Abstract
Purpose of review:
Dementia is a life-course condition with modifiable risk factors from cardiovascular (CV) origin, that disproportionally affects some race/ethnic groups and underserved communities in the U.S. Hypertension is the most common preventable and treatable condition that increases the risk for dementia and exacerbates dementia pathology. Epidemiological studies beginning in midlife provide strong evidence for this association. This study provides an overview of the differences in the associations across the lifespan, and the role of social determinants of health (SDoH).
Recent findings:
Clinical trials support hypertension management in midlife as an avenue to lower the risk for late-life cognitive decline. However, the association between hypertension and cognition differs over the life course SDoH including higher education modify the association between hypertension and cognition which may differ by race and ethnicity. The role of blood pressure variability, interactions among CV risk factors, and cognitive assessment modalities may provide information to better understand the relationship between HTN and cognition.
Summary:
Adopting a life-course approach that considers SDoH, may help develop tailored interventions to manage hypertension and prevent dementia syndromes. Where clinical trials to assess blood pressure management from childhood to late-life are not feasible, observational studies remain the best available evidence.
Keywords: Hypertension, cognition, disparities, lifespan
Introduction
Along with cardiovascular (CV) disease, late-life dementia syndromes, including Alzheimer’s Disease (AD), are among the ten leading causes of morbidity and mortality worldwide. (1,2) These conditions disproportionally affect some racial/ethnic groups as well as underserved communities in the U.S. (3,4,5*,6*) Dementia is a public health priority and a life-course condition with potentially modifiable risk factors, many from CV origin. (7,8,9**) Among older adults, hypertension (HTN) is the most common preventable and treatable condition that increases the risk for dementia and exacerbates dementia pathology. (10,11) However, evidence supports structural and functional disruption in the brain much earlier in the lifespan promoted by chronic exposure to CV risk factors (CVRF), including elevated blood pressure (BP). (12–14)
Life-course social factors like educational attainment may protect the brain from neurodegenerative processes and preserve cognitive function. (15,16,17*) Through modifying the relationship between CVRFs and cognitive function, life-course social factors offer a potential explanation by which marginalized populations are disproportionality impacted by both CV disease and cognitive impairment. (17*,18**,19,20**,21) Therefore, HTN is also a potential target for strategies to prevent and delay the onset of dementia syndromes and minimize disparities. (8)
Here, we provide an overview of the mechanisms linking HTN and cognition and the differences in this association across the lifespan. We discuss the importance of social determinants as primary contributors to disparities in HTN and cognition. We then focus on relevant gaps in knowledge, including the role of BP variability (BPV), the potential synergistic effects with other cardiometabolic risk factors, and the poor precision of traditional cognitive assessments.
The vascular hypothesis and heterogeneity of cognitive syndromes
Dementia in the elderly can result from multiple coexistent cerebral and systemic disorders. (22) Although AD is the most common dementia diagnosis, individuals with this condition often have accompanying vascular neuropathology and deficits in cognitive domains other than memory, including executive function, often thought of as predominantly related to vascular pathology. (23*–25) Vascular and AD neuropathological processes often exist side-by-side and exacerbate each other, creating a continuum of causative processes that may be more heavily weighted toward one or the other, but not entirely due to one or the other. (26,27) Recently, data-driven classification approaches have been used to examine the heterogeneity of dementia syndromes, uncovering disease subtypes characterized by distinct patterns of neurodegeneration. (23*) This has resulted in greater recognition of the mixed nature of most cognitive syndromes, leading to a re-examination of the crucial role of CVRFs in the development of cognitive impairment. (9,28)
The vascular hypothesis posits that CVRFs exposure promotes structural and functional disruption of normal hemodynamics in the brain. (29**,30*) The brain depends on cerebral blood flow (CBF) to receive energy substrates and eliminate toxic byproducts. (31) The endothelial lining of the cerebral vasculature serves as the seat of the well-known blood-brain barrier (BBB) and, together with specialized membrane transporters, regulates the transfer of molecules between blood and brain. (32) Endothelial dysfunction induced by vascular oxidative stress and inflammation promotes vascular leakage and extravasation of plasma proteins triggering axonal injury. (33) CVRF exposure commonly leads to endothelial dysfunction with particular impact on the brain. (34) The subsequent alterations in CBF contribute to the initiation and promotion of neurodegeneration and cognitive impairment; therefore, classifying dementia as purely vascular or purely AD is arbitrary and can be misleading. (35–37)
