, 8 weeks | Knee, 1-4 | ①④⑤⑫ | 1 month |
| Karakas
41
-2020 |
Turkey Age:≥40 years M/F: 12/63 |
(I) LIPUS + Self-discipline n = 36 (C) Sham LIPUS + Self-discipline n = 36 |
1MHZ, 1W/cm2 | 10 min, 3 times a week 8 weeks |
Knee, 2-3 | ①④⑨ | 12 weeks |
LIPUS: low-intensity pulsed ultrasound, TENs: Transcutaneous Electrical Nerve Stimulation, (I): Intervention group, (c): Comparison group, M: male, F: female, NSAID: non-steroidal anti-inflammatory drug, NSAID (a): Celecoxib capsules NSAID (b): Diclofenac sodium sustained-release tablets, n: participants, OARSI: Osteoarthritis Research Society International, JSN: joint space narrowing, ICRS: International cartilage repair society.
①Visual analog scale (VAS) ②Lequesne index ③Lysholm score ④Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score ⑤short form 36 item general health questionnaire (SF-36) ⑥Activity of daily living (ADL) ⑦Noyes articular cartilage defects ⑧score evaluation of clinical symptoms ⑨Timed up and go test (TUG) ⑩Range of motion (ROM) ⑪Magnetic Resonance Imaging (MRI) T2 weighted image ⑫Femoral articular cartilage (FAC) thickness⑬50 meters walking time ⑭6 min walk test⑮20 meters walking time ⑯muscle peak torques during knee flexion (MPT).
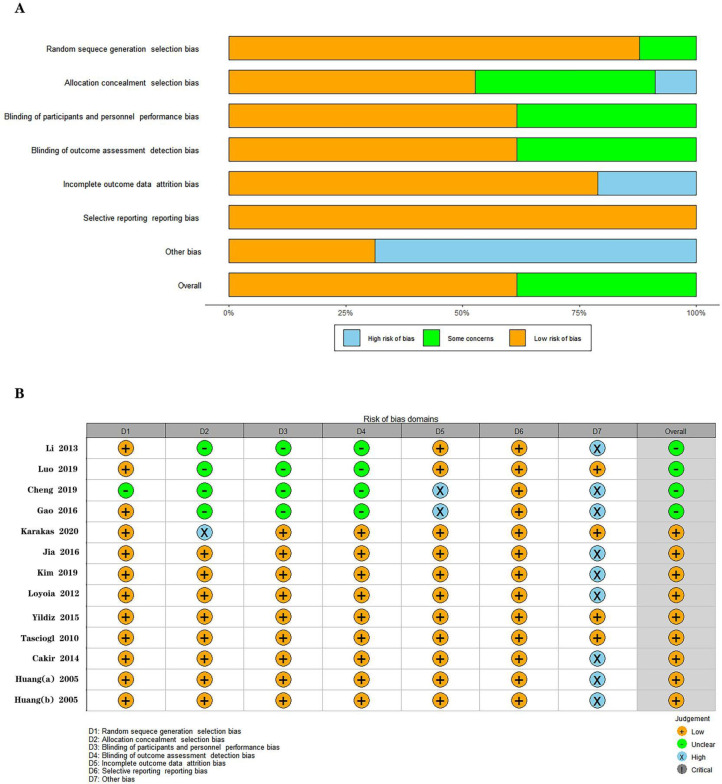
Three studies in the intervention group used LIPUS as monotherapy regarding the interventions.31,32,35 And ten studies used it as a combination therapy.29,30,33,34,36–41 The outcome indicators of the included studies were: the Visual analog scale score, the Western Ontario and McMaster Universities Osteoarthritis Index score, the Lysholm score, the Lequesne index, Range of motion, 20 meters and 50 meters walking time. The follow-up period ranged from 2 weeks to 1 year. The overall bias of the included studies is shown in Figure 2.
Figure 2.
Risk of bias for the included randomized controlled trials. A.Risk of bias graph, B.Risk of bias summary.
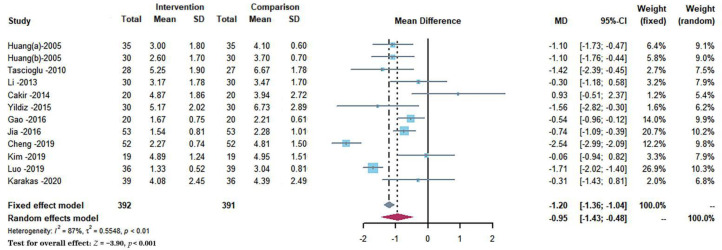
Twelve studies with 783 participants reported the effects of different interventions on the Visual analog scale score outcome. As shown in Figure 3.29–31,33–41 The synthesized data indicated that the intervention group had significantly alleviated pain as compared with a comparison group (MD = −0.95, 95% CI: −1.43 to −0.48, P < 0.001), and a random-effect model was used because of a substantial heterogeneity for this synthesized outcome (I2 = 86.6%). For the subgroups of trials with therapeutic periods of ≤ 4 weeks and > 4 weeks, no differences in pain relief were found when comparing the effect between them (P = 0.6443), and all subgroups show heterogeneity (I2 > 50%). The comparison between subgroups in the therapeutic protocol showed that LIPUS monotherapy produced a more significant effect on pain relief (MD = −1.96, 95% CI: −2.79 to −1.14, P = 0.0213), and all subgroups show heterogeneity (I2 > 60%). In the subgroup analysis for the duration time of every therapy, no significant differences in pain relief were found between groups (P = 0.7619). The longer duration time (≥20 min) shows high heterogeneity (I2 = 92.9%), while the shorter duration time (< 20 min) shows moderate significant heterogeneity (I2 = 49.8%). Subgroup analysis is shown in Table 2.
