Abstract
Objective
The aim of the study was to evaluate the diagnostic potential of placental shear wave elastography in predicting preeclampsia at 16 to 20 weeks of gestation.
Materials and methods
A total of 230 pregnant women between 16 and 20 weeks of gestation were observed for the study. These women underwent shear wave elastography ElastPQ (Philips Healthcare, Bothell, Washington, USA) of the placenta. The mean value of placental shear modulus was obtained for each participant. These participants were followed up for the development of preeclampsia and were divided into two groups; group A included those who developed preeclampsia and group B included those who remained normotensive until delivery. The elasticity values of the two groups were compared, and the ROC curve was drawn to obtain the best cut-off value that would predict the onset of preeclampsia.
Results
Placental shear modulus varied from 1.03 kPa to 7.4 kPa at 16 to 20 weeks of gestation with an average of 2.74 ± 0.87 kPa. There was a statistically significant difference in the mean value of elasticity between two groups, being 4.61 kPa in group A and 2.51 kPa in group B. Maximum diagnostic accuracy was obtained at 2.9667 kPa with area under the curve 0.970, sensitivity 92%, specificity 91.71%, positive predictive value 57.5% and negative predictive value 98.9%.
Conclusion
Stiffness of placenta, quantitatively measured by SWE at 16 to 20 weeks of gestation, is higher in the women who develop preeclampsia and hence may be used for predicting preeclampsia.
Keywords: Shear wave elastography, ElastPQ, pregnancy, gestational hypertension
Introduction
Preeclampsia (PE) is a leading cause of maternal and perinatal morbidity. 1 It is a disorder characterized by new onset hypertension and proteinuria which occurs most often after 20 weeks of gestation. 2 However, proteinuria may be absent in some women with PE. 3 The American College of Obstetricians and Gynecologists (ACOG) recommends the following diagnostic criteria for preeclampsia:
1(a). Systolic blood pressure of 140 mmHg or more or diastolic pressure of 90 mmHg or more on two occasions at least 4 hours apart after 20 weeks of gestation in a woman with a previously normal blood pressure or
Systolic pressure of 160 mmHg or more or diastolic blood pressure of 110 mmHg or more.
1(b). And proteinuria – 300 mg or more per 24 hours’ urine collection or protein/creatinine ratio of 0.3mg or more, or dipstick reading of 2+
- 2. In the absence of proteinuria, new onset hypertension with the new onset of any of these:
- (a) thrombocytopenia: platelet count <100,000 × 109/L,
- (b) renal insufficiency,
- (c) impaired liver function,
- (d) pulmonary edema. 4
Preeclampsia affects 2–8% pregnancies globally. 5 Its incidence is estimated to be seven times higher in developing countries compared to developed countries. 6 Preeclampsia can be divided into early and late onset, depending upon its occurrence before or after 34 weeks of gestation. These two entities differ in their etiologies and in pregnancy outcome. 7 Early onset preeclampsia is thought to be predominantly due to defective implantation of placenta within the uterine endometrium. Abnormal placentation leads to maldevelopment of utero-placental perfusion, an increased inflammatory response and endothelial dysfunction. Eventually, the unsuccessful trophoblast invasion of the muscular spiral arteries into the myometrial layer causes failure of transformation of spiral arteries into “low-resistance” capacitance vessels, thus resulting in placental ischemia and hypoxia which leads to reduced nutrient supply to the fetus. 8 , 9 The late onset type is associated with no change or shallow modification of the spiral arteries and is possibly linked to maternal constitutional factors. 7
Early prediction and subsequent management of preeclampsia are imperative to improve maternal and fetal outcome. Screening for preeclampsia based on maternal characteristics and maternal history like past or family history of PE, nulliparity, age more than 35 years, multiple pregnancy, chronic kidney disease, diabetes and obesity can identify 35% at a false-positive rate of 10%. 10 Various maternal serum biochemical markers including activin A, inhibin A, placental growth factor and pregnancy-associated plasma protein A (PAPP-A) have been used for prediction of preeclampsia, but these have low predictive value. 11 Similarly, uterine artery Doppler alone has low predictive value for early onset preeclampsia and even lower value for the late onset type. 12
A combination of maternal biophysical markers like uterine artery Doppler and mean arterial pressure along with maternal serum biochemical markers have been found to improve the performance of screening for preeclampsia. 13 Spencer et al. reported a detection rate of 66% with uterine artery Doppler alone and more than 90% by combination of Doppler with maternal serum activin A and inhibin A levels for a false-positive rate of 10% in pregnancies between 22 weeks and 24+6 weeks. 14 Gorman et al. have reported a detection rate of 60% using a combination of maternal mean arterial pressure and PAPP-A at a false-positive rate of 10% in pregnancies at 11–13 weeks’ gestation. This increased to 75% by combination of uterine artery pulsatility index, mean arterial pressure and serum placental growth factor. 13 However, there is no consensus on the type of combination of the parameters and gestational age for the assessment.
