Abstract
Objectives
Bedside lung ultrasound has been indispensable during the coronavirus disease 2019 (COVID-19) pandemic, allowing us to rapidly assess critically unwell patients. We demonstrate the unique application of contrast-enhanced ultrasound with the aim of further understanding this disease.
Methods
Patient demographics were recorded alongside recent cross-sectional imaging and inflammatory markers. Ultrasound was conducted by experienced operators in a portable setting. Conventional six-point lung ultrasound method was used to evaluate B-lines, small (subpleural) consolidation and the pleura. Areas of small consolidation were targeted after intravenous administration of ultrasound contrast.
Results
The areas of small consolidations, a potential sign of pneumonia on B-mode lung ultrasound, usually enhance on contrast-enhanced ultrasound. Our study revealed these areas to be avascular, indicating an underlying thrombotic/infarction process. Findings were present in 100% of the patients we examined. We have also shown that the degree of infarction correlates with CT severity (r = 0.4) and inflammatory markers, and that these areas improve as patients recover.
Conclusions
We confirmed the theory of immune thrombus by identifying the presence of microthrombi in the lungs of 100% of our patients, despite 79% having had a recent negative CT pulmonary angiogram study. contrast-enhanced ultrasound can be utilised to add confidence to an uncertain COVID-19 diagnosis and for prognosticating and monitoring progress in confirmed COVID-19 patients. Contrast-enhanced ultrasound is clearly very different to CT, the gold standard, and while there are specific pathologies that can only be detected on CT, contrast-enhanced ultrasound has many advantages, most notability the ability to pick up microthrombi at the periphery of the lungs.
Keywords: Coronavirus, consolidation, microthrombi, computed tomography, bedside ultrasound, critical care, microbubble
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has resulted in a global pandemic and unparalleled strain on healthcare systems worldwide. Reverse‐transcriptase polymerase chain reaction (RT-PCR) is currently the gold standard for diagnosis, but it has variable detection rates and its sensitivity is estimated to reach as low as 38%. 1 Predicting the sensitivity and specificity of such tests, and therefore reliably diagnosing COVID-19, has presented a challenge due to the prevalence of asymptomatic carriers along with mild, moderate, severe and critically ill stages of disease, all of which have still not been fully characterised.
After a positive diagnosis, assessing the severity of disease is key in order to tailor management and predict outcomes for each patient. There have been a number of articles published that have correlated specific biochemical markers with disease severity, but there has been limited confirmation.2–5 Currently, lymphopenia is recognised as a reliable prognosticator for developing severe COVID-19. 3 It is also widely accepted that increasing age, high serum lactate dehydrogenase (LDH), C-reactive protein (CRP) and D-dimer, as well as low albumin are tied to a poorer outcome.4,5 There have also been attempts to use imaging to prognosticate. 6 Computed tomography (CT), chest radiographs (CXR) and lung ultrasound (LUS) findings have been retrospectively analysed and scoring systems created to try to understand patterns that can allow us to predict outcomes and better manage patients.
It is now well recognised that COVID-19 predisposes an individual to a thrombotic state, with reports of high numbers of pulmonary emboli (PE) and deep vein thrombosis (DVT), (compounded by D-dimer levels of >1 μg · mL−1 identified as a risk factor for poor outcome 5 ) The number of patients with a diagnosis of venous thromboembolism (VTE) is higher than what would normally be expected in the general population, even taking into account long intensive care (ICU) stays. 5 While PE and DVT have been reported in other viral pneumonias, the rate is higher still in COVID-19. 5 In COVID-19 patients requiring ICU support, cumulative incidence of VTE has been reported to be as high as 59%, despite thrombosis prophylaxis (the normal incidence of VTE in ICU varies hugely from study to study but it is predicted to be anything from 22% to 80%).7,8 Rates of VTE, both in patients with and without COVID-19, are likely to be underestimated, as many ICU patients are too unstable to have diagnostic imaging and the signs and symptoms of PE may be missed as they mimic many other conditions. Another reason VTE may be missed in COVID-19 is because of the unique disease process that predisposes patients to microthrombi, which may go undetected as resolution limitations of conventional CT techniques does not allow detection of microthrombi.
