Abstract
The methylated form of the Ada protein (meAda) activates transcription from the Escherichia coli ada, aidB, and alkA promoters with different mechanisms. In this study we identify amino acid substitutions in region 4 of the RNA polymerase subunit ς70 that affect Ada-activated transcription at alkA. Substitution to alanine of residues K593, K597, and R603 in ς70 region 4 results in decreased Ada-dependent binding of RNA polymerase to the alkA promoter in vitro and impairs alkA transcription both in vivo and in vitro, suggesting that these residues define a determinant for meAda-ς70 interaction. In a previous study (P. Landini, J. A. Bown, M. R. Volkert, and S. J. W. Busby, J. Biol. Chem. 273:13307–13312, 1998), we showed that a set of negatively charged amino acids in ς70 region 4 is involved in meAda-ς70 interaction at the ada and aidB promoters. However, the alanine substitutions of positively charged residues K593, K597, and R603 do not affect meAda-dependent transcription at ada and aidB. Unlike the ς70 amino acids involved in the interaction with meAda at the ada and aidB promoters, K593, K597, and R603 are not conserved in ςS, an alternative ς subunit of RNA polymerase mainly expressed during the stationary phase of growth. While meAda is able to promote transcription by the ςS form of RNA polymerase (EςS) at ada and aidB, it fails to do so at alkA. We propose that meAda can activate transcription at different promoters by contacting distinct determinants in ς70 region 4 in a manner dependent on the location of the Ada binding site.
Exposure of Escherichia coli cells to sublethal concentrations of DNA-methylating agents such as methyl methanesulfonate (MMS) triggers the expression of a set of genes which allows E. coli to tolerate the toxic and mutagenic effects of such agents. This process is called the adaptive response (10, 29). The Ada protein plays a dual role in the adaptive response, being both a DNA repair protein and a transcription activator. Ada is a 354-amino-acid methyltransferase able to remove methyl groups from both the DNA strand and damaged nucleotides. Methyl groups are transferred to two cysteine residues in the protein itself, at positions 69 and 321 (7). Methylation of cysteine at position 69 triggers a conformational change in the Ada protein; cysteine-69-methylated Ada (meAda) is a specific DNA binding protein able to promote transcription of its own gene, ada, and of the alkB gene, whose transcription is also driven by the ada promoter (25, 32). In addition, meAda activates transcription from the promoters of at least two more genes, alkA and aidB (14, 25, 32). The AlkA protein is a DNA repair protein able to excise methylated bases from DNA (32), while AidB appears to be involved in metabolic detoxification of some alkylating agents, such as MNNG (N-methyl-N′-nitro-N-nitrosoguanidine) (13). AlkB is a membrane protein, and its function is unknown (34). All Ada-dependent promoters display a common sequence, AAT(N)6GCAA, thought to be the recognition sequence for meAda (14).
A number of observations show that the mechanism of transcription activation by Ada at the alkA promoter differs from that at the ada and aidB promoters. Both the unmethylated and the methylated forms of the Ada protein can activate alkA transcription in vivo and in vitro (9, 27). In contrast, the unmethylated Ada protein activates very poorly, if at all, transcription from the ada and aidB promoters, and it has been shown to be inhibitory for the ada promoter at high concentrations (26). Moreover, Ada mutants capable of activating alkA but not ada, and vice versa, have been isolated (1, 27, 30). These observations strongly suggest that different determinants in the Ada protein are involved in the interaction with RNA polymerase at different adaptive response promoters.
