ABSTRACT
Background
Tendinopathy is a painful condition that is prevalent in athletes as well as the general human population, and whose management is challenging.
Objective
This systematic review aimed to evaluate the impact of nutrition on the prevention and treatment of tendinopathy.
Methods
Searches were conducted in PubMed, EMBASE, Web of Science, and SPORTDiscus without restriction to year of publication. Studies examining the impact of exposure to nutrient intake in an adult human population on 1) prevalence/incidence of tendinopathy, 2) clinical outcomes of tendinopathy, 3) structural changes in the tendon by imaging modalities. Experimental and observational study designs written in English, Dutch, or German were eligible.
Results
Nineteen studies met the inclusion criteria. The effects of the habitual diet were investigated in one study. Four studies examined the effects of exposure to alcohol. Alcohol consumption can be a potential risk factor associated with Achilles tendinopathy and rotator cuff tears, although findings were inconsistent. The use of dietary supplements was examined in fourteen studies. Among these, collagen-derived peptides were most often part of the supplements evaluated. Combining training and dietary supplements seems to induce better clinical and functional outcomes in tendinopathy.
Conclusion
This review demonstrates the paucity of high-quality studies and a wide variety among studies regarding nutrients, tendon location, study population, and reported outcome measures. Individual studies showed promising clinical implications for the use of dietary supplements, particularly those containing collagen-derived peptides. However, giving any definitive dietary recommendations on the prevention and treatment of tendinopathy remains elusive.
KEYWORDS: Tendon, tendinopathy, diet, supplements, collagen
1. Introduction
Tendinopathy, which involves persistent tendon pain and loss of function related to mechanical loading [1], is common in athletes as well as in the general population [2,3]. Mechanical overuse is seen as the key initial trigger in the multifactorial etiology of tendinopathy [3], hence it is a common cause of injury in sports that involve exposure to high forces and repetitive movements, such as running, volleyball, and tennis [3–5]. Tendinopathy is also prevalent in workers exposed to monotonous repetitive work tasks [2], and is associated with a number of medical conditions such as obesity and diabetes mellitus [6–8]. Other intrinsic risk factors are age, sex, and genetics [9]. The potential suffering from pain and loss of function may impact health, sports performance, and work ability [10]. Also, the impact of tendinopathy on quality of life is substantial, specifically on the domains mobility, pain/discomfort, and usual activities [11].
There is only limited evidence for the efficacy of preventive interventions for tendinopathy [12]. Numerous treatment options for tendinopathy have been described [9], but convincing evidence of success of many common therapies is lacking. Hence the management of tendinopathy remains a challenging and often time-consuming process [9]. This stresses the relevance of additional strategies for both prevention and treatment of tendinopathy.
The pathological tendon is characterized by an altered tissue homeostasis [13]. Given that diet plays a major role in the homeostasis of all tissues and poor nutrition is one of the extrinsic factors that contributes to the development of tendinopathy [9], nutritional interventions, e.g. intake of collagen, are a plausible, potential strategy to improve the prevention and healing of tendinopathy.
Adequate intake of nutrients – macronutrients as well as micronutrients – is of great importance, especially for populations with specific demands such as athletes. This primarily entails consumption of a healthy habitual diet, but also sport-specific nutritional strategies as well as dietary supplements may be used to optimize intake in specific situations. A dietary supplement is defined as a food, food component, nutrient, or non-food compound that is purposefully ingested in addition to the habitually consumed diet with the aim of achieving a specific health and/or performance benefit [14]. The use of supplements is widespread among athletes as well as the general population [15]. However, specific recommendations of dietary strategies for preventing or treating tendon injuries, either as part of the habitual diet or with additional dietary supplements, are lacking [16].
The potential benefits of nutrition on tendon health have been described in only a few reviews [17–19]. A recent short review identified various nutrients, including amino acids, vitamins, and trace minerals, as being potentially useful in improving tendon growth and healing [17]. It has additionally been suggested that nutritional interventions involving multiple nutrients, e.g. collagen combined with vitamin C, may be more effective than single-nutrient strategies, as many nutrients are involved in tendon and collagen metabolism [17]. So far, only evidence for effects of vitamin and amino acid supplements on tendon tissue healing has systematically been evaluated, but hardly any clinical studies are included [20,21]. No previous study has systematically synthesized the evidence of nutritional exposure for clinical outcomes on physical, psychosocial and overall life impact, or the risk of tendinopathies in relation to nutrition. The aim of this systematic review was therefore to evaluate the impact of nutrition on the prevention and treatment of tendinopathy in a general human population. This will guide future studies on directions of research toward evidence-based nutritional recommendations to prevent and treat tendinopathy, which ultimately leads to a lower prevalence and better management of this bothersome condition.
2. Methods
This systematic review complied with the PRISMA guidelines [22]. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 29 June 2020 (CRD42020189773).
Eligibility criteria
Studies were eligible if they investigated the impact of exposure to nutrient intake (either as part of the habitual diet or in the form of specific dietary supplements) in an adult (>18 years) human population, using at least one of the following outcome measures: prevalence/incidence of tendinopathy, a clinical outcome that captures one of the tendinopathy-related core domains as established by the ICON group [23] (see Additional file 1), structural changes in the tendon identified by imaging modalities such as magnetic resonance imaging (MRI) or ultrasound imaging (USI) [24]. Randomized and non-randomized intervention studies and cohort, case-control, cross-sectional, and case studies written in English, Dutch, or German were included. Reviews, letters, and editorials were excluded. There was no restriction with regard to year of publication.
Search strategy
We searched the electronic databases PubMed, EMBASE, Web of Science and SPORTDiscus in June 2020 for eligible studies. The specific search strategies were created by a health science librarian with expertise in systematic review searching and had three components: Nutrition, Tendinopathy, and Human. The PubMed search strategy was adapted to the syntax of other databases and is presented in Additional file 2. In addition to the database search, reference lists of included studies as well as relevant reviews were manually checked to identify additional studies for inclusion.
Study selection
The records were imported into Endnote X9, where duplicates were removed. Two reviewers (AH, JZ) independently screened the titles and abstracts from the identified articles for eligibility, followed by full-text evaluation for final study inclusion. Any disagreements about inclusion/exclusion were discussed between the reviewers and a final decision was made by all authors.
Data extraction and analysis
Data was extracted from the included papers using a spreadsheet prepared in Microsoft Excel. We extracted publication details, study design, study aim, population characteristics, type of tendinopathy/site of interest, exposure and comparator details, relevant outcome measures and results, and conclusion with respect to nutritional exposure. The study characteristics and results are presented in tables and summarized semi-narratively.
Risk of bias assessment
Two reviewers (AH, CR) independently assessed risk of bias of the included studies. The revised Cochrane risk-of-bias tool (RoB 2) was used to assess the quality of randomized trials [25]. We assessed risk of bias on a per-protocol basis for all five domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) outcome measurement, and (5) selection of the reported result. Other intervention, cohort, case-control, and cross-sectional studies were assessed using the ROBINS-I tool [26]. Bias was assessed for the following domains: (1) confounding, (2) selection of participants into the study, (3) classification of interventions, (4) deviations from intended interventions, (5) missing data, (6) measurement of outcomes, and (7) selection of the reported result.
An overall level of certainty in the evidence for clinical outcomes and for the occurrence/prevalence of tendinopathy was rated using the GRADE approach for systematic reviews in which only a narrative summary of the effect across studies is available [27].
3. Results
Figure 1 shows the study selection process. A total of 8618 records were retrieved from the database and manual searches. After duplicates were removed, 6538 records were screened for eligibility, of which 89 were assessed in full-text. Of these studies, nineteen met the inclusion criteria. Seventy articles were excluded because they did not meet the language (n = 3), study design (n = 37), study population (n = 5), exposure (n = 11) or outcome (n = 9) criteria, or were not available (n = 5). Details of the included studies are provided in Tables 1 and 2. Five studies investigated the effect of the habitual diet (Table 1). The use of dietary supplements was examined by fourteen studies (Table 2), one of which evaluated an intervention of supplement use combined with habitual dietary changes [28].
Figure 1.