To date, only DVT and PE (of which some are likely to develop as a consequence of the widespread microthrombotic disease) have been identified on imaging. However, studies by pathologists indicate that all patients are developing thrombosis at the microvascular level. Post-mortem studies have found that up to 100% of patients have thrombotic features in at least one major organ at autopsy, predominantly in the lung (89% of patients).9,10 These figures are likely to be an underrepresentation due to the limitations of post-mortem investigations; only a small portion of each organ is analysed. The presence of thrombosis (both micro and macro) at autopsy is one of the most consistent features of COVID-19 infection, second only to diffuse alveolar damage. 9 Autopsies in patients with acute respiratory distress syndrome (ARDS) of various aetiologies demonstrated that only 24% of patients had thrombi within the lung, indicating that this phenomenon is unique to the COVID-19 disease state rather than ARDS itself. 9
The underlying pathogenesis of this severe systemic response is felt to be due to a process that mimics a cytokine storm/macrophage activation syndrome (MAS) and thus creates an immune-mediated thrombotic state. 11 MAS is most commonly seen in the context of rheumatic diseases as a potentially fatal complication caused by widespread systemic hyper-inflammation. Laboratory abnormalities typically seen include a decrease in white blood cells, platelets and haemoglobin, and a concomitant marked increase in ferritin, with evidence of intravascular coagulation activation. 12 These are all features seen in COVID-19 infection, although SARS-CoV-2 has 100% pulmonary involvement whereas MAS is typically a multi-organ disease process involving the lungs in only 50% of cases. 11 It is this MAS-like picture that ultimately interferes with normal haemostasis, activating the coagulation cascade and causing vasculopathy, ventilation perfusion mismatch and refractory ARDS.
The use of point-of-care LUS has increased dramatically during the COVID-19 pandemic due to its ease of use, low cost, reproducibility and the ability to perform it by the bedside of unstable patients. LUS is already established as an effective way to diagnose pneumonia, with a diagnostic accuracy that approaches CT chest (the gold standard). 13 The characteristic finding of pneumonia on B-mode ultrasound is B-lines and small consolidation (also known as subpleural consolidation), and these have been characterised further by Lichtenstein in the BLUE-protocol which allows for the examination of most acute respiratory disorders. 14
Contrast-enhanced ultrasound (CEUS) is a technique that involves the intravenous introduction of microbubbles consisting of a phospholipid shell surrounding a perfluorocarbon gas (sulphur hexafluoride) the approximate size of red blood cells that cross the capillary bed with transpulmonary stability. The result is a truly intravascular contrast agent, which can be detected using contrast-specific modes on ultrasound. As a result, areas without contrast can be identified as being avascular. The addition of contrast during a LUS study is expected to demonstrate a short wash-in period and then intense enhancement of the areas of consolidation, 15 thereby confidently diagnosing pneumonia in real time. There are studies proposing the replacement of CXR with LUS in the acute setting, and with increased use and understanding, this may also reduce the need for CT.16,17 We have previously demonstrated that in COVID-19 pneumonia small consolidation is avascular (i.e. infarction rather than infective consolidation),18,19 and this diagnostic study has allowed us to compare LUS with other imaging techniques and markers of COVID-19.
Methods
This study was a retrospective assessment of the data collected during the course of routine LUS examinations and other imaging procedures performed on patients with COVID-19, admitted to the intensive care or high dependency unit (HDU). All imaging was part of routine patient care, with the addition of CEUS which is normal practice in our hospital, as a point-of-care problem solving tool. This study was not defined as a research study according to the National Health Service (NHS) ‘defining research' decision tool, and therefore in the United Kingdom, there was no requirement for a submission to be made for ethics committee approval.
Patient selection
Patient demographics were recorded alongside recent imaging (CXR, LUS and CT) and inflammatory markers (CRP, ferritin, D-dimer, troponin and lymphocytes). A total of nine patients were in ICU, four patients were in HDU and one patient was on a medical ward.