Figure 3.
Forest plots demonstrated the effect of low-intensity pulsed ultrasound on pain relief evaluated by the Visual analog scale score.
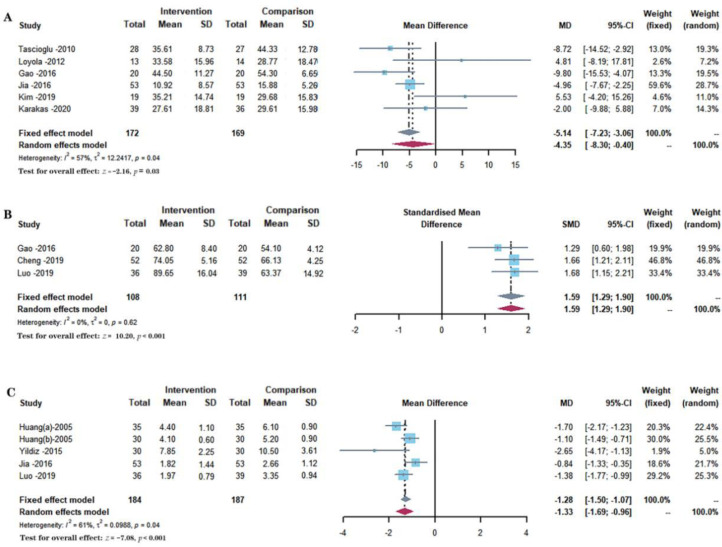
Six studies with 341 participants reported the effects of different interventions on the Western Ontario and McMaster Universities Osteoarthritis Index score, as shown in Figure 4A.31,32,36,37,40,41 The synthesized data indicated that the intervention group had significantly promoted the functional recovery as compared with a comparison group (MD = −4.35, 95% CI: −8.30 to −0.40, P = 0.0309), and a random-effect model was used because of a substantial heterogeneity for this synthesized outcome (I2 = 56.5%). The comparison between subgroups of trials with therapeutic periods of ≤4 weeks and > 4 weeks showed that the shorter therapeutic period (≤4 weeks) produced a more significant effect on reducing the Western Ontario and McMaster Universities Osteoarthritis Index score (P = 0.0083). The longer therapeutic periods (> 4 weeks) show no heterogeneity (I2 = 0.0%), and the shorter therapeutic periods (≤4 weeks) show moderate heterogeneity (I2 = 34.2%). In a subgroup analysis, there was no significant difference between the effects of LIPUS monotherapy, LIPUS combined with pharmacotherapy, and LIPUS combined with non-pharmacological treatment on the Western Ontario and McMaster Universities Osteoarthritis Index scores (P = 0.1948). There was heterogeneity in the LIPUS monotherapy regimens and LIPUS combined with pharmacological treatment (I2 > 25%). However, there is moderate heterogeneity in the regimen of LIPUS combined with non-pharmacological treatments (I2 = 28.1%). For the subgroups of the duration time of every therapy (≥20 min and < 20 min), no differences were found when comparing the effect (P = 0.7266). The longer duration time (≥20 min) showed significant heterogeneity (I2 = 72.1%), while the shorter duration time (< 20 min) showed moderate heterogeneity (I2 = 44.8%). Subgroup analysis is shown in Table 2.
Figure 4.
Forest plots demonstrated the effect of low-intensity pulsed ultrasound on functional recovery evaluated by several scores. A. Western Ontario and McMaster Universities Osteoarthritis Index score, B. Lysholm score, C. Lequesne index.
Three studies with 219 participants reported the effects of different interventions on the Lysholm score, as shown in Figure 4B.36,38,39 The synthesized data indicated that the intervention group had significantly promoted the functional recovery as compared with a comparison group (SMD = 1.59, 95% CI: 1.29 to 1.90, P < 0.001), and a fixed-effect model was used because of no heterogeneity for this synthesized outcome (I2 = 0%).
Five included studies with 371 participants reported the effects of different interventions on the Lequesne index, as shown in Figure 4C.29,30,35,37,39 The synthesized data indicated that the intervention group had significantly promoted the functional recovery as compared with a comparison group (MD = −1.33, 95% CI: −1.69 to −0.96, P < 0.001), and a random-effect model was used because of a substantial heterogeneity for this synthesized outcome (I2 = 61.3%). No differences were found for the subgroups of the therapeutic periods (≤4 weeks and > 4 weeks) when comparing their effect (P = 0.8055). A therapeutic period (≤4 weeks) showed high heterogeneity (I2 = 79.7%), but a therapeutic period (> 4 weeks) showed moderate heterogeneity (I2 = 46.6%). In the subgroup analysis for the Lequesne index, no significant differences were found among the different groups (P > 0.05). There was heterogeneity in the regimes of LIPUS combined with pharmacological therapy and LIPUS combined with non-pharmacological therapy (I2 > 60%). However, no data were available for subgroup analysis of LIPUS monotherapy treatment. For the comparison between subgroups in terms of duration time of every therapy, no significant differences were found when comparing the effect (P = 0.3147), and all subgroups show significant heterogeneity (50% < I2 < 75%). Subgroup analysis is shown in Table 2.
