Abstract
Introduction
Patients with pre-existing cardiovascular disease may carry a higher risk for mortality from COVID-19. This study examined the association between individuals with pre-existing cardiovascular disease admitted for COVID-19 and their clinical outcomes.
Methods
A retrospective cohort study was conducted on patients admitted with COVID-19 to Rush University System for Health (RUSH) to identify cardiovascular risk factors associated with increased mortality and major adverse cardiovascular events (MACE; a composite of cardiovascular death, stroke, myocardial injury, and heart failure exacerbation). Multivariable logistic regression was used to adjust for demographic data and comorbid conditions.
Results
Of the 1682 patients who met inclusion criteria, the median age was 59. Patients were predominantly African American (34.4 %) and male (54.5 %). Overall, 202 (12 %) patients suffered 60-day mortality. In the multivariable model that assessed risk factors for 60-day mortality, age 60–74 (adjusted odds ratio [aOR] 3.30 [CI: 1.23–10.62]; p < 0.05) and age 75–100 (aOR 4.52 [CI: 1.46–16.15]; p < 0.05) were significant predictors when compared to those aged 19 to 39. This model also showed that those with past medical histories of atrial fibrillation (aOR 2.47 [CI: 1.38–4.38]; p < 0.01) and venous thromboembolism (aOR 2.00 [CI: 1.12–3.50]; p < 0.05) were at higher risk of 60-day mortality.
Conclusion
In this cohort, patients over 60 years old with a pre-existing history of atrial fibrillation and venous thromboembolism were at increased risk of mortality from COVID-19.
Keywords: COVID-19, Major adverse cardiovascular events, Venous thromboembolism, Atrial fibrillation, 60-day outcomes
1. Introduction
The coronavirus disease of 2019 (COVID-19) continues to pose major public health implications. The COVID-19 disease caused by SARS-CoV-2 has infected >335 million and resulted in 6.3 million fatalities as of July 2022 [1]. The total cost of the pandemic is estimated at more than $16 trillion, or approximately 90 % of the annual gross domestic product of the US [2]. Risk factors associated with increased mortality include older age, male sex, obesity, myocardial infarction, congestive heart failure, dementia, chronic pulmonary disease, renal disease, and solid metastatic tumor [3], [4], [5], [6]. Regarding cardiovascular-specific risk factors, a study by Pareek et al. demonstrated that age and previous ventricular arrhythmia were associated with increased mortality [7]. Further, after adjusting for confounding variables, atrial fibrillation has been associated with increased mortality [8]. However, data on 60-day outcomes in patients with pre-existing cardiovascular conditions are still limited.
To further elucidate comorbidities associated with poor COVID-19 prognosis, this retrospective cohort study of 1682 hospitalized COVID-19 patients utilized multivariable logistic regression to identify independent risk factors for 60-day mortality.
2. Methods
This was a retrospective cohort study of patients ≥18 years old with COVID-19, confirmed by polymerase chain reaction, March to November 2020 who were admitted to Rush University System for Health (RUSH), a 664-bed academic medical center and two affiliated community hospitals with 411 beds. Data were collected through a combination of automatic and manual extraction methods. The medical record of each patient included in the study was followed and reviewed by physician investigators for a minimum of 60 days from the first day of their COVID-19 admission by chart review of both the RUSH system. Records from local hospitals that use EPIC electronic medical records (EPIC Systems, Verona, WI) were also reviewed.
Comorbidities were defined with automatically extracted data from the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes.
Patient records were manually reviewed for major complications up to 60 days from hospital admission, which included myocardial injury defined as cardiac troponin I > 0.09 ng/mL (Troponin I; Roche Diagnostics™, normal limits 0.0 ng/ml to 0.09 ng/ml), sustained ventricular arrhythmia, deep venous thrombosis, symptoms of acute heart failure, acute renal failure requiring renal replacement therapy, or pulmonary embolism. Readmission and mortality data were also collected from the chart review. If there was no mortality documented, the patient was counted as alive. Race was self-identified and extracted as such from the medical record. Patients of Hispanic and Latino origin were categorized as “Other” in the medical records and further identified into Hispanic and Latino ethnicity.
The study's primary outcome was 60-day mortality. The secondary outcomes were in-hospital mortality, intubation, and 60-day major adverse cardiovascular events (MACE), a composite outcome consisting of cardiovascular death, stroke, myocardial injury, and heart failure exacerbation (Table 1).
Table 1.