Ultrasound technique
Conventional B-mode imaging was undertaken using either Siemens Redwood™ (Siemens Acuson, Mountain View, CA) or GE Logiq E9™ (GE Healthcare, Milwaukee, WI) with a curvilinear transducer (5C1 or C1-6, respectively) in a portable setting. Conventional six-point sonography of the lungs was conducted. 14 Each zone was evaluated for the presence of pleural thickening and irregularity. B-lines were assessed and counted, and hypoechoic areas of consolidation were also evaluated.
Following this, the largest area of consolidation was targeted with CEUS, followed by subsequent examination of all zones. Once a target area for CEUS was selected, a split-screen mode was initiated to allow a simultaneous B-mode and contrast-specific image. Low mechanical index imaging was used, <0.2. CEUS was performed with 2.4 mL Sonovue/Lumason™ (Bracco SpA, Milan) via a venous line with cine clips and still images obtained. The initial area targeted was closely inspected for early arterial flow in the lesion within the first 30 seconds to allow visualisation of arterial phase enhancement. The contrast agent persisted for several minutes, allowing re-imaging of all areas in the late phase. Follow-up imaging was conducted where possible.
Two radiologists and two clinicians with a special interest in LUS were involved in the practical scanning and all other aspects of this study. Additional radiologists were involved with the data analysis and writing. All operators were experienced with ultrasound with specific focused training in CEUS by an operator with 10 years’ experience.
Ultrasound analysis
All images were reviewed live and retrospectively by an experienced radiologist. B-lines were assessed and categorized as none, < 3, ≥3 or confluent. Hypoechoic areas of small consolidation were a key finding defined as none, focal (<2 per single ultrasound field) or multiple (≥2).
Each of the six zones was then scored using the system detailed in Table 1 (maximum score 30). All patients had pleural irregularity and pleural thickening, and therefore these findings were not included in the LUS score. The values were then documented as mild (0–10), moderate (11–20) or severe (21–30).
Table 1.
The six zones of the lung were assessed using ultrasound and a score was given for each area depending on the findings as listed above.
| B-lines | Sub pleural consolidation |
|---|---|
| Confluent 3 | Multiple (≥2) 2 |
| ≥3 2 | Focal (<2) 1 |
| <3 1 | None 0 |
| None 0 |
The absence of enhancement of small consolidation was documented as avascular, and any contrast enhancement was documented by comparing the area of small consolidation to surrounding structures, i.e. hypo or hyper enhancement. The dominant lesion enhancement was described and was used as the index lesion. Other examined lesion characteristics were noted if different from the index lesion. The addition of CEUS was not included in the LUS score but deployed to further characterise the small consolidation.
CT and X-ray analysis
The most contemporaneous CT chest (preferably a CT pulmonary angiogram (PA) within the last 24–48 hours) was then evaluated using a system adapted from the British Thoracic Imaging Society (BTS) guidelines. 20 CT scores were calculated by estimating the percentage of abnormal lung in each lobe and then calculating an average of all lobes. The final percentage was then rounded to the nearest 10%. The values were then documented as mild (0–30%), moderate (40–60%) or severe (70–100%). Abnormal lung features included ground glass opacification, consolidation and interstitial abnormality.
Typical CXR appearances of COVID-19 have been relatively well described, with classical features including widespread bilateral peripheral airspace opacification.21,22 An atypical CXR might include unilateral dense focal consolidation or pleural effusion. Using this, the most recent CXR was evaluated and recorded as either typical, atypical or normal.
Statistical analysis
The Pearson correlation coefficient is a mathematical measure of the linear correlation between two sets of data. We used this equation to assess the relationship between LUS and the other parameters, where r = –1 is a perfect negative correlation, r = 1 is a perfect positive correlation and 0 is no correlation.
Results
A total of 14 patients with COVID-19 (confirmed with positive RT-PCR) were examined (eight male, average age 63 years). The patients had varying disease severity; one patient was ambulant and requiring minimal oxygen, four patients were on CPAP and nine were intubated and ventilated (and two of these were on CPAP the first time we assessed them).