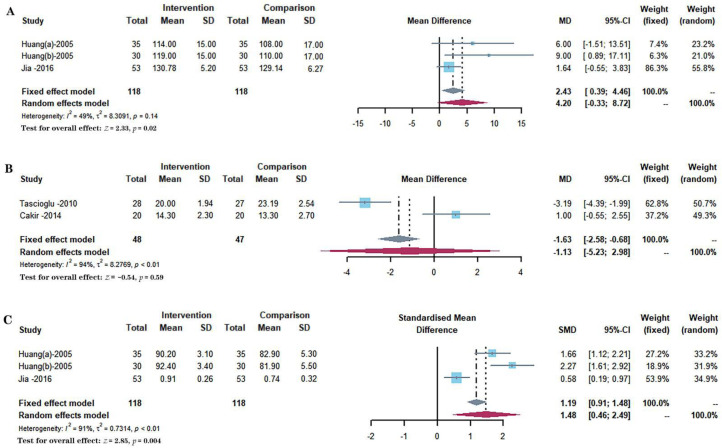
Three included studies with 236 participants reported the effects of different interventions on the Range of motion, as shown in Figure 5A.29, 30, 37 A fixed-effect model was used. The synthesized data indicated that knee range of motion significantly increased in the intervention group compared with a comparison group (MD = 2.43, 95% CI: 0.39 to 4.46, P = 0.0197).
Figure 5.
Forest plots demonstrated the effect of low-intensity pulsed ultrasound on joint mobility and walking ability by range of motion and walking tests. A.Range of motion, B. 20 meters walking time, C. 50 meters walking time.
Two included studies with 95 participants reported the effects of different interventions on the 20 meters walking time.31, 34 And three included studies totaling 236 participants reported the effects of different interventions on 50 meters walking time, as shown in Figure 5 B-C.29, 30, 37 A random-effect model was used, and the synthesized data indicated that 20 meters walking time were no significant increase in the intervention group compared with a comparison group (MD = −1.13, 95% CI: −5.23 to 2.98, P = 0.5911), while 50 meters walking time was a significant decrease in the intervention group compared with a comparison group (SMD = 1.48, 95% CI: 0.46 to 2.49, P = 0.0044).
The publication bias of primary outcomes (Visual analog scale score, Western Ontario and McMaster Universities Osteoarthritis Index score, and Lequesne index) was evaluated using funnel plots based on 13 studies. The results showed P = 0.2324 for Visual analog scale score, P = 0.3877 for Western Ontario and McMaster Universities Osteoarthritis Index score, and P = 0.3670 for Lequesne index by Egger's test, respectively, demonstrating that no publication bias was found for the primary outcomes of LIPUS therapy in patients with KOA .
Table 2.
Subgroup analysis of visual analog scale, Western Ontario and McMaster Universities Osteoarthritis Index score, and Lequesne index in the intervention and comparison groups.
| Outcome | Subgroup | Article numbers | participants | MD | Heterogeneity | Subgroup difference |
|---|---|---|---|---|---|---|
| (95% CI) | I2% (P value ) | Q (P value ) | ||||
| VAS | Therapeutic periods | |||||
| ≤4 weeks | 4 | 261 | −0.79 [−1.57 to 0.00] | 65.0% (P = 0.04) | 0.21 (P = 0.6443) | |
| > 4 weeks | 8 | 522 | −1.02 [−1.63 to −0.41] | 89.0% (P < 0.01) | ||
| Therapeutic schedule | ||||||
| LIPUS monotherapy | 3 | 219 | −1.96 [−2.79 to −1.14] | 63.7% (P = 0.06) | 7.70 (P = 0.0213) | |
| LIPUS + pharmacotherapy | 4 | 281 | −0.87 [−1.53 to −0.21] | 89.7% (P < 0.01) | ||
| LIPUS + non-pharmacological treatment | 5 | 283 | −0.51 [−1.14 to 0.13] | 61.4% (P = 0.03) | ||
| Time per treatment | ||||||
| < 20 min | 6 | 360 | −0.89 [−1.43 to −0.35] | 49.8% (P = 0.08) | 0.09 (P = 0.7619) | |
| ≥20 min | 6 | 423 | −1.03 [−1.74 to −0.32] | 92.9% (P < 0.01) | ||
| WOMAC | Therapeutic periods | |||||
| ≤4 weeks | 3 | 201 | −6.92 [−10.10 to −3.74] | 34.2% (P = 0.22) | 6.96 (P = 0.0083) | |
| > 4 weeks | 3 | 140 | 1.68 [−3.86 to 7.22] | 0.0% (P = 0.44) | ||
| Therapeutic schedule | ||||||
| LIPUS monotherapy | 2 | 82 | −3.26 [−16.27 to 9.76] | 71.2% (P = 0.06) | 3.27 (P = 0.1948) | |
| LIPUS + pharmacotherapy | 2 | 146 | −6.69 [−11.24 to −2.14] | 55.3% (P = 0.13) | ||
| LIPUS + non-pharmacological treatment | 2 | 113 | 1.20 [−6.09 to 8.50] | 28.1% (P = 0.24) | ||
| Time per treatment | ||||||
| < 20 min | 2 | 130 | −5.91 [−12.41 to 0.59] | 44.8% (P = 0.18) | 0.12 (P = 0.7266) | |
| ≥20 min | 3 | 184 | −4.32 [−10.45 to 1.81] | 72.1% (P = 0.03) | ||
| Lequesne | Therapeutic periods | |||||
