Abstract
Background
The treatment paradigm of unresectable malignant pleural mesothelioma (MPM) has changed in recent years. Checkmate 743 demonstrate that nivolumab plus ipilimumab showed good clinical benefits compared with chemotherapy in the treatment of MPM. The study is aim to evaluate the cost-effectiveness of Nivolumab plus ipilimumab vs. platinum plus chemotherapy for the first-line treatment of unresectable MPM.
Methods
A Markov model was developed to compare the cost and quality-adjusted life-year (QALY) of nivolumab plus ipilimumab and chemotherapy over a 10-year time horizon. Clinical efficacy and safety data were extracted from the CheckMate 743 trials. Health state utilities were obtained from published literature. Costs were collected from an US payer perspective. One-way and probabilistic sensitivity analyses were conducted to explore the impact of uncertainties on the cost-effectiveness's results.
Results
In the base case analysis, the incremental healthcare costs and QALYs for Nivolumab plus Ipilimumab vs. chemotherapy are $196,604.22 and 0.53, respectively, resulting an incremental cost-effectiveness ratio (ICER) of $372,414.28/QALYs for the model cohort of patients with locally advanced or metastatic MPM. However, Probabilistic sensitivity analysis showed that there was no probability that Nivolumab plus ipilimumab was cost-effective within the fluctuation range of other model parameters in first-line in unresectable MPM. The results of one-way sensitivity analysis showed that the cost of Nivolumab was the most sensitive parameter.
Conclusions
The ICER of Nivolumab plus ipilimumab is above the theoretical willingness-to-pay threshold in the U.S, which suggests that first-line nivolumab plus ipilimumab for unresectable MPM may be not a cost-effective choice.
Keywords: cost-effectiveness, nivolumab, ipilimumab, malignant pleural mesothelioma, Markov model
Introduction
Malignant pleural mesothelioma (MPM) is an uncommon but fatal malignancy with high aggressiveness. The number of new cases of MPM globally is 30,870 and the number of deaths is 26,278 according to the statistics in 2020 (1). Its pathogenesis is strongly associated with prior exposure to asbestos, and that caused high incidence of MPM in developed countries (2). Despite the widespread prevention, the incidence and mortality of MPM is still increasing and will reach its maximum after about 20 years, especially in developing countries, because of the long latency of asbestos exposure (3). The prognosis of malignant mesothelioma is poor. The survival rate at 5 years is <10% and the median overall survival of the diagnosed patients is 7.9 months (4, 5).
The great majority of patients of MPM are not diagnosed until advanced stage, so only a minority of patients can be treated with surgery. For a long time in the past, the only first-line treatment that FDA (U.S. Food and Drug Administration) approved for unresectable MPM is a platinum-based chemotherapy combined with pemetrexed. However, the efficacy is modest (6). A better treatment is urgently needed. Biological targeted therapy for MPM is not recommended because of the lack of known oncogenic driver alterations (7). Clinical studies of MPM immunotherapy has underwent a transition from relapse to second-line, monotherapy to combination, and now seemed promising in some phase II clinical studies (8). Based on these trials, the NCCN guidelines (2018) recommend nivolumab ± ipilimumab or pembrolizumab as subsequent systemic therapy (9).
The phase III randomized trial Checkmate 743 (NCT02899299), in which compare the curative effect and safety of cisplatin (or carboplatin)-pemetrexed and that of nivolumab-ipilimumab as first-line treatment, also show a promising result. It demonstrated that nivolumab-ipilimumab prolonged overall survival in comparison with the chemotherapy group [median 18.1 vs. 14.1 months; hazard ratio (HR), 0.74 (96.6% CI 0.60–0.91); p = 0.0020]. Two-year overall survival rates were 41% (95% CI 35.1–46.5) vs. 27% (21.9–32.4). In addition, among all treated patients, 91 (30%, nivolumab plus ipilimumab) and 91 (32%, chemotherapy) patients had grade 3–4 treatment-related adverse events. The overall incidence of treatment-related adverse events adjusted for exposure in nivolumab-ipilimumab group is lower than that in chemotherapy group (10). According to the results, nivolumab plus ipilimumab has been approved by the FDA to treat the unresectable MPM as a first-line therapy in October 2020 (11).
Despite the efficiency and safety of nivolumab-ipilimumab combination therapy for unresectable MPM, what we need to evaluate further is the cost-effectiveness of these drugs when considering both drugs have high unit prices which may add heavy financial burden to the patients (12). The objective of this study is to evaluate the cost-effectiveness of nivolumab plus ipilimumab vs. chemotherapy as the first-line treatment for unresectable MPM from the US payer perspective.