Baseline characteristics stratified by 60-day survival versus mortality.
| Survived | 60-Day Mortality | ||
|---|---|---|---|
| n | 1480 | 202 | |
| Age (median [IQR]) | 57.00 [45.00, 69.00] | 67.00 [58.00, 76.00] | <0.001 |
| Male Sex (%) | 799 (54.0) | 117 (57.9) | 0.328 |
| BMI (median [IQR]) | 30.90 [26.37, 37.02] | 30.30 [26.20, 36.50] | 0.281 |
| Race (%) | 0.029 | ||
| White | 382 (25.8) | 68 (33.7) | |
| African American | 527 (35.6) | 52 (25.7) | |
| Asian | 21 (1.4) | 5 (2.5) | |
| Other | 464 (31.4) | 63 (31.2) | |
| Unknown or patient declined to provide | 86 (5.8) | 14 (6.9) | |
| Current smoker (%) | 71 (5.2) | 6 (3.6) | 0.474 |
| Atrial fibrillation (%) | 183 (12.4) | 71 (35.1) | <0.001 |
| Coronary artery disease (%) | 364 (24.6) | 82 (40.6) | <0.001 |
| Hypertension (%) | 927 (62.6) | 147 (72.8) | 0.006 |
| COPD (%) | 109 (7.4) | 27 (13.4) | 0.005 |
| Diabetes mellitus (%) | 673 (45.5) | 101 (50.0) | 0.256 |
| Asthma (%) | 216 (14.6) | 19 (9.4) | 0.059 |
| Cancer (%) | 182 (12.3) | 30 (14.9) | 0.361 |
| Ventricular ARRHYTHMIA (%) | 53 (3.6) | 26 (12.9) | <0.001 |
| Stroke (%) | 212 (14.3) | 35 (17.3) | 0.305 |
| Peripheral artery disease (%) | 136 (9.2) | 17 (8.4) | 0.820 |
| Myocardial infarction (%) | 202 (13.6) | 57 (28.2) | <0.001 |
| Venous thromboembolism (%) | 193 (13.0) | 52 (25.7) | <0.001 |
| Life-threatening arrhythmia (%) | 16 (1.1) | 10 (5.0) | <0.001 |
| Heart failure (%) | 299 (20.2) | 69 (34.2) | <0.001 |
| Hyperlipidemia (%) | 696 (47.0) | 113 (55.9) | 0.021 |
| Obstructive sleep apnea (%) | 231 (15.6) | 203 (11.4) | 0.142 |
| Pacemaker or ICD (%) | 49 (3.3) | 10 (5.0) | 0.325 |
| Interstitial lung disease (%) | 29 (2.0) | 11 (5.4) | 0.005 |
| HIV (%) | 12 (0.8) | 1 (0.5) | 0.958 |
| Dementia (%) | 119 (8.0) | 21 (10.4) | 0.317 |
| Peptic ulcer disease (%) | 51 (3.4) | 7 (3.5) | 1.000 |
| Cirrhosis (%) | 36 (2.4) | 15 (7.4) | <0.001 |
| Pulmonary hypertension (%) | 67 (4.5) | 18 (8.9) | 0.013 |
| Chronic kidney disease (%) | 345 (23.3) | 78 (38.6) | <0.001 |
Abbreviations: IQR = interquartile range; BMI = body mass index; COPD = chronic obstructive pulmonary disorder; HIV = human immunodeficiency virus.
All data analysis, including statistical analyses, was performed using RStudio version 1.3 (Boston, Massachusetts). Continuous variables were compared with t-tests or Wilcoxon rank-sum test, and categorical variables with the Pearson chi-square test. Continuous variables are reported with mean and standard deviation for normally distributed variables and with median and interquartile range for variables not normally distributed. Categorical variables are reported as counts and proportions.
Logistic regression was performed between the comorbidities as predictors for 60-day mortality in one model, in-hospital mortality in a second model, MACE in a third model, and intubation in a fourth model. All covariates present in Table 2, Table 3, Table 4, Table 5 were included in a single model to predict 60-day mortality, in-hospital mortality, MACE, and intubation respectively. Including all the comorbidities into a single model for each outcome allows for assessing each covariate independently by adjusting the other variables in the model. Covariates for each model were chosen after review of existing literature describing risk factors for adverse outcomes in COVID-19 with preference given to variables with previously significant findings. Adjusted odds ratios (aOR) with 95 % confidence intervals (CI) are reported for logistic regression. The threshold for statistical significance was set to a p-value <0.05.
Table 2.
Multivariable model for risk factors of 60-day mortality.