Hypertension is a proposed risk factor for BBB damage, microvascular hemodynamic instability, and neuroinflammation leading to accumulation of neurotoxic molecules, including amyloid beta (Ab) precursors (11,38,39). Exposure to elevated BP promotes microvascular damage to the brain arteries leading to endothelial dysfunction and impaired cerebrovascular autoregulation (the capacity to maintain CBF constant despite BP changes).(32,40) Chronic exposure to high BP leads to continuous disruptions in CBF, causing chronic hypoperfusion, further damaging the endothelium.(40,41) Additionally, HTN increases the risk for atherosclerosis, an independent contributor to vascular and AD pathology. (42) Arterial parameters like carotid intima-media thickness and aortic stiffness have been reliable surrogates of atherosclerosis and strongly associate with cognitive dysfunction. (43,44) Altogether, these changes result in chronic cerebral hypoperfusion, neuronal injury, and neurodegeneration, all of which are key mechanisms for the development of dementia. (45–47**)
The life-course relationship between HTN and cognitive function
The relationship between BP levels and cognitive function in observational studies differs across the lifespan. (48) In late-life, a paradoxical relationship whereby higher BP is associated with better cognitive function has been found in several studies, and leads to the question of whether elevated BP in late-life could be protective.(49–51) Indeed, in epidemiological studies among those ≥ 80 years, including the Australian Centenarian Study, the OCTO-Twin Study, and The Kungsholmen Project, higher SBP associated with better cognition. (32,51,52) Earlier in late-life (≥ 60 years old), cross-sectional cohorts like The Baltimore Study have found U-shaped associations between BP and cognition, in the ranges of SBP 90 – 200 mmHg.(49) In contrast, longitudinal analyses showed that higher SBP at baseline associated with higher rates of cognitive decline later in life (age ≥80). (49) Similarly, results from six years of follow-up in the ARIC study showed that HTN at baseline (age 50–60) was associated with cognitive decline. (13) The inconsistency of late-life findings has emphasized the importance of longitudinal measurements that align with the timing of pathophysiological processes in cognitive impairment. (53**,54**)
Longer follow-up periods better capture the influence of BP on prodromal stages beginning in midlife and demonstrate strong associations between BP and cognitive function. (9) Studies with more than 20 years of follow-up have shown that elevated SBP and HTN associate with worse cognitive performance and dementia. (55–59) Furthermore, results from the CARDIA study demonstrated that a higher burden of SBP from young adulthood is associated with worse cognitive performance as early as midlife (age ~50). (60) Thus, providing information that better demonstrates a time-dependent effect of BP on cognition.
In children and adolescents elevated BP is associated with cognitive dysfunction both during youth and later in life, likely as manifestations of target-organ damage due to disruptions in cerebral microvasculature. (61*–64) Cross-sectional results from the NHANES III study demonstrated that SBP ≥ the 90th percentile in children 6–16 years associated with decreased performance on the Digit Span Task. (65) Similar results were found in the Generation R Study. (66) In a prospective multicenter study in the US, children ages 10–18 with uncontrolled HTN had sustained decreased performance on NP tests compared to normotensive controls after 1-year. (67) The long-term effects of elevated BP from childhood were demonstrated in the Young Finns Study, where a cumulative burden of higher SBP was associated with poorer performance on NP tests in midlife. (68) These findings provide evidence for the possibility of targeting BP during childhood and adolescence as a potential pathway to prevent cognitive decline.
Evidence from Clinical Trials
Most clinical trials have studied individuals in late-life when neuropathological changes may have already been established. Therefore, any BP management interventions at this point might be too late to provide benefits. (69) A recent meta-analysis of BP treatment interventions in adults of ≥60 years failed to provide evidence of benefit but also no evidence of adverse cognitive outcomes. (70) Despite this, multicenter clinical trials targeting BP control, including FINGER*, PROGRESS, and SPRINT-MIND, have shown positive results. (71**–73) The SPRINT-MIND trial found that intensive BP control (SBP <120mmHg) reduced the incidence of MCI after five years. (71**) This suggests that management of HTN in midlife may be an avenue to lower the risk for late-life cognitive decline. Still, managing BP to maintain cognitive health remains a challenge; the benefits vary from person to person, and that variability could possibly be influenced by earlier exposure to CVRFs. Given that randomized clinical trials that last the decades necessary to assess HTN treatment from childhood and late-life cognitive function are not feasible, observational studies remain the best available evidence.
Social determinants and disparities in hypertension and cognition
There are racial, geographic, and socioeconomic differences in the prevalence of HTN and dementia syndromes. (74,75*) The exact mechanisms driving such disparities and the connection between these conditions are complex. Yet, the interaction of neurobiological processes with life-course social determinants of health (SDoH) – defined as the various circumstances in which people are born, developed, and grow – are strongly implicated on the causal pathway (Figure 1.) (76,77*,78) Socioeconomic status in the US is influenced by education. (79) Together, they impact the development of vascular conditions such as HTN and late life cognitive syndromes including dementia, which is compounded by racial inequities in both conditions.
Figure 1.