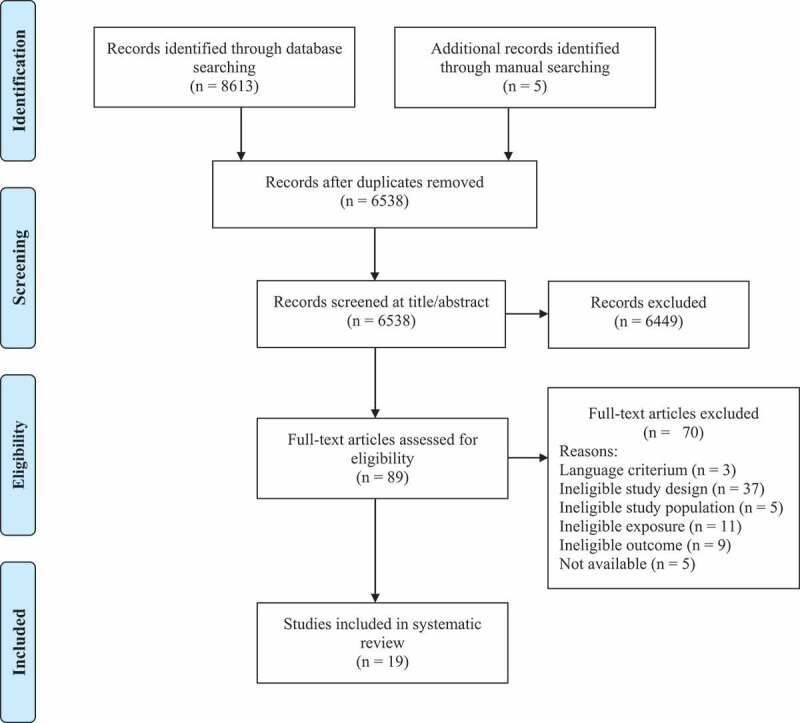
PRISMA flow diagram of the study selection process.
Table 1.
Details of the studies examining exposure to the habitual diet (n = 5).
| Study | Design | Aim | Population | Nutritional exposure | Outcome measure(s) | Results | Conclusion |
|---|---|---|---|---|---|---|---|
| Hjerrild et al. (2019) [31] | Cross-sectional study | To investigate the effects of life-long physical activity on skin autofluorescence (SAF) and AT structure, and to determine if SAF and tendon structure are influenced by dietary factors | 182 athletes + 24 sedentary persons (54 ±18 y, male) | Diet (fruit, vegetables, fish, bread, cereals, coffee, wine, beer, liqueurs, total fluid, pure water) as well as overall dietary pattern (Western vs. Mediterranean) currently and during youth | Anteroposterior AT thickness (USI) | None of the dietary parameters was a significant predictor of AT thickness | Diet did not affect AT thickness |
| Jain et al. (2018) [29] | Prospective cohort study | To assess predictors of better shoulder pain and function after surgery | 50 patients with symptomatic RC tears undergoing operative treatment (59 ±9 y, 62% male) | Alcohol (habitual consumption <2-3/month vs. >1-2/week) | Shoulder pain and function (SPADI) at 3, 6, 12 and 18 months follow-up | Those consuming alcohol >1-2 times/week had lower SPADI scores (less shoulder pain and better function) than those consuming alcohol <2-3 times/month (p = 0.017) | Alcohol use is a longitudinal predictor of pain and functional outcomes after operative treatment for RC tears |
| Owens et al. (2013) [30] | Prospective cohort study | To prospectively identify risk factors for the development of lower extremity tendinopathy and plantar fasciitis in United States military personnel | 80,106 US active-duty military personnel (70.1% male) | Alcohol (none vs. light/moderate/heavy) | Risk of AT and PT tendinopathy (OR) | Moderate weekly alcohol consumption was marginally associated with increased risk for AT tendinopathy (OR = 1.33 (1.00-1.76), but not for PT tendinopathy (OR = 0.93 (0.71-1.21)) | Alcohol consumption is a potentially modifiable risk factor associated with AT tendinopathy |
| Passaretti et al. (2016) [33] | Case-control study | To investigate the association between alcohol consumption and RC tears | 249 patients treated arthroscopically for RC repair + 356 controls without RC tears (cases: 64 (54-78) y, 56% male; controls: 66 (58-82) y, 52% male) | Alcohol (nondrinkers vs. moderate/excessive drinkers) | Risk of RC tears (OR) | Significant risks of RC tears for excessive drinkers (men: OR = 1.7, p = 0.04; women: OR = 1.9, p = 0.04) | Long-term alcohol intake is a significant risk factor for onset and severity of rotator cuff tears |
| Rechardt et al. (2010) [32] | Cross-sectional study | To assess the associations of lifestyle factors, metabolic factors and carotid intima-media thickness with shoulder pain and chronic (>3 months) RC tendinitis. | 6237 participants (male: 50.8 y; female: 52.9 y, 46% male) | Alcohol (none/light/moderate/heavy) | Risk of RC tendinitis (OR) | Alcohol consumption was not associated with chronic RC tendinitis in either gender (data not shown) | (no conclusion with regard to alcohol) |
AT, Achilles tendon; CG, control group; OR, odds ratio; PT, patellar tendon; RC, rotator cuff; SPADI, Shoulder Pain and Disability Index; TG, treatment group; USI, ultrasound imaging
Table 2.
Details of the studies examining exposure to dietary supplements (n = 14).
| Study | Design | Aim | Population | Nutritional exposure | Concurrent exposure | Comparator | Outcome measure(s) | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Arquer et al. (2014) [44] | Non-comparative intervention study | To evaluate the efficacy and safety of a nutritional supplement on the clinical and structural evolution of AT, PT and LET tendinopathies | 98 tendinopathy patients, AT (n = 32): 49.2 ±3.64 y; PT (n = 32): 47.7 ±1.69 y; LET (n = 34): 39.0 ±2.44 y, both sexes | 3 capsules Tendoactive (mucopolysaccharides (435 mg), type I collagen (75 mg), vitamin C (60 mg)) per day for 90 consecutive days | None | Pre-measurements | Pain intensity at rest and when active (VAS); joint function (VISA-A/VISA-P/PRTEE); tendon cross-sectional thickness (USI) | After 90 days: Pain at rest decreased by 80% (AT), 71% (PT) and 91% (LET) (p <0.001). Pain when active decreased by 82% (AT), 73% (PT) and 81% (LET) (p <0.001). Functional scores improved by 38% (AT), 46% (PT) and 77% (LET) (p <0.001). Thickness reduced by 12% (AT), 10% (PT) and 20% (LET) (p <0.05). | Administration of Tendoactive is effective for improving the clinical symptoms and structural evolution of tendinopathies |
| Balius et al. (2016) [36] | RCT | To determine the additional benefit of mucopolysaccharides, collagen and vitamin C (MCVC) to a physical therapy program in patients with AT tendinopathy | 58 reactive or degenerative AT tendinopathy patients (18-70 y, both sexes) | 3 capsules MCVC (mucopolysaccharides (435 mg), type I collagen (75 mg), vitamin C (60 mg)) per day for 3 months | Eccentric training (EC+MCVC) or passive stretching (PS+MCVC) | Eccentric training only (EC) | VISA-A; pain at rest and during activity (VAS); tendon bilateral thickness (USI) | After 12 weeks: Statistically and clinically significant improvement in VISA-A scores in all groups without between-group effect (p >0.1). VAS scores decreased in all groups with a difference for pain at rest between PS+MCVC (−3.7(0.8) and EC (−2.7(1.3), p <0.05). Bilateral thickness remained constant in EC and EC+MCVC, and reduced in PS+MCVC (−0.63(0.3) mm, p <0.05). | MCVC seems to be therapeutically useful for the management of tendinopathies |
| Praet et al. (2019) [39] | RCT (cross-over) | To investigate whether oral supplementation of specific collagen peptides improves symptoms and tendon vascularization in patients with chronic mid-portion AT tendinopathy in combination with structured exercise | 20 mid-portion AT tendinopathy patients (44 ±8 y, 65% male) | Two daily sachets Tendoforte (2.5 g hydrolyzed specific collagen peptides) for 3 months | Eccentric and running exercises for 6 months | Placebo + eccentric and running exercises for 6 months | Pain and functional limitations (VISA-A); vascularization (USI) | The group receiving the supplement in the first 3 months improved by 12.6 (9.7-15.5) in the supplemental phase and 5.9 (2.8-9.0) in the placebo phase. The other group improved by 5.3 (2.3-8.3) and 17.7 (14.6-20.7). There was a difference between groups in evolution of the VISA-A scores over time (p <0.0001). No difference in vascularization between groups. | Supplementation of specific collagen peptides may accelerate the clinical benefits of exercise program in AT patients. |
| Vitali (2019) [43] | Non-randomized controlled study | To determine the efficacy of Extracorporeal Shock Wave Therapy (ESWT) in combination with the dietary supplement Tendisulfur Forte in the treatment of shoulder, LET and AT tendinopathies | 90 AT, shoulder or LET tendinopathies (39-69 y, 50% male) | Tendisulfur Forte (containing methyl-sulfonyl-methane (MSM), hydrolyzed swine collagen (Type I and Type II), L-arginine and L-lysine, vitamin C, chondroitin sulfate, glucosamine, Curcuma longa extracted to obtain curcuminoids, dry Boswellia serrata extracted to obtain acetyl-11-keto-b-boswellic acid (AKBA), and myrrh) 2x day for 1 month, 1x day for 1 month | ESWT | ESWT | Pain (VAS); clinical functional evaluation (VISA-A, UCLA shoulder score, MEPS) | After 60 days: UCLA scores were higher in TG [27] compared to CG (23, p = 0.0002). MEPS was higher in TG compared to CG (p <0.00001). VISA-A scores improved more in TG (+27, +39%) compared to CG (+7, 17%). VAS scores were lower in TG compared to CG for all tendinopathy types (p <0.0001). | Combined treatment of ESWT and oral supplementation leads to a faster recovery and better outcomes of AT, shoulder and LET tendinopathy. |