Sonoelastography is a technique which is sensitive to tissue stiffness or elasticity. Altered soft tissue stiffness is the result of various physiological or pathological processes. In the placenta, ischemia-related changes like inflammation, necrosis, infarction and fibrosis lead to increased stiffness. 15 , 16
Shear wave elastography (SWE) is a type of elastography which is feasible in the placenta. It uses acoustic radiation forces to transiently deform tissues in the region of interest (ROI), and the dynamic displacement response of tissues is measured ultrasonically. For a given impulsive radiation force, softer tissues move further, take longer to reach a peak displacement and recover more slowly than stiffer tissues. Absolute quantification of soft tissue stiffness is presented as shear wave velocity in meters per second or as shear modulus in kilopascals (kPa). 17
Sugitani et al. measured the shear wave velocity of placenta ex vivo using acoustic radiation force impulse and reported increased values or firmer placenta in fetal growth restriction. Significant histological changes such as placental infarction and inflammation were seen in some of these cases with increased shear wave velocity. 18 Subsequently, in vivo study of placental tissue using virtual touch tissue quantification demonstrated increased shear wave velocity in fetal growth restriction and pregnancy-induced hypertension. 19 A similar finding of increased placental elasticity in hypertensive disorder of pregnancy has been reported by various authors. 16 , 20 , 21 However, most of these studies included pregnant women in the late second or third trimester with pre-existing hypertensive disorder of pregnancy.
It has been further observed that there is no significant correlation between the shear wave velocity of placenta and gestational age in normal pregnancy. 16 , 19 Also there is no significant difference in the elasticity in different areas of placenta in normal pregnancies. 20 , 22 However, placenta in preeclampsia has been reported to have different elasticity values in different areas. 20 , 21
Based on these observations, we evaluated placental elasticity using ElastPQ shear wave elastography at 16–20 weeks’ gestation and correlated the values with the development of preeclampsia at a later date.
Materials and methods
This prospective observational cohort study was conducted between October 2016 and June 2018. The study protocol was approved by the institutional ethical committee. Written informed consent was obtained from all participants before recruiting them into the study. A total of 413 pregnant women with a gestation between 16 and 20 weeks were enrolled. All women were normotensive at the time of enrollment. All women were of Indian origin, and there was no history of smoking. The following exclusion criteria were used: women over the age of 40 years, and those with medical conditions like chronic hypertension, chronic heart or renal disease, diabetes mellitus or severe anaemia, and women with a posterior placenta, twin pregnancy, fetal or placental structural abnormality. Based on these, 170 women with a posterior placenta were excluded. Four more women were excluded due to presence of one of these – twin pregnancy, fetal structural anomaly, pre-existing maternal diabetes and cardiac disease. A total of nine women were lost to follow-up. The remaining 230 participants underwent ultrasound examination with a Philips iU22, using a 2–5 MHz convex transducer with shear wave elastographic capability (ElastPQ) (Philips Healthcare, Bothell, Washington, USA).
Gestational age was determined based on the sure date of last normal menstrual period and confirmed by sonography in the first trimester or early second trimester. In four participants, who were unsure of dates, gestational age was calculated based on ultrasound biometry in the late first trimester.
Fetal biometry was performed by obtaining the biparietal diameter, head circumference, abdominal circumference and femur length. Grayscale examination of the placenta was performed to look for relation to the uterus and exclude morphological abnormalities like focal anechoic or hyperechoic areas, retroplacental hematoma and tumour. Sonoelastography was performed on anterior or anterolateral placentae in the same sitting using shear wave elastographic technique (ElastPQ). All the examinations were conducted by the same radiologist to ensure same technique. The elastographic examination time was kept to a minimum. With the participant lying in the supine position, the placenta was centred in the field of view and its right to left length was divided into four equal parts by imaginary lines; the middle two parts with the cord insertion were taken as the central part. One fourth part of placenta on either side of central part was considered as the right and left peripheral part. The fixed-size ROI was placed in the central part and the placental elasticity was displayed as shear modulus in kPa along with the grayscale image. Three readings each were obtained from the central part, and the right and the left peripheral parts of the placenta. The average quantitative value at each site was calculated from these three measurements. The average placental elasticity was calculated from all nine measurements.