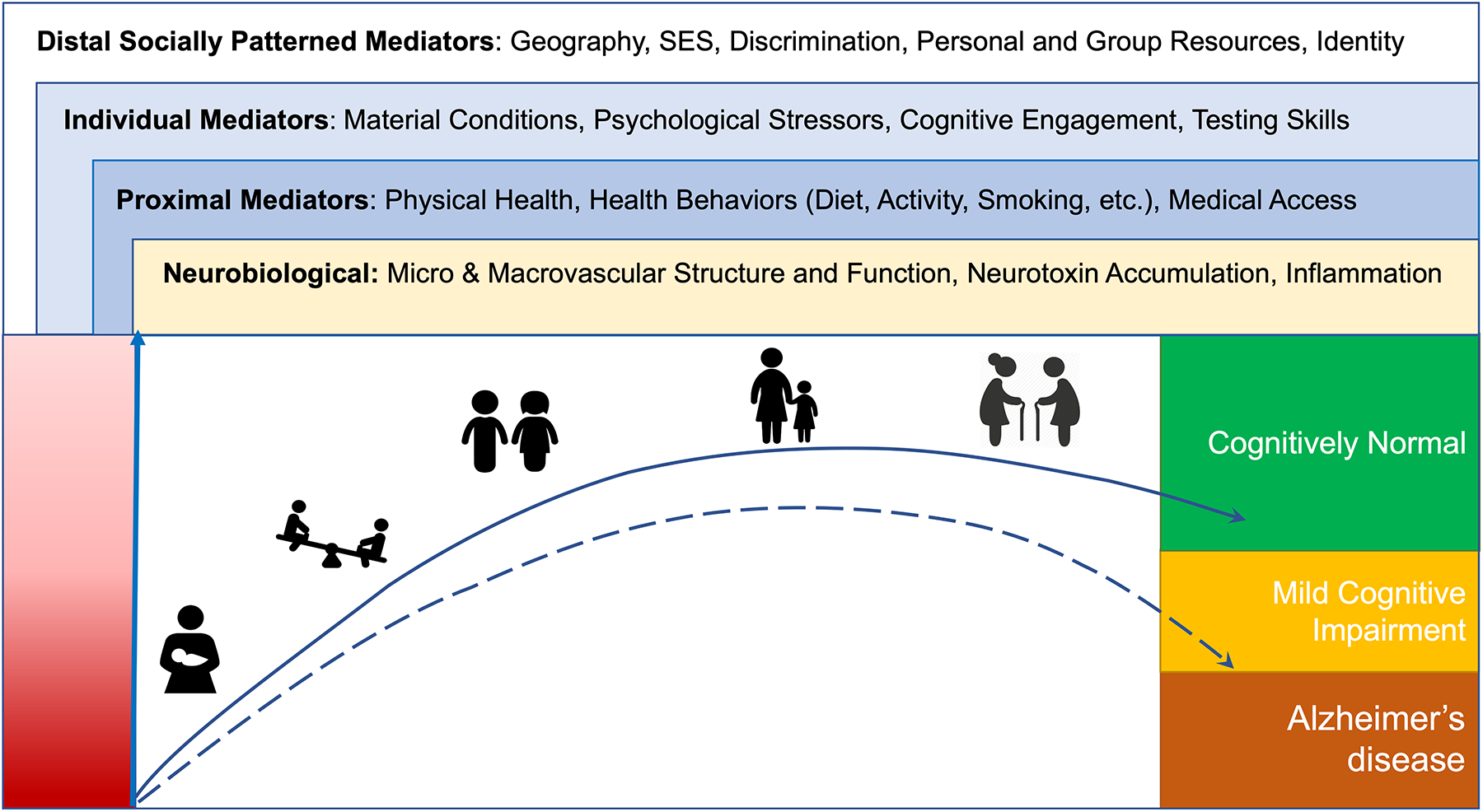
Life-course factors impacting hypertension and cognition
Adapted from Glymour and Manly, 2008; Figure 1. depicts neurobiological parameters, and social factors impacting hypertension, cognitive function and the association between both across the life-course, from the prenatal period to old age. Social factors are divided according to their mediator role in the causal pathway to cognitive outcomes.
The relationship between HTN and cognitive function over the life-course may differ by race and ethnicity. (80) Individuals from five well-characterized prospective cohorts demonstrated a steeper decline in cognition among African Americans compared to their White counterparts, and the difference was eliminated when HTN was accounted for in the multivariate model. (81**) This strongly suggests that higher BP levels contributed to racial differences in late-life cognitive decline. Similar results were found in the REGARDS study. (82) In studies, with smaller sample sizes and shorter duration, differences by race were not apparent or were confined to baseline cognition rather than cognitive change. (75*) Some of these inconsistencies may result from selective survival and differential attrition, whereby African Americans with a higher burden of HTN who may be experiencing cognitive issues are lost or withdrawn from longitudinal studies. (83,84)
Education has a substantial impact on cognitive outcomes across the life-course and has been shown to modify the pathway between CVRFs and cognition. (20) Higher levels of education protect the brain through bolstering cognitive reserve or the ability to sustain better cognitive function despite brain pathology. (17*) Thus, individuals in the same age-span can have the similar levels of pathobiological indicators such as Aβ in the brain but have different cognitive outcomes. (15,17*,20).Greater cognitive reserve, associated with higher levels of education, decreases the incidence and prevalence of dementia. (86) Education may also indirectly impact cognition by promoting better lifestyle choices and greater levels of health literacy, both of which may improve the management of chronic conditions such as HTN.