| Adjusted OR (CI) | p-value | |
|---|---|---|
| Age | ||
| 18–39 | 1 [Ref] | – |
| 40–59 | 1.42 (0.54–4.47) | 0.507 |
| 60–74 | 3.30 (1.23–10.62) | 0.027 |
| 75–100 | 4.52 (1.46–16.15) | 0.013 |
| Race | ||
| White | 1 [Ref] | – |
| African American | 0.60 (0.33–1.09) | 0.093 |
| Asian | 0.36 (0.02–2.37) | 0.376 |
| Other | 1.14 (0.65–2.04) | 0.643 |
| BMI | ||
| <25 | 1 [Ref] | – |
| 25–30 | 0.98 (0.47–2.08) | 0.955 |
| >30 | 1.37 (0.71–2.79) | 0.362 |
| Comorbidities | ||
| Atrial fibrillation | 2.47 (1.38–4.38) | 0.002 |
| CAD | 0.87 (0.41–1.77) | 0.712 |
| Hypertension | 0.69 (0.38–1.26) | 0.225 |
| COPD | 1.00 (0.43–2.21) | 0.993 |
| Asthma | 0.63 (0.28–1.30) | 0.232 |
| Ventricular arrhythmia | 2.19 (0.97–4.73) | 0.050 |
| Myocardial infarction | 1.58 (0.74–3.46) | 0.241 |
| Venous thromboembolism | 2.00 (1.12–3.50) | 0.017 |
| Heart failure | 0.83 (0.45–1.49) | 0.532 |
| Hyperlipidemia | 0.81 (0.48–1.38) | 0.444 |
| Interstitial lung disease | 1.51 (0.45–4.46) | 0.475 |
| Cirrhosis | 2.68 (0.93–7.13) | 0.055 |
| Pulmonary hypertension | 0.84 (0.31–2.09) | 0.718 |
| Chronic kidney disease | 0.78 (0.44–1.37) | 0.397 |
| Prior glycemic control | ||
| HbA1c < 6.5 | 1 [Ref] | – |
| HbA1c 6.5–7.4 | 1.95 (1.06–3.58) | 0.031 |
| HbA1c 7.5–8.4 | 2.01 (0.97–4.07) | 0.056 |
| HbA1c ≥ 8.5 | 1.15 (0.62–2.10) | 0.654 |
Abbreviations: OR = odds ratio; CI = confidence interval; BMI = body mass index; COPD = chronic obstructive pulmonary disorder; DM = diabetes mellitus; HbA1c = hemoglobin A1c.
Table 3.
Multivariable model for risk factors of in-hospital mortality.
| Adjusted OR (CI) | p-value | |
|---|---|---|
| Age | ||
| 18–39 | 1 [Ref] | – |
| 40–59 | 1.03 (0.41–3.00) | 0.946 |
| 60–74 | 2.46 (0.96–7.24) | 0.077 |
| 75–100 | 1.82 (0.57–6.29) | 0.326 |
| Race | ||
| White | 1 [Ref] | – |
| African American | 0.64 (0.33–1.24) | 0.187 |
| Asian | 0.38 (0.02–2.61) | 0.413 |
| Other | 1.29 (0.71–2.40) | 0.408 |
| BMI | ||
| <25 | 1 [Ref] | – |
| 25–30 | 1.00 (0.46–2.25) | 0.999 |
| >30 | 1.08 (0.53–2.32) | 0.838 |
| Comorbidities | ||
| Atrial fibrillation | 2.52 (1.35–4.65) | 0.003 |
| CAD | 0.67 (0.28–1.50) | 0.345 |
| Hypertension | 0.57 (0.31–1.05) | 0.070 |
| COPD | 1.14 (0.45–2.67) | 0.776 |
| Asthma | 0.79 (0.34–1.66) | 0.554 |
| Ventricular arrhythmia | 1.88 (0.78–4.24) | 0.141 |
| Myocardial infarction | 2.02 (0.86–4.99) | 0.115 |
| Venous thromboembolism | 2.10 (1.14–3.78) | 0.015 |
| Heart failure | 0.85 (0.44–1.60) | 0.621 |
| Cirrhosis | 2.13 (0.64–6.03) | 0.179 |
| Chronic kidney disease | 0.65 (0.34–1.20) | 0.178 |
| Prior glycemic control | ||
| HbA1c < 6.5 | 1 [Ref] | – |
| HbA1c 6.5–7.4 | 1.54 (0.79–2.94) | 0.195 |
| HbA1c 7.5–8.4 | 1.66 (0.75–3.53) | 0.195 |
| HbA1c ≥ 8.5 | 0.87 (0.45–1.64) | 0.667 |
Abbreviations: OR = odds ratio; CI = confidence interval; BMI = body mass index; HbA1c = hemoglobin A1c.
Table 4.
Multivariable model for risk factors of 60-day major adverse cardiovascular events.
| Adjusted OR (CI) | p-value | |
|---|---|---|
| Age | ||
| 18–39 | 1 [Ref] | – |
| 40–59 | 2.27 (0.55–15.66) | 0.315 |
| 60–74 | 2.50 (0.62–17.16) | 0.257 |
| 75–100 | 2.17 (0.44–16.46) | 0.382 |
| Race | ||
| White | 1 [Ref] | – |
| African American | 0.98 (0.44–2.24) | 0.961 |
| Asian | 1.36 (0.07–9.37) | 0.792 |
| Other | 0.65 (0.25–1.68) | 0.377 |
| BMI | ||
| <25 | 1 [Ref] | – |
| 25–30 | 0.79 (0.25–2.63) | 0.692 |
| >30 | 1.26 (0.47–3.82) | 0.661 |
| Comorbidities | ||
| Atrial fibrillation | 0.64 (0.26–1.46) | 0.302 |
| Ventricular arrhythmia | 2.19 (0.77–5.65) | 0.121 |
| Myocardial infarction | 5.98 (2.95–12.35) | < 0.001 |
| Venous thromboembolism | 1.24 (0.57–2.6) | 0.573 |
| Chronic kidney disease | 0.73 (0.33–1.56) | 0.420 |
| Heart failure | 2.00 (0.96–4.16) | 0.062 |
| Prior glycemic control | ||
| HbA1c < 6.5 | 1 [Ref] | – |
| HbA1c 6.5–7.4 | 1.03 (0.42–2.36) | 0.952 |
| HbA1c 7.5–8.4 | 1.04 (0.32–2.86) | 0.949 |
| HbA1c ≥ 8.5 | 0.57 (0.23–1.34) | 0.208 |
Abbreviations: OR = odds ratio; CI = confidence interval; BMI = body mass index; HbA1c = hemoglobin A1c.