Materials and Methods
Population
CheckMate 743 is a randomized phase 3 trial involving 713 patients from November 19, 2016 to April 28, 2018 in 21 countries. Among enrolled patients, 605 were randomized to receive nivolumab plus ipilimumab (n = 303) or chemotherapy (n = 302). Median age of participants was 69 years (IQR 64–75) and ECOG was 0–1. The median follow-up was 29·7 months (IQR 26.7–32.9). Overall survival [18.1 months (95% CI 16.8–21.4)] was significantly longer in the nivolumab plus ipilimumab group than in the chemotherapy group [14.1 months (95% CI 12.4–16.2)]. Our data were based on clinical characteristics of Checkmate 743 subjects aged 18 years or older with unresectable MPM, regardless of PD-L1 expression.
In the total number of people in this trial, 303 participants were in the experimental group (Nivolumab plus ipilimumab) and 302 in the control group (chemotherapy). We conducted a cost-effectiveness analysis for the Nivolumab plus ipilimumab group and chemotherapy group to provide a foundation for their different treatment. The research methods refer to the consolidated health economic evaluation reporting standards (CHEERS) (see Supplementary Material S1).
The model's structure
The study used TreeAge Software 2021 (TreeAge Software, Inc., Williamstown, Massachusetts) to program a multi-state Markov model. The purpose was to evaluate the cost-effectiveness of Nivolumab plus ipilimumab and chemotherapy in patients with unresectable malignant pleural mesothelioma. The multiple health states include PFS, progressive disease state (PD) and death. Assuming that patients in a certain state only make one state transition in a cycle, once the patients are in the PD state, they cannot return to the PFS state. Similarly, the patients in the dead state cannot transition to other states. The specific transition relationships are shown in Supplementary Figure S1. We assumed that all the patients were in a PFS healthy state at the model's initial stage. The patients were treated with Nivolumab plus ipilimumab or chemotherapy according to their groupings. When the disease progresses, the follow-up treatment plan in the CheckMate 743 clinical trial is used for treatment until the patient's death.
We developed a Markov model to simulate the patient's entire life course and evaluate the cost and effectiveness of first-line therapy for patients with unresectable malignant pleural mesothelioma. In the CheckMate 743 clinical trial, the median survival time of the experimental group was 18.1 months, the control group was 14.1 months. The effect of immunotherapy has a delayed effect and may continue to work beyond the treatment period. It should be analyzed from long-term data to avoid inaccuracy and uncertainty of results (13, 14). As the result of that, with reference to the dosing cycle of the CheckMate 743 clinical trial, we set the cycle of the Markov model to 3 weeks and the time range was 10 years. Approximately 99% of patients were in the absorption state (15). A half-circle correction was conducted to simulate the transfer process more accurately. Simultaneous simulation analysis of the cost and utility is performed to estimate the cumulative total cost and health outcomes within the cohort's time frame (16, 17). The research was based on the American payers' perspectives, with a 3% discount on costs and utilities (18). According to the World Health Organization, ICER is acceptable when it is below three times GDP per capita (19). This study will use three times of the United States's triple GDP per capita in 2021 is $69,231 as the threshold (World Economic Outlook Database, April 2022, https://www.imf.org/en/Publications/WEO/weo-database/2022/April). The WTP is assumed to be $207,659. The research indicators include the costs, life-years (LYs), quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratios (ICERs).
The model's survival and progression risk estimates
The original data for constructing the model were obtained from the CheckMate 743 clinical trial. When some data were unavailable, we referred to the related published literature. The GetData Graph Digitiser (version 2.26; http://getdata-graph-digitizer.com/download.php) was used to extract the Kaplan–Meier curve's data of the PFS and OS in the Nivolumab plus ipilimumab group and the chemotherapy group. We also referred to the algorithm of Guyot et al. who refers to the pseudo-individual patient's data reconstructed by R software (version 4.1.0; https://www.r-project.org/) (20). This was combined with the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) to select the Log-logistic, Weibull and Log-norm distribution that fitted the survival curve for Nivolumab plus ipilimumab and chemotherapy, respectively, after the reconstruction (21). The distribution has a higher flexibility and estimated distributions (22, 23). Details of model extrapolation are shown in Supplementary Table S1.
The utility and cost estimates
During the follow-up, the CheckMate 743 trial used the Mesothelioma LungCancer Symptom Scale (LCSS-Meso) to compare quality of life after nivolumab plus ipilimumab vs. chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. The average health utility (0.65 for PFS and 0.47 for PD) of the patients with unresectable malignant pleural mesothelioma in the PFS and PD was obtained by the published values for non-small cell cancer (NSCLC) (24), because there is no previously published study mentioning precise utility scores. The top three incidence adverse events (AEs) with grade 3 or above were selected in Nivolumab plus ipilimumab [nivolumab (3 mg/kg intravenously once every 2 weeks) plus ipilimumab (1 mg/kg intravenously once every 6 weeks)] and platinum plus pemetrexed chemotherapy [pemetrexed (500 mg/m2 intravenously) plus cisplatin (75 mg/m2 intravenously) or carboplatin (area under the concentration-time curve 5 mg/mL per min intravenously)] to be considered to evaluate the loss of the health utility caused by the three to five adverse events (AEs) for simplifying the calculation. The top three incidence adverse events (AEs) with grade 3 or above in Nivolumab plus ipilimumab and chemotherapy are Diarrhea (0.3%), Increased lipase (0.5%), Increased amylase (0.3%) and Anemia (36%), Nausea (36%), Decreased appetite (18%), respectively.