In clinical trials targeting BP control, individuals from diverse backgrounds account for less than 5% of participants, and far less come from rural or segregated communities. (88) Stratification of results and consideration of SDoH are useful methodologies to better understand the relationship of BP and cognition among subgroups. However, most studies to date have failed to present stratified results and or include a more comprehensive assessment of SDoH.(71**,89,90) Community-based interventions have proven to be a critical resource in HTN management by reaching out to populations with geographic or socioeconomic access barriers to health care and might be an ideal avenue to achieve BP control to prevent cognitive impairment. (91*) These interventions are often tailored to the beliefs and traditions of specific groups and thus may be more effective for HTIN control. (75*)
Current knowledge gaps –
Blood pressure variability
Measurement of mean BP has been historically used as an indicator of risk; however, evidence supports BPV as an independent risk factor for CV events. (92–94) The dose and duration of BPV, usually measured as visit-to-visit BPV, has been strongly correlated with atherosclerotic changes and stroke, suggesting that cerebrovascular injury over time might be the primary driver for its relationship with adverse cognitive outcomes. (95*,96**) In the elderly, BPV is correlated with brain atrophy and a higher burden of white matter hyperintensities, which associate with cognitive impairment and dementia. (97) Studies aiming to disentangle the exact mechanisms linking BPV with cognitive function suggest that arterial stiffness and atherosclerosis contribute to BPV, leading to extreme fluctuation of BP levels that disrupt the continuous flow to small vessels in the brain. (98,99) As a result, the extreme pulsatility damages brain microvasculature causing endothelial dysfunction and chronic inflammation, key components of the vascular hypothesis. (101) These results support the importance of expanding from traditional in-clinic BP measurements to include ambulatory BP monitoring that can capture not only mean BP levels but also BP rhythm and BPV.
Interaction with cardiometabolic risk factors
Individuals with HTN have double the risk of presenting with obesity and diabetes mellitus (DM). In epidemiological studies, these conditions are included as covariates in regression models, but their synergistic effect with HTN across the lifespan is often not captured. (102*) The cumulative burden of elevated fasting glucose has been associated with worse NP performance across different cognitive domains, and the potential mechanisms for this association include CVRFs. (60,103) DM is also directly related to chronic kidney disease and heart failure, which exacerbate damage to cerebral microvasculature and CBF. (104) Additionally, the combination of smoking, BP, and hypercholesterolemia has been shown to predict cognitive function independent of other cardiometabolic conditions in early life. (60) These findings support the need for clinical trials, such as PREVENTABLE, that assess the impact of treating other CVRF on cognitive function.
Consistency of cognitive assessment
Single cognitive-domain assessment has received greater attention in contrast to previously used global neurocognitive score-composites. (105) The hope for domain-specific analysis is to gain an understanding of tests most sensitive to HTN that would indicate underlying vascular neuropathology. (48) In particular, impairment in the cognitive domains of processing speed and executive function have been associated with CVRFs; however, the NP tests are not specific enough to test only these domains. (60) Establishing HTN-sensitive testing could have clinical applications for early detection and treatment of cognitive decline. Data-derived NP profiles or cognitive endophenotypes have been associated with distinct underlying neuropathology and, therefore, could provide useful information regarding the underlying distribution of clinical characteristics (106,107*).
Furthermore, cultural experiences and socioeconomic factors highly influence NP test performance. Even when NP tests measure the same construct across racial/ethnic groups, individuals from socially and geographically disadvantaged environments are often misclassified.(108,109) Evidence shows that despite age and years of education adjustment, African Americans with normal cognition scored lower than their White counterparts, likely due to education quality in Southern, rural, and segregated schools.(21,110) To cope with this, considering the quality of education and the use of demographically standardized NP test scores has been strongly supported as a measure to maintain consistency in test scores across different groups. (109)
Conclusion
In summary, chronic exposure to HTN promotes microvascular damage to the brain arteries leading to BBB dysfunction, neuroinflammation, and the accumulation of neurotoxic molecules, initiating and promoting neurodegeneration and cognitive impairment. The association between HTN and cognitive function is different across the lifespan. Observational and clinical trial evidence supports midlife as a critical time window for BP management to prevent or delay cognitive decline. Marginalized populations based on race, ethnicity, or geographic location in the U.S. have the highest prevalence of HTN and dementia syndromes; thus, adequate BP control may be a potential avenue to minimize disparities. Because life-course determinants like educational quality are linked to racial inequities in cognition outcomes, adopting a life-course approach that carefully considers the impact of social determinants and the cumulative burden of BP and BPV from childhood, together with precise cognitive assessments, may help develop more effective and targeted interventions to manage BP and prevent dementia syndromes.