The alkA promoter also differs from the ada and aidB promoters in its architecture and in the location of the Ada binding site relative to the promoter elements. In ada and aidB, the Ada binding site is, respectively, 5 and 7 bp upstream of a fairly well conserved −35 sequence (14, 28); at the alkA promoter, the Ada binding site overlaps a −35 hexamer which bears no similarity to the consensus sequence (1, 9). The adaptive response promoters also differ in their interaction with RNA polymerase: at ada and aidB, RNA polymerase binds the promoters in the absence of Ada, mostly through α subunit/UP-like element contacts, to form an unusual binary complex which is poorly active in transcription initiation (15). Binding of meAda results in the formation of a transcription initiation-competent ternary complex. In a recent study (18), we showed that the target for activation by meAda is in region 4 of the ς70 subunit of RNA polymerase (amino acids 574 to 613); several negatively charged amino acids in this region (E574, E575, E591, E605, and D612, as well as hydrophobic residue I590) appear to be involved in meAda-ς70 interaction. At alkA, no RNA polymerase-promoter binary complex in the absence of Ada is detectable, and both forms of the Ada protein appear to increase RNA polymerase binding affinity to the promoter region (17). Finally, meAda is able to activate transcription by RNA polymerase containing ςS (EςS) at ada and aidB (16, 31) while no such evidence has been shown for alkA. ςS is the main ς subunit during stationary phase (19).
In this report, we present evidence that region 4 of ς70 is also the target of activation by the Ada protein at the alkA promoter; however, different determinants are involved in Ada-ς70 interaction. Three positively charged amino acids in ς70 region 4 (K593, K597, and R603) play a major role in Ada-dependent transcription at alkA. Moreover, the Ada protein is unable to activate transcription by EςS at the alkA promoter, consistent with the fact that the K593, K597, and R603 residues are not conserved in ςS. Based on these observations, we conclude that meAda can activate transcription via interaction with distinct determinants in ς70 region 4, depending on the location of its binding site.
MATERIALS AND METHODS
In vivo transcription from the alkA promoter.
A plasmid library carrying mutations resulting in single alanine substitutions at 17 amino acids of ς70 region 4 (obtained from C. Gross, University of California—San Francisco; plasmids are derivatives of pGEX-2Tς70 [8]) was used to transform strain MV3764. MV3764 carries a lacZ fusion within the chromosomal alkA gene. The strains obtained from transformation of MV3764 were tested for β-galactosidase activity to quantify the effects of the alanine substitutions in ς70 region 4 on alkA transcription. Cells were grown overnight in Luria broth supplemented with 20 μg of tetracycline per ml, 25 μg of chloramphenicol per ml, and 80 μg of ampicillin per ml, diluted 1:100 in fresh medium, and grown to an optical density at 600 nm of 0.1. Ada-dependent transcription of alkA was induced by the addition of 0.02% MMS. Samples were taken after 2 h, and specific β-galactosidase activity was determined as described in reference 23.
In vitro transcription.
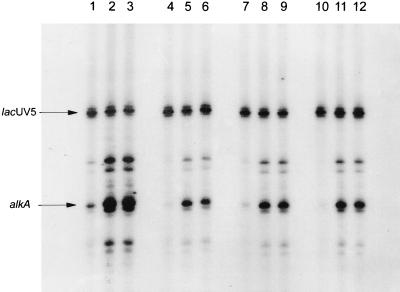
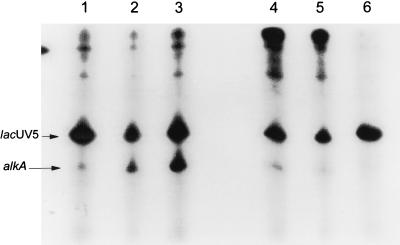
Mutated ς70 subunits affecting alkA transcription in vivo, as well as wild type ς70, were purified as described previously (8). ςS (obtained from A. Kolb, Institut Pasteur) was purified as described previously (6). RNA polymerase holoenzymes were reconstituted by adding the purified ς subunits to RNA polymerase core enzyme (Epicentre Technology) at a 5:1 ratio (except for ς70RA603, which was added at a 20:1 ratio because of its lower affinity for core RNA polymerase [21]). Core enzyme and the required ς factor were incubated for 20 minutes at 37°C in a solution of 50 mM Tris-HCl (pH 7.6), 100 mM KCl, and 50% glycerol; the reconstituted RNA polymerase holoenzymes were then used for in vitro transcription. Reconstituted RNA polymerases were incubated for 15 min at 37°C in a solution of 40 mM Tris-HCl (pH 8.0), 7.5% (wt/vol) glycerol, 30 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol, and 20 μg of bovine serum albumin per ml in the presence or absence of either form of the Ada protein. Transcription reactions were started by the addition of 0.1 mM (each) GTP, ATP, and CTP, 0.002 mM UTP, 10 μCi of [α-32P]UTP, and 250 μg of heparin per ml. The reaction was stopped after 5 min at 37°C, and samples were analyzed on a denaturing polyacrylamide gel. The DNA fragments used as templates were a 205-bp EcoRI DNA fragment from pYN3066 (25) carrying the lacUV5 promoter (used as an internal control) and a 166-bp HindIII/EcoRI fragment from pMV466 carrying the alkA promoter (17). The two fragments were obtained by PCR amplification with Pfu enzyme. PCR products were separated by electrophoresis in 1% agarose and recovered with the Geneclean kit (Bio 101). The amount of transcription was quantified after normalization to the lacUV5 transcript by a PhosphorImager (Molecular Dynamics). In the experimental results shown in Fig. 2, three Ada-dependent transcripts from the alkA promoter can be detected; the presence of more than one transcript is likely to be due to the production of different-length DNA fragments in the PCR amplification of the alkA promoter region rather than to the presence of alternative transcription start points in the alkA promoter (cf. reference 17).