During acquisition of placental elasticity, the participants were asked to breathe lightly and refrain from moving. Myometrium and placental sinusoids were excluded from the ROI.
All participants were followed up in the antenatal clinic at an interval of four weeks during the second trimester until 32 weeks, thereafter every two weeks until 36 weeks. After this, the follow-up was weekly. Clinical examination including recording of blood pressure along with dipstick urine examination for proteins was done at every visit. If the dipstick showed traces or 1+, the proteinuria was further evaluated in a 24-hour urine sample. Preeclampsia was diagnosed on the basis of ACOG criteria. 4 The participants who developed preeclampsia were admitted and managed further by the obstetrician as per the institutional protocol.
Statistical analysis
The data were entered in MS EXCEL spreadsheet, and analysis was done using Statistical Package for Social Sciences v21.0. (SPSS Inc., Chicago, USA). Categorical variables were presented in number and percentage and continuous variables were presented as mean ± SD and median.
Statistical tests were applied as follows:
Quantitative variables were compared using independent t test/Mann-Whitney Test between the two groups.
Qualitative variables were correlated using Chi-Square test/Fisher exact test.
Receiver operating characteristic curve was used to determine cut-off point of shear modulus for predicting PE.
p-Value of <0.05 was considered statistically significant.
Results
Of 230 participants who underwent placental elastography between 16 and 20 weeks’ gestation, 25 (10.87%) developed preeclampsia on subsequent follow-up and formed group A. The remaining 205 participants (89.13%) were normotensive until delivery and formed group B.
The age of the participants ranged from 16 to 35 years and 88.26% (n = 203) were in the age range 21 to 30 years. The average age of participants in group B was 24.89 years, and in group A, it was 23.92 years. Nullips comprised 140 participants (60.9%), while 90 (39.1%) were multiparous.
There was no significant difference in the age and gestational period in the two groups at the time of imaging assessment (Table 1). However, nulliparity was more commonly associated with the development of preeclampsia. Of 25 patients who developed PE later in the pregnancy, 17 were nulliparous and only eight were multiparous.
Table 1.
Demographic and obstetric data (n = 230).
| Group An = 25 | Group Bn = 205 | p | |
|---|---|---|---|
| Age (years) | 23.92 | 24.89 | 0.52 |
| Period of gestation at time of screening | 18.25 | 18.26 | 0.9 |
| Parity | |||
| Nulliparous | 68% | 60% | <0.001 |
| Multiparous | 32% | 40% |
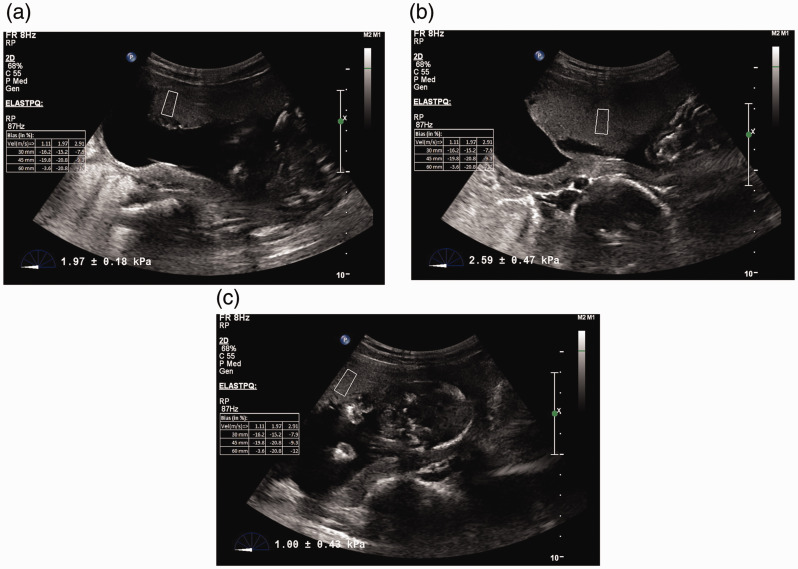
The SWE value of the placenta was calculated from the average of measurements obtained from at least three places: E1 – Right peripheral part of placenta, E2 – Center of placenta and E3 – Left peripheral part of placenta (Figures 1 and 2). Table 2 shows the distribution of elasticity values of the placenta at three sites in our study. The average shear modulus value was 2.74 kPa.
