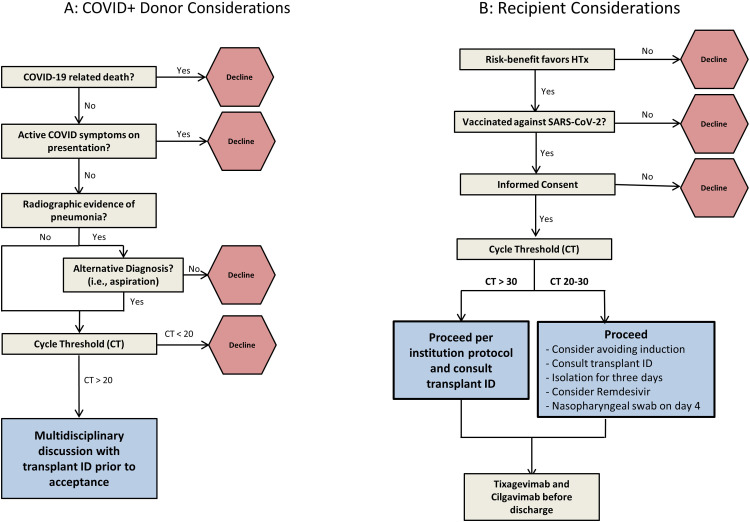
The ongoing COVID-19 pandemic has profoundly impacted many aspects of patient care, including heart transplantation (HTx). Early in the pandemic, several transplant societies, recommended against transplanting grafts from SARS-CoV-2-positive (SARS-CoV-2+) donors given the potential risk for transmission of the virus and risk of allograft dysfunction.1 , 2 However, multiple recent case reports in solid organ transplants have noted nontransmission of the virus.3
We report our single-center outcomes experience of eight HTx recipients of SARS-CoV-2+ donors as well as our proposed protocol for donor evaluation and recipient monitoring. (Figure 1 A: donor selection, 1B: recipient workflow). Data points for donors include cause of death, current or prior symptoms of COVID-19, sample source (nasal swab versus sputum versus bronchial alveolar lavage), type of test (polymerase chain reaction or PCR test vs antigen test), cycle threshold (CT) value and radiographic review. Albeit conservatively, we tended to decline the offer if the CT value was <20, while unknown or higher values would trigger further, multidisciplinary discussion. For recipients, the key considerations are assessment of the risk-benefit ratio of accepting a SARS-CoV-2+ donor, vaccination status, informed consent, adjustment of immunosuppression (if applicable) and post-transplant monitoring. Lastly, a protocol for the procurement team, implanting surgical team, and the post-operative team were developed. Post-transplant, patients are isolated for 3 days and a nasopharyngeal PCR test for SARS-CoV-2 is performed on day 4.
Figure 1.
Title: SARS-CoV-2 positive donor considerations and recipient considerations.
Figure 1 Description.
Figure 1A: Proposed algorithm to evaluate donor information if they test positive for SARS-CoV-2; Figure 1B: Proposed algorithm for post-transplant management of the recipient if the donor tested positive for SARS-CoV-2. CT, cycle threshold; ID, infectious diseases team; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Table 1 lists information about our recipients who received a SARS-CoV-2+ donor including the UNOS listing status, immunosuppression strategy, and outcomes. All our recipients were vaccinated at the time of HTx, as per our institution policy. Post HTx, they were tested with a nasopharyngeal swab for SARS-CoV-2 on day 4 and were negative. Four of 8 patients received basiliximab, in addition to the standard triple regimen of tacrolimus, mycophenolate and prednisone but these decisions were made to account for their individual risk of rejection/infection or need to delay tacrolimus, unrelated to the donor being SARS-CoV-2+. At 90 days post-HTx, all recipients were doing well, with no evidence of viral transmission, clinically significant graft dysfunction, rejection, or other major adverse events. Median duration of follow-up is 204 days. There was no transmission of the virus to any member of the procurement or implanting surgical team. All patients receive 1 dose of tixagevimab and cilgavimab (Evushield, AstraZeneca Pharmaceuticals LP, Wilmington, DE) before hospital discharge. Table 2 lists donor information. COVID-19 was diagnosed as part of routine testing at the donor centers but was not the admitting/clinical diagnosis or cause of death for any of them. The vaccination status of these donors was often unknown.
Table 1.
Recipient Information
| Case number | UNOS status | SARS COV-2 antibody level (> 80 = positive) | Immunosuppression | Clinically significant rejection at any period (> Grade 1R ACR or any AMR) | Graft function - 90 days | Outcome till date |
|---|---|---|---|---|---|---|
| 1 | 3 | Basiliximab, Tacrolimus, prednisone | No | Normal LV, RV dysfunction | Alive at 1 year | |
| 2 | 6 | Basiliximab, Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 1 year | |
| 3 | 4 | Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 9 months | |
| 4 | 2 | Basiliximab, Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 6 months | |
| 5 | 1 | Basiliximab, Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 5 months | |
| 6 | 3 | Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 5 months | |
| 7 | 6 | >250 | Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 4 months |
| 8 | 3 | >250 | Tacrolimus, mycophenolate, prednisone | No | Normal | Alive at 3 months |
Abbreviations: ACR, acute cellular rejection; AMR, antibody-mediated rejection; BAL: Bronchoalveolar lavage COVID-19 PCR test; CT, PCR cycle threshold number; NP: nasopharyngeal COVID-19 PCR test; TA, Tracheal Aspirate COVID 19 PCR Test.