The costs are reported in 2021 US dollars (US $1.0 = CNY 6.4). Only the direct costs of the medical expenses were considered. This included the cost of the drugs, subsequent treatment costs, management costs, follow-up costs, laboratory examination costs, and the major adverse reactions with grade 3 or above had the top three incidence rates according to CheckMate 743 trial.
The estimated cost of each drug during the set period is listed in Table 1. The probability that different treatment groups intend to receive different follow-up treatment and the treatment mode of specific subsequent therapies [systemic therapy other than PD-(L)1 inhibitors, local regional therapy, radiation therapy, surgery, PD-(L)1 inhibitors] are derived from CheckMate 743 trial (10, 24–33).
Table 1.
Baseline value.
| Variable | Baseline value | Range | Distribution | References | ||
|---|---|---|---|---|---|---|
| Minimum | Maximum | |||||
| Weilbull OS survival model in Nivolumab plus ipilimumab | Shape = 1.19536, scale = 25.48855 | - | - | Weilbull | Model fitting | |
| Log-normal PFS survival model in Nivolumab plus ipilimumab | Meanlog = 1.88660, sdlog = 1.23141 | - | - | log-normal | Model fitting | |
| Log-logistic OS survival model in chemotherapy | Shape = 1.7027, scale = 14.1088 | - | - | log-logistic | Model fitting | |
| Log-logistic PFS survival model in chemotherapy | Shape = 2.185, scale = 7.392 | - | - | log-logistic | Model fitting | |
| Risk for main adverse events | ||||||
| Nivolumab plus ipilimumab group | ||||||
| Diarrhea | 0.003 | 0.0024 | 0.0036 | Beta | Checkmate 743 | |
| Increased lipase | 0.005 | 0.004 | 0.006 | Beta | Checkmate 743 | |
| Increased amylase | 0.003 | 0.0024 | 0.0036 | Beta | Checkmate 743 | |
| Chemotherapy | ||||||
| Anemia | 0.36 | 0.288 | 0.432 | Beta | Checkmate 743 | |
| Nausea | 0.36 | 0.288 | 0.432 | Beta | Checkmate 743 | |
| Decreased appetite | 0.18 | 0.144 | 0.216 | Beta | Checkmate 743 | |
| Health utility scores | ||||||
| Utility of PFS | 0.65 | 0.52 | 0.78 | Beta | (24) | |
| Utility of PD | 0.47 | 0.376 | 0.564 | Beta | (24) | |
| Cost, $/per cycle | ||||||
| Cisplatin | 48.6108 | 38.88864 | 58.33296 | 9.72216 | Gamma | 2022 Payment allowance limits for Medicare |
| Carboplatin | 55.80288 | 44.6423 | 66.963456 | 11.160576 | Gamma | 2022 Payment allowance limits for Medicare |
| Pemetrexed | 12,832.512 | 10,266.01 | 15,399.0144 | 2,566.5024 | Gamma | 2022 Payment allowance limits for Medicare |
| Nivolumab | 18,419.94 | 14,735.95 | 22,103.928 | 3,683.988 | Gamma | 2022 Payment allowance limits for Medicare |
| Ipilimumab | 11,258.31 | 9,006.648 | 13,509.972 | 2,251.662 | Gamma | 2022 Payment allowance limits for Medicare |
| Laboratory_test | 157.5 | 126 | 189 | 31.5 | Gamma | |
| Follow-up | 59.2 | 47.36 | 71.04 | 11.84 | Gamma | |
| Administration | 69.81 | 55.848 | 83.772 | 13.962 | Gamma | |
| Best supportive care | 117.1 | 93.68 | 140.52 | 23.42 | Gamma | (25) |
| Pembrolizumab | 21,479.6 | 17,183.68 | 25,775.52 | 4,295.92 | Gamma | 2022 Payment allowance limits for Medicare |
| Vinorelbine | 227.2756 | 181.8205 | 272.73072 | 45.45512 | Gamma | 2022 Payment allowance limits for Medicare |
| Expenditures on main AEs, $ | ||||||
| Diarrhea | 303 | 242 | 363 | Gamma | (26) | |
| Increased lipase | 2,933 | 2,346.4 | 3,519.6 | 586.6 | Gamma | (27) |
| Increased amylase | 2,933 | 2,346.4 | 3,519.6 | 586.6 | Gamma | (27) |
| Anemia | 493.04 | 246.52 | 739.56 | 98.608 | Gamma | (28) |
| Nausea | 218.27 | 174.616 | 261.924 | 43.654 | Gamma | (29) |
| Decreased appetite | 115.4 | 103.8 | 126.9 | 23.08 | Gamma | (30) |
| Disutility due to AEs | ||||||
| Diarrhea | −0.047 | −0.0564 | −0.0376 | −0.0094 | Beta | (31) |
| Increased lipase | −0.47 | −0.564 | −0.376 | −0.094 | Beta | (27) |
| Increased amylase | −0.47 | −0.564 | −0.376 | −0.094 | Beta | (27) |
