Abstract
Introduction
Hearing loss is one of the self-reported symptoms of Long COVID patients, however data from objective and subjective audiological tests demonstrating diminished hearing in Long COVID patients has not been published.
Materials and methods
Respondents of a large Long COVID online survey were invited to the ENT-department for an otologic exam. The participants were split into three groups based on their history of SARS-CoV-2 infection and persistence of symptoms. Respondents with a history of a SARS-CoV-2 infection were allocated to the Long COVID group, if they reported persistent symptoms and to the Ex COVID group, if they had regained their previous level of health. Participants without a history of SARS-CoV-2 infection made up the No COVID control group. In total, 295 ears were examined with otoscopy, tympanograms, pure tone audiometry and otoacoustic emissions. Ears with known preexisting hearing loss or status post ear surgery, as well as those with abnormal otoscopic findings, non-type A tympanograms or negative Rinne test were excluded.
Results
Compared to the No COVID and Ex COVID groups, we did not find a clinically significant difference in either hearing thresholds or frequency specific TEOAEs. However, at 500 Hz the data from the left ear, but not the right ear showed a significantly better threshold in the Ex COVID group, compared to Long COVID and No COVID groups. Any of the other tested frequencies between 500 Hz and 8 kHz were not significantly different between the different groups. There was a significantly lower frequency-specific signal-to-noise-ratio of the TEOAEs in the Long COVID compared to the No COVID group at 2.8 kHz. At all other frequencies, there were no significant differences between the three groups in the TEOAE signal-to-noise-ratio.
Conclusion
This study detected no evidence of persistent cochlear damage months after SARS-CoV-2 infection in a large cohort of Long COVID patients, as well as those fully recovered.
Keywords: Long COVID, COVID-19, SARS-CoV-2, Hearing loss
1. Introduction
Hearing loss is a known complication of many viral infections like measles, varicella, lassa virus or HIV [1]. Since the beginning of the SARS-CoV-2 pandemic there have been numerous reports of COVID patients with acute sensorineural hearing loss [2], [3]. Histological temporal bone studies offered a compelling mechanistic narrative for SARS-CoV-2-mediated inner ear damage, by showing the expression of receptors in inner ear tissues [4]. However, studies investigating inner ear function in SARS-CoV-2 positive patients with and without COVID have yielded conflicting results [5], [6], [7].
Long COVID is a term used when symptoms persist more than four weeks after a SARS-CoV-2 infection or reappear after an initial phase of recovery and cannot be explained otherwise [8]. More than 200 different symptoms have been associated with Long COVID. An investigation into symptom clusters in Long COVID patients found that Long COVID patients had an increasing probability of reporting hearing loss in the months after the acute SARS-CoV-2 infection [9].
To our knowledge no study has used objective or subjective audiological tests to investigate the audiological profile of Long COVID patients so far. We therefore sought to determine, whether there is any evidence of persistent or late-onset, clinically significant or subclinical inner ear damage in a large cohort of Long COVID patients.
2. Materials and methods
Adult participants with and without Long COVID were recruited via an online survey on the project website (http://www.defeat-corona.de/) in Long COVID forums, as well as waiting rooms, public areas, and the websites of the of participating medical and research centers. Survey participants entered whether and when they tested positive for SARS-CoV-2, whether they suffered any persistent symptoms and whether they were open to receiving invitations to in-person follow-up research. All respondents gave written informed consent before participating in the study. Adult participants with any persistent symptom more than four weeks after SARS-CoV-2 infection (PCR-test), not explained by any other health condition, were allocated to the Long COVID cohort. The control group was divided into participants who reported never having tested positive for SARS-CoV-2 (No COVID) and those who had recovered without any persistent symptoms (Ex COVID). Ears with preexisting hearing loss or previous ear surgery were excluded. In total, 127 Long COVID and 11 Ex COVID patients as well as 28 No COVID controls underwent otologic examinations including bilateral otoscopy, tympanograms, Rinne test, pure tone audiometry (PTA) and otoacoustic emissions. Those with abnormal findings in the otoscopic exam, non-type-A tympanogram (Interacoustics AT235 Impedance Audiometer) or negative Rinne test were excluded from further analysis. The air conduction threshold was determined using the Interacoustics AC33 Clinical Audiometer. Transient evoked otoacoustic emissions (TEOAE) were measured using a Interacoustics DP Echoport ILO292 device. TEOAE measurements were excluded from the final analysis if the reproducibility was below 60 %. The audiological measurements were conducted from January to March 2022. Participant data was processed and saved using pseudonyms. The study was approved by the ethics committee of Hannover Medical School (No. 9948_BO_K_2021) and University Medical Center Göttingen (29/3/21).