Key points:
The association between hypertension and cognitive function differs across the lifespan.
Midlife is a critical time window for hypertension management to prevent or delay cognitive decline.
Adequate blood pressure control may be a potential avenue to minimize disparities
Observational studies remain the best available evidence to assess the benefits of hypertension control from youth in late-life cognition.
Financial support and sponsorship:
This research was supported by the National Institute on Aging and National Heart, Lung, and Blood Institute of the NIH under grant RF1AG041200-06 (principal investigator, Dr. Bazzano) and the American Heart Association (AHA) award number 20SFRN35490098 (principal investigators, Dr. Au and Dr. Bazzano).
Footnotes
Conflict of interest:
The authors disclose no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Kochanek KD. Mortality in the United States, 2019. 2020;(395):8. [PubMed] [Google Scholar]
- 2.Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional Mortality Data — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021. Apr 9;70(14):519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Alzheimer Report 2010: The Global Economic Impact of Dementia - Executive Summary. 2010;12. [Google Scholar]
- 4.Bouldin ED, Shaull L, Andresen EM, Edwards VJ, McGuire LC. Financial and Health Barriers and Caregiving-Related Difficulties among Rural and Urban Caregivers. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Oba H, Kadoya Y, Okamoto H, Matsuoka T, Abe Y, Shibata K, et al. The Economic Burden of Dementia: Evidence from a Survey of Households of People with Dementia and Their Caregivers. Int J Env Res Public Health. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study found that people with dementia had more financial problems even when being assisted and managed by their caregivers.
- 6*.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019 Jan;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that, in the US, the prevalence of ADRD was higher for women. Black individuals had the highest prevalence followed by Hispanics and non-Hispanic whites.
- 7.Baumgart M Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. 2015;9. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. [Internet]. 2019. [cited 2020 Dec 21]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542796/ [PubMed]
- 9**.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020. Aug;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an overview of the modifiable risk factors for dementia across the lifespan and provides the percentage reduction in dementia prevalence if the risk factor is eliminated.
- 10.Chartbook and Charts | CMS [Internet]. [cited 2021 Oct 6]. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts
- 11.Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016. Jan;36(1):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abell JG, Kivimäki M, Dugravot A, Tabak AG, Fayosse A, Shipley M, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018. Sep 1;39(33):3119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: The ARIC study. Neurology. 2011. May 31;76(22):1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infante-Garcia C, Ramos-Rodriguez JJ, Galindo-Gonzalez L, Garcia-Alloza M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology. 2016. Mar;65:15–25. [DOI] [PubMed] [Google Scholar]
- 15.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. :7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettigrew C, Soldan A. Defining Cognitive Reserve and Implications for Cognitive Aging. Curr Neurol Neurosci Rep. 2019. Jan;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Zahodne LB, Mayeda ER, Hohman TJ, Fletcher E, Racine AM, Gavett B, et al. The role of education in a vascular pathway to episodic memory: brain maintenance or cognitive reserve? Neurobiol Aging. 2019. Dec;84:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence for the role of education to attenuate the effect of white matter hyperintensities on memory among individuals less likely to progress to dementia. Providing evidence for a cognitive reserve mechanism.
- 18**.Avila JF, Rentería MA, Jones RN, Vonk JMJ, Turney I, Sol K, et al. Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement. 2021. Jan;17(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence to support that education does not contribute to cognitive reserve to the same extent across racial and ethnic groups.
- 19.Dubal DB. Sex difference in Alzheimer’s disease: An updated, balanced and emerging perspective on differing vulnerabilities. In: Handbook of Clinical Neurology [Internet]. Elsevier; 2020. [cited 2021 Oct 7]. p. 261–73. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780444641236000187 [DOI] [PubMed] [Google Scholar]
- 20**.Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM. Education and Cognitive Functioning Across the Life Span. Psychol Sci Public Interest. 2020. Aug;21(1):6–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an overview of education attainment and its influences on late-life cognitive function, by contributing to individual differences in cognitive skills in early adulthood that persist until older age.
- 21.Sisco S, Gross AL, Shih RA, Sachs BC, Glymour MM, Bangen KJ, et al. The Role of Early-Life Educational Quality and Literacy in Explaining Racial Disparities in Cognition in Late Life. J Gerontol B Psychol Sci Soc Sci. 2015. Jul;70(4):557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale SA, Acar D, Daffner KR. Dementia. Am J Med. 2018. Oct;131(10):1161–9. [DOI] [PubMed] [Google Scholar]
- 23*.Habes M, Grothe MJ, Tunc B, McMillan C, Wolk DA, Davatzikos C. Disentangling Heterogeneity in Alzheimer’s Disease and Related Dementias Using Data-Driven Methods. Biol Psychiatry. 2020. Jul;88(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides and overview of data-driven methods to disentangle heterogeneity in Alzheimer’s disease. Highlighting the contribution from these studies, as well as the challenges and future directions of these novel methodologies.
