Abstract
Objective
To describe the successful implementation of an enhanced public health surveillance system based on early detection, tracing contacts, and patient follow-up and support.
Study design
A prospective observational cohort study conducted in Serrana, São Paulo State, Brazil.
Methods
The implementation was based on four axes: increasing the access to SARS-CoV-2 testing; correct swab collection; testing patients with mild symptoms; and patient follow-up. Positivity rate, patient demographic and clinical characteristics, dynamics of disease severity, SARS-CoV-2 genome evolution, and the impact on COVID-19 research were assessed from August 23, 2020 to February 6, 2021 (between epidemiological week 35/2020 and 5/2021, a total of 24 weeks).
Results
The number of sites collecting rt-PCR for SARS-CoV-2 was increased from one to seven points and staff was trained in the correct use of personal protective equipment and in the swab collection technique. During the study period, 6728 samples were collected from 6155 participants vs. 2770 collections in a similar period before. SARS-CoV-2 RNA was detected in 1758 (26.1%) swabs vs. 1117 (36.7%) before the implementation of the surveillance system (p < 0.001). Positivity rates varied widely between epidemiological weeks 35/2020 and 5/2021 (IQR, 12.8%–31.3%). Out of COVID-19 patients, 91.1% were adults at a median age of 35 years (IQR, 25–50 years), 42.6% were men and 57.4% were women, with a SARS-CoV-2 positivity rate of 28.6% and 24.4% (p < 0.001), respectively. The most common symptoms were headache (72.6%), myalgia (65.0%), and cough (61.7%). Comorbidities were found in 20.8% of patients, the most common being hypertension and diabetes. According to the World Health Organization clinical progression scale, 93.5% of patients had mild disease, 1.6% were hospitalized with moderate disease, 3.2% were hospitalized with severe disease, and 1.4% died. The enhanced surveillance system led to the development of COVID-19 related research.
Conclusions
The enhanced surveillance system in Serrana improved COVID-19 understanding and management. By integrating community and academic institutions, it was possible to monitor SARS-CoV-2 positive cases and variants, follow the epidemic trend, guide patients, and develop relevant research projects.
Keywords: COVID-19, SARS-CoV-2, Public health, Public health surveillance
1. Introduction
In December 2019, in Wuhan, China, a group of patients with pneumonia was diagnosed with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection [1]. The World Health Organization (WHO) declared the Coronavirus Disease 2019 (COVID-19) a pandemic on March 11, 2020 [2]. Brazil's first case was identified on February 26 in a resident of São Paulo city who travelled to Italy (Lombardy region) in early February 2020 [3]. By the end of May 2021, over 30 million confirmed cases of COVID-19 had been reported in Brazil, with almost 665,000 deaths, the third- and second-largest number worldwide, respectively. Approximately a third of the cases in Brazil are concentrated in the southeast region, which includes São Paulo State [4]. The rate of SARS-CoV-2 infection spread in Brazil elicits that current interventions are insufficient to control the pandemic [5].
At least 186 countries have implemented different non-pharmaceutical interventions (NPIs) to control COVID-19 spread, from few restrictions to lockdowns [1,6,7]. Although extreme restrictions can reduce cases, hospital admissions, and deaths, they may have an immediate negative impact on the economy. Regardless of the restriction degree adopted, a core strategy should be a coordinated surveillance system that identifies and tests actively all suspected people, traces contacts, and isolates and supports patients with confirmed infection [2,6].
Surveillance strategies should consider cultural characteristics, social factors, and economic conditions. In some Asian countries, people with confirmed infection were isolated in hospitals or other facilities. In Hong Kong, patients with COVID-19 were isolated in hospitals or community treatment facilities until they recover, depending on the phase of the pandemic and the healthcare system burden [8,9]. Similarly, in South Korea, asymptomatic or mild patients were isolated in isolation-and-care centres and patients with moderate or severe disease were monitored in hospitals [6,10]. Whereas in the western countries, patients with mild disease usually stayed at home [2,3,6]. Confirmatory tests and contact tracing strategies also vary among countries [3]. Testing strategies vary from a broader indication for suspected patients even with mild symptoms or mass testing (e.g., South Korea, New Zealand, Germany, and Slovakia) to a more limited strategy, focused on patients with severe disease or high-risk patients (e.g., Mexico, Nigeria, Egypt) [11,12]. To decrease virus transmission, the contact tracing capacity needed to scale up substantially. Countries manage this with different strategies, such as manual contact tracing by health workers, increasing the existing capacity with contact tracer, hiring outsourcing corporations, or with digital methods (e.g., police supercomputer system to track and map transmission, smartphone apps, credit cards, and electronic health records) [6,12].