| Merolla et al. (2015) [35] | RCT | To assess the analgesic effect of a dietary supplement containing Boswellia serrata and Curcuma longa in a population of subjects with full-thickness SSP tendon tear treated arthroscopically | 100 patients who underwent surgical SSP tendon repair (TG: 53.3 ±7.6 y, 54% male; CG: 55.4 ±9.4 y, 56% male) | Two daily sachets for 15 days, 1 sachet Tendisulfur (methyl-sulfonyl-methane, type I and II collagen, glycosaminoglycans, L-arginine, L-lysine, Boswellia serrata dry extract titrated to 30% inacetyl-1 1-keto-B-boswellic acid, Curcuma longa dry extract titrated to 95% curcuminoids) for 45 days | Conventional analgesic therapy | Placebo + conventional analgesic therapy | Overall pain, and pain at night, during activity and at rest (VAS); CMS; shoulder function (SST) | Lower overall and night pain scores in TG compared to CG at week 1 (p = 0.0477, p = 0.0113), but not for other pain scores or subsequent time points (p >0.05). CMS in TG (60.3 ±8.6) was not different from CG (59.3 ±8.8, p = 0.884) after 12 weeks or 24 weeks (71.6 ±8.1 vs. 69.9 ±7.2, p = 0.352). SST in TG (7.7 ±1.8) was not different from CG (6.9 ±2.7, p = 0.523) after 12 weeks or after 24 weeks (8.2 ±1.7 vs. 8.1 ±0.9, p = 0.292) | Tendisulfur alleviated short and partially mid-term pain after SSP tendon repair, while long-term pain was unchanged. |
| Gumina et al. (2012) [34] | RCT | To determine whether the intake of an oral integrator might mitigate shoulder pain and improve repair integrity of RC shoulder tear after arthroscopic repair | 87 RC patients who underwent surgical repair (47-69 y, 48% male) | Two daily sachets Tenosan (arginine-L-alpha-ketoglutarate, methyl-sulfonyl-methane, hydrolyzed type I collagen and bromelain) for 3 months starting from postoperative day 1 | Motion and strengthening exercises | Motion and strengthening exercises | Shoulder pain (VAS); CMS; shoulder function (SST); maximum strength; repair integrity according to Sugaya’s classification (MRI) | After 6 months: Pain decreased more in TG (−6.7) compared to CG (−5.0, p <0.001). After 12 months: no differences between groups in CMS (TG: 21.3 ±4.6, CG: 22.6 ±6.6, p = 0.329) and SST (TG: 6.9 ±1.4, CG: 7.0 ±1.9, p = 0.072). The groups were different in terms of repair type (I, II, III) (p = 0.045). | Use of the supplement for 3 months after RC repair decreases postoperative shoulder pain and leads to slight improvement in repair integrity. |
| Notarnicola et al. (2012) [38] | RCT | To assess the clinical efficacy and perfusion effects of oral dietary supplements in association with ESWT for insertional AT tendinopathy | 64 insertional AT tendinopathy patients (55.8 ±13.2 y, 53% male) | Two daily sachets Tenosan (500 mg arginine-L-alpha-ketoglutarate, 550 mg methyl-sulfonyl-methane, 300 mg hydrolyzed collagen type I, 125 mg Vinitrox, 50 mg bromelain, 60 mg vitamin C) for 60 days | ESWT | Placebo + ESWT | Pain (VAS); subjective scores of pain and function and objective scores of physical examination (Ankle-Hindfoot Scale); pain and limitations of activity (Roles and Maudsley score) | VAS scores were lower in TG compared to CG after 2 months (3.9 ±3.2 vs. 5.1 ±2.7, p = 0.07) and 6 months (2.9 ±2.3 vs. 2.0 ±1.8, p = 0.04). Values for the Ankle-Hindfoot Scale were higher in TG compared to CG after 2 months (85 ±12.4 vs. 72.1 ±23.1, p = 0.0035) and 6 months (92.4 ±8.5 vs. 76.5 ±21.6, p = 0.0002). Roles and Maudsley scores were lower in TG compared to CG after 2 months (1.7 ±0.9 vs. 2.8 ±0.4, p <0.0001) and 6 months (1.5 ±0.6 vs. 2.3 ± 0.8, p <0.0001). | Dietary supplement plus ESWT can induce better clinical and functional outcome in AT patients. |
| Baar (2019) [45] | Case study | To determine whether a targeted loading and nutritional program could enhance the outcomes of a PT tendinopathy rehabilitation program | 1 professional basketball player (21 y, male) with PT tendinopathy | 15 g gelatine + 225 mg vitamin C twice a week for 18 months (one hour before every PT targeted training session) | Strength-based rehabilitation program | Pre-measurements | Maximal single-leg isometric hamstring strength; isometric leg extension strength; leg press strength; tendon thickness (MRI) | After 18 months: Increased hamstring (196%), leg extension (156%) and leg press (187%) strength. Thickness of proximal end of the tendon decreased by 25%. Thickness at tendon midpoint increased by 10%. | A nutritional intervention combined with a rehabilitation program can improve clinical outcomes in elite athletes |
| Mavrogenis et al. (2004) [37] | RCT | To evaluate the effect of essential fatty acids, antioxidants and physiotherapy on chronic tendon disorder | 31 active recreational athletes with chronic tendon disorder (TG: 31 y, 76% male; CG: 32 y, 86% male) | 8 capsules/day 376 mg eicosapentaenoic acid (EPA), 264 mg docosahexaenoic acid (DHA) and 672 mg gamma-linolenic acid (GLA) + 1 antioxidant-complex tablet 100 µg selenium, 15 mg zinc, 1 mg vitamin A, 2.2 mg vitamin B6, 90 mg vitamin C and 15 mg vitamin E for 32 days | Physiotherapy (therapeutic ultrasound), 16 sessions x 5 min | Placebo + physiotherapy (as TG) | Pain during sporting activity and after an isometric test (VAS); quantification of sports activity | After 32 days: Pain during sporting activity and after an isometric test decreased more in TG (99%, 99%) compared to CG (31%, 37%, p <0.001). Sports activity increased by 53% in TG and 11% in CG. | Essential fatty acids and antioxidants in combination with physiotherapy have beneficial effects in treating chronic tendon disorders. |
| Sandford et al. (2018) [41] | RCT | To compare the effectiveness of long chain omega-3 polyunsaturated fatty acids (PUFAs) as part of the management for people diagnosed with RC-related shoulder pain | 73 patients with RC-related shoulder pain (TG: 52.2 ±12.0 y, 45% male; CG: 52.0 ±16.2 y, 57% male) | 9 daily capsules MaxEPA (170 mg eicosapentaenoic acid, 115 mg docosahexaenoic acid, 2 units/g tocopherols acetate (vitamin E)) for 2 months | Weekly exercise and education groups for 8 weeks | Placebo (with same amount of vitamin E + antioxidants as TG) + weekly exercise and education groups for 8 weeks | Disability (OSS, SPADI); Pain (NRS, SF-36 bodily pain domain); Quality of life (SF-36, Euro QoL 5D-3 L); Function (PSFS); global perception of change; impairment measures (shoulder range of motion, strength). | Improved OSS scores of 25% in both groups, without differences between groups at 2 (−0.1, p = 0.95) and 12 months (−0.3, p = 0.82). SPADI scores differed only at 3 months between TG (25.3 ±21.1) and CG (13.9 ±18.1). Other outcomes improved in both groups without statistically significant differences between groups. | Omega-3 PUFA supplementation may have a modest effect on disability and pain outcomes in patients with RC-related shoulder pain at 3 months, but not over the course of one year. |