To test two categorical variables, the Fisher Exact Test or Fisher-Freeman-Halton Exact test was used. Metrical variables were tested between two groups using the Mann-Whitney U Test and three groups using the Kruskal-Wallis-Test. For data yielding a significant result from the Kruskal-Wallis-test a Bonferroni adjusted post-hoc Mann-Whitney U Test was performed to determine which groups differ significantly. If not stated otherwise, results were considered statistically significant if the p value was ≤0.05. The open source program R (version 4.1.2) was used for calculations.
3. Results
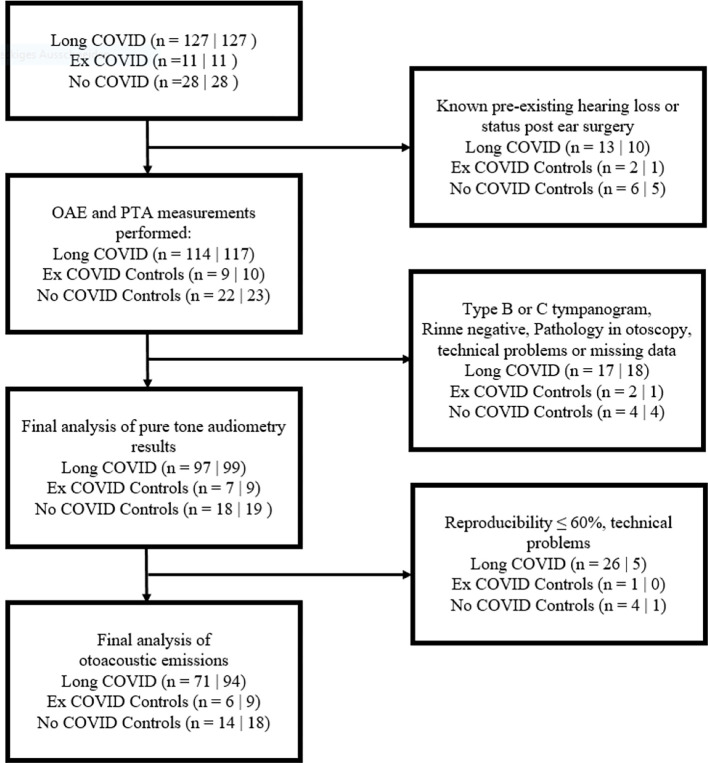
Of the 166 participants (332 ears) recruited through the online questionnaire, 37 ears were excluded due to known preexisting hearing loss or previous ear surgery (Fig. 1 ). Of the 295 ears that underwent otoscopic and audiologic examinations, 249 had a type A tympanogram, positive Rinne test and otoscopically normal tympanic membrane and were thus included in the final analysis of the pure tone audiometry threshold. The average age of those allocated to the three study groups was not significantly different. Participants in the Long COVID group were in average 47 weeks post SARS-CoV-2 infection, while participants in the Ex COVID group were on average infected more recently. Further demographic data is shown in Table 1 .
Fig. 1.
Inclusion and exclusion criteria for study participants: n(ears) = R|L.
Table 1.