In Brazil, there is a socialized unified health system (SUS) with diagnostic tests for SARS-CoV-2 widely distributed. However, heterogeneous access to testing across different regions, delays in testing results and reporting, and changes in notification led to an uncontrolled SARS-CoV-2 transmission [5,[13], [14], [15], [16]]. Additionally, surveillance systems and NPIs are distinct among Brazilian regions and even between nearby cities. A survey with 4027 mayors (72.3% of all municipalities in the country) from all five Brazilian regions showed that NPIs were different among cities and have been implemented asynchronously [17].
We report the successful implementation of an enhanced and low-cost public health surveillance system based on early detection, tracing contacts, and patient follow-up and support. The epidemiology, transmission dynamics and changes in virus strain in Serrana were summarized from September 2020 to February 2021.
2. Methods
2.1. Study design
This is a prospective observational cohort study conducted in Serrana, São Paulo State, Brazil. All data were collected from the official database of the Epidemiological Surveillance of Serrana.
2.2. Study population and period
Serrana, nestled in the southeast of Brazil, is a small town in the state of São Paulo with an estimated population of 45,000 people and approximately 33,000 adults [18,19]. It is a sleepy town with an economy based on sugarcane agriculture. More than one-third of the adult population commutes daily to work in nearby cities, such as Ribeirão Preto, favouring the transmission of infectious contagious disease. All residents in Serrana were the eligible population and the residents who had a swab collected were defined as the study population.
Serrana health care system has 10 units, distributed as follows: four units of Family Health Strategies (FHS), covering approximately 30% of the population; two Basic Healthcare Units (UBS); one Emergency Care Unit (UPA); one Specialty Outpatient Clinic; one Philanthropic Hospital; and a State Hospital (HE Serrana).
The program implementation took place on August 23, 2020 (epidemiological week 35, 2020). All analyses were conducted from epidemiological week 35, 2020 to epidemiological week 5, 2021 (from August 23, 2020, to February 6, 2021, a total of 24 weeks). We also compared the program results with a similar period before its implementation, from epidemiological week 11/2020 to epidemiological week 34/2020 (from March 8, 2020, to August 22, 2020, 24 weeks).
2.3. The enhanced public health surveillance system
The implementation of the enhanced surveillance system was based on four main axes: increasing the access to diagnostic testing for SARS-CoV-2; adequacy of the structure and technique for swab collection, and personal protective equipment (PPE) training; sample collection for SARS-CoV-2 testing even for patients with mild symptoms; and follow-up of confirmed COVID-19 patients.
To expand and facilitate the population access to testing, the number of sites to collect rt-PCR for SARS-CoV-2 was increased from one to seven points. Before the enhanced surveillance system implementation (from epidemiological week 11/2020 to epidemiological week 34/2020), sample collection was centralized in only one healthcare unit. With the enhanced surveillance system, seven healthcare units distributed in town were equipped and reorganized to collect nasopharyngeal and oropharynx swabs from COVID-19 suspected patients.
Separate flows were created in each healthcare unit and adequate PPE was provided to healthcare workers. Additionally, administrative meetings were performed with staff and training was carried out for the proper use of PPE and for swab collection technique. A routine for cleaning and handling infectious waste was also defined.
Criteria to swab collection for SARS-CoV-2 was expanded from only severe cases to mild cases with the presence of one or more symptoms: fever, headache, cough, rhinorrhoea, diarrhoea, nausea, anosmia (smell impairment), ageusia (taste impairment), dyspnoea (respiratory difficulty), myalgia (muscle pain), or odynophagia (painful swallowing), for at least two days until the seventh day after the onset of symptoms. All residents that had a swab collected for SARS-Cov-2 nucleic acid detection were recruited for the study.
2.4. Case definition
The definition of case adopted in this study was the presence of one or more symptoms and the detection of SARS-CoV-2 nucleic acids in a clinical sample. Then, a possible case was anyone who met clinical criteria, and a confirmed case, anyone who met laboratory criteria.
Confirmed cases were followed with phone calls or home visits, when necessary. The follow-up was done at the beginning of the symptoms and at days 5, 10, 14, and 28 after symptoms onset. We prospectively collected information on sociodemographic characteristics, clinical symptoms, and chronic comorbidities. Disease severity was classified according to the WHO clinical progression scale (WHO-CPS) [19]. Additionally to symptoms evolution, patients were instructed regarding isolation measures, screening symptomatic residents in the same home, and warning symptoms. Those who needed hospitalization were followed up daily through institutional records.
2.5. Communication strategy
The population was informed about the enhanced surveillance system through sound cars, posters and flyers distributed door-to-door, and advertisements on municipal social media.