| Farup et al. (2014) [42] | Non-randomized controlled study | To investigate the effect of 12 weeks of either maximal eccentric or concentric resistance training combined with either a high-leucine whey protein hydrolyzate + carbohydrate supplement or placebo, on quadriceps muscle and PT hypertrophy | 22 healthy young recreationally active men (23.9 ±0.8 y) | A drink containing 19.5 g high-leucine (14.2%) whey protein hydrolyzate + 19.5 g carbohydrate (glucose) on all training days (33x in 12 weeks) | Eccentric training with one leg, concentric training with the other leg | Placebo (isoenergetic carbohydrate (glucose)) + training (as TG) | PT CSA (MRI); isometric strength (MVC, RFD) | After 12 weeks: Greater increase in PT CSA at proximal level in TG (14.9 ±3.1%) compared to CG (8.1 ±3.2%, p = 0.054). MVC and RFD increased by 15.6 ±3.5% (p <0.001) and 12-63% (p <0.05) without group effects. | Training-induced hypertrophy of the PT was augmented with a high-leucine whey protein hydrolyzate supplement. |
| Saggini et al. (2010) [40] | RCT (two-arm) | To evaluate the efficacy of a specific rehabilitative, therapeutic protocol integrated with administration of a supplement in both conservative rehabilitation treatment and post-surgery, in patients with RC lesions | Arm A: 30 RC lesion patients, treated conservatively (45 ±10 y, 37% male). Arm B: 50 RC lesion patients, treated conservatively (59.5 (30-80) y (female), 58.4 (28-78) y (male), 48% male) | 1 sachet/day 3.5 g Amedial BF (glucosamine sulfate, chondroitin sulfate, hydrolyzed type II collagen, hydrolyzed hyaluronic acid, L-carnitine fumarate) for 1 month (Arm A) for 60 days (Arm B) | Arm A: 3 shock waves + 9 sittings Multi Joint System; Arm B: rehabilitation treatment | Arm A: 3 shock waves + 9 sittings Multi Joint System (CG1) or 3 shock waves (CG2); Arm B: rehabilitation treatment | Arm A: ROM; pain (VAS); Arm B: UCLA (pain, functionality, active frontal flexion, strength in frontal flexion, satisfaction) | Arm A, after 1 month: VAS scores reduced by 45% in TG, 22% in CG1 and 45% in CG2. Flexion, extension, abduction and external rotation increased by 38%, 57%, 47% and 52% in TG, 28%, 40%, 42% and 40% in CG1 and 26%, 23%, 36% and 25% in CG2. Arm B, after 60 days: Higher improvement in TG compared to CG for pain (73% vs. 70%), function (49% vs. 36%), flexion (41% vs. 29%) and strength (39% vs. 30%) scores. Satisfaction was higher in TG (92%) than in CG (84%). | Supplementation of natural substances is a conservative treatment for RC lesions to consider. Quicker functional recovery with post-surgical supplementation. |
| Schneider et al. (2009) [46] | Case series | To identify characteristics associated with bilateral ruptures of the distal biceps tendons | 10 patients with sustained non-simultaneous bilateral distal biceps brachii tendon ruptures, surgically repaired (49.5 (27.7-76.2) y, male) | Nutritional supplements (multivitamins and omega 3 oils) | NA | NA | Disability (DASH) | DASH scores were not significantly related to using nutritional supplements at the time of injury (p = .145) | No correlation found between outcome following surgical treatment and use of nutritional supplements. |
| Szczurko et al. (2009) [28] | RCT | To evaluate the potential for the combined efficacy of a naturopathic approach including acupuncture, dietary advice and hydrolytic enzymes in the treatment of RC tendinitis | 85 Canadian postal employees with RC tendinitis (TG: 50.7 ±8.16 y, 42% male; CG: 50.9 ±7.86 y, 40% male) | 6 tablets/day of Phlogenzym (90 mg bromelain, 48 mg trypsin, 100 mg rutin) + patient-customized dietary counseling, with special emphasis on reducing alcohol consumption and increasing consumption of fish, berries, fruits, vegetables, nuts, and whole grains for 12 weeks | Acupuncture | Placebo + physical exercise | Disability (SPADI); health-related QoL (SF-36); pain over the last week (VAS); patient experiences (MYMOP); flexion, extension, abduction, adduction, internal rotation and external rotation of affected shoulder | After 12 weeks: SPADI scores improved more in TG (54.5%) compared to CG (18%, p <0.0001). TG also showed superiority in SF-36, VAS, MYMOP scores and range of motion. | Naturopathic treatments including dietary changes, acupuncture and Phlogenzym have a significant effect on decreasing RC tendinitis symptoms. |
AT, Achilles tendon; CG, control group; CMS, Constant-Murley score; CSA, cross-sectional area; DASH, Disabilities of Arm, Shoulder, and Hand; ESWT, Extracorporeal Shock Wave Therapy; LET, lateral epicondyle tendon; MEPS, Mayo Elbow Performance Score; MMYOP, Measure Yourself Medical Outcomes Profile; MRI, magnetic resonance imaging; MVC, maximal voluntary contraction; NRS, Numerical Rating Scale; OSS, Oxford Shoulder Score; PRTEE, Patient-rated Tennis Elbow Evaluation; PSFS, Patient-Specific Functional Scale; PT, patellar tendon; RC, rotator cuff; RCT, randomized controlled trial; RFD, rate of force development; SF-36, Short-Form Health Survey; SPADI, Shoulder Pain and Disability Index; SSP, supraspinatus; SST, Simple Shoulder Test; TG, treatment group; RC, rotator cuff; VAS, Visual Analogue Scale; VISA-A, Victorian Institute of Sports Assessment – Achilles questionnaire; VISA-P, Victorian Institute of Sports Assessment – Patellar questionnaire; USI, ultrasound imaging.
Study designs and populations
All studies examining habitual dietary intake had an observational design; there were two prospective cohort studies [29,30], two cross-sectional studies [31,32] and one case-control study [33]. The number of people included in these studies ranged from 50 to 80,106 (Table 1).
Among the fourteen studies examining the use of dietary supplements, thirteen were experimentally designed: nine RCTs [28,34–41], two non-randomized controlled studies [42,43], one non-comparative intervention study [44] and one case study [45]. In addition, there was one retrospective case series [46]. The number of subjects in these studies ranged from 1 to 100 (Table 2).
Although there was a wide age range within the study populations, they mainly comprised middle-aged (40-60 years) and older (>60 years) non-athletic adults. The participants in three studies examining athletes or active persons were younger [37,42,45]. Also, one study among military personnel had a large proportion of young adults [30]. Most studies included both men and women, but some included relatively more [29,37] or only men [31,42,45,46].
Location of tendon/tendinopathy
Tendons of the rotator cuff were investigated in nine studies [28,29,32–35,40,41,43]. The Achilles tendon was examined in seven studies [30,31,36,38,39,43,44], the patellar tendon in four [30,42,44,45], the lateral elbow tendon in two [43,44] and the biceps brachii tendon in one study [46]. One study did not specify the tendons of interest, but included several types [37]. The effect of nutritional exposure to healthy tendons was investigated in two studies [31,42]. A total of seventeen studies examined people with prevalent tendinopathy who are at risk of tendinopathy [28–30,32–41,43–46].
Nutritional exposure
In the studies investigating the impact of the habitual diet, questionnaires were used to assess nutritional exposure. One study examined the intake of several foods as well as the overall dietary pattern (Western vs Mediterranean) [31], and four studies examined exposure to alcohol [29,30,32,33]. The majority of studies examined the effect of a dietary supplement that contained multiple ingredients, including collagen [34–36,38–40,43,44], vitamin C [36–38,43–45], methyl-sulfonyl-methane [34,35,38,43], arginine-L-alpha-ketoglutarate [34,38], mucopolysaccharides [36,44], bromelain [28,34,38] and essential fatty acids [37,41]. One study evaluated an intervention combining supplements and habitual dietary changes [28]. Duration of supplement use ranged from 1 to 18 months.