All 14 patients demonstrated the presence of B-lines and small consolidation on regular B-mode ultrasound imaging. One (7.1%) had a pleural effusion and all patients had pleural thickening and irregularity. The addition of CEUS revealed avascularity in the areas of small consolidation in all 14 (100%) patients despite only three (21%) having a contemporaneous positive CT PA.
A total of 16 (80%) of the ultrasounds performed (including follow-ups) had contemporaneous CT imaging for direct comparison (see online Appendix 1) and 11 of the 16 (69%) revealed ‘severe’ LUS findings; seven (64%) of these also had ‘severe’ CT findings and the other four (36%) had ‘moderate’ CT findings. The remaining five LUS studies demonstrated ‘moderate’ findings; three (60%) had ‘severe’ CT findings and two (40%) had ‘mild’ CT findings.
Using the correlation coefficient, we found a positive linear relationship between LUS scores and CT scores (r = 0.4) (Table 2). When comparing LUS scores with inflammatory markers, we found that the best relationship was with D-Dimer which showed a positive linear relationship (r = 0.4). Troponin had a weaker positive linear relationship (r = 0.3) as did CRP (r = 0.2) and ferritin (r = 0.1). Lymphocytes had a negative (r = –0.2) linear relationship (lymphopenia is associated with a poorer outcome).
Table 2.
R values demonstrate correlation between LUS, CT and inflammatory markers.
| CT score | CRP | Ferritin | D-Dimer | Lymphocyte count | Troponin | |
|---|---|---|---|---|---|---|
| LUS score | 0.4 | 0.2 | 0.1 | 0.4 | –0.2 | 0.3 |
All three patients who had a positive CT PA also had ‘severe’ LUS findings; however, when using the CT scoring, only one had a ‘severe’ CT and two had ‘moderate’ changes. In 13/16 cases (81%), the LUS score was either the same as the CT score or higher.
A ‘positive’ D-dimer is widely accepted to be a value above 500 μg · L−1 (plus 100 μg · L−1 for each decade above age 50). Using this standard, all of our patients had a raised D-dimer except one (who had a D-dimer of 593 μg · L−1 but at age 75 years an upper limit of 700 μg · L−1) who still had microthrombi on CEUS evaluation.
Of the 14 patients, five (36%) were followed up with a repeat ultrasound scan. Follow-up cases showed progressive findings in one (Figure 1), improvement in three (60%) and stable appearances in one (20%). Two out of five (40%) showed resolution of small consolidation but persistent B-lines. In one case, minimal enhancement was seen in a lesion in recovery phase (Figure 2).
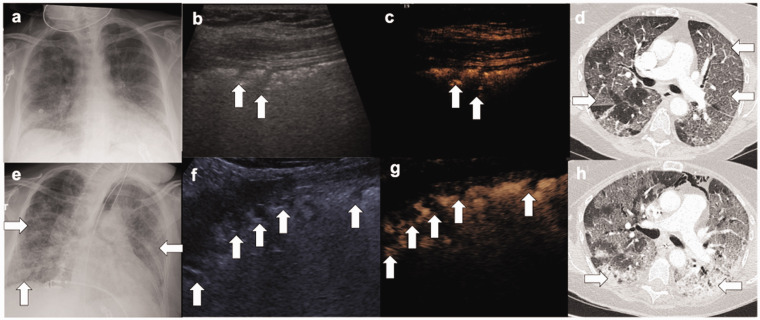
Figure 1.
(a) Chest radiograph show no dense consolidation or pleural effusion, (b, c) Simultaneous B-mode and CEUS show multiple hypoechoic areas (arrows) with no enhancement, (d) contemporaneous CTPA show typical features of severe COVID-19 with peripheral ground glass opacity (arrows). There was no pulmonary embolism. (e) Follow-up chest radiograph demonstrates bilateral, peripheral airspace opacification (arrows). (f, g) Simultaneous ultrasound performed show an increased number of hypoechoic areas throughout all six zones (arrows), all of which showed no enhancement. (h) Contemporaneous CTPA show progression of disease, with areas of dense consolidation (arrows) in addition to ground glass opacification. There was no pulmonary embolism.