| Anemia | −0.09 | −0.108 | −0.072 | −0.018 | Beta | (29) |
| Nausea | −0.048 | −0.0576 | −0.0384 | −0.0096 | Beta | (29) |
| Decreased appetite | −0.038 | −0.0456 | −0.0304 | −0.0076 | Beta | (31) |
| Risk for Subsequent therapy | ||||||
| Nivolumab plus ipilimumab group | ||||||
| Immunotherapy | 0.033 | 0.0264 | 0.0396 | 0.0066 | Beta | Checkmate 743 |
| Chemotherapy | 0.432 | 0.3456 | 0.5184 | 0.0864 | Beta | Checkmate 743 |
| Chemotherapy | ||||||
| Immunotherapy | 0.202 | 0.1616 | 0.2424 | 0.0404 | Beta | Checkmate 743 |
| Chemotherapy | 0.315 | 0.252 | 0.378 | 0.063 | Beta | Checkmate 743 |
The calculated drug dose are based on the actual clinical trials. In the Nivolumab plus ipilimumab group, the patients received nivolumab (3 mg/kg intravenously once every 2 weeks) plus ipilimumab (1 mg/kg intravenously once every 6 weeks). In the chemotherapy group, the patients received platinum plus pemetrexed chemotherapy [pemetrexed (500 mg/m2 intravenously) plus cisplatin (75 mg/m2 intravenously) or carboplatin (area under the concentration-time curve 5 mg/mL per min intravenously)]. We assumed that the average body surface area was 1.68 m2 (34). When patients disease progressed, we assumed that all patients who disease progressed had follow-up treatment. It is important to note that the systemic therapy other than PD-(L)1 inhibitors in the subsequent therapies for unresectable MPM, which we chose based on the NCCN 2022.1 guideline (https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf), is oxaliplatin, leucovorin plus fluorouracil therapy (oxaliplatin 85 mg/m2 IV on day 1, Leucovorin 200 mg/m2 IV on day 1,2,fluorouracil 1,000 mg/m2 IV continuous infusionover 22 h on day 1, 2).
Sensitivity analyses
A one-way sensitivity analysis was performed to explore the influence of uncertain parameters on the ICER. Each parameter was independently changed by assuming ±20% of the expected value to determine the obvious influence on decision-making. Probabilistic analysis (PSA) was used to randomly sample all the parameters from a specified distribution to further explore the uncertainty and relevance of the model's parameters. According to the parameter type, we selected the appropriate distribution for each uncertain parameter: the cost of the adverse reactions to drugs and treatment is the gamma distribution. The risk of adverse reactions, and the health utility scores including PFS, OS, and AE are the beta distribution. We performed a second-order Monte Carlo simulation of 10,000 iterations and generated a cost-benefit acceptability curve (CEAC) to show that Nivolumab plus ipilimumab is cost-effective with different WTP thresholds.
Results
Base-case analysis
The result of base-case analysis about the cost and effectiveness of Nivolumab plus Ipilimumab group and chemotherapy group in patients with unresectable malignant pleural mesothelioma was shown in Table 1. According to our analysis, the incremental cost of Nivolumab plus Ipilimumab ($292,319.48, 1.11 QALYs) vs. chemotherapy ($95,715.26, 0.58 QALYs) is $196,604.22 and the QALYs is 0.53. The ICER values ($371,861.36) are higher than the United States's triple GDP per capita threshold in 2021 ($207,659) (Table 2).
Table 2.
Base-case analysis results.
| Strategies | Cost | Incr cost | LYs | Incr LYs | ICER/LYs | QALYs | Incr QALYs | ICER/QALYs |
|---|---|---|---|---|---|---|---|---|
| All patients group | ||||||||
| Chemotherapy | 95,715.26 | 0.65 | 0.58 | |||||
| Nivolumab plus ipilimumab | 292,319.48 | 196,604.22 | 1.58 | 0.92 | 213,082.80 | 1.11 | 0.53 | 371,861.36 |
Incr cost, incremental cost; Lys, life-years; Incr Lys, incremental life-years; QALYs, quality-adjusted life-years; Incr QALYs, incremental quality-adjusted life-years; ICER, incremental cost-effectiveness ratio.