Concurrent interventions
Twelve out of the thirteen experimental studies implemented other interventions in addition to the use of dietary supplements. The concurrent exposures were training or exercises [34,36,39,41,42,45], analgesic therapy [35], Extracorporeal Shock Wave Therapy (ESWT) [38,40,43], physiotherapy [37], multi-joint system [40], rehabilitation treatment [40] and acupuncture [28]. In addition, six studies considered patients that underwent surgical repair of either the rotator cuff tendon [29,33–35,40] or the biceps brachii tendon [46].
Outcome measures
Clinical outcomes were reported in fifteen studies. Pain was the clinical outcome reported most often, and was measured by the visual analogue scale (VAS) [28,34–38,40,43,44], numerical rating scale (NRS) [41] or Short-Form 36 bodily pain (SF-36 BP) [41]. Disability was captured by many different outcomes, including the Shoulder Pain and Disability index (SPADI) [28,29,41], Victorian Institute of Sport Assessment for the Achilles (VISA-A) [36,39,43,44] or patellar tendon (VISA-P) [44], Patient-rated Tennis Elbow Evaluation (PRTEE) [44], Roles and Maudsley score [38], Oxford Shoulder Score (OSS) [41] and Disabilities of Arm, Shoulder and Hand (DASH) score [46]. Physical function capacity was examined by measuring strength [34,41,42,45], range of motion [28,40,41] and function with the simple shoulder test (SST) [34,35] and Patient-Specific Functional Score (PSFS) [41]. Combinations of clinical outcomes were assessed by the UCLA shoulder score [40,43], Mayo Elbow Performance Score (MEPS) [43], Ankle-Hindfoot Scale [38] and Constant-Murley score [34,35]. Other outcomes were global perception of change [41] and Measure Yourself Medical Outcomes Profile (MYMOP) [28] to measure patient rating of condition, SF-36 [28,41] and Euro QoL 5D-3 [41] to measure quality of life, and quantification of sports activity [37] (participation in life activities). None of the studies assessed psychological factors.
Three studies calculated odds ratios as a measure of association between exposure to alcohol and occurrence/prevalence of tendinopathy [30,32,33]. Structural changes of the tendon by MRI or USI were examined in seven studies [31,34,36,39,42,44,45].
Risk of bias
The risk of bias assessment of all nineteen eligible studies, including the most important sources of bias, is presented in Tables 3 and 4. For the nine RCTs this was evaluated with the RoB 2 tool (Table 3). Two studies were judged at low risk of bias [38,41]; both were double-blinded, placebo-controlled, and included several outcome measures for which the results were reported adequately. However, one of these studies [38] raised some small concerns because baseline characteristics were not presented. Six studies expressed ‘some concerns’ [28,34–37,39]. Main aspects that raised concerns were no inclusion of placebo treatment [34,36], small study populations [36,37,39], unclear or imbalance of baseline comparison and/or other confounding factors [36,39], and mainly presenting participant-reported outcomes [35,37]. Additional concerns, involving judgment tending toward high risk of bias, were raised by the absence of intention-to-treat analyses, assessment of limited outcomes and short follow-up [37], and a cross-over design without a wash-out period [39]. One study had a high risk of bias due to poor clarity of the randomization process, participant characteristics, study protocol and analyses, and selective reporting of results [40].
Table 3.
Overall quality judgment of each study assessed by the RoB 2 tool.
| Author | Overall Risk of Bias Judgment* | Main Sources of Bias |
|---|---|---|
| Balius et al. [36] | Some concerns | No placebo treatment Small study population No baseline comparison |
| Gumina et al. [34] | Some concerns (high) | No placebo treatment |
| Mavrogenis et al. [37] | Some concerns (high) | No intention-to-treat analyses Small study population with broad inclusion criteria Only participant-reported outcomes Short follow-up |
| Merolla et al. [35] | Some concerns | Mainly participant-reported outcomes No between-group comparison of change |
| Notarnicola et al. [38] | Low risk (some concerns) | No baseline characteristics presented |
| Praet et al. [39] | Some concerns (high) | No wash-out period Small study population Potential of selection bias |
| Saggini et al. [40] | High risk | Insufficient information about randomization, group comparison, protocol and analyses Incomplete and unclear reporting of results |
| Sandford et al. [41] | Low risk | |
| Szczurko et al. [28] | Some concerns | High drop-out rate Individual variability in the multiple components of the intervention delivered |
*Possible judgments are: Low risk, Some concerns, High risk
Table 4.
Overall quality judgment of each study assessed by the ROBINS-I tool.
| Author | Overall Risk of Bias Judgment* | Main Sources of Bias |
|---|---|---|
| Arquer et al. [44] | Serious | No control group High drop-out rate Limited participant information |
| Baar [45] | Serious | One participant |
| Farup et al. [42] | Low | Small study population |
| Hjerrild et al. [31] | Serious | Cross-sectional design Incomprehensive exposure assessment Not all confounders were taken into account |
| Jain, et al. [29] | Moderate/Serious | Small study population Participant-reported outcome Incomplete follow-up |
| Owens et al. [30] | Moderate | Not all confounders were taken into account Only severe cases were identified Inappropriate statistical adjustments |
| Passaretti et al. [33] | Serious | Potential of selection bias Potential for underreporting of alcohol consumption |
| Rechardt et al. [32] | Moderate | Cross-sectional design Data of association not shown |
| Schneider et al. [46] | Critical | Small study population Selection bias No quantification and qualification of exposure Single participant-reported outcome measure |
| Vitali et al [43]. | Moderate | No placebo Mainly participant-reported outcomes |
*Possible judgments are: Low, Moderate, Serious, Critical
The ROBINS-I tool was used to assess the risk of bias of the ten remaining studies (Table 4). One study was judged at low risk of bias, although the study population was small [42]. Other studies presented limitations as a result of their observational study design [29–33], small study populations [29,45], incomplete follow-up [29,44] and inappropriate exposure or outcome assessment [29–31,33,43], and were therefore judged at moderate [29,30,32,43] or serious [31,33,44,45] risk of bias. The case series [46] was judged as critical because of selection bias in its small study population, insufficient information about exposure, and assessment of one single outcome measure.
The overall level of certainty in the evidence for clinical outcomes was based on fourteen studies that examined the effect of a dietary supplement (Additional file 5). For the occurrence/prevalence of tendinopathy, the overall assessment of certainty included three studies that examined the effect of alcohol intake (Additional file 6). The summary of findings regarding the judgments of the certainty in evidence is presented in Additional file 7. For the clinical outcomes, the certainty in the evidence was judged at low. There was a very low level of certainty for occurrence/prevalence of tendinopathy.
4. Discussion
This systematic review aimed to evaluate the potential impact of nutrition on the prevention and treatment of tendinopathy. The majority of the included studies investigated the use of dietary supplements. Only a limited number of studies examining the effect of the habitual diet on tendon outcomes were identified. Overall, there was insufficient high-quality data available to enable meta-analyses as a result of the considerable variation in study design, nutritional exposure, concurrent exposure, outcome measures, and risk of bias.
Habitual diet
Only one study was identified that investigated the habitual intake of several foods and type of diet [31]. This study did not find an association between any of the dietary parameters and Achilles tendon thickness, but no other clinical outcomes were assessed. Considering the serious risk of bias, firm conclusions about the impact of the habitual diet from this single study are not possible.
Alcohol
Findings of the four studies examining the impact of alcohol intake varied for effect on tendinopathy. No associations were found between alcohol consumption and chronic rotator cuff tendinitis [32]. Moderate weekly alcohol consumption (men: 7-13 drinks, women: 4-6 drinks) was associated with a modest increased risk for Achilles tendinopathy, but not with patellar tendinopathy [30]. Excessive alcohol intake (men: >13 drinks, women: >6 drinks) was a significant risk factor for the occurrence and severity of rotator cuff tears [33]. By contrast, Jain et al. [29] found a positive association between alcohol consumption and less shoulder pain and better function after rotator cuff repair. However, it was suggested that alcohol use may be a proxy for another variable that was not captured in the study. In addition, differences in study design, population, and classification into categories for amount of alcohol consumption (light, moderate, heavy) limit comparability across these studies. Despite inconsistent findings on the risk and severity of tendinopathy in humans, there is evidence that alcohol may inhibit collagen synthesis through toxic effects [47].