Table 5.
Multivariable model for risk factors of intubation.
| Adjusted OR (CI) | p-value | |
|---|---|---|
| Age | ||
| 18–39 | 1 [Ref] | – |
| 40–59 | 1.12 (0.59–2.20) | 0.728 |
| 60–74 | 1.30 (0.65–2.65) | 0.463 |
| 75–100 | 0.46 (0.19–1.11) | 0.085 |
| Race | ||
| White | 1 [Ref] | – |
| African American | 0.87 (0.52–1.43) | 0.572 |
| Asian | 1.52 (0.36–6.11) | 0.557 |
| Other | 2.38 (1.47–3.92) | 0.001 |
| BMI | ||
| < 25 | 1 [Ref] | – |
| 25–30 | 1.57 (0.84–3.02) | 0.165 |
| > 30 | 1.88 (1.07–3.43) | 0.034 |
| Comorbidities | ||
| Atrial fibrillation | 1.96 (1.17–3.29) | 0.011 |
| CAD | 0.85 (0.45–1.55) | 0.606 |
| Hypertension | 1.11 (0.70–1.76) | 0.652 |
| COPD | 1.23 (0.61–2.45) | 0.554 |
| Asthma | 1.17 (0.66–2.04) | 0.586 |
| Ventricular arrhythmia | 2.17 (0.99–4.79) | 0.053 |
| Myocardial infarction | 4.80 (2.55–9.29) | <0.001 |
| Venous thromboembolism | 4.42 (2.73–7.23) | <0.001 |
| Heart failure | 0.96 (0.58–1.55) | 0.860 |
| Cirrhosis | 1.37 (0.52–3.50) | 0.519 |
| Chronic kidney disease | 0.89 (0.55–1.41) | 0.607 |
| Prior glycemic control | ||
| HbA1c < 6.5 | 1 [Ref] | – |
| HbA1c 6.5–7.4 | 1.83 (1.10–3.04) | 0.020 |
| HbA1c 7.5–8.4 | 1.23 (0.66–2.27) | 0.507 |
| HbA1c ≥ 8.5 | 0.69 (0.44–1.10) | 0.120 |
Abbreviations: OR = odds ratio; CI = confidence interval; BMI = body mass index; HbA1c = hemoglobin A1c.
Due to the significant association between glycemic control and cardiovascular disease, glycemic control was assessed regardless of diabetes mellitus status with the last hemoglobin A1c within one year before patients' COVID-19 admission. To evaluate the relationship between glycemic control and the outcomes of this study, HbA1c was additionally plotted using univariable local regression with locally estimated scatterplot smoothing (LOESS) to graphically display estimated outcome probabilities across the range of values.
3. Results
A total of 1682 patients were included in our cohort with a median age of 59 years old (interquartile range [IQR] 46–71), and BMI was 30.8 (IQR 26.3–37.0). In terms of race, 579 (34.4 %) were African American, 450 (26.6 %) White, 26 (1.5 %) Asian, and 527 (31.3 %) patients identified as “Other” race. For 100 (5.9 %) patients, race was either unknown or the patient declined to answer when asked. There were 77 (4.6 %) patients who were current smokers. The median length of stay was 6 days (IQR 3–11). A total of 153 patients (9.1 %) in the cohort died during their index COVID-19 hospitalization.
In the overall cohort, the incidence of various cardiovascular comorbidities included atrial fibrillation in 254 (15.1 %), hypertension in 608 (36.1 %), history of ventricular arrhythmias in 79 (4.7 %), history of myocardial infarction in 259 (15.4 %), history of heart failure in 368 (21.9 %), and hyperlipidemia in 809 (48.1 %). In terms of chronic respiratory disease, chronic obstructive pulmonary disease, asthma, and interstitial lung disease were each present in 136 (8.1 %), 235 (14.0 %), and 40 (2.4 %) patients, respectively.