The core information was that in the presence of any symptoms related to COVID-19, patients should seek the healthcare unit closest to their home or work to undergo the diagnostic test.
An online meeting was held with local leaders, including religious leaders, politicians, business people, health professionals, municipal secretaries, prosecutors, and university and school directors. We presented the project objectives, clarified the doubts, and asked them to disseminate the information in the community.
2.6. Specimen collection and viral detection
Nasopharyngeal and oropharyngeal swabs were collected, stored in a sterile tube with viral transport media between 2 °C and 8 °C, and processed following the CDC guideline for detection of SARS-CoV-2 [20]. Briefly, SARS-CoV-2 RNA detection was performed using Gene FinderTM COVID-19 Plus RealAmp kit (OSang Healthcare Co. Ltd.) targeting viral RdRp, E, and N genes.
After collection, specimens were centralized in the Epidemiological Surveillance of Serrana and sent to the Ribeirão Preto Blood Center for processing. Results were released in the next working day.
2.7. RNA sequencing
All positive samples showing a cycle threshold (Ct) value < 35 underwent complete genome sequencing. SARS-CoV-2 genomic libraries were generated using the COVIDSeq kit (Illumina, San Diego, CA), following the manufacturer's specifications. The normalized sample libraries were sequenced on an Illumina MiSeq instrument (Kit v2, 2 × 300; Illumina, San Diego, CA, USA). The obtained sequences were of high quality, mean read number of 462,050, mean depth of 1,656, and 99.8% coverage.
2.8. Statistical analysis and ethical aspects
Data were obtained from the Brazilian public health system databases (e-SUS and SIVEP-Gripe). Categorical variables were expressed as a percentage. Continuous variables were expressed as mean and standard deviation (normal distribution) and median and interquartile range (non-normal distribution). For categorical variables, the Fisher test was used. For continuous variables with normal distribution, Student's t-test was used, and for variables with non-normal distribution, the Mann Whitney non-parametric test was used. A value of p < 0.05 was considered significant. The analysis was performed using GraphPadPrism®. The local research ethics committee approved this analysis as a public health investigation and surveillance and waived the requirement for informed consent (CAAE 51760221.2.0000.5440).
3. Results
3.1. Administrative measures and training
The Epidemiological Surveillance, Health Department, and mayor of Serrana provided political and administrative support to the program. We performed an initial meeting in each unit with administrative and health care workers, explaining its objectives and importance.
Staff was trained in the correct use of PPE (masks, goggles, gowns, or gloves) during the care of suspected patients and in the swab collection technique. Environmental cleaning and disinfection practices were reviewed. Materials replacement was defined not to run out PPE and material for rt-PCR assays. An intermediate meeting with each unit was held to review progress and present preliminary results.
3.2. Decentralization of sample collection for rt-PCR
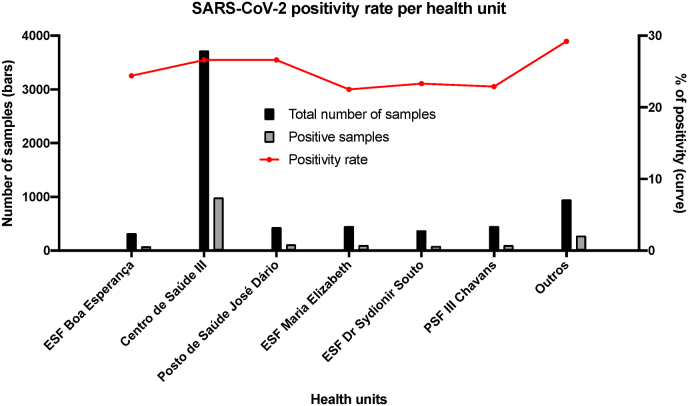
Before the program, sample collection was centralized in a central UBS. After its implementation, seven health units distributed in the town started to collect samples for rt-PCR. However, even with the decentralization, most of the collection continued to be performed in the same health unit as before. The central UBS was responsible for 55.4% of all samples for rt-PCR and the other units, from 4.8% to 6.8% (Fig. 2). The mean positivity rate did not differ significantly among units, from 22.5% to 29.2% (Fig. 2).
Fig. 2.
SARS-CoV-2 positivity rate per health unit in Serrana, Brazil, from epidemiological week 35, 2020, to epidemiological week 5, 2021.
3.3. Specimen collection and kinetics of SARS-CoV-2 positivity rate
After program implementation (during 24 weeks), 6728 samples for rt-PCR for SARS-CoV-2 were collected from 6155 participants. The median testing frequency per patient was 1.1 times (ranging from 1 to 6 times). Among the total samples, SARS-CoV-2 RNA was detected in 1758 (26.1%) swabs. In a similar period (24 weeks) before implementing the enhanced surveillance system, only 2770 collections were done with a significant higher mean positivity of 36.7% (p < 0.001) (Table 3).