| ≤4 weeks | 2 | 166 | −1.60 [−3.35 to 0.15] | 79.7% (P = 0.03) | 0.06 (P = 0.8055) | |
| > 4 weeks | 3 | 205 | −1.37 [−1.70 to −1.04] | 46.6% (P = 0.15) | ||
| Therapeutic schedule | ||||||
| LIPUS monotherapy | 1 | 60 | −2.65 [−4.17 to −1.13] | — | 3.47 (P = 0.1766) | |
| LIPUS + pharmacotherapy | 2 | 181 | −1.13 [−1.66 to −0.60] | 64.8% (P = 0.09) | ||
| LIPUS + non-pharmacological treatment | 2 | 130 | −1.38 [−1.97 to −0.80] | 73.1% (P = 0.05) | ||
| Time per treatment | ||||||
| < 20 min | 3 | 190 | −1.55 [−2.19 to −0.92] | 69.0% (P = 0.04) | 1.01 (P = 0.3147) | |
| ≥20 min | 2 | 181 | −1.13 [−1.66 to −0.60] | 64.8% (P = 0.09) |
MD: random effects mean difference, 95% CI: 95% confidence interval, LIPUS: low-intensity pulsed ultrasound, VAS: Visual analog scale score, Lequesne: Lequesne index, WOMAC: Western Ontario and McMaster Universities Osteoarthritis index score, —: None.
Due to the high heterogeneity of the meta-analysis results, the sensitivity analysis was performed by deleting each study to determine the robustness. The statistical heterogeneity of this synthesized outcome decreased after removing one study from the 12 studies participating in the Visual analog scale score (MD = −0.81, 95% CI: −1.22 to −0.40, I2 = 77.3%). 38 After removing one of the six studies involved in the Western Ontario and McMaster Universities Osteoarthritis Index score, the statistical heterogeneity of this combined result showed a significant decrease in the Western Ontario and McMaster Universities Osteoarthritis Index score (MD = −5.76, 95% CI: −9.12 to −2.40, I2 = 39.8%). 40 The sensitivity analysis table is available in Appendix 2.
Discussion
This meta-analysis included 13 studies involving 807 patients to explore the efficacy of LIPUS for the treatment of KOA. In this study, the data suggested that LIPUS could significantly ameliorate pain and promote functional recovery in KOA individuals. LIPUS may be available as an alternative non-pharmacological therapy in the rehabilitation program of KOA.
KOA is characteristic of knee cartilage damage. Degeneration and inflammation can lead to knee pain and dysfunction. Currently, the primary goals in KOA management are to relieve arthritic symptoms, including pain and stiffness, to avoid disability. 38 Our findings showed significant improvements in pain and physical function with the LIPUS, consistent with the results of the two similar previous studies in 2018 and 2019.14, 15 However, the meta-analysis in 2018 only included five studies, with high heterogeneity existed and without subgroup analysis due to the limited number of studies. 14 Another meta-analysis in 2019 only assessed the effect of LIPUS therapeutic ultrasound on the pain in patients with KOA. 15 On the contrary, our meta-analysis included more studies and participants and performed more subgroup analyses.
Our meta-analysis demonstrated that LIPUS therapeutic ultrasound significantly alleviated pain (Visual analog scale score) and improved physical function (Western Ontario and McMaster Universities Osteoarthritis Index score, Lequesne index, and Lysholm score). The benefits of these treatments were the same as those of the included randomized controlled trials. In addition, several randomized controlled trials have evaluated the effectiveness of LIPUS to other outcomes (Range of motion and Walking tests) for patients with KOA. So, we pooled randomized controlled trials’ data to analyze the effectiveness of LIPUS on Range of motion and walked ability. Fortunately, our meta-analysis demonstrated that LIPUS improved the Range of motion and walking ability.