Figure 1.
ElastPQ shear wave elastography images of a 25-year-old pregnant woman at 17 weeks of gestation. (a) ROI box placed near the right edge of placenta gives an elasticity value of 1.97 kPa. (b) ROI box placed at the centre of placenta gives an elasticity value of 2.59 kPa. (c) ROI box placed near the left edge of placenta gives an elasticity value of 1.0 kPa. This woman remained normotensive throughout the pregnancy.
Figure 2.
ElastPQ shear wave elastography images of a 27-year-old pregnant woman at 19 weeks of gestation. (a) ROI box is placed near the right edge of placenta where the elasticity value is 5.54 kPa. (b) ROI box is placed at the centre of placenta where the elasticity value is 3.06 kPa. (c) ROI box is placed near the left edge of placenta where the elasticity value is 4.75 kPa. This woman developed preeclampsia later in pregnancy.
Table 2.
Distribution of elasticity values of placenta at three sites (n = 230).
| Elastography | Mean±SD | Minimum to maximum | Inter quartile range |
|---|---|---|---|
| E1 | 2.69 ± 0.78 | 1.1–7.1 | 2.200–2.900 |
| E2 | 2.82 ± 1.22 | 0.8–15 | 2.400–2.900 |
| E3 | 2.72 ± 0.89 | 1.1-8.3 | 2.200–2.900 |
| Average of E | 2.74 ± 0.87 | 1.03–7.4 | 2.333–2.867 |
E1: elasticity value of right peripheral part of placenta; E2: elasticity value at centre of placenta; E3: elasticity value of Left peripheral part of placenta.
Table 3 shows the shear modulus at three sites of placenta for the two groups. Distribution of elasticity measurements of the two groups and different sampling sites is depicted in Figure 3. The average value of placental shear modulus in group A was 2.51 kPa and in group B it was 4.61 kPa (p value <0.0001).
Table 3.
Correlation of placental elasticity values with development of PE in both groups (n = 230).
| Placental elasticity values | Group |
||
|---|---|---|---|
| A | B | ||
| E1 | 2.5 | 4.27 | p <0.0001 |
| E2 | 2.54 | 5.06 | |
| E3 | 2.50 | 4.51 | |
| Average | 2.51 | 4.61 | |
Figure 3.
Distribution of elasticity values of the two groups at three different sites of placenta.
Receiver operating characteristic (ROC) curve was used to determine the cut-off value of placental shear modulus for predicting PE (Figure 4). The cut-off value obtained was 2.9667 kPa with area under curve being 0.970 with standard error 0.017 and 95% confidence interval (0.94 to 0.99). At this cut-off, sensitivity, specificity, positive predictive value and negative predictive value were 92%, 91.71%, 57.5% and 98.9%, respectively.
Figure 4.
Receiver operating characteristic curve for prediction of preeclampsia based on ElastPQ shear wave elastographic values. The cut-off value and area under curve were 2.96 kPa and 0.97, respectively.
Discussion
Among the risk factors for preeclampsia, we observed a more than twofold increase in the incidence of PE in the nulliparous patients, which is concordant with the result of a previous study which showed that nulliparity increases the risk of PE by three times. 23 This coupled with the high percentage (60.9%) of nulliparous participants is the possible reason for the higher rate of preeclampsia noted in our study.
We found significantly higher values of placental elasticity in women who later developed PE (mean 4.61 kPa) compared to women who did not have PE (mean 2.51 kPa). The elasticity values in preeclampsia group were maximal at the centre of placenta compared to the peripheral part near the edges and this difference was significant. However, no significant difference was observed in the elasticity values of the placenta at different sites in normal pregnancy. Similar findings have been reported by previous studies. 20 , 22 Cimsit et al. measured placental elasticity in 101 normal women and 28 women with gestational hypertension with onset as early as between 20 and 23 weeks. The mean placental elasticity values of 101 women with normal outcome and 28 women with early onset PE were 2.53 kPa and 7.10 kPa respectively. 24 The higher value in Cimsit et al.’s study could be due to the inclusion of women who had onset of PE before 23 weeks, whereas we did not differentiate between women with early and late onset PE. This may indicate higher placental stiffness in early onset PE compared to late onset PE.