Table 3.
Sample and patient characteristics in Serrana, Brazil, before (epidemiological week 11, 2020, to 34, 2020) and after (epidemiological week 35, 2020, to 5, 2021) the implementation of an enhanced public health surveillance system.
| Characteristics | Epidemiological week 11,2020 to 34, 2020 | Epidemiological week 35, 2020 to 5, 2021 | P-value |
|---|---|---|---|
| Samples | |||
| Number of collection (n) | 2770 | 6728 | |
| Positivity (%) | 36.7 | 26.1 | <0.001 |
| Age | |||
| Median (IQR), y | 40 (30–54) | 35 (25–50) | <0.001 |
| <18 years (%) | 3.1 | 8.5 | <0.001 |
| ≥18 years (%) | 95.9 | 91.1 | |
| Unknown (%) | 1.0 | 0.4 | |
| Sex | |||
| Female (%) | 56.2 | 57.4 | >0.288 |
| Male (%) | 43.8 | 42.6 | |
| Symptoms at disease onset | |||
| At least one symptom (%) | 84.4 | 97.1 | <0.001 |
Source: Epidemiological Surveillance of Serrana.
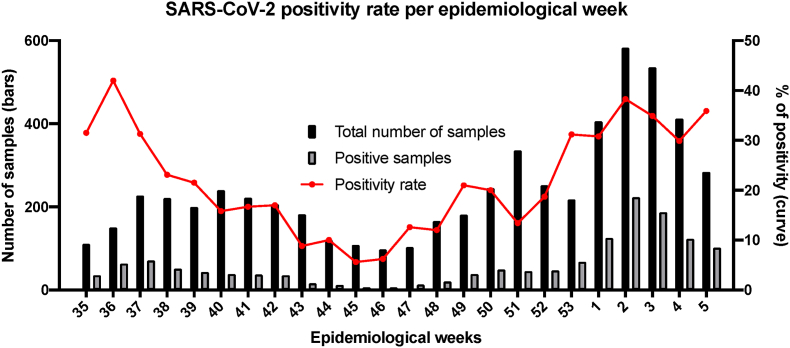
Positivity rates varied widely among epidemiological weeks (IQR, 12.8%–31.3%), reaching its peak in epidemiological week 36, 2020 (42%) (Fig. 1). However, in the epidemiological week 2, 2021, there was the greatest number of positive samples (223 samples, positivity rate of 38.3%). COVID-19 confirmed cases were not static, then with the program we could follow the epidemic trend and adapt the necessary measures. Of note, we could easily document a persistent increase in the positive samples even before the cities nearby.
Fig. 1.
SARS-CoV-2 positivity rate per epidemiological week in Serrana, Brazil, from epidemiological week 35, 2020, to epidemiological week 5, 2021.
3.4. Patient demographic and clinical characteristics
Patient demographic characteristics are shown in Table 1. In this prospective study, we included 6155 patients; 24 (0.4%) cases did not have age information available; 91.1% were adults and 8.5% children; and the median age was 35.0 years (range, 0–112 years; IQR, 25.0–50.0 years). Among these patients, 97.4% were symptomatic for COVID-19 at the time of swab collection; 2622 (42.6%) were men and 3533 (57.4%) were women, with a SARS-CoV-2 positivity rate of 28.6% and 24.4% (p < 0.001), respectively (Table 3).
Table 1.
Characteristics of patients with COVID-19 in Serrana, Brazil, from epidemiological week 35, 2020, to epidemiological week 5, 2021.
| Characteristics | Patients |
|---|---|
| Age | |
| Median (IQR), y | 36 (25–50) |
| <18 years (%) | 8.5 |
| ≥18 years (%) | 91.1 |
| Unknown (%) | 0.4 |
| Sex | |
| Female (%) | 57.4 |
| Male (%) | 42.6 |
| Comorbid conditions (%) | |
| Any comorbidity | 20.8 |
| Hypertension | 63.8 |
| Diabetes | 30.4 |
| Chronic lung disease | 13.7 |
| Chronic renal failure | 13.0 |
| Cardiovascular disease | 10.2 |
| Symptoms at disease onset (%) | |
| Any symptom | 79.4 |
| Headache | 72.6 |
| Myalgia | 65.0 |
| Cough | 61.7 |
| Sore throat | 51.5 |
| Rhinorrhoea | 48.7 |
| Fatigue | 47.2 |
| Nasal congestion | 46.9 |
| Fever | 42.4 |
| Anosmia | 41.1 |
| Dysgeusia | 40.3 |
| Diarrhoea | 28.5 |
| Nausea or vomiting | 22.8 |
Source: Epidemiological Surveillance of Serrana.