Demographics of study participants included in the final analysis of pure tone audiometry results.
| Variable/data | Long COVID | Ex COVID | No COVID | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Number of participants n (ears: right|left) | 97|99 | 7|9 | 18|19 | Right|left | ||||
| Time from beginning of the infection to survey (weeks) | 46.7 (22.4) | 47.7 (22.3) | 28.2 (22.5) | 23.5 (22.1) | – | – | <0.001a | <0.001a |
| Gender n (%) | ||||||||
| Female | 77 (79.4)|80 (80.8) | 4 (57.1)|5 (55.6) | 18 (100.0)|19 (100.0) | 0.02b | 0.01b | |||
| Male | 19 (19.6)|18 (18.2) | 3 (42.9)|4 (44.4) | 0 (0.0)|0 (0.0) | |||||
| Not answered | 1 (1.0)|1 (1.0) | 0|0 (0.0) | 0 (0.0)|0 (0.0) | |||||
| Age (years) median (25th–75th percentile) | 44.2 (33.3–51.6) | 44.2 (33.2–51.5) | 47.7 (41.9–56.6) | 48.8 (47.4–54.6) | 43.0 (29.5–49.8) | 44.1 (30.3–50.7) | 0.37c | 0.29c |
| Not answered | 2 | 2 | 0 | 0 | 0 | 0 | ||
| Hospitalization n (%) | ||||||||
| No | 76 (78.4) | 78 (78.8) | 4 (57.1) | 5 (55.6) | – | – | 1.0 | 1.0 |
| Yes, regular ward | 9 (9.3) | 9 (9.1) | 0 (0.0) | 0 (0.0) | – | – | ||
| Yes, intensive care unit | 5 (5.2) | 5 (5.0) | 0 (0.0) | 0 (0.0) | – | – | ||
| Unknown/not answered | 7 (7.2) | 7 (7.1) | 3 (42.9) | 4 (44.4) | – | – | ||
| Vaccination status against Sars-CoV-2 n (%) | ||||||||
| Yes (overall) | 83 (85.6) | 85 (85.6) | 2 (28.6) | 2 (22.2) | 3 (16.7) | 5 (26.3) | 0.045b | 0.001b |
| No | 7 (7.2) | 7 (7.1) | 2 (28.6) | 4 (44.4) | 0 | 0 | ||
| Yes (prior infection) | 9 (9.3) | 8 (80.1) | 1 (14.3) | 1 (1.1) | – | – | 0.37b | 0.45b |
| Unknown/not answered | 7 (7.2) | 7 (7.1) | 3 (42.6) | 3 (33.3) | 15 (83.3) | 14 (73.7) | ||
Multiple selection possible.
Mann–Whitney U test.
Fisher Exact Test or Fisher-Freeman-Halton Exact test.
Kruskal-Wallis-Test.
The air conduction thresholds of all study groups showed a typical morphology for the average age of the participants studied with only mild hearing loss at 6 kHz (Fig. 2 ). At 500 Hz the statistical analysis of data from the left ear, but not the right ear showed a significantly better threshold for the Ex COVID group, compared to the Long COVID and No COVID groups (Table 2 ). Any of the other tested frequencies between 250 Hz and 8 kHz were not significantly different between study participants from the Ex COVID, No COVID and Long COVID groups (Table 2).
Fig. 2.
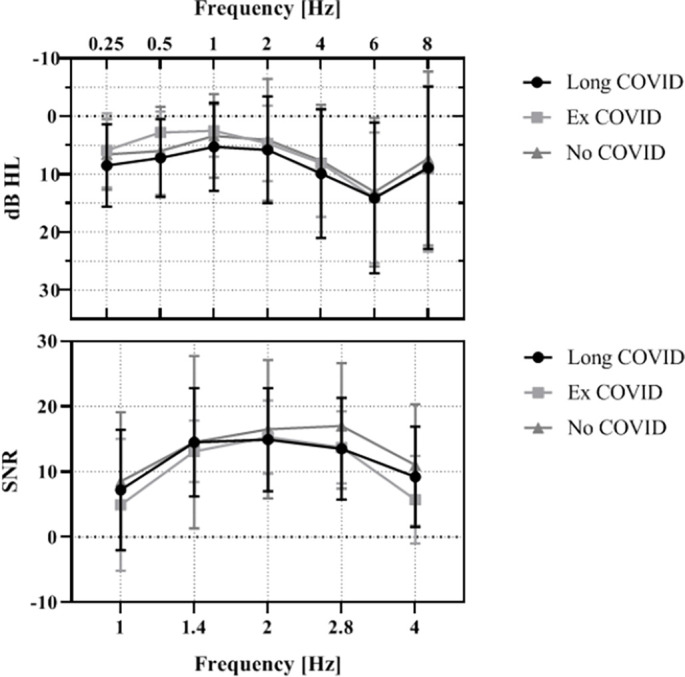
Top: Pure tone audiometry air conduction threshold: n (Long COVID) = 194; n (Ex COVID) = 16; n (No COVID) = 37 ears. Bottom: Signal-to-noise-ratio (SNR) of transient evoked otoacoustic emissions (TEOAEs): n (Long COVID) = 164; n(Ex COVID) = 15; n(No COVID) = 32 ears. Error bars ± 1SD.