Our present study included substantial heterogeneity. To investigate the source of the heterogeneity, we next carried out the subgroup analysis. Subgroup analyses indicated no statistically significant difference among single LIPUS, LIPUS combined drug therapy, LIPUS combined non-drug therapy in Western Ontario and McMaster Universities Osteoarthritis Index score, and Lequesne index for different treatment protocols. Compared with a control group, the longer duration time of every therapy (≥20 min) showed a significant improvement in the Visual analog scale score and Western Ontario and McMaster Universities Osteoarthritis Index scores. In comparison, the shorter duration time of every therapy (< 20 min) showed no significant difference. In addition, for different therapeutic periods, subgroup analyses indicated the shorter therapeutic periods (≤4 weeks) produced a more significant effect on reducing the Lequesne index. In contrast, more extended therapeutic periods (> 4 weeks) showed no significant difference, which could explain the source of the heterogeneity. Therefore, it is necessary to increase the number of randomized controlled trials to investigate the treatment period of LIPUS and the effectiveness of treating KOA symptoms.
Researchers have carried out studies on the potential mechanisms of LIPUS on KOA. For one thing, LIPUS can induce extracellular matrix synthesis, and the migration and proliferation of chondrocytes can also be increased.42, 43 For another thing, the synthesis of type II collagen in articular cartilage can be increased by LIPUS and shows the ability to slow down cartilage degeneration.44, 45 These data supported that LIPUS could improve KOA by inducing extracellular matrix synthesis and chondrocyte proliferation.
The present study has several limitations. (1) Only studies published in English and Chinese were considered in the present study, and consequently, we were unable to analyze further. (2) KOA is common in older women and is more common with advancing age. 46 Due to the differences in the effects of pain treatment and functional recovery among patients of different gender and age, more high-quality studies need to be carried out on more men or patients of different age groups.47, 48 (3) The severity of KOA was not consistent in the included population, so the effect of LIPUS treatment on pain relief and functional recovery may have been overestimated. (4) The parameters of the LIPUS device and intervention protocols differed in the included studies, contributing to heterogeneity. (5) The long-term efficacy of LIPUS treatment cannot be determined as there are only two weeks to 1 year of follow-up. Moreover, due to the lack of follow-up studies, it is unclear how long the therapeutic effect of LIPUS on KOA will last. (6) There may be publication bias in the included trials, as positive trials are more likely to be published than negative results. Furthermore, this study focuses only on pain relief and functional recovery in patients and needs further investigation for other metrics, such as changes in radiological parameters. (7) There is still a lack of data on the adverse effects of LIPUS on patients with KOA. (8) Only studies from countries and regions in Asia were included in our meta-analysis. However, different national customs and even religious practices can also affect the extent of the disease and the outcome of the patient's recovery.49, 50
In conclusion, current evidence suggests that LIPUS guides the short-term clinical use of KOA patients. However, In the future, new and high-quality randomized controlled trials should be conducted to determine the cost and safety of LIPUS for KOA, as well as the possible long-term side effects. When interpreting the results, evidence of lower or moderate improvement in outcomes should be considered, and sufficiently broad sample sizes and inadequate follow-up should be taken into account. At the same time, the study should have the selection criteria of LIPUS in favor of the type, intensity, frequency, and duration of KOA patients so that clinicians and patients can effectively implement these findings in the real world.
Clinical messages.
-
Low-intensity pulsed ultrasound could significantly ameliorate pain and promote functional recovery for patients with KOA.
Monotherapy of LIPUS produced a better effect on ameliorating pain, and the shorter therapeutic period (≤4 weeks) produced a more significant effect on promoting functional recovery for patients with KOA.
Appendix
Appendix 1
Search Strategies for Pubmed, Cochrane Library, Physiotherapy Evidence Database (PEDro), Web of Science, and China National Knowledge Infrastructure (CNKI)
We included papers published from their inception to 11 February 2022 in English and Chinese.
PubMed
1.”Osteoarthritis, Knee”[Mesh]
2.((((Knee Osteoarthritis[Title/Abstract]) OR (Knee Osteoarthritides[Title/Abstract])) OR (Osteoarthritis of Knee[Title/Abstract])) OR (Osteoarthritis of the Knee[Title/Abstract])) OR (Osteoarthritis, Knee[Title/Abstract])
3.1 OR 2