Out of 1759 positive samples, we prospectively collected clinical characteristics of 1410 patients (80.1%). The most common symptoms at onset of illness were headache (72.6%), myalgia (65.0%), cough (61.7%), sore throat (51.5%), rhinorrhoea (48.7%), fatigue (47.2%), nasal congestion (46.9%), fever (42.4%), anosmia (41.1%), dysgeusia (40.3%), diarrhoea (28.5%), and nausea or vomiting (22.9%). Comorbidities were found in 20.8% of patients, the most common being hypertension (63.8%), diabetes (30.4%), chronic lung disease (13.7%), chronic renal failure (13.0%), and cardiovascular disease (10.2%).
3.5. Dynamics of disease severity
Regarding warning signs (persistent fever, shortness of breath, or hypotension), 13.4% of patients had at least one at the onset of illness. During follow-up, according to WHO-CPS, 93.5% of patients had a mild disease, 1.6% were hospitalized and had a moderate disease, 3.2 were hospitalized and had a severe disease, and 1.4% died (Table 2). Then, during the 22 weeks, 74 (5.1%) patients needed to be hospitalized, and case-fatality ratio was 1.4% (21 patients died) during the study period.
Table 2.
Disease severity of COVID-19 patients in Serrana, Brazil, from epidemiological week 35, 2020, to epidemiological week 5, 2021.
| Characteristics | Patients |
|---|---|
| WHO-CPSa(%) | |
| Ambulatory mild disease | 93.5 |
| Hospitalized: moderate disease | 1.6 |
| Hospitalized: severe diseases | 3.2 |
| Dead | 1.4 |
| Hospitalization n (%) | 74 (5.1) |
| Case-fatality ratio (%) | 1.4 |
World Health Organization-Clinical Progression Scale.
Source: Epidemiological Surveillance of Serrana.
3.6. SARS-CoV-2 genome evolution
Out of 1759 positive samples, 109 (6.2%) were sequenced, varying from 4.0% to 38.4% of the samples each month. Interestingly, in September 2020, all cases were caused by parental lineages such as B.1.1.28 and B.1.1.33; in December 2020, the P.2 interest variant became predominant, accounting for 60% of the cases; and in early February 2021, there was the emergence of the P1 gamma variant. We noticed that the number of positive cases increased with the variant changes.
3.7. Impact on COVID-19 research
The enhanced surveillance system led to the development of COVID-19 related research and contributed to the public health policy. The study “Assessment of Incidence of SARS-CoV-2 Infection and COVID-19 in Brazil (AVISA)" (NCT04355338, ClinicalTrials.gov) is an observational study to evaluate the incidence and immune response of SARS-CoV-2 and COVID-19 in several age groups of 11 cities in Brazil, including Serrana. Another project entitled “An Effectiveness Study of the Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine'', also called “Projeto S" (NCT04747821, ClinicalTrials.gov) is a stepped-wedge cluster randomized trial to assess the effectiveness of vaccination. In this stepped-wedge trial, approximately 81% of the adult population of Serrana were vaccinated with two doses of CoronaVac, and the city population is being followed-up for one year. Another study, called “Evaluation of seroconversion of adults and elderly vaccinated with CoronaVac in the municipality of Serrana- São Paulo'', assesses the humoral and cellular response to CoronaVac during one year.
4. Discussion
In the present study, we showed the process and impact of an enhanced public health surveillance system implemented in a small town in São Paulo State, Brazil.
Surveillance is one of the cornerstones to control infectious diseases. Many types of surveillance have been described, such as routine, active, syndromic, sentinel, sentinel-syndromic, laboratory, and hospital-based surveillance [21]. According to WHO, the objectives of COVID-19 surveillance involve: “monitor SARS-COV-2 incidence and COVID-19 morbidity and mortality; track potential epidemiological changes over time; detect and contain outbreaks of new SARS-CoV-2 variants; guide the implementation and adjustment of COVID-19 control measures including isolation of cases, contact tracing and quarantine of contacts; evaluate the impact of the pandemic on health care systems and society; and contribute to the understanding of the co-circulation of SARS-CoV-2, influenza, other respiratory viruses, and other pathogens” [22].