Sensitivity analyses
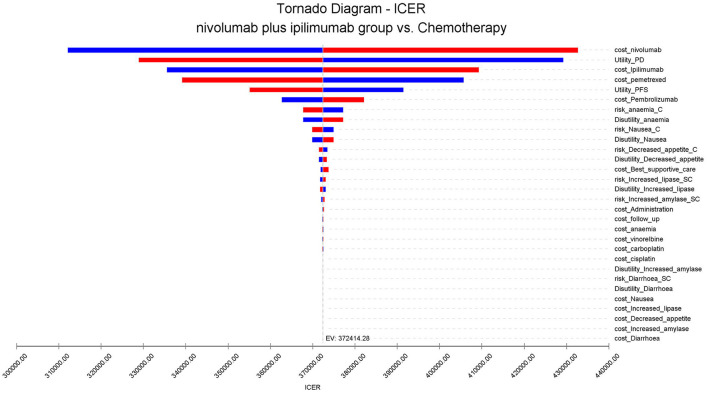
A one-way sensitivity analysis was used to test the robustness of the two population model outputs. Under the condition that the input model parameters change by ±20%, the influence of each parameter on the analysis results is explored. The results are presented in the tornado diagram (Figure 1). The sensitivity analysis results demonstrated that the cost of Nivolumab has the most contributed to it.
Figure 1.
Tornado diagram for one-way sensitivity analysis.
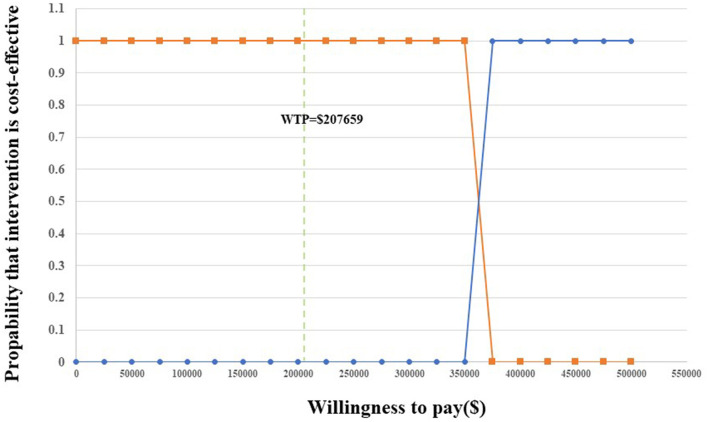
Probability sensitivity analysis
Probability sensitivity analysis (PSA) is applied to test the bias of the multiple model parameters on the analysis results when the multiple model parameters change simultaneously.
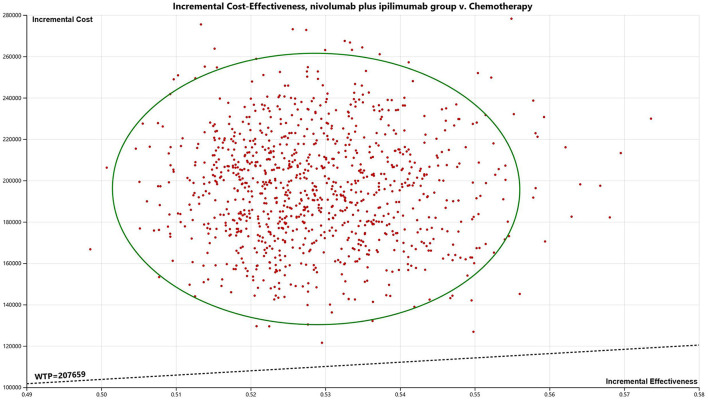
The results are presented through cost-effectiveness acceptability curves (Figure 2) and incremental cost-effectiveness scatterplots (Figure 3). According to the results, we found that the higher the average social willingness to pay, the higher the probability of Nivolumab plus Ipilimumab producing the cost effect. Under the condition of a payment threshold of $207,659 per QALY, Probabilistic sensitivity analysis showed that there was no probability that Nivolumab plus ipilimumab was cost-effective within the fluctuation range of other model parameters in first-line in unresectable malignant pleural mesothelioma.
Figure 2.
Acceptability curves for the choice of nivolumab plus ipilimumab vs. chemotherapy at different WTP thresholds.
Figure 3.
Incremental cost-effectiveness scatter plot (nivolumab plus ipilimumab vs. chemotherapy).