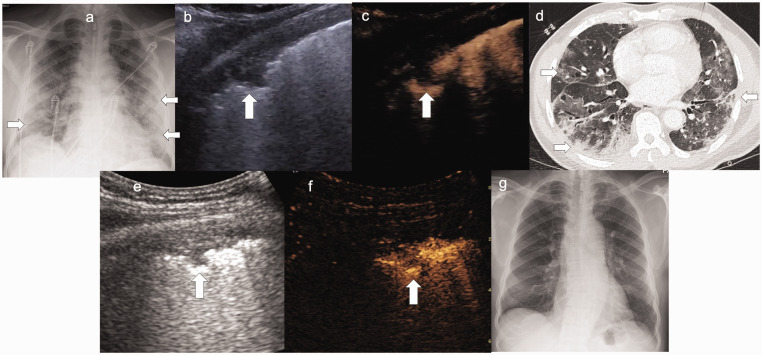
Figure 2.
(a) Chest radiograph shows typical features of COVID-19 with bilateral peripheral airspace opacification (arrows). (b, c) Simultaneous B-mode and CEUS demonstrates a hypoechoic area (arrow) with no enhancement, and simultaneous CT (d) demonstrated findings of severe disease. (e, f) B-mode and CEUS follow-up after clinical improvement showed resolution of previous hypoechoic areas. One area had retracted with delayed hypoenhancement, signal was seen from individual microbubbles in the capillary bed implying reperfusion (arrow). (g) Follow-up chest radiograph several weeks later shows total resolution of COVID-19 infection.
While statistically there was not a perfect correlation between LUS scoring, CT scoring and inflammatory markers, there was a strong relationship between our findings and clinical progression (online Appendix 2). Therefore, while individual figures alone may not be diagnostic, following trends of LUS scores (alongside inflammatory markers) could be a useful prognosticator. In all of the four patients scanned twice, an increase in LUS score (i.e. worsening appearances of their lung parenchyma and clot burden) coincided with an increase in ferritin, D-dimer, troponin and CRP and a decrease in lymphocytes. One patient was scanned three times, with an increase in lung ultrasound score each time which matched a subsequent clinical deterioration and increasing respiratory support from CPAP to mechanical ventilation (online Appendix 2).
Discussion
LUS has come to the forefront during the COVID-19 pandemic and has been proposed as a tool for immediate assessment and monitoring of disease progression. This has proved of particular importance given the vast numbers of cases seen, especially within the economically less developed world. Ultrasound is an inexpensive bedside imaging modality and allows for rapid assessment without the logistical difficulties of other imaging modalities. Historically, there have been concerns over the user dependant nature of LUS and the limited views that can be obtained (LUS can only assess lung peripheries); however, our experience is that it is a teachable skill and provides excellent clinical information.
To date, existing research into LUS findings in COVID-19 has lacked specificity and typical features previously seen in viral and bacterial pneumonias have been described. The addition of contrast to LUS has allowed us to more confidently identify COVID-19 by the bedside (along with an appropriate clinical history and inflammatory markers). The application of a scoring system then allowed for direct comparison with CT, the current gold standard. Our results did show correlation between LUS findings and CT scores, but the correlation between our follow-up imaging and inflammatory markers was even more striking. Therefore, while our dataset does not allow us to independently predict outcomes, it can be used to monitor progress alongside trends in blood results, which could subsequently reduce the need for CT.
Pathologists have already identified the presence of microthrombi during autopsy, and for the first time, we have been able to demonstrate this during the acute phase of the illness. Like pathologists, who found microthrombi in 100% of COVID-19 patients at autopsy, we identified the presence of microthrombi in the lungs of 100% of our patients, despite 79% having had a recent negative CTPA study which confirms the theory of immune thrombus. We have also shown for the first time that these areas of microthrombi resolve as patients recover; two patients, who had previously had multiple areas of avascular small consolidation, had no small consolidation on repeat ultrasound performed after improving clinically. Secondly, one patient had areas of minimally enhancing small consolidation on follow-up study which we have ascribed to a resolving infarct. These three patients show that these avascular lesions can resolve, and that this happens in line with clinical and biochemical improvement.