3.1. Primary 60-day mortality outcome
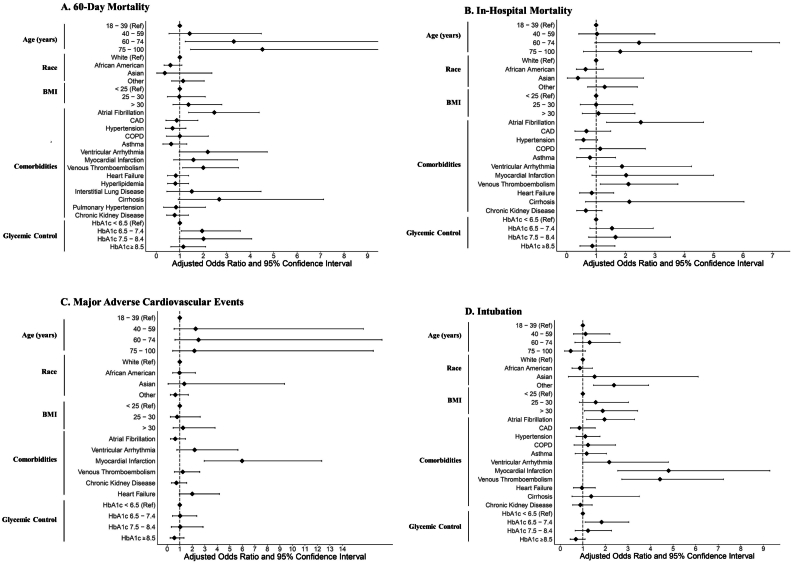
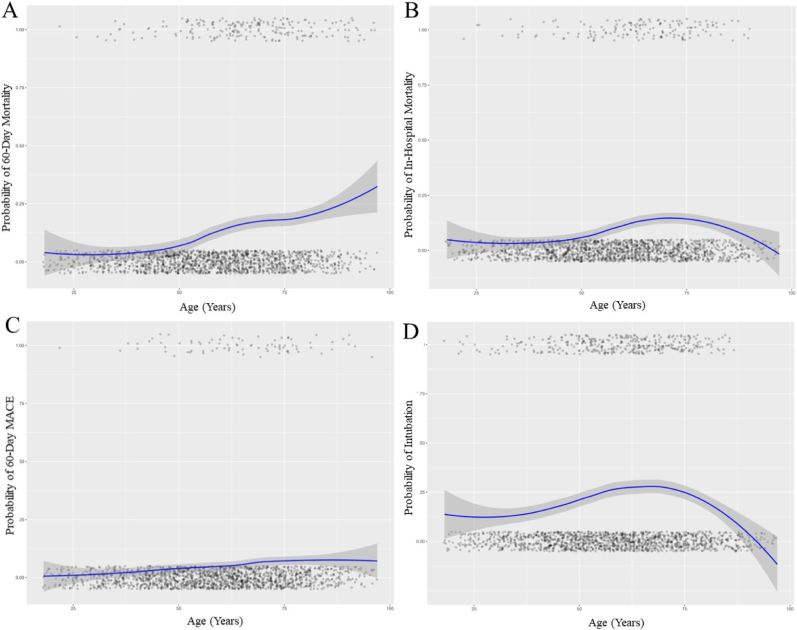
A total of 202 (12.0 %) patients in the cohort died within 60 days of their COVID-19 admission. Compared to the age 18–39 group, 60 to 74-year-old patients (aOR 3.30 [CI: 1.23–10.62]; p < 0.05) and 75 to 100-year-old patients (aOR 4.52 [CI: 1.46–16.15]; p < 0.05) had increased 60-day mortality (Fig. 1A; Table 2). The probability of mortality was plotted across the continuous range of ages using LOESS, demonstrated in Fig. 2A. Race and body mass index were not significant predictors.
Fig. 1.
Forest plots depicting adjusted odds ratios and 95 % confidence intervals for (A) 60-day mortality, (B) in-hospital mortality, (C) major adverse cardiac events, and (D) intubation.
Fig. 2.
LOESS curve (blue) demonstrating the probability of (A) 60-day mortality, (B) in-hospital mortality, (C) major adverse cardiovascular events, and (D) intubation by age. Gray shading indicates 95 % confidence intervals. Each point represents an individual patient within the dataset. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Compared to patients without each individual cardiovascular comorbidity, those with a history of atrial fibrillation (aOR 2.47 [CI: 1.38–4.38]; p < 0.01) and venous thromboembolism (aOR 2.00 [CI: 1.12–3.50]; p < 0.05) were associated with increased 60-day mortality. A statistically trending effect was found for those with a history of cirrhosis (aOR 2.68 [CI: 0.93–7.13]; p = 0.06) and ventricular arrhythmia (aOR 2.19 [CI: 0.97–4.73]; p = 0.05). The other comorbidities in the model, including coronary artery disease (CAD), chronic obstructive pulmonary disease, prior myocardial infarction, and chronic kidney disease, were not significant predictors.
In our cohort, 751 (44.6 %) patients had HbA1c measurements within one year of hospitalization. The median HbA1c value was 6.9 (IQR 6.00–9.00) with a range of 4.20 to 14.71. These patients were clustered by HbA1c into the following groups: <6.5 (299 patients, 39.8 %), 6.5–7.4 (131 patients, 17.4 %), 7.5–8.4 (82 patients, 10.9 %), and ≥8.5 (239 patients, 31.8 %).