Discussion
The phase III randomized trial Checkmate 743 showed a positive result after comparing the efficiency and safety of nivolumab plus ipilimumab and that of chemotherapy as first-line therapy for unresectable MPM. Beforehand, the only one first-line treatment approved by FDA is a platinum-based chemotherapy combined with pemetrexed. Based on Checkmate 743, nivolumab plus ipilimumab has been approved as a first-line therapy for advanced MPM by FDA in October 2020 (35).
There is no doubt that this is a giant leap forward in terms of immunotherapy for MPM. However, the high price has been the spotlight, the financial toxicity will affect the patients' compliance and even leads to termination and abandonment of treatment (36). Especially for the patients with poor ECOG performance status and patients aged 75 years or older on the account of this subgroup didn't benefit from nivolumab plus ipilimumab in current data from Checkmate 743. So, further consideration needs to be given to whether nivolumab plus ipilimumab have a cost-effectiveness advantage as first-line treatment for patients with unresectable MPM.
Based on CheckMate 743 clinical trials and the latest population data and drug prices in USA, our study is the first analysis to evaluate whether Nivolumab plus ipilimumab is more cost-effective than chemotherapy as first-line treatment for treatment-naive advanced MPM with different histology subtypes and PD-L1 expressions from the American payers' perspectives. We obtained the data of Nivolumab plus ipilimumab group and chemotherapy group through the clinical trial CheckMate 743, so we could only conduct cost-effectiveness analysis on these two groups. Our analysis shows that with a WTP threshold of $207,659/QALY for the two groups, the Nivolumab plus ipilimumab group may not be cost-effective to be the first-line for unresectable MPM. We also performed subgroup analyses for all the subgroups mentioned in the clinical trials, unfortunately, the subgroup analysis implied that Nivolumab plus ipilimumab was not a cost-effective strategy across all the patient subgroups. Yet in Checkmate 743, there was a big gap between non-epithelioid [HR 0.46 (95% CI 0.31–0.68)] and epithelioid histology [HR 0.86 (0.69–1.08)] in overall survival of nivolumab plus ipilimumab group and expression level of PD-L1 affects the prognosis of the chemotherapy group, which also called for the further stratified analysis of more heterogeneous epithelioid subtypes.
One-way sensitivity analysis demonstrated that reducing the cost of drugs was the most influential factor to the result of cost-effectiveness. Since the therapeutic effect of the experimental group was better than that of the control group, and chemotherapy was in the control group, the price largely determines the cost-effectiveness. The lower the price of cisplatin, carboplatin, pemetrexed, the lower the cost-effectiveness of the experimental group. There also have been some economic studies on chemotherapy for MPM. A comprehensive review of cost-effectiveness analysis published in 2013 by CHRISTEL C.L.M. BOONS shows that PC (pemetrexed/cisplatin) was not regarded to be cost-effective for the treatment of MPM in the UK. However, the increased cost is considered to be reasonable on account of the lack of efficient treatment (24). After the exploration of targeted therapy for MPM, a study by Mei Zhan on the cost-effectiveness of additional bevacizumab to pemetrexed plus cisplatin in the treatment of unresectable MPM predicted to increase the cost by $81,446.69 with a gain of 0.112 QALYs, which is equal to an ICER of $727,202.589/QALY (37). The study found that additional bevacizumab to PC is not a cost-effective option in China, putting the choice of treatment for advanced MPM into an awkward position to some extent. So, people turn to put focus more on immunotherapy. It has been shown that nivolumab plus ipilimumab is cost-effective for patients suffered from cancer, such as advanced NSCLC with PD-L1 expression level <1%, PD-L1-positive advanced renal-cell carcinoma from the US payer's perspective (34, 36). However, most of the current cost-effectiveness analysis data show that chemotherapy is the more preferred choice than nivolumab plus ipilimumab to treat advanced NSCLC (38). Chemotherapy is much less expensive than immunotherapy in most cases, so it is easier to show cost-effectiveness. Therefore, we conclude that PD-1 inhibitors may be difficult to recommend as cost-effectiveness options for first-line recommendations in unresectable MPM.
We found that when the cost of Nivolumab plus ipilimumab was reduced by 34% (ICER/QALY = 205,679), the ICER was $207,659/QALY which was cost-effective. Therefore, changing the price of Nivolumab plus ipilimumab is an effective feasible strategy to achieve efficient use of them. Medical insurance authority will negotiate with pharmaceutical companies to ensure reasonable drug prices and adjust the medical insurance list to reduce the medical burden on patients.