FIG. 2.
In vitro transcription with reconstituted RNA polymerases (50 nM). Lanes 1 to 3, wild-type Eς70; lanes 4 to 6, Eς70KA593; lanes 7 to 9, Eς70KA597; lanes 10 to 12, Eς70RA603. Lanes 1, 4, 7, and 10, no Ada protein; lanes 2, 5, 8, and 11, 0.2 μM unmethylated Ada; lanes 3, 6, 9, and 12, 0.2 μM methylated Ada. The positions of the main lacUV5 and alkA transcripts are indicated.
Gel retardation assays.
The 166-bp HindIII/EcoRI fragment carrying the alkA promoter was also used for gel retardation. The fragment was labeled with [α-32P]dATP (3,000 Ci/mmol) (Amersham) by filling in with DNA polymerase I Klenow fragment (Pharmacia). A 10,000-cpm sample (corresponding to ca. 5 fmol) of labeled fragment was used in a 20-μl final volume of reaction buffer (50 mM Tris-HCl [pH 7.6], 50 mM NaCl, 2.5 mM dithiothreitol, 6.25% glycerol, 25 μg of herring sperm DNA per ml). When necessary, meAda was present at 0.2 μM. Reconstituted RNA polymerase was added to a final concentration of 50 nM, and samples were incubated for 30 min at 37°C prior to the addition of 250 μg of heparin per ml. After 5 min of incubation, samples were loaded onto a 4% native polyacrylamide gel. Gels were run at 10 V/cm in 0.25× TBE (22.5 mM Tris-borate, 0.5 mM EDTA)–1.25% glycerol, and bands were visualized by autoradiography. Quantification of retarded bands was performed with a PhosphorImager.
RESULTS
Effects of mutations in rpoD on alkA transcription in vivo.
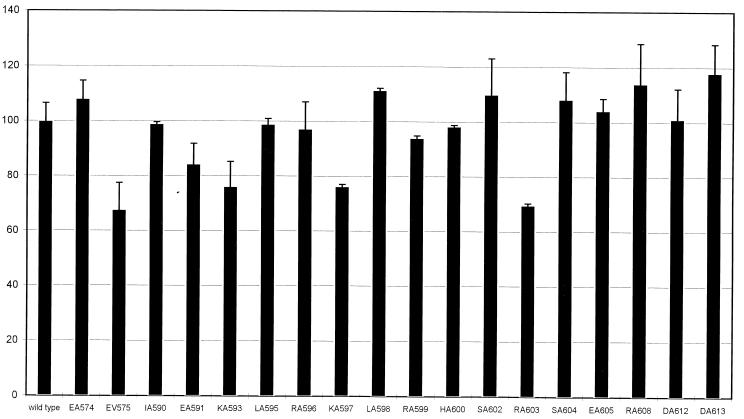
We transformed strain MV3764 (alkA::lacZ) with a set of plasmids carrying rpoD alleles with single alanine substitutions at 17 amino acids of ς70 region 4. β-Galactosidase assays were performed to quantify the effects of the alanine substitutions, as well as the effect of the EV575 substitution, which we had found to affect transcription from the ada promoter (18). In strain MV3764, wild-type ς70 from the chromosomal rpoD gene is expressed at normal levels; since in the absence of IPTG (isopropyl-β-d-thiogalactopyranoside) the mutated rpoD alleles from the plasmid are expressed at a low level and mutated ς subunits are present in only a slight excess to wild-type ς70 (data not shown), a reduction of alkA expression to below 80% of the wild type was considered significant (Fig. 1). Expression of four mutant rpoD alleles resulted in lower levels of in vivo transcription at the alkA promoter: EV575 (down to 67.7% of the level observed with wild-type ς70-encoding plasmid), KA593 (76.1%), KA597 (76.3%), and