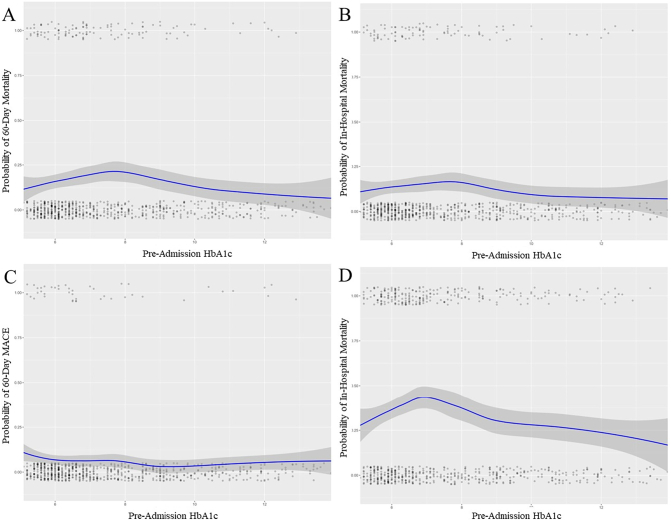
When compared to patients with a HbA1c < 6.5, a HbA1c of 6.5–7.4 was a significant predictor of 60-day mortality (aOR 1.95 [CI: 1.06–3.58]; p < 0.05). A statistically trending effect was found for those with a HbA1c of 7.5–8.4 (aOR 2.01 [CI: 0.97–4.07]; p = 0.06), and no predictive value was identified in those with an HbA1c ≥ 8.5 (aOR 1.15 [CI: 0.62–2.10]; p = 0.65). The probability of mortality was plotted across the continuous range of HbA1c levels using LOESS, demonstrated in Fig. 3A.
Fig. 3.
LOESS curve (blue) demonstrating the probability of (A) 60-day mortality, (B) in-hospital mortality, (C) major adverse cardiovascular events, and (D) intubation by pre-hospitalization hemoglobin A1c level. Gray shading indicates 95 % confidence intervals. Each point represents an individual patient within the dataset.
Abbreviations: MACE = major adverse cardiovascular events. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Secondary in-hospital mortality outcome
A total of 153 patients (9.1 %) suffered in-hospital mortality during their admission. There were no significant differences in the risk of in-hospital mortality across age or BMI groups and race (Table 3, Fig. 1B). The probability of mortality was plotted across the continuous range of ages using LOESS, demonstrated in Fig. 2B. Those with a pre-existing history of atrial fibrillation (aOR 2.52 [CI: 1.35–4.65]; p < 0.05) and venous thromboembolism (aOR 2.10 [CI: 1.14–3.78]; p < 0.05) were at increased risk for in-hospital mortality compared to patients without each comorbidity.
When examining the degree of glycemic control among patients with DM, no significantly increased risk was present for each HbA1c when compared to diabetic patients with an HbA1c < 6.5. The probability of in-hospital mortality was plotted across the continuous range of HbA1c levels using LOESS, demonstrated in Fig. 3B.
3.3. Secondary 60-day MACE outcome
A total of 124 (7.3 %) patients in the cohort suffered MACE within 60-days of hospitalization. When the individual components of the composite MACE outcome were assessed, heart failure exacerbation was the most common individual outcome (49 events, 2.9 % incidence rate in the cohort) followed by myocardial injury (44, 2.6 %; Supplemental Table 1). Of note, nonfatal stroke only occurred four times (0.2 %) in the cohort.
In a multivariable model to assess predictors for 60-day MACE, age, BMI, and race were not statistically significant (Fig. 1c; Table 4). The probability of mortality was plotted across the continuous range of ages using LOESS, demonstrated in Fig. 2C.
Of the comorbidities included in the model, prior myocardial infarction (aOR 5.98 [CI: 2.95–12.35]; p < 0.001) was a statistically significant predictor of 60-day MACE when compared to those without a prior myocardial infarction. A statistically trending effect was present in patients with a history of heart failure (aOR 2.00 [CI: 0.96–4.16]; p = 0.06).
No significant difference was present across the HbA1c groups. The probability of 60-day MACE was plotted across the continuous range of HbA1c levels using LOESS as shown in Fig. 3C.
3.4. Secondary intubation outcome
A total of 352 patients (20.9 %) required intubation and mechanical ventilation during their admission. There were no significant differences in the risk of intubation across ages (Table 5, Fig. 1D). The probability of intubation was plotted across the continuous range of ages using LOESS, demonstrated in Fig. 2D. Compared the White patients, the category of “other” races was more likely to require intubation (aOR 2.38 [CI: 1.47–3.92]; p < 0.01). Those with a history of atrial fibrillation (aOR 1.96 [CI: 1.17–3.29]; p < 0.05), myocardial infarction (aOR 4.80 [CI: 2.55–9.29]; p < 0.001), and venous thromboembolism (aOR 4.42 [CI: 2.73–7.23]; p < 0.001) were at higher risk for intubation compared to those without each comorbidity.