Table 2.
Donor Information
| Donor number | History of vaccination | Symptoms of COVID | Clinical context | Cause of death related to COVID? | Donor testing | LVEF of donor heart >55% |
|---|---|---|---|---|---|---|
| 1 | N/A | yes - fever and cough | Lower lobe consolidation related to bacterial pneumonia | No | NP + (D-2; CT 40), TA - (D-2), TA - (D-2), NP - (D-2) | Yes |
| 2 | N/A | No | Bilateral consolidations, repeat bronchoscopy negative | No | BAL - (D-5), BAL + (D-3), NP - (D-3), BAL - (D-3) | Yes |
| 3 | N/A | No | Bilateral perihilar ground-glass opacities, 2 negative bronchoscopies | No | NP - (D-3), BAL - (D-3), NP - (D-2), BAL - (D-2), BAL - right lung (D-1), BAL + left lung (D-1) | Yes |
| 4 | Yes | No | No | No | NP- (D-4), NP + (D-3) (CT 30.8 and 33.3), BAL - (D-2) | Yes |
| 5 | N/A | No | No | No | NP Antigen - (D-4), NP + (D-4), NP - (D-3), BAL - (D-3) | Yes |
| 6 | N/A | No | Bilateral multilobar consolidation; likely aspiration, negative bronchoscopy | No | NP + (D-1), BAL - (D-1) | Yes |
| 7 | N/A | No | Bilateral patchy, lower lobe consolidation, likely aspiration | No | NP (D-2) (CT 27.1), BAL (D-1) (CT 27.7) | Yes |
| 8 | Yes | No | No | No | NP + (CT 36), NP + (CT 36) | Yes |
Abbreviations: BAL, bronchoalveolar lavage; CT, cycle threshold; D, day of transplantation; N/A, not available; NP, nasopharyngeal swab; TA, tracheal aspirate.
Although recommendations from transplant societies are evolving,4 data regarding outcomes is sparse and recommendations are largely driven by expert recommendations. While there are reports of viral detection in the myocardium based on autopsy studies, there are no reported cases of SARS-CoV-2 infection transmission from a donor to HTx recipients to our knowledge.5 There is no data regarding the usage of CT values to guide clinical decision making, however, lower CT values are considered to reflect a higher viral load and vice versa and should be used as one of many data points when considering donors. We did not have the information of donor vaccination status in all cases but did not feel strongly that it would preclude our decision to consider those donors.
Understanding the safety and feasibility of SARS-CoV-2+ donors for HTx at individual centers will allow us to develop appropriate best practices and selection strategies, as the pandemic rages on. We propose that the use of hearts from asymptomatic SARS-CoV-2+ donors could be safe and effective and that such donors should be carefully evaluated in a multidisciplinary fashion. Our small sample-size results and proposed algorithm should be considered with caution, especially as new variants continue to mutate. Ongoing experience and evaluation of long-term outcomes will determine future best-practices in considering such donors.
Disclosure statement
The authors have no conflicts of interest to declare.
Anantha Sriharsha Madgula, Michael Nestasie, Christopher Link, Matthew M Lander, Deeksha Jandhyala and Candice Lee: None; Manreet K. Kanwar, MD: Advisory Board for Abiomed and CareDx.
References
- 1.Weiss MJ, Hornby L, Foroutan F, et al. Clinical practice guideline for solid organ donation and transplantation during the COVID-19 pandemic. Transpl Direct. 2021;7:e755. doi: 10.1097/txd.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.article CttC C4 article: implications of COVID-19 in transplantation. Am J Transpl. 2021;21:1801–1815. doi: 10.1111/ajt.16346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichenberger EM, Coniglio AC, Milano C, et al. Transplanting thoracic COVID-19 positive donors: an institutional protocol and report of the first 14 cases. J Heart Lung Transpl. 2022 doi: 10.1016/j.healun.2022.06.018. 2022/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss MJ, Lalani J, Patriquin-Stoner C, et al. Summary of international recommendations for donation and transplantation programs during the coronavirus disease pandemic. Transplantation. 2021;105:14–17. doi: 10.1097/tp.0000000000003520. [DOI] [PubMed] [Google Scholar]
- 5.Gaussen A, Hornby L, Rockl G, et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. doi: 10.1097/tp.0000000000003744. [DOI] [PubMed] [Google Scholar]