Collagen
As tendons are mainly composed of collagen, whose ongoing synthesis is required to maintain a healthy extracellular matrix, it is not surprising that collagen-derived peptides, including gelatin and hydrolyzed collagen, were most often a component of the dietary supplements evaluated. The majority of these supplements were found to improve clinical and/or structural outcomes in the treatment of tendinopathy [34,36,38–40,43–45]. Merolla et al. [35] showed only short-term effectiveness for pain reduction after supraspinatus tendon repair, while long-term pain was unchanged. This could be addressed by increased dosage and treatment duration. Thus, in the study of Gumina et al. [34], an extended treatment of a similar preparation in rotator cuff patients showed improvement in pain after six months. However, both studies did not find a better physical function capacity after surgical repair as a result of the supplement intake. In all other studies examining the effect of a supplement containing collagen, participants were not treated surgically and benefits for various clinical outcomes were found [36,38–40,43–45]. Although most studies showed improvement after 2-3 months of supplement use, the daily dose as well as the type of collagen varied among interventions. Tendon is mainly composed of type I, while cartilage contains type II. Most tendon studies supplemented type I, although some combined with type II [35,43], or type II only [40], which can explain some of the variation in outcome. All in all, collagen seems to be beneficial in the treatment of tendinopathy, but conclusions about optimal dosage, timing, duration, and type of collagen supplementation cannot be drawn yet. Also, because many interventions implemented collagen supplementation with concurrent treatment, the specific benefits of collagen alone remain unknown.
Other nutrients
Many other nutrients may contribute to collagen synthesis or may have anti-inflammatory effects, so most dietary supplements used in the different studies contained multiple substances. In four studies a similar preparation was used, as they all comprised methyl-sulfonyl-methane and arginine in addition to hydrolyzed collagen [34,35,38,43]. Curcumin and Boswellia serrata were only used by Merolla et al. [35] and Vitali et al. [43], while the preparations used by Gumina et al. [34] and Notarnicola et al. [38] contained bromelain. All these compounds were found to be associated with improvement in pain, but a specific evaluation of each nutrient is difficult because of the multiple nutrients within a single supplement.
Also, the amino acid leucine may have exerted a stimulatory effect on collagen synthesis. A single study in healthy young men found that tendon hypertrophy was augmented with high-leucine whey protein supplementation (providing 19.5 g amino acids of which 2.77 g leucine on training days) in addition to resistance exercise [42]. Although findings from this small single study are not conclusive, they may have important clinical implications. Leucine-induced tendon hypertrophy may lead to relatively less mechanical stress on the tendon during exercise, which may assist in tendon rehabilitation.
Two studies investigated the potential role of essential fatty acids in the treatment of tendinopathy because of their anti-inflammatory properties [48]. In the high-quality trial of Sandford et al. [41], eight weeks of Omega 3 PUFA supplementation (daily providing 1530 mg of EPA and 1035 mg of DHA) was found to have a modest improvement on disability and pain outcomes in patients with rotator cuff-related shoulder pain after 3 months, but not after one year. Mavrogenis et al. [37] used supplements for only 32 days but at a higher dose, and reported a significant improvement in pain. However, the short follow-up and other methodological concerns limit the potential impact of these findings. Current evidence does not support the use of essential fatty acids, but further research is warranted to assess the potential impact of higher-dose and longer-duration interventions.
The intervention evaluated by Szczurko et al. [28] consisted of multiple components, including supplementation of hydrolytic enzymes (bromelain, trypsin, rutin), individual dietary counseling, and acupuncture. This naturopathic treatment showed clinically significant improvement in shoulder pain and quality of life compared with standardized physical exercise. Although they raised only small concerns with respect to risk of bias, their study is of limited evidence for the effectiveness of dietary supplements and/or dietary changes on tendinopathy, because the effects of the individual components cannot be established.
Study quality and limitations
Despite the broad inclusion criteria, the total number of eligible studies was relatively low. In addition, the overall quality of studies was poor. Evidence for the effects of nutrition results from limited high-quality studies. An overall rating of the certainty in the evidence was only provided for clinical outcomes and for occurrence/prevalence of tendinopathy.
Another limitation resulting from the inclusion criteria is the heterogeneity among studies. This is why the rating of quality of evidence needs to be interpreted with caution. Especially the evidence from studies on the effects of nutrition in healthy human tendons was scarce. We also included studies that did not primarily aim to investigate nutritional exposure or one of the eligible outcomes, and therefore did not assess or report this in much detail. Nevertheless, this review provides an overview of the evidence for effects of any nutritional exposure and is the first study to systematically synthesize the findings for clinical outcomes on physical, psychosocial and overall life impact, and the risk of tendinopathies in relation to nutrition.
A limitation of the included studies is that supplement use was often combined with several types of interventions (e.g. exercises, physiotherapy, shockwaves, surgery). Although these interventions were similar in the control group, it cannot be ruled out that the concurrent treatment supported the effect of the nutritional treatment. The results show that combining supplement use with other treatments provides further benefits than the treatment alone. It would be interesting to see what effects are induced by taking the dietary supplement alone. Nevertheless, nutritional strategies do not interfere and can easily be implemented in combination with other interventions.
Another barrier in the synthesis of findings from the different studies is that many disparate clinical outcomes were reported. Many studies rely on a limited number of outcomes and were mainly participant-reported. Pain measured by the VAS was an outcome reported in most studies, but referred to a different activity or timeframe, or rated pain without further specification. As recommended by the ICON group, clinical trials should include a measure for each of the nine core domains at a minimum [23]. However, from the studies identified in this review that of Sandford et al. [41] captured six domains. Other studies reported even less. This stresses the need to determine a core outcome set that should be adopted widely in tendinopathy research.
Recommendations for future research
More knowledge is required on the impact of habitual dietary exposures on tendon health, as a healthy habitual diet is the basis for adequate nutrient intake. Improving the habitual intake should be the main focus of athletes rather than the intake of dietary supplements. High-quality studies with extensive dietary intake assessment are needed to examine this association in tendinopathy patients as well as in healthy populations, to determine the role of nutrition in preventing tendinopathy. Research should be conducted specifically in athletes and active populations and by assessing core clinical outcome measures to enable future meta-analyses.
5. Conclusion
Due to the limited scientific quality and variety among studies on nutrient intake, tendon location, study population and reported outcome measures, it is impossible to draw definitive conclusions and formulate dietary recommendations on the prevention and treatment of tendinopathy. Findings on alcohol intake were inconsistent. Individual studies present important clinical implications for the use of dietary supplements on tendon health, of which especially those containing collagen-derived peptides seem to be beneficial in the treatment of tendinopathy. Also, methyl-sulfonyl-methane, arginine, bromelain, curcumin, and Boswellia were present in supplements that showed clinical improvements. Future clinical studies considering nutritional intake should use standardized dietary assessment methods, adopt the core domains for tendon research and report a core outcome set for each tendinopathy, in order to synthesize findings from different studies.
Supplementary Material
Additional file 1.
Table 1 Core domains of tendinopathy as defined by the ICON group [2019, 23].
| Domain | Description/definition | Example outcome |
|---|---|---|
| Patient rating of condition | A single assessment numerical evaluation | Rate your tendon status where 100% is no problems and 0% worst case scenario, global rating of change, patient acceptable symptom status |
| Participating in life activities | Patient rating of the level of participating | Ratings of level of sport and time to return to sport |
| Pain on activity/loading | Patient reported intensity of pain on performing a task/activity that loads the tendon | VAS or NRS for pain intensity when the patient performs a tendon-specific pain-provocative task |
| Function | Patient rated level of function (and not referring to the intensity of their pain) | Patient Specific Function Scale on a VAS or NRS |
| Psychological factors | Psychology | Pain self-efficacy, pain catastrophisation, kinesiophobia, anxiety or depression scales |
| Physical function capacity | Quantitative measures of physical tasks performed in clinic | Number of hops, timed stair walk, number of single limb squats, including dynamometry and wearable technology |
| Disability | Composite scores of a mix of patient-rated pain and disability due to the pain, usually to tendon-specific activities/tasks | VISA scales, patient-rated tennis elbow evaluation, disability of the arm, shoulder and hand |
| Quality of life | The general well-being of the individual | Specific QoL questionnaires such as European QoL – 5 Dimension (EQ-5D) Australian QoL (AQoL), 36-item Short Form survey (SF-36) |
| Pain over a specified time | Participant reported pain intensity over a period of time (morning, night, 24 hours, a week) | VAS, NRS |
Additional file 2.