- 24.Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013. Mar;136(3):844–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, Cohn-Sheehy BI, La Joie R, Vogel JW, Möller C, Lehmann M, et al. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease: Origin and Spread of Atrophy in AD Variants. Hum Brain Mapp. 2015. Nov;36(11):4421–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of Cerebral Vessel Disease to Alzheimer’s Disease Dementia and Cognitive Function in Older Persons: A Cross-sectional Study. Lancet Neurol. 2016. Aug;15(9):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006. Dec;60(6):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004. May;5(5):347–60. [DOI] [PubMed] [Google Scholar]
- 29**.Yaffe K, Vittinghoff E, Hoang T, Matthews K, Golden SH, Zeki Al Hazzouri A. Cardiovascular Risk Factors Across the Life Course and Cognitive Decline: A Pooled Cohort Study. Neurology. 2021. Apr 27;96(17):e2212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study provides pooled-cohort results for the association between life course cardiovascular risk factors and cognitive decline later in life. Suggesting that early adulthood management of these conditions could benefit cognition in late life.
- 30*.Scheffer S, Hermkens DMA, van der Weerd L, de Vries HE, Daemen MJAP. Vascular Hypothesis of Alzheimer Disease: Topical Review of Mouse Models. Arterioscler Thromb Vasc Biol. 2021. Apr;41(4):1265–83. [DOI] [PubMed] [Google Scholar]; Provides a review of the role of cardiovascular disease and chronic cerebral hypoperfusion in AD development from animal models.
- 31.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2010. Jan;30(1):2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011. Sep;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrall AJ, Wardlaw JM. Blood–brain barrier: Ageing and microvascular disease – systematic review and meta-analysis. Neurobiol Aging. 2009. Mar;30(3):337–52. [DOI] [PubMed] [Google Scholar]
- 34.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler Thromb Vasc Biol. 2005. May;25(5):932–43. [DOI] [PubMed] [Google Scholar]
- 35.Zlokovic BV. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron. 2008. Jan 24;57(2):178–201. [DOI] [PubMed] [Google Scholar]
- 36.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008. Mar;9(3):169–81. [DOI] [PubMed] [Google Scholar]
- 37.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta BBA - Mol Basis Dis. 2016. May;1862(5):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnevale D, Mascio G, D’Andrea I, Fardella V, Bell RD, Branchi I, et al. HYPERTENSION INDUCES BRAIN β-AMYLOID ACCUMULATION, COGNITIVE IMPAIRMENT AND MEMORY DETERIORATION THROUGH ACTIVATION OF RAGE IN BRAIN VASCULATURE. Hypertension. 2012. Jul;60(1):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gentile MT, Poulet R, Pardo AD, Cifelli G, Maffei A, Vecchione C, et al. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009. Feb;30(2):222–8. [DOI] [PubMed] [Google Scholar]
- 40.Walker KA, Power MC, Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep. 2017. Mar;19(3):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller M, van der Graaf Y, Visseren FL, Mali WPThM, Geerlings MI, for the SMART Study Group. Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann Neurol. 2012. Jun;71(6):825–33. [DOI] [PubMed] [Google Scholar]
- 42.Johnston SC, O’Meara ES, Manolio TA, Lefkowitz D, O’Leary DH, Goldstein S, et al. Cognitive Impairment and Decline Are Associated with Carotid Artery Disease in Patients without Clinically Evident Cerebrovascular Disease. :12. [DOI] [PubMed] [Google Scholar]
- 43.Della-Morte D, Dong C, Markert MS, Elkind MSV, Sacco RL, Wright CB, et al. Carotid Intima-Media Thickness Is Associated With White Matter Hyperintensities: The Northern Manhattan Study. Stroke. 2018. Feb;49(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arntzen KA, Mathiesen EB. Subclinical carotid atherosclerosis and cognitive function: Subclinical carotid atherosclerosis and cognitive function. Acta Neurol Scand. 2011. Aug;124:18–22. [DOI] [PubMed] [Google Scholar]
- 45**.Cortes-Canteli M, Iadecola C. Alzheimer’s Disease and Vascular Aging. J Am Coll Cardiol. 2020. Mar;75(8):942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review that provides evidence of the alterations in cerebral and systemic vasculature, the heart, and hemostasis that occur in Alzheimer’s disease and the relationship with cognitive function.