Fujita et al. evaluated placental elasticity in pregnant women between 16 and 32 weeks and observed development of PE. Shear wave velocity values, which directly correlate with tissue stiffness, were significantly higher in the PE onset group compared to the non-onset group. The study reported a cut-off value of 1.188 m/s for predicting PE with sensitivity, specificity, PPV and NPV of 92.3%, 91.3%, 40.0% and 99.5% respectively. 25 In our study, the best cut-off value of shear modulus was 2.96 kPa with sensitivity, specificity, PPV and NPV of 92%, 91.75%, 57.5% and 98.9%, respectively, and area under ROC 0.97.
There are concerns regarding the safety of use of SWE in pregnant women. Some authors argue that it generates a considerably higher thermal index compared to conventional ultrasound. 20 , 26 According to a study by Herman and Harris, any transient temperature rise due to pulse bursts of ARFI may still be within the safe limit determined by the US Food and Drug Administration. 27 Sugitani et al. investigated the biological effects of ARFI on placental tissue and did not observe any histological evidence of thermal damage. 18 Although no harmful effects have been reported, judicious use of ARFI is recommended along with the least possible time of examination. Considering the safety issue, interobserver variability was not evaluated in the present study to avoid repeated examinations of the same fetus. Also, the wave-path of ARFI did not pass through the fetus because of the anterior location of placenta.
There are some limitations to our study. Due to the fixed dimensions of the SWE sample box, information about a small area only could be obtained for the elastographic values. As the maximum depth of SWE is limited to 8 cm, posterior placentae and morbidly obese pregnant patients could not be included in the study. We did not evaluate the relation between placental elasticity and the severity or type of preeclampsia based on the time of onset. We did not obtain the body mass index of participants, hence the effect of obesity and thickness of maternal abdominal wall, if any, on placental elasticity calculation was not taken into consideration. Another limitation is the lack of histopathological evaluation of placenta, which might have shown structural changes in relation to elasticity values.
Conclusion
Our study demonstrated that there is significant difference in the elasticity values of the placenta in women who develop preeclampsia at a later date and those who do not. Therefore, ARFI appears to be a useful tool for predicting preeclampsia at 16 to 20 weeks’ gestation, but its universal application in pregnant women would not be possible due to technical issues related to depth as in posterior placentae and obesity. Also, larger studies are required to confirm its safety, to establish normal values of placental elasticity and the cut-off value for prediction of preeclampsia.
Supplemental Material
Supplemental material, sj-pdf-1-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound
Supplemental material, sj-pdf-2-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound
Supplemental material, sj-pdf-3-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound
Acknowledgments
We acknowledge the contribution of Simran Malik in editing the grammar and language of article.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Institute Ethics Committee, VMMC and Safdarjung Hospital, New Delhi 110029.
Contributorship: All authors were involved in conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content and final approval of the version to be published.