RA603 (69.6%). No mutations resulted in significantly increased levels of alkA transcription (more than 120% of the wild type [Fig. 1]), nor was alkA transcription in the absence of MMS significantly altered by any rpoD allele tested (data not shown). In a previous study, we had found that a set of negatively charged residues in ς70 (E574, E575, E591, E605, and D612) negatively affects transcription of the ada promoter in vivo (18); of these substitutions, only EV575 significantly affected alkA promoter activity (Fig. 1). Expression of the KA593, KA597, and RA603 mutated ς subunits did not result in decreased transcription from the ada promoter in vivo (18).
FIG. 1.
In vivo transcription from the alkA promoter. The effects of the substitutions in ς70 region 4 on alkA expression are shown as percentages of alkA expression with wild-type ς70. Values (± standard deviations) are averages of four independent experiments. The average value for the wild type was 342 Miller units.
Mutant ς70 subunits affect alkA transcription in vitro.
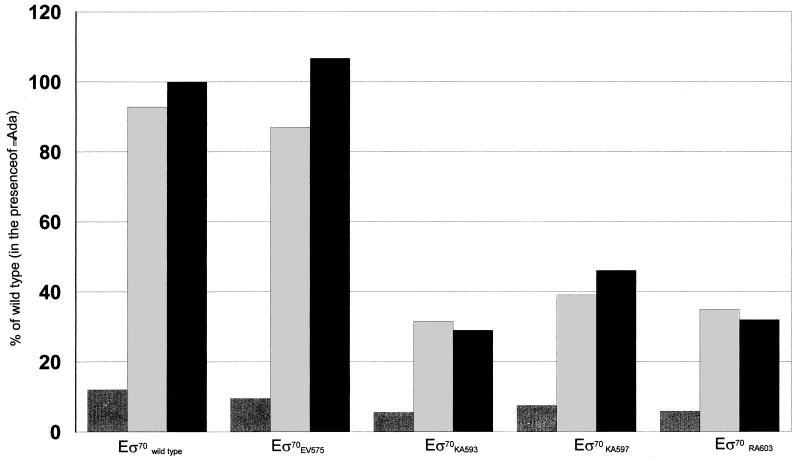
Purified wild-type and mutant ς subunits EV575, KA593, KA597, and RA603 were used for reconstitution of RNA polymerase and in vitro transcription (Fig. 2 and 3). The Eς70EV575, Eς70KA593, Eς70KA597, and Eς70RA603 forms of RNA polymerase were all able to carry out transcription from the lacUV5 promoter with roughly the same efficiency as the wild type, suggesting that these mutant forms of ς70 are not affected in factor-independent transcription. In contrast, transcription from the alkA promoter by Eς70KA593 (Fig. 2, lanes 4 to 6) Eς70KA597 (lanes 7 to 9), and Eς70RA603 (lanes 10 to 12), in the presence of either Ada or meAda, was only 28 to 45% of the transcription obtained with wild-type ς70 (Fig. 3). These results suggest that these mutant ς subunits are indeed defective in their interaction with the Ada protein and that both methylated and unmethylated Ada activate transcription by contacting the same determinants in ς70. Surprisingly, Ada-dependent transcription of alkA by Eς70EV575 was not impaired (Fig. 3) despite the fact that expression of ς70EV575 had the strongest effect on alkA expression in vivo. A possible reason for this discrepancy is the very strong negative effect of ς70EV575 overexpression on transcription of ada in vivo (18), which results in lower intracellular concentrations of the Ada protein; this, in turn, would result in suboptimal activation of the alkA promoter.