- 46.Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci. 2017. Sep 28;131(19):2451–68. [DOI] [PubMed] [Google Scholar]
- 47.Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019. Feb;25(2):270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement from the American Heart Association. Hypertension. 2016. Dec;68(6):e67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear Relations of Blood Pressure to Cognitive Function: The Baltimore Longitudinal Study of Aging. Hypertension. 2005. Mar;45(3):374–9. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson SE, Read S, Berg S, Johansson B, Melander A, Lindblad U. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res. 2007. Feb;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 51.Richmond R, Law J, Kay-Lambkin F. Higher blood pressure associated with higher cognition and functionality among centenarians in Australia. Am J Hypertens. 2011. Mar;24(3):299–303. [DOI] [PubMed] [Google Scholar]
- 52.Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood Pressure and Performance on the Mini-Mental State Examination in the Very Old: Cross-sectional and Longitudinal Data from the Kungsholmen Project. Am J Epidemiol. 1997. Jun 15;145(12):1106–13. [DOI] [PubMed] [Google Scholar]
- 53**.Porsteinsson AP, Isaacson RS, Knox S, Sabbagh MN, Rubino I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J Prev Alzheimers Dis. 2021;1–16. [DOI] [PubMed] [Google Scholar]; A review that highlights the importance of an early Alzheimer’s disease diagnosis, providing a guide and practical considerations.
- 54**.Vermunt L, Sikkes SAM, Hout A, Handels R, Bos I, Flier WM, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019. Jul;15(7):888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study estimates the duration of the preclinical, prodromal and dementia stages of Alzheimer’s disease and the influence of sex and Apolipoprotein E on the duration of the disease.
- 55.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated Blood Pressure Level Is Inversely Related to Cognitive Functioning: The Framingham Study. Am J Epidemiol. 1993. Sep 15;138(6):353–64. [DOI] [PubMed] [Google Scholar]
- 56.Launer LJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA J Am Med Assoc. 1995. Dec 20;274(23):1846–51. [PubMed] [Google Scholar]
- 57.Chen KHM, Henderson VW, Stolwyk RJ, Dennerstein L, Szoeke C. Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing. 2015. May;44(3):439–45. [DOI] [PubMed] [Google Scholar]
- 58.Gabin JM, Tambs K, Saltvedt I, Sund E, Holmen J. Association between blood pressure and Alzheimer disease measured up to 27 years prior to diagnosis: the HUNT Study. Alzheimers Res Ther. 2017. Dec;9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottesman RF, Schneider ALC, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014. Oct 1;71(10):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer RA, et al. Early Adult to Midlife Cardiovascular Risk Factors and Cognitive Function. Circulation. 2014. Apr 15;129(15):1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Lande MB, Kupferman JC. Blood Pressure and Cognitive Function in Children and Adolescents. Hypertension. 2019. Mar;73(3):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review that provides evidence for the association between early life hypertension and lower cognitive function in childhood, and later in midlife.
- 62.Urbina EM, Lande MB, Hooper SR, Daniels SR. Target Organ Abnormalities in Pediatric Hypertension. J Pediatr. 2018. Nov;202:14–22. [DOI] [PubMed] [Google Scholar]
- 63.Wong LJ, Kupferman JC, Prohovnik I, Kirkham FJ, Goodman S, Paterno K, et al. Hypertension Impairs Vascular Reactivity in the Pediatric Brain. Stroke. 2011. Jul;42(7):1834–8. [DOI] [PubMed] [Google Scholar]
- 64.Páll D, Lengyel S, Komonyi É, Molnár C, Paragh G, Fülesdi B, et al. Impaired cerebral vasoreactivity in white coat hypertensive adolescents: Impaired cerebral vasoreactivity in WCH adolescents. Eur J Neurol. 2011. Apr;18(4):584–9. [DOI] [PubMed] [Google Scholar]
- 65.Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school-age children and adolescents in the United States. J Pediatr. 2003;143(6):720–4. [DOI] [PubMed] [Google Scholar]
- 66.Lamballais S, Sajjad A, Leening MJG, Gaillard R, Franco OH, Mattace-Raso FUS, et al. Association of Blood Pressure and Arterial Stiffness With Cognition in 2 Population-Based Child and Adult Cohorts. J Am Heart Assoc [Internet]. 2018. Nov 6 [cited 2022 Mar 6];7(21). Available from: https://www.ahajournals.org/doi/10.1161/JAHA.118.009847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lande MB, Batisky DL, Kupferman JC, Samuels J, Hooper SR, Falkner B, et al. Neurocognitive Function in Children with Primary Hypertension. J Pediatr. 2017. Jan;180:148–155.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rovio SP, Pahkala K, Nevalainen J, Juonala M, Salo P, Kähönen M, et al. Cardiovascular Risk Factors From Childhood and Midlife Cognitive Performance. J Am Coll Cardiol. 2017. May;69(18):2279–89. [DOI] [PubMed] [Google Scholar]
- 69.Gottesman RF. Should Hypertension Be Treated in Late Life to Preserve Cognitive Function? Hypertension. 2018. May;71(5):787–92. [DOI] [PubMed] [Google Scholar]
- 70.Weiss J, Freeman M, Low A, Fu R, Kerfoot A, Paynter R, et al. Benefits and Harms of Intensive Blood Pressure Treatment in Adults Aged 60 Years or Older: A Systematic Review and Meta-analysis. Ann Intern Med. 2017. Mar 21;166(6):419. [DOI] [PubMed] [Google Scholar]
- 71**.The SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019. Feb 12;321(6):553. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides results from a multi-centered randomized clinical trial among adults 50 years and older. Intervention included strict SBP management <120mm Hg. The outcomes were mild cognitive impairment and probable dementia.