Table 2.
Pure tone audiometry results.
| Frequency [kHz] |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 |
0.5 |
1 |
2 |
4 |
6 |
8 |
|||||||||||||||
| Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | |
| Long COVID (n = 194) | 8.5 | 7.1 | 0.12 | 7.2 | 6.7 | 0.011 | 5.3 | 7.6 | 0.07 | 5.8 | 9.2 | 0.23 | 9.9 | 11.1 | 0.45 | 14.1 | 13.0 | 0.92 | 8.9 | 14.0 | 0.75 |
| Ex COVID (n = 16) | 5.9 | 6.4 | 2.8 | 3.6 | 2.5 | 4.5 | 4.7 | 6.5 | 8.1 | 9.3 | 14.1 | 11.3 | 9.1 | 14.3 | |||||||
| No COVID (n = 37) | 6.6 | 6.1 | 6.0 | 7.6 | 3.4 | 7.2 | 4.1 | 10.5 | 7.7 | 9.7 | 13.1 | 12.8 | 7.3 | 15.0 | |||||||
SD; standard deviation; p < 0,05 = statistically significant difference between groups.
Post hoc Wilcox-test (Bonferroni adjusted): Long COVID vs. Ex COVID p = 0.011, Ex COVID vs. No COVID p = 0.444 Long COVID vs. No COVID p = 0.676.
A further 37 ears were excluded from the final analysis of the TEOAE frequency-specific signal-to-noise ratio due to low reproducibility (<60 %) of the otoacoustic emissions, mostly due to a technical problem with the right ear measurement probe (Fig. 1). The final analysis of 212 ears showed a significantly lower frequency-specific signal-to-noise-ratio of the TEOAEs in the Long COVID group compared to the No COVID control group at 2.8 kHz (Table 3 ). The No COVID group demonstrated a higher signal-to-noise ratio compared to both COVID groups at 4 kHz as well. This difference was not statistically significant. At all other frequencies, there were no differences between the three groups in the signal-to-noise-ratio.
Table 3.
TEOAE results.
| Frequency[kHz] |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
1.4 |
2 |
2.8 |
4 |
|||||||||||
| Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | |
| Long COVID (n = 164) | 7.2 | 9.2 | 0.68 | 14.5 | 8.3 | 0.17 | 14.9 | 7.9 | 0.31 | 13.5 | 7.8 | 0.021 | 9.2 | 7.7 | 0.06 |
| Ex COVID (n = 15) | 4.9 | 10.1 | 13.1 | 4.7 | 15.3 | 5.6 | 13.7 | 5.5 | 5.7 | 6.7 | |||||
| No COVID (n = 32) | 8.5 | 10.6 | 14.5 | 13.2 | 16.5 | 10.6 | 17.0 | 9.6 | 11.0 | 9.3 | |||||
SD = standard deviation; p < 0,05 = statistically significant difference between groups.
Post hoc Wilcox-test (Bonferroni adjusted): Long COVID vs. Ex COVID p = 1, Ex COVID vs. No COVID p = 0.119 Long COVID vs. No COVID p = 0.015.