FIG. 3.
Ratio of alkA/lacUV5 transcripts. Values are percentages of transcription by wild-type Eς70 in the presence of meAda and are averages of three independent experiments. Dark grey bars, transcription levels in the absence of Ada; light grey bars, transcription levels in the presence of unmethylated Ada; black bars, transcription levels in the presence of methylated Ada. Standard deviations were less than 15%.
Gel retardation assays.
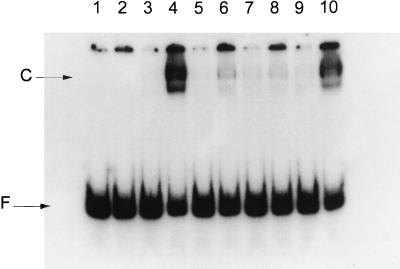
In the presence of either form of Ada, a stable ternary complex, (me)Ada-RNA polymerase-alkA, which is resistant to challenge with heparin is formed and can be detected in gel retardation assays (Fig. 4). In order to investigate whether the KA593, KA597, and RA603 substitutions in ς70 region 4 affect the formation of the ternary complex, gel retardation assays were performed with RNA polymerase reconstituted with mutated ς subunits. The results in Fig. 4 show that Eς70KA593, Eς70KA597, and Eς70RA603 are all impaired in formation of the ternary complex with the alkA promoter and meAda. However, the defect was more dramatic for Eς70KA593 (Fig. 4, lanes 5 and 6) and Eς70KA597 (lanes 7 and 8) than for Eς70RA603, which was still able to promote formation of the ternary complex at about 50% of wild-type levels (lanes 9 and 10). This result was unexpected, since the KA593, KA597, and RA603 substitutions have very similar effects on alkA transcription both in vitro and in vivo (Fig. 1 to 3). It is possible that in addition to interaction with Ada, Eς70RA603 is also impaired in transcription initiation at alkA at a later step, such as promoter clearance, which is likely to be Ada-independent. Interestingly, Eς70RA603 has been shown to be defective in transcription initiation at the factor-independent promoter RNA-I (21).
FIG. 4.
Gel retardation assays performed with reconstituted RNA polymerase. Odd-numbered lanes, no meAda; even-numbered lanes, 0.2 μM meAda. Lanes 1 and 2, no RNA polymerase; lanes 3 and 4, wild-type Eς70; lanes 5 and 6, Eς70KA593; lanes 7 and 8, Eς70KA597; lanes 9 and 10, Eς70RA603. F, unbound alkA promoter DNA; C, RNA polymerase-alkA complex.
Eς70- and EςS-dependent transcription in vitro.
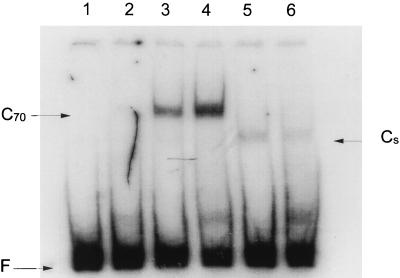
Although interaction with ς70 region 4 is the principal mechanism of transcription activation by meAda, the results of the in vitro transcription experiments strongly suggest that the target of its activation at alkA differs from that at ada and aidB at the amino acid level. At the ada and aidB promoters, the E574, E575, I590, E591, E605, and D612 side chains are important for activation by meAda (18). With the sole exception of D612, these residues are conserved in ςS, consistent with the ability of meAda to promote transcription by EςS at both ada and aidB (16, 31). In contrast, the K593, K597, and R603 residues, which appear to be the target amino acids for activation by Ada at the alkA promoter, are not conserved in ςS. Thus, we tested EςS for Ada-dependent transcription at alkA. The results of this experiment are shown in Fig. 5. While either form of the Ada protein clearly activated transcription by Eς70 (lanes 1 to 3), no stimulation of transcription by EςS was detected (lanes 4 to 6); in fact, meAda displayed a weak negative effect on alkA transcription by EςS (compare lane 4 to lane 6). We also tested EςS in gel retardation assays: as expected from the results of the in vitro transcription experiments, meAda failed to promote the formation of a stable heparin-resistant ternary complex with EςS and the alkA promoter (Fig. 6).