This is not the first time that areas of infarction in the lung parenchyma have been identified. Caremani et al. have previously used CEUS to distinguish pneumonia (viral or bacterial) from other pathologies, such as neoplasms and avascular lesions (infarction, cysts or foci of necrosis) by assessing the enhancement patterns of the areas of consolidation. 23 Most lung pathology is enhancing and they demonstrated that they could successfully identify pneumonia by its uniform enhancement pattern. Complete absence of vascularity in small consolidation is unusual and not often seen; therefore, it is generally considered to be a feature of pulmonary embolism or microthrombi. 24
As described in our results, we found a positive correlation (r = 0.4) between LUS score and D-dimer results. There are currently no guidelines for the interpretation of D-dimer levels in the context of COVID-19 infection, but the European Society of Cardiology (ESC) recommends performing a CTPA when unenhanced CT findings cannot explain the severity of respiratory failure. 25 Two out of the three patients from our study who had a positive PE identified on CTPA had very high D-dimer levels (above 10,000 μg · mL−1) demonstrating that there is likely to be a correlation between burden of thrombus and D-dimer results. However, unlike during routine practice when an age adjusted D-dimer is used to confidently exclude VTE, that is not possible with COVID-19 infection.
Screening and diagnosing COVID-19 has proven challenging, and the current gold standard RT-PCR method has poor sensitivity and a high false-negative rate. As a result, patients with a high clinical suspicion of COVID-19 who repeatedly test negative on RT-PCR present a logistical challenge in already stretched hospitals due to isolation requirements. In China, there have been attempts to overcome the problems with RT-PCR tests by using CT as a first-line investigation. Ai et al. reported a CT sensitivity of 97% when compared with RT-PCR from their Wuhan cohort; 26 however, false-negative CT rates are also documented in the literature and findings, although sensitive, lack the specificity required for screening. 27 Furthermore, there is inherent logistical limitation transferring a large number of potentially infected patients to a static CT scanner, difficulty allocating patients to ‘infected’ or ‘clean areas’ and increased workloads and costs for radiology departments.
Currently, because of the aforementioned problems with testing, even without a positive result on RT-PCR, a patient is treated as ‘positive’ if their clinical assessment, radiological appearances and blood tests fit with a COVID-19-like picture. CEUS could be utilised to increase diagnostic certainty, alongside other LUS protocols being proposed such as the one by Volpicelli et al. 28 CEUS can be performed at the bedside in seriously unwell and unstable patients, and it can also be performed in an outpatient setting. This allows a wide range of healthcare professionals to perform this examination. LUS is straightforward and already routinely used by clinicians in ICU and the addition of CEUS requires limited experience. The rate of adverse events has been estimated to be 0.0086% 29 and lacks the risk of iodine induced nephrotoxicity. 30 LUS is rapid, reproducible and allows for continued assessment of all areas of the lung periphery (whereas post-mortem only samples a limited tissue and CT only shows a snapshot in time and lacks the resolution for viewing microthrombi).
We recognise that there are limitations to our research in that 14 is a small study size and only five patients were followed up. In addition, we are comparing our results to CT, a well-established gold standard. We also did not assess any patients with asymptomatic disease due to nature of the patients in hospital at the time.
Conclusion
We have demonstrated that CEUS can be used to add confidence to an uncertain COVID-19 diagnosis and also has potential for prognosticating and monitoring progress. LUS is an established bedside tool used daily in ICU settings, 31 and with basic training, CEUS becomes a highly useful tool that can be utilised in diagnosing and managing this disease. CEUS is clearly very different to CT, the gold standard, and while there are specific pathologies that can only be detected on CT, CEUS has many advantages, most notability the ability to pick up microthrombi at the periphery of the lungs, the hallmark of this disease.