Table 2 Search strategy in PubMed.
| Concept | Search terms |
|---|---|
| Tendinopathy | (((‘Tendinopathy’[Mesh] OR tendinopathy[tiab] OR tendinopathies[tiab] OR tendinosis[tiab] OR tendinoses[tiab] OR tendinitis[tiab] OR tendonitis[tiab] OR tendonosis[tiab] OR tendonitides[tiab] OR ‘Tendon Injuries’[Mesh] OR tendon injuries[tiab] OR tendon injury[tiab] OR tendon healing[tiab] OR tendon disorder*[tiab] OR tendon repair[tiab]) OR ((‘Tendons’[Mesh] OR tendon*[tiab]) AND (‘prevention and control’ [Subheading] OR prevention[tiab] OR preventive therapy[tiab]))) |
| Nutrition | AND (curcumin[tiab] OR boswellic acid[tiab] OR arginin*[tiab] OR tendisulfur[tiab] OR bromelain[tiab] OR methylsulfonylmethane[tiab] OR ‘Amino Acids, Peptides, and Proteins’[Mesh] OR amino acid[tiab] OR protein[tiab] OR proteins[tiab] OR leucine[tiab] OR glutamine[tiab] OR arginine[tiab] OR taurine[tiab] OR gelatin[tiab] OR ‘Collagen’[Mesh] OR collagen[tiab] OR ‘Phytochemicals’[Mesh] OR phytochemicals[tiab] OR phytonutrients[tiab] OR ‘coenzyme Q10’[Supplementary Concept] OR coenzyme Q10[tiab] OR co-enzyme Q10[tiab] OR ‘Fatty Acids, Omega-3’[Mesh] OR omega 3[tiab] OR omega-3[tiab] OR ‘Lipids’[Mesh] OR lipids[tiab] OR fatty acids[tiab] OR fish oils[tiab] OR plant oils[tiab] OR ‘Nutrition Therapy’[Mesh] OR nutrition therapy[tiab] OR diet therapy[tiab] OR nutrient intake[tiab] OR ‘Nutrients’[Mesh] OR nutrient*[tiab] OR macronutrient*[tiab] OR ‘Diet, Food, and Nutrition’[Mesh] OR nutrition[tiab] OR ‘Micronutrients’[Mesh] OR micronutrient*[tiab] OR vitamin*[tiab] OR ‘Ascorbic Acid’[Mesh] OR ascorbic acid[tiab] OR vitamin c[tiab] OR antioxidant*[tiab] OR ‘Vitamin D’[Mesh] OR vitamin d[tiab] OR cholecalciferol[tiab] OR ergocalciferols[tiab] OR ‘Minerals’[Mesh] OR minerals[tiab] OR calcium[tiab] OR manganese[tiab] OR copper[tiab] OR zinc[tiab] OR magnesium[tiab] OR iron[tiab] OR molybdenum[tiab] OR silicon[tiab] OR calories[tiab] OR ‘Dietary Supplements’[Mesh] OR dietary supplement*[tiab] OR food supplement*[tiab] OR food additives[tiab] OR fortified food[tiab] OR nutraceutical[tiab] OR nutritional[tiab] OR ‘Glycerol’[Mesh] OR glycerin[tiab] OR glycerol[tiab])) |
| Human | NOT ((animals[mh] NOT (animals[mh] AND humans[mh])) NOT rat[tiab] NOT rats[tiab] NOT mice[tiab] NOT rabbit*[tiab]) |
Additional file 3.
Table 3 Rating of the certainty of evidence for clinical outcomes.
| GRADE domain | Judgment | Concerns about certainty domains |
|---|---|---|
| Methodological limitations of the studies | Among the nine RCTs, the majority expressed ‘some concerns’ with respect to the risk of bias. Two studies were judged at low risk of bias and one study had a high risk of bias. The risk of bias of the remaining five intervention and observational studies was judged at low or moderate for two studies, and serious or critical for three studies. Main aspects that raise concerns were reporting participant-reported outcomes, incomplete or unclear reporting of methods or results, and small study populations. Therefore, we judged the studies to have serious methodological limitations. | Serious |
| Indirectness | Most studies were primarily aimed to investigate the effect of the dietary supplement on clinical outcomes, but often in combination with other treatments. We judged the evidence to have moderate indirectness. | Moderate |
| Imprecision | The total number of participants included in all studies was 819. We judged the evidence to have moderate imprecision. | Moderate |
| Inconsistency | The majority of the studies found a beneficial effect of the supplement intake on one or more of the clinical outcomes. There is inconsistency in the effects on different time points, but this could be addressed by variation in study protocol. We judged the evidence to have moderate inconsistency. | Moderate |
| Publication bias | Some studies are commercial studies. We found no commercial studies without effect. | Potential |
Additional file 4.
Table 4 Rating of the certainty of evidence for occurrence/prevalence of tendinopathy.
| GRADE Domain | Judgment | Concerns About Certainty Domains |
|---|---|---|
| Methodological limitations of the studies | The risk of bias was judged at moderate for two out of three studies. One study was judged at serious risk of bias, but this study was smaller compared to the other two (605 vs. 80,106/6237). All studies had an observational design, which involves several limitations. In addition, sources of bias were inappropriate statistical adjustments, potential underreporting of intake and incomplete reporting of results. Therefore, we judged the studies to have serious methodological limitations. | Serious |
| Indirectness | Only one study primarily aimed to investigate the association between the intake of alcohol and the risk of tendinopathy. In the other two studies, alcohol consumption was only one of many factors that were investigated to find an association. One study did not even report data with regard to alcohol consumption. Therefore, we judged the evidence to have serious indirectness. | Serious |
| Imprecision | The total number of participants included in all studies was 86,948. This is a large number, but this is mainly due to one large cohort study with relatively low number of cases identified. We judged the evidence to have moderate imprecision. | Moderate |
| Inconsistency | The studies reported either a positive association or no association between alcohol consumption and the risk of tendinopathy. One study found a marginal association for moderate weekly alcohol consumption and Achilles tendinopathy, but not for heavy weekly alcohol consumption or patellar tendinopathy. Another study found significant risks of rotator cuff tears for excessive drinkers. We judged the evidence to have moderate inconsistency. | Moderate |
| Publication bias | We do not suspect publication bias, taking into account that we have few studies | Not suspected |
Additional file 5.
Table 5 Summary of findings regarding the GRADE judgments.