Several countries and even regions in the same country adopted different strategies to implement surveillance systems, ranging from a proactive control to a delayed intervention or a suppression strategy [2,6,23]. These approaches also changed according to the pandemic phase. In China, a strategy called “zero-COVID” was implemented with strict lockdowns, mass testing, and patient isolation in quarantine centres or hospitals [24]. Lucero-Prisno III et al. reviewed the surveillance strategies in 13 African countries and detected significant variations in the implementation level among these countries [25]. Using an explanatory mixed-methods study, Assefa et al. demonstrated that differences in surveillance strategies among nine countries could be explained by “leadership, governance, and coordination of response; communication; community engagement; multisector actions; public health capacity; universal health coverage; medical services and hospital capacity; demography; and burden of non-communicable diseases” [23]. Brazil has a large territory marked by social contrasts among different regions and states, leading to differences in surveillance systems. Many differences have been reported among regions, states, and cities, e.g., the number of ICU beds, lack of diagnostic tests and massive testing, ineffective collective health policies and anti-science actions, and lack of standardization of COVID-19 databases and data sharing [26].
In Serrana, prior to the implementation of this system, sample collection for SARS-CoV-2 occurred mainly for hospitalized patients at UPA and HE Serrana, and for few suspected patients referred to Epidemiological Surveillance. Consequently, a more restrict indication for rt-PCR might have led to undetected cases of COVID-19, especially, mild or paucisymptomatic infections, and contributed to rapid SARS-CoV-2 dissemination [27,28]. With the enhanced surveillance system, testing access was facilitated and a more significant number of samples for SARS-CoV-2 was collected, helping to better understand the COVID-19 pandemic situation in Serrana.
To decentralize sample collection for rt-PCR, it was necessary to organize previously the structure, technique for swab collection, and personnel training in all health units in Serrana. Although SUS was planned to have the UBS and FHS as “entrance door”, in the real world, there is a gap between the SUS principles and the UBS and FHS performance [29]. Moreover, not all healthcare workers received adequate training for dealing with COVID-19 infected patients. This reinforces the need for a central organization to standardize procedures in health units and train healthcare workers, which could have been made through any online platform [30,31].
Clear communication plays a crucial role in any health program, especially in infectious disease outbreaks. In the current pandemic, communication is critical to prevent SARS-CoV-2 dissemination and reinforce treatment and vaccines' effectiveness [32]. Only when people clearly understand their risk and that their attitude helps to control viral transmission they can adhere to prevention practices [32]. One strategy is to use a graded, individual-level pandemic notification system that could allow the population to be informed in real-time about the pandemic situation and to facilitate a coordinated response [33]. In Brazil, as in other countries, we faced misinformation (fake news) about the number of cases and deaths and the correct prevention measures and treatment [[34], [35], [36], [37]]. Several people became “experts” and reproduced faked or unproven preventive measures and treatments as well as anti-vaccination positions, with all disastrous consequences [38]. Then, we opted to include local leaders, politicians, health professionals, influencers, and public workers to disseminate the information in the community. Although it has not been measured objectively, we did not have any critical incidents related to fake news, as seen in other Brazilian cities.
Another challenge for the system implementation was the necessity to work in a network. Due to the complexity of managing COVID-19, especially in small towns such as Serrana, and following the SUS principles, we established a network of academic and community institutions. In this network, rt-PCR and genome viral sequencing were performed in the Ribeirão Preto Blood Center with the support of Butantan Institute, protocols and administrative measures were done with the assistance of experts from the HE Serrana and Butantan Institute, and the Ribeirão Preto Medical School of University of São Paulo and Clinical Hospital of Ribeirão Preto gave all necessary support. Only with the cooperation of all health units, it was possible to implement the enhanced epidemiological surveillance and, consequently, to develop essential COVID-19 related research, such as the Projeto S.
As several measures were implemented with the enhanced public health surveillance system in Serrana, we could not measure their direct effect on the occurrence of natural infection and herd immunity. Then, the impact of the surveillance system might be confounded by changes in disease dynamics.
Our study has limitations. First, due to the relatively short period (24 weeks), we were unable to assess how long the strategies lasted as they were implemented. Second, we could not objectively compare its impact with the surveillance systems of other Brazilian cities or other countries. Third, although we decentralized sample collection, traced contacts, and gave patient follow-up and support, we did not assess patient perception and adherence. Finally, we did not evaluate the impact of the enhanced public health surveillance on the health care workers. However, once Serrana is a small town, we established a network of academic and community institutions, and there was clear communication with the population, therefore, we were able to implement all the strategies quickly and to evaluate their impact as well.
In conclusion, we described the implementation process of an enhanced and low-cost surveillance system in Serrana, a small town in São Paulo State, based on the principles of SUS. By integrating community and academic institutions, we were able to monitor positive cases and variants of SARS-CoV-2, follow the epidemic trend, guide patients, and develop relevant research projects related to COVID-19.