4. Discussion
To date, it is unclear whether there is a single common underlying pathophysiological explanation for the broad spectrum of symptoms Long COVID patients experience. A recent report from our group showed a high prevalence of neurotological symptoms like tinnitus and vertigo or dizziness among Long COVID survey participants [10]. In contrast, hearing loss is less frequently reported, but previous studies suggested evidence of subclinical cochlear damage in audiological tests during or a few weeks after the acute phase of a SARS-CoV-2 infection. Mustafa reported significant differences in pure tone audiometry hearing thresholds and otoacoustic emissions of asymptomatic SARS-CoV-2-positive patients, compared to SARS-CoV-2-negative controls [6].
Performing the same tests on controls and COVID patients one and three months after infection, Yildiz found no significant difference in pure tone audiometry thresholds and otoacoustic emissions [7]. However, patients reporting symptomatic acute hearing loss were excluded from that study and there is no mention of whether the number of exclusions was higher in the COVID- or control group.
A more recent study by Öztürk et al. showed a significantly elevated toneaudiometric threshold in the high frequencies in COVID patients at least one month after infection compared to a control group [11]. However, in the study by Öztürk et al., the control group was significantly younger than the test group, which may have confounded the results. In contrast, our study showed a balanced age average across the study groups and no clinically significant difference in hearing thresholds or otoacoustic emissions between participants who had COVID-19 and those who did not. Only the left ear 500 Hz PTA-threshold of the Ex COVID group showed a statistically significant difference to the other groups. This is most likely due to the relatively small number of left ears in the Ex COVID group (n = 9), compared to the Long COVID (n = 98) and No COVID groups (n = 19). The large difference in group sizes is a limitation of this trial. However, from our large number of Long COVID patients we conclude that clinically significant hearing loss is not a hallmark of Long COVID.
5. Conclusions
This study measured the pure tone audiometric thresholds and otoacoustic emissions of Long COVID, Ex COVID and No COVID participants. There was no evidence that the pathophysiological processes underlying Long COVID negatively affect hearing.
Acknowledgments
Acknowledgements
Funding
This study was part of the DEFEAT Corona project, funded by the European Regional Development Fund (EFRE, Funding No: ZW7-85152953). The funding source had no influence on the design or execution of the study, data analysis or interpretation.
Appendix A.
Table 4.
Pure tone audiometry results of the left and right ear separately.
| Frequency [kHz] |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 |
0.5 |
1 |
2 |
4 |
6 |
8 |
||||||||||||||||
| Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | ||
| Right ear | Long COVID (n = 96) | 10.0 | 7.0 | 0.18 | 7.8 | 6.5 | 0.24 | 5.0 | 7.2 | 0.29 | 6.0 | 9.1 | 0.34 | 9.8 | 9.9 | 0.17 | 13.3 | 11.7 | 0.93 | 8.7 | 13.5 | 0.86 |
| Ex COVID (n = 7) | 7.9 | 4.9 | 4.3 | 3.5 | 3.6 | 6.3 | 5.0 | 5.8 | 4.3 | 7.3 | 12.1 | 13.8 | 12.1 | 18.2 | ||||||||
| No COVID (n = 18) | 6.9 | 4.9 | 6.1 | 5.6 | 2.2 | 4.3 | 2.8 | 6.5 | 6.4 | 6.6 | 12.5 | 9.6 | 6.1 | 11.6 | ||||||||
| Left ear | Long COVID (n = 98) | 7.1 | 7.1 | 0.34 | 6.6 | 7.0 | 0.041 | 5.6 | 8.1 | 0.17 | 5.7 | 9.5 | 0.61 | 9.9 | 12.4 | 0.71 | 15.0 | 14.2 | 0.69 | 9.0 | 14.5 | 0.86 |
| Ex COVID (n = 9) | 4.4 | 7.3 | 1.7 | 3.5 | 1.7 | 2.5 | 4.4 | 7.3 | 11.1 | 9.9 | 15.6 | 9.5 | 6.7 | 10.9 | ||||||||
| No COVID (n = 19) | 6.3 | 7.2 | 5.8 | 9.3 | 4.5 | 9.1 | 5.3 | 13.4 | 9.0 | 12.0 | 13.7 | 15.5 | 8.4 | 17.9 | ||||||||
SD; standard deviation; p < 0,05 = statistically significant difference between groups.