FIG. 5.
In vitro transcription with Eς70 (lanes 1 to 3) and EςS (lanes 4 to 6) forms of RNA polymerase. Lanes 1 and 4, no Ada protein; lanes 2 and 5, 0.2 μM Ada; lanes 3 and 6, 0.2 μM meAda. The positions of the main lacUV5 and alkA transcripts are indicated.
FIG. 6.
Gel retardation assays performed with Eς70 and EςS forms of RNA polymerase. Odd-numbered lanes, no meAda; even-numbered lanes, 0.2 μM meAda. Lanes 1 and 2, no RNA polymerase; lanes 3 and 4, Eς70; lanes 5 and 6, EςS. F, unbound alkA promoter DNA; C70 and CS, Eς70-alkA and EςS-alkA complexes, respectively.
DISCUSSION
The results presented in this report show that substitution to alanine of amino acids K593, K597, and R603 in region 4 of the ς70 subunit of RNA polymerase disrupts Ada-dependent transcription from the alkA promoter (Fig. 1 to 3). Thus, we propose that Ada activates alkA transcription via direct interaction with ς70 region 4. The target for activation by Ada is located immediately downstream of the helix-turn-helix DNA binding motif, which is responsible for recognition of the −35 promoter element (20) and is the target of several transcription activators (12, 21, 22). Interestingly, although ς70 region 4 is also the target for Ada activation at the ada and aidB promoters, two different sets of amino acids are involved. A striking difference between the two sets is the nature of the amino acids involved: all except one of the ς70 residues necessary for meAda-dependent transcription at ada and aidB are negatively charged (18), in contrast to the positive charges of K593, K597, and R603.
It is noteworthy that K593, K597, and R603 are also targets for other activator proteins: substitution to alanine of any of these residues severely affects transcription activation by FNR and by cyclic AMP receptor protein (CRP), respectively, at the dmsA and melR(Con) promoters (21). Although the three-dimensional structure of ς70 has not yet been solved, Lonetto et al. (21) recently proposed two alternative model structures based on the highly similar DNA binding regions of the NarL and Cro proteins (3, 24). According to both models, residues K593 and K597 belong to a surface-exposed patch, thus providing a target for interaction with activator proteins such as Ada, while R603 is removed from the protein surface and in closer contact with the helix-turn-helix DNA binding motif (21). The location of the R603 residue would suggest that the RA603 substitution can affect alkA transcription by altering the general conformation of region 4, consistent with the effects of the RA603 mutation on factor-independent transcription at some promoters (21) and with the results of gel retardation experiments at the alkA promoter (Fig. 4).
To our knowledge, the Ada protein is the first example of a transcription activator able to contact two distinct determinants in the ς70 subunit of RNA polymerase in a promoter-specific fashion. The use of different targets in region 4 of ς70 appears to depend upon the location of the Ada binding site, which in the alkA promoter is positioned between −47 and −35, i.e., one helical turn downstream compared to the Ada binding site in ada and aidB (1, 9, 14, 25). Additional contributions to transcription activation of the alkA promoter are provided by interactions between the α subunit of RNA polymerase and the Ada protein (17). It is significant that other transcription activators, such as the Mor protein of bacteriophage Mu and CRP, also display the ability to interact with both α and ς70 subunits, suggesting that this might be a common feature for activators whose binding site overlaps the −35 sequence (2, 5, 11).
The presence of two distinct activation targets in ς70 determines the ability by Ada to activate transcription by RNA polymerase containing ςS (EςS). ςS is the main ς subunit of RNA polymerase during stationary phase (4, 19). The methylated form of the Ada protein is able to activate transcription by EςS as well as Eς70 at both the ada and aidB promoters (16, 31). These observations are consistent with the fact that the ς70 amino acids important for activation by meAda are also conserved in ςS (18). In contrast, we have shown in this study that meAda is unable to recruit EςS to the alkA promoter (Fig. 6), thus failing to stimulate alkA transcription by EςS (Fig. 5), consistent with the lack of conservation of K593, K597, and R603 in ςS.