Limitations
Our study has some limitations. First, our research is based on Checkmate 743, where there are some inadequacy itself, including the very limited beneficiaries of immunotherapy and the lack of further stratified analysis of epithelioid subtypes (39). Second, CheckMate 743 is a phase three randomized controlled trial, and we used this model to simplify the study. For instance, regarding the adverse reactions, we selected the three to four main AEs that would lead to errors. Third, the study's data originated from the CheckMate 743 trial. Due to the limitation of the number of patients included in the trial, we could not perform a larger-scale analysis, and the trial did not provide the follow-up survival data for patients. We relied on the survival data based on the trial and performed a reasonable extrapolation to predict the long-term survival of patients. This will inevitably be different from the data of real-world patients obtained through regular follow-ups. Fourth, since CheckMate 743 does not disclose the specific health data of patients, our PFS and PD effectiveness were derived from previously published related studies. This may be different from the actual situation of the study. Fifth, we only considered the cost impact and utility reduction caused by the three to four main AEs. The utility reduction caused by specific adverse reactions comes from other published literature, which is in line with the real situation. Sixth, the clinical trial is a multicentred and comprehensive study between different countries and races. The treatment plan of the trial, and especially the follow-up treatment of patients, will be adjusted appropriately according to the specific situation. Therefore, more clinical trials are still required to reduce the study population, follow-up treatment, and other factors that impact the results.
Conclusion
Overall, from the Americans payers' perspectives, compared with chemotherapy, Nivolumab plus ipilimumab a for the first-line treatment of patients aged 18 years or older who were clinically or pathologically diagnosed with locally advanced or metastatic MPM may be not a cost-effective choice at a WTP threshold of $207,659/QALY.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
This study was based on a literature review and modeling techniques; this study did not require approval by an Institutional Research Ethics Board.
Author contributions
Z-mY and ZX had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and concept and design, acquisition, analysis, or interpretation of data. Drafting of the manuscript: Z-QT, Z-mY, and HL. Critical revision of the manuscript for important intellectual content, obtained funding, and supervision: QZ and HL. Statistical analysis: Z-mY, ZX, and Z-QT. Administrative, technical, or material support: Z-QT and HL. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 81900201) and Youth Foundation of Xiangya Hospital (No. 2017Q09) to HL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.947375/full#supplementary-material
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Cantini L, Hassan R, Sterman DH, Aerts J. Emerging treatments for malignant pleural mesothelioma: where are we heading? Front Oncol. (2020) 10:343. 10.3389/fonc.2020.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Xu D, Schmid RA, Peng RW. Biomarker-guided targeted and immunotherapies in malignant pleural mesothelioma. Ther Adv Med Oncol. (2020) 12:1758835920971421. 10.1177/1758835920971421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuribayashi K, Doi H, Kijima T. Types of surgery post-neoadjuvant chemotherapy for pleural mesothelioma. Expert Rev Respir Med. (2019) 13:1189–94. 10.1080/17476348.2019.1679119 [DOI] [PubMed] [Google Scholar]
- 5.Viscardi G, Di Liello R, Morgillo F. How I treat malignant pleural mesothelioma. ESMO Open. (2020) 4:e000669. 10.1136/esmoopen-2019-000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoro A, O'Brien ME, Stahel RA, Nackaerts K, Baas P, Karthaus M, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol. (2008) 3:756–63. 10.1097/JTO.0b013e31817c73d6 [DOI] [PubMed] [Google Scholar]
- 7.Terenziani R, Zoppi S, Fumarola C, Alfieri R, Bonelli M. Immunotherapeutic approaches in malignant pleural mesothelioma. Cancers. (2021) 13:793. 10.3390/cancers13112793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vita E, Stefani A, Di Salvatore M, Chiappetta M, Lococo F, Margaritora S, et al. Oncological frontiers in the treatment of malignant pleural mesothelioma. J Clin Med. (2021) 10:290. 10.3390/jcm10112290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gooijer CJ, Borm FJ, Scherpereel A, Baas P. Immunotherapy in malignant pleural mesothelioma. Front Oncol. (2020) 10:187. 10.3389/fonc.2020.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. (2021) 397:375–86. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 11.Liao D, Yu Y, Mei Q, Wang Z, Li X, Jia Y, et al. Advances in immunotherapy of malignant pleural mesothelioma. Onco Targets Ther. (2021) 14:4477–84. 10.2147/OTT.S317434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilberberg MD, Shorr AF. Understanding cost-effectiveness. Clin Microbiol Infect. (2010) 16:1707–12. 10.1111/j.1469-0691.2010.03331.x [DOI] [PubMed] [Google Scholar]