ORCID iDs
Rajkumar Meena https://orcid.org/0000-0001-9238-7326
Amita Malik https://orcid.org/0000-0003-4658-135X
References
- 1.Backes CH, Markham K, Moorehead P, et al. Maternal preeclampsia and neonatal outcomes. J Pregnancy 2011; 2011: 214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner LK. . Diagnosis and management of preeclampsia. Am Fam Physician 2004; 70: 2317–2324. [PubMed] [Google Scholar]
- 3.Homer CS, Brown MA, Mangos G, et al. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens 2008; 26: 295–302. [DOI] [PubMed] [Google Scholar]
- 4. ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol 2019; 133: e1–e25. [DOI] [PubMed] [Google Scholar]
- 5.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet 2010; 376: 631–644. [DOI] [PubMed] [Google Scholar]
- 6.Malik A, Jee B, Gupta SK. Preeclampsia: disease biology and burden, its management strategies with reference to India. Pregnancy Hypertens 2019; 15: 23–31. [DOI] [PubMed] [Google Scholar]
- 7.Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008; 52: 873–880. [DOI] [PubMed] [Google Scholar]
- 8.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27: 939–958. [DOI] [PubMed] [Google Scholar]
- 9.Devisme L, Merlot B, Ego A, et al. A case-control study of placental lesions associated with pre-eclampsia. Int J Gyneacol Obstet 2013; 120: 165–168. [DOI] [PubMed] [Google Scholar]
- 10.Wright D, Syngelaki A, Akolekar R, et al. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol 2015; 213: 62.e1–62.e10. [DOI] [PubMed] [Google Scholar]
- 11.Espinoza J. Recent biomarkers for the identification of patients at risk for preeclampsia: the role of uteroplacental ischemia. Expert Opin Med Diag 2012; 6: 121–130. [DOI] [PubMed] [Google Scholar]
- 12.Cnossen JS, Morris RK, Ter Riet G, et al. Use of uterine artery doppler ultrasonography to predict preeclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ 2008; 178: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol 2016; 214: 103.e1–103.e12. [DOI] [PubMed] [Google Scholar]
- 14.Spencer K, Yu CK, Savvidou M, et al. Prediction of pre-eclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein-A, free beta-human chorionic gonadotropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks' gestation. Ultrasound Obstet Gynecol 2006; 27: 658–663. [DOI] [PubMed] [Google Scholar]
- 15.Apel-Sarid L, Levy A, Holcberg G, et al. Term and preterm (<34 and <37 weeks gestation) placental pathologies associated with fetal growth restriction. Arch Gynecol Obstet 2010; 282: 487–492. [DOI] [PubMed] [Google Scholar]
- 16.Alan B, Tunc S, Agacayak E, et al. Diagnosis of pre-eclampsia and assessment of severity through examination of the placenta with acoustic radiation force impulse elastography. Int J Gynaecol Obstet 2016; 135: 43–46. [DOI] [PubMed] [Google Scholar]
- 17.Sarvazyan A, Skovoroda AR, Emelianov S, et al. Biophysical bases of elasticity imaging. Acoust Imag 1995; 21: 223–241. [Google Scholar]
- 18.Sugitani M, Fujita Y, Yumoto Y, et al. A new method for measurement of placental elasticity: acoustic radiation force impulse imaging. Placenta 2013; 34: 1009–1013. [DOI] [PubMed] [Google Scholar]
- 19.Ohmaru T, Fujita Y, Sugitani M, et al. Placental elasticity evaluation using virtual touch tissue quantification during pregnancy. Placenta 2015; 36: 915–920. [DOI] [PubMed] [Google Scholar]
- 20.Kiliç F, Kayadibi Y, Yüksel MA, et al. Shear wave elastography of placenta: in vivo quantitation of placental elasticity in preeclampsia. Diagn Interv Radiol 2015; 21: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaman E, Arslan H, Çetin O, et al. Comparison of placental elasticity in normal and pre-eclamptic pregnant women by acoustic radiation force impulse elastosonography. J Obstet Gynaecol Res 2016; 42: 1464–1470 [DOI] [PubMed] [Google Scholar]
- 22.Li WJ, Wei ZT, Yan RL, et al. Detection of placenta elasticity modulus by quantitative real-time shear wave imaging. Clin Exp Obstet Gynecol 2012; 39: 470–473. [PubMed] [Google Scholar]
- 23.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005; 330: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimsit C, Yoldemir T, Akpinar IN. Shear wave elastography in placental dysfunction: comparison of elasticity values in normal and preeclamptic pregnancies in the second trimester. J Ultrasound Med 2015; 34: 151–159. [DOI] [PubMed] [Google Scholar]
- 25.Fujita Y, Nakanishi TO, Sugitani M, et al. Placental elasticity as a new non-invasive predictive marker of pre-eclampsia. Ultrasound Med Biol 2019; 45: 93–97. [DOI] [PubMed] [Google Scholar]
- 26.Simon EG, Callé S, Perrotin F, et al. Measurement of shear wave speed dispersion in the placenta by transient elastography: a preliminary ex vivo study. PLoS ONE 2018; 13: e0194309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman BA, Harris GR. Models and regulatory considerations for transient temperature rise during diagnostic ultrasound pulses. Ultrasound Med Biol 2002; 28: 1217–1224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound
Supplemental material, sj-pdf-2-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound
Supplemental material, sj-pdf-3-ult-10.1177_1742271X211040543 for Placental elastography in second trimester preeclampsia prediction: A prospective study by Rajkumar Meena, Amita Malik, Swarna Jain and Achla Batra in Ultrasound