What is the physiological reason for the lack of alkA transcription by EςS? It has been proposed recently that low levels of endogenous methylation damage of DNA take place during stationary phase, possibly through amino acid nitrosation (31), resulting in weak induction of aidB and ada, but not of alkA, at the onset of stationary phase (12a, 31, 33). In stationary phase, it might be more efficient for E. coli to prevent the accumulation of endogenously produced methylating agents than to repair DNA damage by expression of the alkA gene. Thus, aidB, which is responsible for detoxification of some methylating agents (13), and AlkB, a membrane protein cotranscribed with ada and possibly involved in their excretion (34), would be preferentially expressed during stationary phase.
ACKNOWLEDGMENTS
We thank Mike Volkert for the gift of strain MV3764 and for critical reading of the manuscript, Carol Gross for the alanine scan plasmids, and Annie Kolb for the gift of purified ςS.
This work was supported by a Long Term EMBO Fellowship and a TMR Fellowship from the European Union to P.L.
REFERENCES
- 1.Akimaru H, Sakumi K, Yoshikai T, Anai M, Sekiguchi M. Positive and negative regulation of transcription by a cleavage product of Ada protein. J Mol Biol. 1990;216:261–273. doi: 10.1016/S0022-2836(05)80318-3. [DOI] [PubMed] [Google Scholar]
- 2.Artsimovich I, Murakami K, Ishihama A, Howe M M. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both α and ς70 subunits of Escherichia coli RNA polymerase. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 3.Baikalov I, Schroeder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus R, Dickerson R. Structure of the Escherichia coli response regulator NarL. Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 4.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase inducible “gearbox” promoters: differential effects of katF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busby S, Ebright R H. Transcription activation at class II CAP-dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 6.Colland F, Orsini G, Brody E, Buc H, Kolb A. The bacteriophage T4 AsiA protein: a molecular switch for ς70-dependent promoters. Mol Microbiol. 1998;27:819–829. doi: 10.1046/j.1365-2958.1998.00729.x. [DOI] [PubMed] [Google Scholar]
- 7.Demple B, Sedgwick B, Robins P, Totty N, Waterfield M D, Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci USA. 1985;82:2288–2292. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombroski A, Walter W, Record M T, Siegele D, Gross C. Polypeptides containing highly conserved regions of transcription initiation factor ς70 exhibit specificity of binding to promoter DNA. Cell. 1992;70:501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- 9.Furuichi M, Yu C G, Anai M, Sakumi K, Sekiguchi M. Regulatory elements for expression of the alkA gene in response to alkylating agents. Mol Gen Genet. 1992;236:25–32. doi: 10.1007/BF00279639. [DOI] [PubMed] [Google Scholar]
- 10.Jeggo P. Isolation and characterization of Escherichia coli K-12 mutants unable to induce the adaptive response to simple alkylating agents. J Bacteriol. 1979;139:783–791. doi: 10.1128/jb.139.3.783-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin R, Sharif K, Krakow J. Evidence for contact between the cyclic AMP receptor protein and the ς70 subunit of RNA polymerase. J Biol Chem. 1995;270:19213–19216. doi: 10.1074/jbc.270.33.19213. [DOI] [PubMed] [Google Scholar]
- 12.Kuldell N, Hochschild A. Amino acid substitutions in the −35 recognition motif of ς70 that result in defects in phage λ repressor-stimulated transcription. J Bacteriol. 1994;176:2991–2998. doi: 10.1128/jb.176.10.2991-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Landini, P. Unpublished data.