4.((((((((LIPUS[Title/Abstract]) OR (Low-Intensity Pulsed Ultrasound[Title/Abstract])) OR (Low Intensity Pulsed Ultrasound[Title/Abstract])) OR (Low-Intensity Pulsed Ultrasounds[Title/Abstract])) OR (Pulsed Ultrasound, Low-Intensity[Title/Abstract])) OR (Pulsed Ultrasounds, Low-Intensity[Title/Abstract])) OR (Ultrasound, Low-Intensity Pulsed[Title/Abstract])) OR (Ultrasounds, Low-Intensity Pulsed[Title/Abstract])) OR (Low Intensity Pulsed Ultrasound Radiation[Title/Abstract])
5.3 AND 4
the Cochrane Library
#1 MeSH descriptor: [Osteoarthritis, Knee] explode all trees
#2 (Osteoarthritis, Knee):ti,ab,kw OR (Knee Osteoarthritis):ti,ab,kw OR (Knee Osteoarthritides):ti,ab,kw OR (Osteoarthritis of Knee):ti,ab,kw OR (Osteoarthritis of the Knee):ti,ab,kw
#3 #1 OR #2
#4 (LIPUS):ti,ab,kw OR (Low-Intensity Pulsed Ultrasound):ti,ab,kw OR (Low Intensity Pulsed Ultrasound):ti,ab,kw OR (Low-Intensity Pulsed Ultrasounds):ti,ab,kw OR (Pulsed Ultrasound, Low-Intensity):ti,ab,kw OR (Pulsed Ultrasounds, Low-Intensity):ti,ab,kw OR (Ultrasound, Low-Intensity Pulsed):ti,ab,kw OR (Ultrasounds, Low-Intensity Pulsed):ti,ab,kw OR (Low Intensity Pulsed Ultrasound Radiation):ti,ab,kw
#5 #3 AND #4
Physiotherapy Evidence Database (PEDro)
Abstract & Title: Knee Osteoarthritides Title Only: “ Low-Intensity Pulsed Ultrasound *” Match any search term (OR)
Web of Science
1 ((((TS = (Osteoarthritis, Knee)) OR TS = (Knee Osteoarthritis)) OR TS = (Knee Osteoarthritides)) OR TS = (Osteoarthritis of Knee)) OR TS = (Osteoarthritis of the Knee)
2 ((((((((TS = (LIPUS)) OR TS = (Low-Intensity Pulsed Ultrasound)) OR TS = (Low Intensity Pulsed Ultrasound)) OR TS = (Low-Intensity Pulsed Ultrasounds)) OR TS = (Pulsed Ultrasound, Low-Intensity)) OR TS = (Pulsed Ultrasounds, Low-Intensity)) OR TS = (Ultrasound, Low-Intensity Pulsed)) OR TS = (Ultrasounds, Low-Intensity Pulsed)) OR TS = (Low Intensity Pulsed Ultrasound Radiation)
3 1 AND 2
China National Knowledge Infrastructure (CNKI)
1 SU % = 'Osteoarthritis, Knee' OR TKA = 'Knee Osteoarthritis’ OR TKA = 'Knee Osteoarthritides’ OR TKA = 'Osteoarthritis of Knee' OR TKA = 'Osteoarthritis of the Knee'
2 TKA = 'LIPUS’ OR TKA = 'Low-Intensity Pulsed Ultrasound' OR TKA = 'Low Intensity Pulsed Ultrasound' OR TKA = 'Low-Intensity Pulsed Ultrasounds’ OR TKA = 'Pulsed Ultrasound, Low-Intensity' OR TKA = 'Pulsed Ultrasounds, Low-Intensity' OR TKA = 'Ultrasound, Low-Intensity Pulsed' OR TKA = 'Ultrasounds, Low-Intensity Pulsed' OR TKA = 'Low Intensity Pulsed Ultrasound Radiation'
3 1 AND 2
Appendix 2
Sensitivity analysis table for Visual analog scale score
| Omitting | mean difference (MD) | 95% confidence interval (CI) | P value | Heterogeneity I2 |
|---|---|---|---|---|
| Huang(a)-2005 | -0.9342 | -1.4574; -0.4110 | 0.0005 | 87.8% |
| Huang(b)-2005 | -0.9348 | -1.4561; -0.4136 | 0.0004 | 87.8% |
| Tascioglu -2010 | -0.9141 | -1.4220; -0.4061 | 0.0004 | 87.8% |
| Li -2013 | -1.0109 | -1.5101; -0.5117 | < 0.0001 | 87.2% |
| Cakir -2014 | -1.0657 | -1.5400; -0.5914 | < 0.0001 | 86.4% |
| Yildiz -2015 | -0.9127 | -1.4138; -0.4116 | 0.0004 | 87.8% |
| Gao -2016 | -0.9979 | -1.5104; -0.4854 | 0.0001 | 85.9% |
| Jia -2016 | -0.9693 | -1.5066; -0.4319 | 0.0004 | 86.4% |
| Cheng -2019 | -0.8076 | -1.2153; -0.3999 | 0.0001 | 77.3% |
| Kim -2019 | -1.0337 | -1.5253; -0.5420 | < 0.0001 | 86.7% |
| Luo -2019 | -0.8627 | -1.3909; -0.3345 | 0.0014 | 85.2% |
| Karakas -2020 | -1.0013 | -1.4990; -0.5036 | <0.0001 | 87.4% |
| Pooled estimate | -0.9547 | -1.4339; -0.4755 | <0.0001 | 86.6% |
Sensitivity analysis table for Western Ontario and McMaster Universities Osteoarthritis Index scores
| Omitting | mean difference (MD) | 95% confidence interval (CI) | P value | Heterogeneity I2 |
|---|---|---|---|---|
| Tascioglu −2010 | −3.1271 | −7.8250; 1.5707 | 0.1920 | 59.3% |
| Loyola −2012 | −5.1209 | −9.0205; −1.2214 | 0.0101 | 56.5% |
| Gao −2016 | −3.0351 | −7.4206; 1.3504 | 0.1750 | 53.4% |
| Jia −2016 | −3.4329 | −9.3539; 2.4881 | 0.2558 | 65.1% |
| Kim −2019 | −5.7568 | −9.1161; −2.3974 | 0.0008 | 39.8% |
| Karakas −2020 | −4.5866 | −9.1615; −0.0117 | 0.0494 | 63.1% |
| Pooled estimate | −4.3474 | −8.2954; −0.3993 | 0.0309 | 56.5% |
Appendix 3
Methodological quality assessment for studies by Jadad score
| First author | Randomization | Double blinding | Withdrawals and dropouts | Score |
|---|---|---|---|---|
| Li. | 2 | 1 | 1 | 4 |
| Luo | 2 | 1 | 1 | 4 |
| Chen | 2 | 1 | 1 | 4 |
| Gao | 2 | 1 | 1 | 4 |
| Karakas. | 2 | 2 | 0 | 4 |
| Jia | 2 | 2 | 1 | 5 |
| Kim | 2 | 1 | 1 | 4 |
| Loyoia | 2 | 2 | 1 | 5 |
| Yildiz | 2 | 2 | 1 | 5 |
| Tascioglu. | 2 | 2 | 1 | 5 |
| Cakir. | 2 | 2 | 0 | 4 |
| Huang (a) | 2 | 2 | 1 | 5 |
| Huang (b)() | 2 | 2 | 1 | 5 |
Footnotes
Author contributions: MLS, XAZ contributed to the study concept and design. HQC, ZW, and MLS conducted the literature review and statistical analysis. All authors contributed to the interpretation of data. HQC and MLS contributed to drafting the paper. All authors revised the text for intellectual content and have read and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the The Scientific research funding project of the Department of Education of Liaoning Province, (grant number LJKZ1048).