Post hoc Wilcox-test (Bonferroni adjusted): Long COVID vs. Ex COVID p = 0.039, Ex COVID vs. No COVID p = 0.915; Long COVID vs. No COVID p = 0.953.
Table 5.
TEOAE results of the left and right ear separately.
| Frequency [kHz] |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
1.4 |
2 |
2.8 |
4 |
||||||||||||
| Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | Mean | SD | p-Value | ||
| Right ear | LongCOVID (n = 71) | 4.5 | 10.7 | 0.53 | 11.6 | 10.2 | 0.96 | 13.1 | 9.8 | 0.94 | 11.0 | 9.4 | 0.33 | 6.0 | 7.3 | 0.62 |
| ExCOVID (n = 6) | 5.8 | 15.0 | 12.3 | 4.7 | 14.5 | 5.0 | 14.3 | 6.9 | 6.8 | 7.0 | ||||||
| NoCOVID (n = 14) | 7.6 | 13.0 | 9.6 | 17.2 | 13.4 | 13.6 | 13.5 | 12.0 | 8.2 | 11.6 | ||||||
| Left ear | LongCOVID (n = 93) | 9.2 | 7.2 | 0.09 | 16.4 | 5.9 | 0.12 | 16.3 | 5.9 | 0.19 | 15.3 | 5.9 | 0.011 | 11.6 | 7.1 | 0.032 |
| ExCOVID (n = 9) | 4.3 | 6.1 | 14.7 | 4.9 | 15.8 | 6.3 | 13.2 | 4.9 | 5.0 | 6.9 | ||||||
| NoCOVID (n = 18) | 9.2 | 8.6 | 15.0 | 7.7 | 18.8 | 7.1 | 19.8 | 6.1 | 13.2 | 6.5 | ||||||
SD = standard deviation; p < 0.05 = statistically significant difference between groups.
Post hoc Wilcox-test (Bonferroni adjusted): Long COVID vs. Ex COVID p = 1, Ex COVID vs. No COVID p = 0.130; Long COVID vs. No COVID p = 0.063.
Post hoc Wilcox-test (Bonferroni adjusted): Long COVID vs. Ex COVID p = 0.065, Ex COVID vs. No COVID p = 0.046; Long COVID vs. No COVID p = 1.0.
References
- 1.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18 doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degen C., Lenarz T., Willenborg K. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin Proc. 2020;95:1801–1803. doi: 10.1016/j.mayocp.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jafari Z., Kolb B.E., Mohajerani M.H. Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Can J Neurol Sci. 2021 doi: 10.1017/cjn.2021.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong M., Ocwieja K.E., Han D., Wackym P.A., Zhang Y., Brown A., Moncada C., Vambutas A., Kanne T., Crain R., Siegel N., Leger V., Santos F., Welling D.B., Gehrke L., Stankovic K.M. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun Med. 2021;1 doi: 10.1038/s43856-021-00044-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokten N., Celik S., Mutlu A., Pektas E., Icten S., Kalcioglu M.T. Does COVID-19 have an impact on hearing? Acta Otolaryngol. 2022;142:48–51. doi: 10.1080/00016489.2021.2020897. [DOI] [PubMed] [Google Scholar]
- 6.Mustafa M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yıldız E. Comparison of pure tone audiometry thresholds and transient evoked otoacoustic emissions (TEOAE) of patients with and without Covid-19 pneumonia. Am J Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwan N.A., Johnson L. Defining long COVID: going back to the start. Med. 2021;2:501–504. doi: 10.1016/j.medj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degen C.V., Mikuteit M., Niewolik J., Schröder D., Vahldiek K., Mücke U., Heinemann S., Müller F., Behrens G.M.N., Klawonn F., Dopfer-Jablonka A., Steffens S. Self-reported tinnitus and vertigo or dizziness in a cohort of adult long COVID patients. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.884002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Öztürk B., Kavruk H., Aykul A. Audiological findings in individuals diagnosed with COVID-19. Am J Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103428. [DOI] [PMC free article] [PubMed] [Google Scholar]