- 13.Landini P, Hajec L I, Volkert M R. Structure and transcriptional regulation of the Escherichia coli adaptive response gene aidB. J Bacteriol. 1994;176:6583–6589. doi: 10.1128/jb.176.21.6583-6589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landini P, Volkert M R. Transcriptional activation of the Escherichia coli adaptive response gene aidB is mediated by binding of methylated Ada protein. Evidence for a new consensus sequence for Ada-binding sites. J Biol Chem. 1995;270:8285–8289. doi: 10.1074/jbc.270.14.8285. [DOI] [PubMed] [Google Scholar]
- 15.Landini P, Volkert M R. RNA polymerase α subunit binding site in positively controlled promoters: a new model for RNA polymerase-promoter interaction and transcriptional activation in the Escherichia coli ada and aidB genes. EMBO J. 1995;14:4329–4335. doi: 10.1002/j.1460-2075.1995.tb00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landini P, Hajec L I, Nguyen L H, Burgess R R, Volkert M R. The leucine-responsive regulatory protein (Lrp) acts as a specific repressor for ςS-dependent transcription of the Escherichia coli aidB gene. Mol Microbiol. 1996;20:947–955. doi: 10.1111/j.1365-2958.1996.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 17.Landini P, Gaal T, Ross W, Volkert M R. The RNA polymerase α subunit carboxy-terminal domain is required for both basal and activated transcription from the alkA promoter. J Biol Chem. 1997;272:15914–15919. doi: 10.1074/jbc.272.25.15914. [DOI] [PubMed] [Google Scholar]
- 18.Landini P, Bown J A, Volkert M R, Busby S J W. Ada protein-RNA polymerase ς subunit interaction and α subunit-promoter DNA interaction are necessary at different steps in transcription initiation at the Escherichia coli ada and aidB promoters. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- 19.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 20.Lonetto M, Gribskov M, Gross C. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonetto M, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 22.Makino K, Amemura M, Kim S, Nakata A, Shinagawa H. Role of the ς70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev. 1993;7:149–160. doi: 10.1101/gad.7.1.149. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 24.Mondragon A, Harrison S. The phage 434 Cro/OR1 complex at a 2.5 Angstroem resolution. J Mol Biol. 1991;219:321–334. doi: 10.1016/0022-2836(91)90568-q. [DOI] [PubMed] [Google Scholar]
- 25.Nakabeppu Y, Sekiguchi M. Regulatory mechanisms for induction of synthesis of repair enzymes in response to alkylating agents: Ada acts as a transcriptional regulator. Proc Natl Acad Sci USA. 1986;83:6297–6301. doi: 10.1073/pnas.83.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saget B M, Walker G C. The Ada protein acts as both a positive and negative modulator of Escherichia coli’s response to methylating agents. Proc Natl Acad Sci USA. 1994;91:9730–9734. doi: 10.1073/pnas.91.21.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saget B M, Shevell D E, Walker G C. Alteration of lysine 178 in the hinge region of the Escherichia coli Ada protein interferes with activation of ada, but not alkA, transcription. J Bacteriol. 1995;177:1268–1274. doi: 10.1128/jb.177.5.1268-1274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakumi K, Sekiguchi M. Regulation of the expression of the ada gene controlling the adaptive response: interactions with the ada promoter and RNA polymerase. J Mol Biol. 1989;205:373–385. doi: 10.1016/0022-2836(89)90348-3. [DOI] [PubMed] [Google Scholar]
- 29.Samson L, Cairns J. A new pathway for DNA repair in Escherichia coli. Nature (London) 1977;267:281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- 30.Shevell D E, Walker G C. A region of the Ada DNA-repair protein required for the activation of ada transcription is not necessary for activation of alkA. Proc Natl Acad Sci USA. 1991;88:9001–9005. doi: 10.1073/pnas.88.20.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taverna P, Sedgwick B. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J Bacteriol. 1996;178:5105–5111. doi: 10.1128/jb.178.17.5105-5111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo I, Sedgwick B, Kilpatrick M W, McCarthy T V, Lindahl T. The intracellular signal for induction of resistance to alkylating agents in E. coli. Cell. 1986;45:315–324. doi: 10.1016/0092-8674(86)90396-x. [DOI] [PubMed] [Google Scholar]
- 33.Volkert M R, Nguyen D C. Induction of specific Escherichia coli genes by sublethal treatments with alkylating agents. Proc Natl Acad Sci USA. 1984;81:4110–4114. doi: 10.1073/pnas.81.13.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y-F, Chen B J, Samson L. Suppression of Escherichia coli alkB mutants by Saccharomyces cerevisiae genes. J Bacteriol. 1995;177:5009–5015. doi: 10.1128/jb.177.17.5009-5015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]