ORCID iD: Mingli Sun https://orcid.org/0000-0001-5007-4241
References
- 1.Huang KH, Hsieh RL, Lee WC. Pain, physical function, and health in patients with knee osteoarthritis. Rehabil Nurs 2017; 42: 235–241. [DOI] [PubMed] [Google Scholar]
- 2.Doshi R, Ostrovsky D. Glucosamine may be effective in treating pain due to knee osteoarthritis. Explore (NY) 2019; 15: 317–319. [DOI] [PubMed] [Google Scholar]
- 3.Uebelhart D, Knols R, de Bruin ED, et al. Treatment of knee osteoarthritis with oral chondroitin sulfate. Adv Pharmacol 2006; 53: 523–539. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Zhao L, Yu C, et al. Clinical therapeutic effect and safety of celecoxib in treating knee osteoarthritis. Pak J Pharm Sci 2018; 31): 1629–1632.30203750 [PubMed] [Google Scholar]
- 5.Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage 2005; 13: 20–27. [DOI] [PubMed] [Google Scholar]
- 6.Vincent KR, Vasilopoulos T, Montero C, et al. Eccentric and concentric resistance exercise comparison for knee osteoarthritis. Med Sci Sports Exerc 2019; 51: 1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devrimsel G, Metin Y, Serdaroglu Beyazal M. Short-term effects of neuromuscular electrical stimulation and ultrasound therapies on muscle architecture and functional capacity in knee osteoarthritis: a randomized study. Clin Rehabil 2019; 33: 418–427. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Wang Y, Shi X, et al. Effect of ultrasound-guided acupotomy vs electro-acupuncture on knee osteoarthritis: a randomized controlled study. J Tradit Chin Med 2016; 36: 450–455. [PubMed] [Google Scholar]
- 9.Kheshie AR, Alayat MS, Ali MM. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: a randomized controlled trial. Lasers Med Sci 2014; 29: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 10.Kul'chitskaya DB, Konchugova TV, Luk'yanova TV, et al. The substantiation for the application of high-intensity laser therapy for the treatment of the patients presenting with gonarthrosis. Vopr Kurortol Fizioter Lech Fiz Kult 2015; 92: 23–26. [DOI] [PubMed] [Google Scholar]
- 11.Qi L, Tang Y, You Y, et al. Comparing the effectiveness of electroacupuncture with different grades of knee osteoarthritis: a prospective study. Cell Physiol Biochem 2016; 39: 2331–2340. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Grande EI, Osma-Rueda JL, Serrano-Villar Y, et al. Effects of pulsed therapeutic ultrasound on the treatment of people with knee osteoarthritis. J Phys Ther Sci 2017; 29: 1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin SMZ, Komatsu DE. Therapeutic potential low-intensity pulsed ultrasound for osteoarthritis: pre-clinical and clinical perspectives. Ultrasound Med Biol 2020; 46: 909–920. [DOI] [PubMed] [Google Scholar]
- 14.Zhou XY, Zhang XX, Yu GY, et al. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: a meta-analysis of randomized clinical trials. Biomed Res Int 2018; 2018: 7469197.30105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Zhu S, Lv Z, et al. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clin Rehabil 2019; 33: 1863–1875. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021; 372: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J 2020 Jun; 37(6): 387. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Br Med J 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The R Project for Statistical Computing. http://www.r-project.org/ (accessed 4 February 2022)
- 21.R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria 2019, https://mran.microsoft.com/ (2019, accessed 4 February 2022)
- 22.Shim SR, Kim SJ. Intervention meta-analysis: application and practice using R software. Epidemiol Health 2019; 41: e2019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Medical Biometry and Statistic. R packages. https://www.imbi.uni-freiburg.de/lehre/lehrbuechecher/meta-analysis-with-r/r-packages (accessed 4 February 2022)
- 24.Microsoft R Application Network. https://mran.microsoft.com/packages (accessed 19 February 2022)
- 25.Julian Higgins JT, Chandler J, Cumpston M, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 2022. www.training.cochrane.org/handbook (2022, accessed 9 February 2022)
- 26.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. Br Med J 2011; 342: d549. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test Br Med J 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang MH, Yang RC, Lee CL, et al. Preliminary results of integrated therapy for patients with knee osteoarthritis. Arthritis Rheum 2005; 53: 812–820. [DOI] [PubMed] [Google Scholar]
- 30.Huang MH, Lin YS, Lee CL, et al. Use of ultrasound to increase effectiveness of isokinetic exercise for knee osteoarthritis. Arch Phys Med Rehabil 2005; 86: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 31.Tascioglu F, Kuzgun S, Armagan O, et al. Short-term effectiveness of ultrasound therapy in knee osteoarthritis. J Int Med Res 2010; 38: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 32.Loyola-Sánchez A, Richardson J, Beattie KA, et al. Effect of low-intensity pulsed ultrasound on the cartilage repair in people with mild to moderate knee osteoarthritis: a double-blinded, randomized, placebo-controlled pilot study. Arch Phys Med Rehabil 2012; 93: 35–42. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Chen SR. Clinical therapeutic effect of low-intensity pulsed ultrasound, intra-articular injection of sodium hyaluronate and these two methods combined on the mild and moderate knee osteoartheitis. Master Thesis, ChongQing Medical University, China, 2013.