- 13.Othus M, Bansal A, Koepl L, Wagner S, Ramsey S. Accounting for cured patients in cost-effectiveness analysis. Value Health. (2017) 20:705–9. 10.1016/j.jval.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Bullement A, Latimer NR, Bell Gorrod H. Survival extrapolation in cancer immunotherapy: a validation-based case study. Value Health. (2019) 22:276–83. 10.1016/j.jval.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 15.Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. (2009) 471:469–86. 10.1007/978-1-59745-416-2_24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States Pharmacoeconomics. (2017) 35:831–44. 10.1007/s40273-017-0527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S, Luo S, Zhong L, Lai S, Zeng D, Rao X, et al. Cost-effectiveness of atezolizumab plus chemotherapy for advanced non-small-cell lung cancer. Int J Clin Pharm. (2020) 42:1175–83. 10.1007/s11096-020-01076-3 [DOI] [PubMed] [Google Scholar]
- 18.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. (2016) 316:1093–103. 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 19.Thokala P, Ochalek J, Leech AA, Tong TJP. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. (2018) 36:509–22. 10.1007/s40273-017-0606-1 [DOI] [PubMed] [Google Scholar]
- 20.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Zhang L, Huang Q, Li N, Zheng B, Cai H. Cost-effectiveness analysis of ceritinib and alectinib versus crizotinib in the treatment of anaplastic lymphoma kinase-positive advanced non-small cell lung cancer. Cancer Manag Res. (2019) 11:9195–202. 10.2147/CMAR.S223441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zeng X, Cai H, Li N, Liu M, Qiu L, et al. Cost-effectiveness of second-line nivolumab for platinum-treated advanced non-small-cell lung cancer. J Comp Eff Res. (2020) 9:1301–9. 10.2217/cer-2020-0053 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Jin L, Cui J, Ma K, Chen X, Li W. Mouse double minute-2 homolog (MDM2)-rs2279744 polymorphism associated with lung cancer risk in a Northeastern Chinese population. Thorac Cancer. (2015) 6:91–6. 10.1111/1759-7714.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan M, Zheng H, Xu T, Yang Y, Li Q. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer. (2017) 110:1–6. 10.1016/j.lungcan.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 25.Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. (2000) 9:235–51. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Fu Y, Kumar A, Chen M, Si L, Rojanasarot S. Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Ann Transl Med. (2021) 9:1226. 10.21037/atm-21-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris S, Gurusamy KS, Patel N, Davidson BR. Cost-effectiveness of early laparoscopic cholecystectomy for mild acute gallstone pancreatitis. Br J Surg. (2014) 101:828–35. 10.1002/bjs.9501 [DOI] [PubMed] [Google Scholar]
- 28.Ding D, Hu H, Shi Y, She L, Yao L, Zhu Y, et al. Cost-effectiveness of pembrolizumab plus axitinib versus sunitinib as first-line therapy in advanced renal cell carcinoma in the US. Oncologist. (2021) 26:e290–e7. 10.1002/ONCO.13522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullement A, Nathan P, Willis A, Amin A, Lilley C, Stapelkamp C, et al. Cost effectiveness of avelumab for metastatic merkel cell carcinoma. Pharmacoecon Open. (2019) 3:3779coe 10.1007/s41669-018-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. (2020) 16:1189–98. 10.2217/fon-2019-0821 [DOI] [PubMed] [Google Scholar]
- 31.Li LY, Wang H, Chen X, Li WQ, Cui JW. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China. Chin Med J. (2019) 132:2790–4. 10.1097/CM9.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ASP Drug Pricing Files (2022). Available online at: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2022-asp-drug-pricing-files (accessed May 1, 2022).
- 33.Haddad R, Cohen EEW, Venkatachalam M, Young K, Singh P, Shaw JW, et al. Cost-effectiveness analysis of nivolumab for the treatment of squamous cell carcinoma of the head and neck in the United States. J Med Econ. (2020) 23:442–7. 10.1080/13696998.2020.1715414 [DOI] [PubMed] [Google Scholar]
- 34.Ward MC, Shah C, Adelstein DJ, Geiger JL, Miller JA, Koyfman SA, et al. Cost-effectiveness of nivolumab for recurrent or metastatic head and neck cancer. Oral Oncol. (2017) 74:49–55. 10.1016/j.oraloncology.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 35.Nakajima EC, Vellanki PJ, Larkins E, Chatterjee S, Mishra-Kalyani PS, Bi Y, et al. FDA approval summary: nivolumab in combination with ipilimumab for the treatment of unresectable malignant pleural mesothelioma. Clin Cancer Res. (2022) 28:446–51. 10.1158/1078-0432.CCR-21-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. (2018) 68:153–65. 10.3322/caac.21443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beusterien KM, Davies J, Leach M, Meiklejohn D, Grinspan JL, O'Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. (2010) 8:50. 10.1186/1477-7525-8-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courtney PT, Yip AT, Cherry DR, Salans MA, Kumar A, Murphy JD. Cost-effectiveness of nivolumab-ipilimumab combination therapy for the treatment of advanced non-small cell lung cancer. JAMA Netw Open. (2021) 4:e218787. 10.1001/jamanetworkopen.2021.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceresoli GL, Pasello G. Immune checkpoint inhibitors in mesothelioma: a turning point. Lancet. (2021) 397:348–9. 10.1016/S0140-6736(21)00147-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.