Those with a HbA1c of 6.5–7.4 were at increased risk for intubation when compared to those with a HbA1c < 6.5 (aOR 1.83 [CI: 1.10–3.04]; p < 0.01). No statistically significant differences were present in the other glycemic control groups. The probability of intubation was plotted across the continuous range of HbA1c levels using LOESS, demonstrated in Fig. 3D.
4. Discussion
In this retrospective study of patients admitted with COVID-19, age > 60 years old, prior history of atrial fibrillation, venous thromboembolism, and pre-admission HbA1c value of 6.5–7.4 were each independent risk factors for 60-day mortality. Other pre-existing cardiovascular conditions such as CAD, hypertension, prior myocardial infarction, pre-existing heart failure and hyperlipidemia were not associated with increased mortality. When MACE was used as the outcome, prior history of myocardial infarction was an independent risk factor. Risk factors for intubations included patients with a history of atrial fibrillation, myocardial infarction, venous thromboembolism, and diabetes with a HbA1c of 6.5–7.4.
This observational cohort study of COVID-19 hospitalized patients showed 9.1 % in-hospital mortality and 12 % 60-day mortality at the pandemic onset. This mortality rate was similar to other studies in the literature with in-hospital mortality rates ranging from 10.6 % in March 2020, increasing to 19.7 % in April 2020 and decreasing to 9.3 % in November 2020 [9]. Mortality at 60 days has been reported to be 29.2 % for the months of March to July 2020 [10].
Age > 60 years old was associated with a higher risk of 60-day mortality, as illustrated in Fig. 2A. This is consistent with prior studies demonstrating increased mortality with increasing age compared to younger individuals. [11], [12]. Several hypotheses have been proposed to explain the increase in mortality, including frailty, decreased immune response, age-related decline in respiratory function, and multiple comorbidities [13], [14]. Risk factors such as high systolic pressure, low forced expiratory volume in 1 s, and multiple long-term conditions could partially explain the higher mortality. Nonetheless, older age was an independent risk factor for mortality [15]. Preventive measures should focus on targeting older adults.
Our findings support prior studies that have identified a previous history of atrial fibrillation as a risk factor for increased COVID-19 mortality and morbidity [16], [17], [18]. The etiology of atrial arrhythmia is multifactorial, including inflammatory response secondary to viral injury, intravascular volume depletion, metabolic derangements, and use of vasopressors [16]. Similar to the sepsis literature, atrial fibrillation is an independent risk factor for mortality for patients admitted to the hospital with COVID-19 [19], [20]. The mechanism by which atrial fibrillation contributes to increased mortality is unclear and beyond the scope of this study but could be due to hemodynamic instability from loss of atrial kick or rapid ventricular response [21].
In addition, our study confirms that patients with venous thromboembolic (VTE) disease have a higher risk of 60-day mortality, as shown in previous studies [22]. This could be due to direct endothelial injury by viral particles, stasis from immobilization, and elevated prothrombotic factors [23], [24], [25] Increased mortality could also be secondary to the significant increased microvascular and macrovascular thrombotic burden in nearly every organ, as evidenced by autopsy reports [26], [27], [28].
While other studies have described diabetes mellitus as an independent risk factor for mortality for COVID-19 [[30], [31], [32]], our study went one step further by exploring the effect of pre-admission glycemic control of diabetic patients on COVID-19 outcomes. When adjusting for HbA1c, only patients with A1C of 6.5 to 7.4 % had an increased risk of 60-day mortality. This could be because there is a strong association between acute phase reactants and A1C [33]; not reflecting accurate glycemic control in this patient population.
The study's major limitations include using an electronic health system that has inherent sources for error. The diagnosis of cardiac conditions was made using ICD-10 codes, which are specific but not sensitive [34]. The diagnosis of COVID-19 was based on either positive polymerase chain reaction (PCR) or point of care rapid testing with a sensitivity of 71 % and specificity of 100 % [35]. The dataset captured 60-day events for patients who had a subsequent hospitalization to a health care system in or around Chicago using the EPIC electronic medical record (EPIC Systems, Verona, WI). The HbA1c analysis could have been subjected to bias as patients who had A1C tested could have been more critically ill. In addition, 55.4 % of patients did not have A1C recorded within one year of hospitalization or could have obtained HbA1C outside of the RUSH system. Lastly, 31.3 % of patients were categorized in “Other” race. The large percentage of this “Other” race could be because patients of Hispanic and Latino origin were categorized as “Other” in the electronic medical record and further classified into Hispanic and Latino ethnicity. However, this does not exclude the possibility that races besides Hispanic and Latino origins were included in this category. This represents a limitation in interpreting the outcomes of COVID-19 and race.
5. Conclusion
This study demonstrates that factors associated with increased mortality in COVID-19 were age > 60 years old, pre-existing atrial fibrillation and venous thromboembolism. MACE was significantly associated with increased mortality rates. We found that patients with other pre-existing cardiovascular conditions were not at increased risk of adverse outcomes from COVID-19. Furthermore, these observations were at the beginning of the COVID-19 pandemic and may not represent more recently hospitalized patients with COVID-19. More studies are needed to further elucidate the mechanism of these factors' association with COVID-19 mortality.