- 34.Cakir S, Hepguler S, Ozturk C, et al. Efficacy of therapeutic ultrasound for the management of knee osteoarthritis: a randomized, controlled, and double-blind study. Am J Phys Med Rehabil 2014; 93: 405–412. [DOI] [PubMed] [Google Scholar]
- 35.Yildiz SK, Özkan F, Aktaş I, et al. The effectiveness of ultrasound treatment for the management of knee osteoarthritis: a randomized, placebo-controlled, double-blind study. Turk J Med Sci 2015; 45: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 36.Gao MX, Lin Q, Chen AL, et al. Clinical effectiveness of low intensity pulsed ultrasound with drugs therapy for the treatment of traumatic knee osteoarthritis. Chin J Rehabil Med 2016; 31: 862–867. [Google Scholar]
- 37.Jia L, Wang Y, Chen J, et al. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci Rep 2016; 6: 35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y, Xu Y, Wang F. Study on the low-intensity pulsed ultrasound for relieving knee osteoarthritis pain and repairing articular cartilage injury. Chin Mod Doctor 2019; 57: 32–35. [Google Scholar]
- 39.Luo XW, Li MX. Low-intensity pulsed ultrasound can alleviate knee osteoarthritis pain and promote articular cartilage repair. J Clin Rehabil Tis Eng Res 2019; 23: 348–353. [Google Scholar]
- 40.Kim ED, Won YH, Park SH, et al. Efficacy and safety of a stimulator using low-intensity pulsed ultrasound combined with transcutaneous electrical nerve stimulation in patients with painful knee osteoarthritis. Pain Res Manag 2019; 2019: 7964897.31316682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakaş A, Dilek B, Şahin MA, et al. The effectiveness of pulsed ultrasound treatment on pain, function, synovial sac thickness and femoral cartilage thickness in patients with knee osteoarthritis: a randomized, double-blind clinical, controlled study. Clin Rehabil 2020; 34: 1474–1484. [DOI] [PubMed] [Google Scholar]
- 42.Jang KW, Ding L, Seol D, et al. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med Biol 2014; 40: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uddin SM, Richbourgh B, Ding Y, et al. Chondro-protective effects of low intensity pulsed ultrasound. Osteoarthritis Cartilage 2016; 24: 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurkan I, Ranganathan A, Yang X, et al. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthritis Cartilage 2010; 18: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naito K, Watari T, Muta T, et al. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res 2010; 28: 361–369. [DOI] [PubMed] [Google Scholar]
- 46.Tang X, Wang S, Zhan S, et al. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthritis Rheumatol 2016; 68: 648–653. [DOI] [PubMed] [Google Scholar]
- 47.Denegar CR, Schimizzi ME, Dougherty DR, et al. Responses to superficial heating and cooling differ in men and women with knee osteoarthritis. Physiother Theory Pract 2012; 28: 198–205. [DOI] [PubMed] [Google Scholar]
- 48.Paradowski PT, Bergman S, Sundén-Lundius A, et al. Knee complaints vary with age and gender in the adult population. Population-based reference data for the knee injury and osteoarthritis outcome score (KOOS). BMC Musculoskelet Disord 2006; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Hunter DJ, Nevitt MC, et al. Association of squatting with increased prevalence of radiographic tibiofemoral knee osteoarthritis: the Beijing osteoarthritis study. Arthritis Rheum 2004; 50: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 50.Chokkhanchitchai S, Tangarunsanti T, Jaovisidha S, et al. The effect of religious practice on the prevalence of knee osteoarthritis. Clin Rheumatol 2010; 29: 39–44. [DOI] [PubMed] [Google Scholar]