Ethical approval
The study was approved by the Ethics Committee of the Clinical Hospital, Ribeirão Preto Medical School, University of São Paulo (CAAE 51760221.2.0000.5440).
Funding
This work was supported by Butantan Institute.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was only possible thanks to the support of Butantan Institute, Ribeirão Preto Medical School of University of São Paulo, Clinical Hospital of Ribeirão Preto, Ribeirão Preto Blood Center, Foundation for the Support of Instruction, Research and Treatment, and Serrana State Hospital. We want to underscore the engagement of Serrana mayors, Leonardo Caressato Capitelli and Valerio Galante, and Health Secretary, Leila Gusmão, and local officers.
References
- 1.WHO . World Health Organization; 2020. Coronavirus Disease (COVID-2019) Situation Reports. [Google Scholar]
- 2.The Lancet Infectious Diseases The COVID-19 exit strategy-why we need to aim low. Lancet Infect. Dis. 2021;21(3):297. doi: 10.1016/S1473-3099(21)00080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickens B.L., Koo J.R., Wilder-Smith A., Cook A.R. Institutional, not home-based, isolation could contain the COVID-19 outbreak. Lancet. 2020;395(10236):1541–1542. doi: 10.1016/S0140-6736(20)31016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saúde Md. Ministério da Saúde; 2021. Painel Coronavírus. [Google Scholar]
- 5.Candido D.S., Claro I.M., de Jesus J.G., Souza W.M., Moreira F.R.R., Dellicour S., et al. Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science. 2020;369(6508):1255–1260. doi: 10.1126/science.abd2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han E., Tan M.M.J., Turk E., Sridhar D., Leung G.M., Shibuya K., et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396(10261):1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortaleza C.R., Vilches T.N., Almeida G.B., Ferreira C.P., Souza L.D.R., Fortaleza C. Impact of nonpharmaceutical strategies on trends of COVID-19 in sao Paulo state. Rev. Saude Publica. 2021;55:48. doi: 10.11606/s1518-8787.2021055003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Li J.C.M., Fong M.W., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.C., Leung M., Tong D.W., Lee L.L., Leung W.L., Chan F.W., et al. Infection control challenges in setting up community isolation and treatment facilities for patients with coronavirus disease 2019 (COVID-19): implementation of directly observed environmental disinfection. Infect. Control Hosp. Epidemiol. 2021;42(9):1037–1045. doi: 10.1017/ice.2020.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peck K.R. Early diagnosis and rapid isolation: response to COVID-19 outbreak in Korea. Clin. Microbiol. Infect. 2020;26(7):805–807. doi: 10.1016/j.cmi.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilecco F.B., Coelho C.G., Fernandes Q., Silveira I.H., Pescarini J.M., Ortelan N., et al. The effect of laboratory testing on COVID-19 monitoring indicators: an analysis of the 50 countries with the highest number of cases. Epidemiol Serv Saude. 2021;30(2) doi: 10.1590/S1679-49742021000200002. [DOI] [PubMed] [Google Scholar]
- 12.Rajan S., McKee M., Hernandez-Quevedo C., Karanikolos M., Richardson E., Webb E., et al. What have European countries done to prevent the spread of COVID-19? Lessons from the COVID-19 Health system response monitor. Health Pol. 2022 doi: 10.1016/j.healthpol.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croda J.H.R., Garcia L.P. Immediate health surveillance response to COVID-19 epidemic. Epidemiol Serv Saude. 2020;29(1) doi: 10.5123/S1679-49742020000100021. [DOI] [PubMed] [Google Scholar]
- 14.Croda J., Oliveira W.K., Frutuoso R.L., Mandetta L.H., Baia-da-Silva D.C., Brito-Sousa J.D., et al. COVID-19 in Brazil: advantages of a socialized unified health system and preparation to contain cases. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kameda K., Barbeitas M.M., Caetano R., Lowy I., Oliveira A.C.D., Correa M., et al. Testing COVID-19 in Brazil: fragmented efforts and challenges to expand diagnostic capacity at the Brazilian Unified National Health System. Cad. Saúde Pública. 2021;37(3) doi: 10.1590/0102-311X00277420. [DOI] [PubMed] [Google Scholar]
- 16.Thornton J. Covid-19: lack of testing in Brazil is a "major failure," says MSF. BMJ. 2020;370 doi: 10.1136/bmj.m2659. m2659. [DOI] [PubMed] [Google Scholar]
- 17.de Souza Santos A.A., Candido D.D.S., de Souza W.M., Buss L., Li S.L., Pereira R.H.M., et al. Dataset on SARS-CoV-2 non-pharmaceutical interventions in Brazilian municipalities. Sci. Data. 2021;8(1):73. doi: 10.1038/s41597-021-00859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IBGE Panorama de Serrana: ibge. https://cidades.ibge.gov.br/brasil/sp/serrana/panorama [Available from:
- 19.infection WWGotCCaMoC- A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevention CfDCa . 2020. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- 21.Ibrahim N.K. Epidemiologic surveillance for controlling Covid-19 pandemic: types, challenges and implications. J Infect Public Health. 2020;13(11):1630–1638. doi: 10.1016/j.jiph.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . World Health Organization; 2022. Public Health Surveillance for COVID-19 - Interim Guindance. [Google Scholar]
- 23.Chen Y.Y., Assefa Y. The heterogeneity of the COVID-19 pandemic and national responses: an explanatory mixed-methods study. BMC Publ. Health. 2021;21(1):835. doi: 10.1186/s12889-021-10885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan S. Zero COVID in China: what next? Lancet. 2022;399(10338):1856–1857. doi: 10.1016/S0140-6736(22)00873-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adebisi Y.A., Rabe A., Lucero-Prisno D.E., Iii COVID-19 surveillance systems in African countries. Health Promot. Perspect. 2021;11(4):382–392. doi: 10.34172/hpp.2021.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boschiero M.N., Palamim C.V.C., Ortega M.M., Mauch R.M., Marson F.A.L. One year of coronavirus disease 2019 (COVID-19) in Brazil: a political and social overview. Ann Glob Health. 2021;87(1):44. doi: 10.5334/aogh.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pullano G., Di Domenico L., Sabbatini C.E., Valdano E., Turbelin C., Debin M., et al. Underdetection of cases of COVID-19 in France threatens epidemic control. Nature. 2021;590(7844):134–139. doi: 10.1038/s41586-020-03095-6. [DOI] [PubMed] [Google Scholar]
- 29.Flor C.R., Oliveira C.D.L., Cardoso C.S., Rabelo C.F., Gontijo B.L., Carvalho S.F., et al. Primary health care as assessed by health professionals: comparison of the traditional model versus the Family Health Strategy. Rev. Bras. Epidemiol. 2017;20(4):714–726. doi: 10.1590/1980-5497201700040013. [DOI] [PubMed] [Google Scholar]
- 30.Sharma R., Mohanty A., Singh V., S V.A., Gupta P.K., Jelly P., et al. Effectiveness of video-based online training for health care workers to prevent COVID-19 infection: an experience at a tertiary care level Institute, uttarakhand, India. Cureus. 2021;13(5) doi: 10.7759/cureus.14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celuppi I.C., Lima G.D.S., Rossi E., Wazlawick R.S., Dalmarco E.M. An analysis of the development of digital health technologies to fight COVID-19 in Brazil and the world. Cad. Saúde Pública. 2021;37(3) doi: 10.1590/0102-311X00243220. [DOI] [PubMed] [Google Scholar]
- 32.Skovdal M., Pickles M., Hallett T.B., Nyamukapa C., Gregson S. Complexities to consider when communicating risk of COVID-19. Publ. Health. 2020;186:283–285. doi: 10.1016/j.puhe.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakib M.N., Butt Z.A., Morita P.P., Oremus M., Fong G.T., Hall P.A. Considerations for an individual-level population notification system for pandemic response: a review and prototype. J. Med. Internet Res. 2020;22(6) doi: 10.2196/19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Barcelos T.D.N., Muniz L.N., Dantas D.M., Cotrim Junior D.F., Cavalcante J.R., Faerstein E. Analysis of fake news disseminated during the COVID-19 pandemic in Brazil. Rev. Panam. Salud Públic. 2021;45:e65. doi: 10.26633/RPSP.2021.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paschoalotto M.A.C., Costa E., Almeida S.V., Cima J., Costa J.G.D., Santos J.V., et al. Running away from the jab: factors associated with COVID-19 vaccine hesitancy in Brazil. Rev. Saude Publica. 2021;55:97. doi: 10.11606/s1518-8787.2021055003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galveas D., Barros F., Jr., Fuzo C.A. A forensic analysis of SARS-CoV-2 cases and COVID-19 mortality misreporting in the Brazilian population. Publ. Health. 2021;196:114–116. doi: 10.1016/j.puhe.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiehchen D., Espinoza M., Slovic P. Political partisanship and mobility restriction during the COVID-19 pandemic. Publ. Health. 2020;187:111–114. doi: 10.1016/j.puhe.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasconcellos-Silva P.R., Castiel L.D. COVID-19, fake news, and the sleep of communicative reason producing monsters: the narrative of risks and the risks of narratives. Cad. Saúde Pública. 2020;36(7) doi: 10.1590/0102-311x00101920. [DOI] [PubMed] [Google Scholar]