Supplemental Material
Supplemental material, sj-pdf-1-ult-10.1177_1742271X211047945 for Point-of-care contrast enhanced lung ultrasound and COVID-19 by Alice Tee, Gibran Timothy Yusuf, Adrian Wong, Deepak Rao, Sa Tran and Paul S Sidhu in Ultrasound
Acknowledgments
N/A.
Ethics Approval: Patients consented for ultrasound.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor: AT.
Contributorship: AT: Manuscript preparation, analysis of data, data collection. GTY: Manuscript preparation, analysis of data, data collection, development of concept. AW: Manuscript review and preparation. DR: Manuscript review and preparation. ST: Manuscript review and preparation, analysis of data. PSS: Analysis of data, review of manuscript with final approval.
ORCID iD: Alice Tee https://orcid.org/0000-0003-2842-1280
Supplemental material: Supplemental material for this article is available online.
References
- 1.Dramé M, Tabue Teguo M, Proye E, et al. Should RT-PCR be considered a gold standard in the diagnosis of COVID-19? J Med Virol 2020 Nov; 92(11): 2312–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velavan TP andMeyer CG.. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 2020; 95: 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 2020; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis 2020; 71: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 2020; 56: 2001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli G andGargani L.. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J 2020; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18: 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha AT andTapson VF.. Venous thromboembolism in intensive care patients. Clin Chest Med 2003; 24: 103–122. [DOI] [PubMed] [Google Scholar]
- 9.Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 2020; 18: 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGonagle D, O’Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020; 2: e437–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bracaglia C Prencipe G andDe Benedetti F.. Macrophage activation syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J 2017; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reissig A, Copetti R, Mathis G, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest 2012; 142: 965–972. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care 2014; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo PH, Copetti R, Feller-Kopman D, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med 2019; 45: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 16.Haggag YI, Mashhour K, Ahmed K, et al. Effectiveness of lung ultrasound in comparison with chest X-ray in diagnosis of lung consolidation. Open access Maced J Med Sci 2019; 7: 2457–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amatya Y, Rupp J, Russell FM, et al. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med 2018; 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tee A, Wong A, Yusuf GT, et al. Contrast-enhanced ultrasound (CEUS) of the lung reveals multiple areas of microthrombi in a COVID-19 patient. Intensive Care Med 2020; 46: 1660–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf GT, Wong A, Rao D, et al. The use of contrast-enhanced ultrasound in COVID-19 lung imaging. J Ultrasound 2020; 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BSTI: CT reporting proforma: COVID-19. Version 2 13.04.2020. Accessed from: https://www.bsti.org.uk/media/resources/files/BSTI_COVID_CT_Proforma_v2_13.04.2020.pdf
- 21.Cleverley J Piper J andJones MM.. The role of chest radiography in confirming covid-19 pneumonia. BMJ 2020; 370: m2426. [DOI] [PubMed] [Google Scholar]
- 22.Kong W andAgarwal PP.. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging 2020; 2: e200028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caremani M, Benci A, Lapini L, et al. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: a study of 60 cases. J Ultrasound 2008; 11: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich CF. Contrast-enhanced endobronchial ultrasound: potential value of a new method. Endosc Ultrasound 2017; 6: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The European Society for Cardiology. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Belgium: Author, 2020. [Google Scholar]
- 26.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296: E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavare AN, Braddy A, Brill S, et al. Managing high clinical suspicion COVID-19 inpatients with negative RT-PCR: a pragmatic and limited role for thoracic CT. Thorax 2020; 75: 537–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med 2021; 47: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piscaglia F andBolondi L.. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006; 32: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 30.Hu C, Feng Y, Huang P, et al. Adverse reactions after the use of SonoVue contrast agent: characteristics and nursing care experience. Medicine (Baltimore) 2019; 98: e17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouhemad B, Zhang M, Lu Q, Rouby J-J. Clinical review: bedside lung ultrasound in critical care practice. Crit Care 2007; 11: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ult-10.1177_1742271X211047945 for Point-of-care contrast enhanced lung ultrasound and COVID-19 by Alice Tee, Gibran Timothy Yusuf, Adrian Wong, Deepak Rao, Sa Tran and Paul S Sidhu in Ultrasound