| Outcome | Effect | Number of Participants (Studies) | Certainty in the Evidence |
|---|---|---|---|
| Clinical outcomes | Most studies showed positive effects on one or more clinical outcomes, or found no significant effects | 819 (14 experimental studies including 9 RCTs) |
 Low |
| Occurrence/prevalence of tendinopathy | Two studies found a positive association between alcohol consumption and risk of tendinopathy. One study showed no association. | 86,948 (3 observational studies) |
 Very low |
Funding Statement
This study was financially supported by the EAT2MOVE project (Province of Gelderland, proposal PS2014-49.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
Conception and planning of the work that led to the manuscript and analysis and interpretation of the data was performed by all authors. CR performed the database searches. The study selection was performed by AH and JZ and was approved by all authors. AH and CR judged the risk of bias of studies. A first draft of the manuscript was written by AH. CR, MM, and JZ edited and revised the manuscript. All authors approved the final version before submission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2022.2104130
References
- [1].Scott A, Squier K, Alfredson H, et al. ICON 2019: international scientific tendinopathy symposium consensus: clinical terminology. Br J Sports Med. 2020;54(5):260–262. [DOI] [PubMed] [Google Scholar]
- [2].Hopkins C, Fu SC, Chua E, et al. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2016;4:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Albers IS, Zwerver J, Diercks RL, et al. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC Musculoskelet Disord. 2016;17(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Francis P, Whatman C, Sheerin K, et al. The proportion of lower limb running injuries by gender, anatomical location and specific pathology: a systematic review. J Sports Sci Med. 2019;18(1):21–31. [PMC free article] [PubMed] [Google Scholar]
- [5].Zwerver J, Bredeweg SW, van den Akker-Scheek I.. Prevalence of Jumper’s knee among nonelite athletes from different sports: a cross-sectional survey. Am J Sports Med. 2011;39(9):1984–1988. [DOI] [PubMed] [Google Scholar]
- [6].Scott A, Backman LJ, Speed C. Tendinopathy: update on Pathophysiology. J Orthop Sports Phys Ther. 2015;45(11):833–841. [DOI] [PubMed] [Google Scholar]
- [7].Macchi M, Spezia M, Elli S, et al. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: a systematic review of clinical studies. Clin Orthop Relat Res. 2020;478(8):1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahmed AS. Does diabetes mellitus affect tendon healing? Adv Exp Med Biol. 2016;920:179–184. [DOI] [PubMed] [Google Scholar]
- [9].Steinmann S, Pfeifer CG, Brochhausen C, et al. Spectrum of tendon pathologies: triggers, trails and end-state. Int J Mol Sci. 2020;21(3):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Vries AJ, Koolhaas W, Zwerver J, et al. The impact of patellar tendinopathy on sports and work performance in active athletes. Res Sports Med. 2017;25(3):253–265. [DOI] [PubMed] [Google Scholar]
- [11].Sleeswijk Visser TSO, van der Vlist AC, van Oosterom RF, et al. Impact of chronic Achilles tendinopathy on health-related quality of life, work performance, healthcare utilisation and costs. BMJ Open Sport Exerc Med. 2021;7(1):e001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peters JA, Zwerver J, Diercks RL, et al. Preventive interventions for tendinopathy: a systematic review. J Sci Med Sport. 2016;19(3):205–211. [DOI] [PubMed] [Google Scholar]
- [13].Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262–268. [DOI] [PubMed] [Google Scholar]
- [14].Maughan RJ, Burke LM, Dvorak J, et al. IOC consensus statement: dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52(7):439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wardenaar F, van den Dool R, Ceelen I, et al. Self-reported use and reasons among the general population for using sports nutrition products and dietary supplements. Sports. 2016;4(2). DOI: 10.3390/sports4020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scott A, Nordin C. Do dietary factors influence tendon metabolism? Adv Exp Med Biol. 2016;920:283–289. [DOI] [PubMed] [Google Scholar]
- [17].Curtis L. Nutritional research may be useful in treating tendon injuries. Nutrition. 2016;32(6):617–619. [DOI] [PubMed] [Google Scholar]
- [18].Close GL, Sale C, Baar K, et al. Nutrition for the prevention and treatment of injuries in track and field athletes. Int J Sport Nutr Exerc Metab. 2019;29(2):189–197. [DOI] [PubMed] [Google Scholar]
- [19].Loiacono C, Palermi S, Massa B, et al. Tendinopathy: pathophysiology, therapeutic options, and role of nutraceutics. A narrative literature review. Medicina. 2019;55(8):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DePhillipo NN, Aman ZS, Kennedy MI, et al. Efficacy of vitamin C supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: a systematic review. Orthop J Sports Med. 2018;6(10):2325967118804544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tack C, Shorthouse F, Kass L. The physiological mechanisms of effect of vitamins and amino acids on tendon and muscle healing: a systematic review. Int J Sport Nutr Exerc Metab. 2018;28(3):294–311. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vicenzino B, de Vos RJ, Alfredson H, et al. ICON 2019-international scientific tendinopathy symposium consensus: there are nine core health-related domains for tendinopathy (CORE DOMAINS): Delphi study of healthcare professionals and patients. Br J Sports Med. 2020;54(8):444–451. [DOI] [PubMed] [Google Scholar]
- [24].Docking SI, Ooi CC, Connell D. Tendinopathy: is imaging telling us the entire story? J Orthop Sports Phys Ther. 2015;45(11):842–852. [DOI] [PubMed] [Google Scholar]
- [25].Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [26].Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Murad MH, Mustafa RA, Schunemann HJ, et al. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Szczurko O, Cooley K, Mills EJ, et al. Naturopathic treatment of rotator cuff tendinitis among Canadian postal workers: a randomized controlled trial. Arthritis Rheum. 2009;61(8):1037–1045. [DOI] [PubMed] [Google Scholar]
- [29].Jain NB, Ayers GD, Fan R, et al. Predictors of pain and functional outcomes after operative treatment for rotator cuff tears. J Shoulder Elbow Surg. 2018;27(8):1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Owens BD, Wolf JM, Seelig AD, et al. Risk factors for lower extremity tendinopathies in military personnel. Orthop J Sports Med. 2013;1(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hjerrild JN, Wobbe A, Stausholm MB, et al. Effects of long-term physical activity and diet on skin glycation and Achilles tendon structure. Nutrients. 2019;11(6):1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rechardt M, Shiri R, Karppinen J, et al. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord. 2010;11(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Passaretti D, Candela V, Venditto T, et al. Association between alcohol consumption and rotator cuff tear. Acta Orthop. 2016;87(2):165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gumina S, Passaretti D, Gurzì MD, et al. Arginine L-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: a prospective randomized study. Curr Med Res Opin. 2012;28(11):1767–1774. [DOI] [PubMed] [Google Scholar]
- [35].Merolla G, Dellabiancia F, Ingardia A, et al. Co-analgesic therapy for arthroscopic supraspinatus tendon repair pain using a dietary supplement containing Boswellia serrata and Curcuma longa: a prospective randomized placebo-controlled study. Musculoskelet Surg. 2015;99(Suppl S1):S43–S52. [DOI] [PubMed] [Google Scholar]
- [36].Balius R, Álvarez G, Baró F, et al. A 3-arm randomized trial for achilles tendinopathy: eccentric training, eccentric training plus a dietary supplement containing mucopolysaccharides, or passive stretching plus a dietary supplement containing mucopolysaccharides. Curr Ther Res Clin Exp. 2016;78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mavrogenis S, Johannessen E, Jensen P, et al. The effect of essential fatty acids and antioxidants combined with physiotherapy treatment in recreational athletes with chronic tendon disorders - A randomised, double-blind, placebo-controlled study. Phys Ther Sport. 2004;5(4):194–199. [Google Scholar]
- [38].Notarnicola A, Pesce V, Vicenti G, et al. SWAAT study: extracorporeal shock wave therapy and arginine supplementation and other nutraceuticals for insertional achilles tendinopathy. Adv Ther. 2012;29(11):992. [DOI] [PubMed] [Google Scholar]
- [39].Praet SFE, Purdam CR, Welvaert M, et al. Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in achilles tendinopathy patients. Nutrients. 2019;11(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Saggini R, Cavezza T, Di Pancrazio L, et al. Treatment of lesions of the rotator cuff. J Biol Regul Homeost Agents. 2010;24(4):453–459. [PubMed] [Google Scholar]
- [41].Sandford FM, Sanders TA, Wilson H, et al. A randomised controlled trial of long-chain omega-3 polyunsaturated fatty acids in the management of rotator cuff related shoulder pain. BMJ Open Sport Exerc Med. 2018;4(1):e000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Farup J, Rahbek SK, Vendelbo MH, et al. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. 2014;24(5):788–798. [DOI] [PubMed] [Google Scholar]
- [43].Vitali M, Rodriguez NN, Pironti P, et al. ESWT and nutraceutical supplementation (Tendisulfur Forte) vs ESWT-only in the treatment of lateral epicondylitis, Achilles tendinopathy, and rotator cuff tendinopathy: a comparative study. J Drug Assess. 2019;8(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arquer A, García M, Laucirica JA, et al. Efficacy and safety of an oral treatment based on mucopolysaccharides, collagen type i and vitamin C in patients with tendinopathies. Apunts Medicina de l’Esport. 2014;48(182):31–36. [Google Scholar]
- [45].Baar K. Stress Relaxation and Targeted Nutrition to Treat Patellar Tendinopathy. Int J Sport Nutr Exerc Metab. 2019;29(4):453–457. [DOI] [PubMed] [Google Scholar]
- [46].Schneider A, Bennett JM, O’Connor DP, et al. Bilateral ruptures of the distal biceps brachii tendon. J Shoulder Elbow Surg. 2009;18(5):804–807. [DOI] [PubMed] [Google Scholar]
- [47].Hapa O, Cakici H, Gideroğlu K, et al. The effect of ethanol intake on tendon healing: a histological and biomechanical study in a rat model. Arch Orthop Trauma Surg. 2009;129(12):1721–1726. [DOI] [PubMed] [Google Scholar]
- [48].Lewis JS, Sandford FM. Rotator cuff tendinopathy: is there a role for polyunsaturated fatty acids and antioxidants? J Hand Ther. 2009;22(1):49–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.