The following is the supplementary data related to this article.
Individual components of the composite MACE outcome by 60-day survival versus mortality.
Funding
The authors received no financial support for the research, authorship, or publication of this manuscript.
CRediT authorship contribution statement
Mina Medhat Kerolos: Methodology, Validation, Investigation, Writing – original draft, Project administration. Max Ruge: Methodology, Formal analysis, Writing – original draft. Ahmad Gill: Investigation, Writing – original draft. Maria Isabel Planek: Investigation, Writing – review & editing. Annabelle Santos Volgman: Conceptualization, Writing – review & editing, Supervision. Jeanne M. Du-Fay-De-Lavallaz: Methodology, Validation, Writing – review & editing. Joanne Michelle D. Gomez: Writing – review & editing. Tisha Marie Suboc: Writing – review & editing. Kim A. Williams: Validation, Writing – review & editing. Salaheldin Abusin: Conceptualization, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Center Johns Hopkins Coronavirus Resource. https://coronavirus.jhu.edu/ Available at.
- 2.Cutler D.M., Summers L.H. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–1496. doi: 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., GYH Lip. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003321. Published 2020 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izcovich A., Ragusa M.A., Tortosa F., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241955. Published 2020 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins J.L., Masoli J.A.H., Delgado J., et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kompaniyets L., Goodman A.B., Belay B., et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):355–361. doi: 10.15585/mmwr.mm7010e4. Published 2021 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pareek M., Singh A., Vadlamani L., et al. Relation of cardiovascular risk factors to mortality and cardiovascular events in hospitalized patients with coronavirus disease 2019 (from the Yale COVID-19 cardiovascular Registry) Am. J. Cardiol. 2021;146:99–106. doi: 10.1016/j.amjcard.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris S., Inciardi R.M., Lombardi C.M., et al. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID-19 patients: results of the cardio-COVID-Italy multicentre study. Europace. 2021;23(10):1603–1611. doi: 10.1093/europace/euab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finelli L., Gupta V., Petigara T., Yu K., Bauer K.A., Puzniak L.A. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw. Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6556. Published 2021 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann. Intern. Med. 2021;174(4):576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanez N.D., Weiss N.S., Romand J.A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1) doi: 10.1186/s12889-020-09826-8. Published 2020 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonanad C., García-Blas S., Tarazona-Santabalbina F., et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. Correction to: SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(3):1013. doi: 10.1007/s11357-020-00193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montero-Odasso M., Hogan D.B., Lam R., Madden K., MacKnight C., Molnar F., Rockwood K. Age alone is not adequate to determine health-care resource allocation during the COVID-19 pandemic. Can. Geriatr. J. 2020 Mar 1;23(1):152–154. doi: 10.5770/cgj.23.452. PMID: 32550953; PMCID: PMC7279701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho F.K., Petermann-Rocha F., Gray S.R., et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241824. Published 2020 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peltzer B., Manocha K.K., Ying X., et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31(12):3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merino J.L., Caro J., Rey J.R., Castrejon S., Martinez-Cossiani M. Cardiac arrhythmias in COVID-19: mechanisms, outcomes and the potential role of proarrhythmia. Europace. 2021;23(Suppl 3) doi: 10.1093/europace/euab116.115. Published 2021 May 24. [DOI] [Google Scholar]
- 18.Coromilas E.J., Kochav S., Goldenthal I., et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 2021;14(3) doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G.L., Morris P.E. Incidence and prognosis of atrial fibrillation in patients with sepsis. Cardiol. Res. 2011;2(6):293–297. doi: 10.4021/cr108w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mountantonakis S.E., Saleh M., Fishbein J., et al. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm. 2021;18(4):501–507. doi: 10.1016/j.hrthm.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip R.J., Ali A., Baloch Z.Q., et al. Atrial fibrillation as a predictor of mortality in high risk COVID-19 patients: a multicentre study of 171 patients. Heart Lung Circ. 2021;30(8):1151–1156. doi: 10.1016/j.hlc.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollias A., Kyriakoulis K.G., Lagou S., Kontopantelis E., Stergiou G.S., Syrigos K. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc. Med. 2021;26(4):415–425. doi: 10.1177/1358863X21995566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. Published 2020 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barron E., Bakhai C., Kar P., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 32.Bode B., Garrett V., Messler J., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States [published correction appears in J Diabetes Sci Technol. 2020 Jun 10;:1932296820932678] J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan K.C., Chow W.S., Tam S., Bucala R., Betteridge J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes Care. 2004;27(1):223–228. doi: 10.2337/diacare.27.1.223. [DOI] [PubMed] [Google Scholar]
- 34.Quan H., Li B., Saunders L.D., et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv. Res. 2008;43(4):1424–1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson K.E., Caliendo A.M., Arias C.A., et al. The Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing [published online ahead of print, 2021 jan 22] Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual components of the composite MACE outcome by 60-day survival versus mortality.