- 72.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015. Jun;385(9984):2255–63. [DOI] [PubMed] [Google Scholar]
- 73.Effects of Blood Pressure Lowering With Perindopril and Indapamide Therapy on Dementia and Cognitive Decline in Patients With Cerebrovascular Disease. Arch Intern Med. 2003. May 12;163(9):1069. [DOI] [PubMed] [Google Scholar]
- 74.Al Kibria GM. Racial/ethnic disparities in prevalence, treatment, and control of hypertension among US adults following application of the 2017 American College of Cardiology/American Heart Association guideline. Prev Med Rep. 2019. Jun;14:100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement. 2019. Feb;15(2):292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]; A white paper that provides state-of-the-art summary and recommendations to address the immediate needs of to advance ADRD science across diverse ethnic and racial populations.
- 76.Marmot M, Bell R, Goldblatt P. Action on the social determinants of health. Rev DÉpidémiologie Santé Publique. 2013. Aug;61:S127–32. [DOI] [PubMed] [Google Scholar]
- 77*.Ma J, Yang Y, Wan Y, Shen C, Qiu P. The influence of childhood adversities on mid to late cognitive function: From the perspective of life course. Liu SY, editor. PLOS ONE. 2021. Aug 16;16(8):e0256297. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that childhood adversities, including socioeconomic status and depressive symptoms, had negative effects on cognitive function in midlife.
- 78.Glymour MM, Manly JJ. Lifecourse Social Conditions and Racial and Ethnic Patterns of Cognitive Aging. Neuropsychol Rev. 2008;32. [DOI] [PubMed] [Google Scholar]
- 79.Stormacq C, Van den Broucke S, Wosinski J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot Int. 2019. Oct 1;34(5):e1–17. [DOI] [PubMed] [Google Scholar]
- 80.Byrd DR, Gee GC, Tarraf W. Black-white mental status trajectories: What ages do differences emerge? SSM - Popul Health. 2018. Dec;6:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81**.Levine DA, Gross AL, Briceño EM, Tilton N, Kabeto MU, Hingtgen SM, et al. Association Between Blood Pressure and Later-Life Cognition Among Black and White Individuals. JAMA Neurol. 2020. Jul 1;77(7):810. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study looks at the association between cumulative BP levels and global cognition among black and white individuals to determine whether BP potentially explains racial differences in cognitive decline.
- 82.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Blood Pressure and Cognitive Decline Over 8 Years in Middle-Aged and Older Black and White Americans. Hypertension. 2019. Feb;73(2):310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manly JJ, Mungas D. JGPS Special Series on Race, Ethnicity, Life Experiences, and Cognitive Aging. J Gerontol B Psychol Sci Soc Sci. 2015. Jul;70(4):509–11. [DOI] [PubMed] [Google Scholar]
- 84.Whitfield KE, Allaire JC, Belue R, Edwards CL. Are Comparisons the Answer to Understanding Behavioral Aspects of Aging in Racial and Ethnic Groups? J Gerontol B Psychol Sci Soc Sci. 2008. Sep 1;63(5):P301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu Y, An Y, Yu H, Che F, Zhang X, Rong H, et al. Sex-specific nonlinear associations between serum lipids and different domains of cognitive function in middle to older age individuals. Metab Brain Dis. 2017. Aug;32(4):1089–97. [DOI] [PubMed] [Google Scholar]
- 86.Meng X, D’Arcy C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. Laks J, editor. PLoS ONE. 2012. Jun 4;7(6):e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arce Rentería M, Vonk JMJ, Felix G, Avila JF, Zahodne LB, Dalchand E, et al. Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology. 2019. Dec 10;93(24):e2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Increasing Ethnic Minority Participation in Alzheimer Diseas... : Alzheimer Disease & Associated Disorders [Internet]. [cited 2022 Feb 14]. Available from: https://journals.lww.com/alzheimerjournal/Abstract/2002/00002/Increasing_Ethnic_Minority_Participation_in.9.aspx [DOI] [PubMed] [Google Scholar]
- 89.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015. Sep;11(9):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015. Nov 17;85(20):1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91*.Ferdinand DP, Nedunchezhian S, Ferdinand KC. Hypertension in African Americans: Advances in community outreach and public health approaches. Prog Cardiovasc Dis. 2020. Jan;63(1):40–5. [DOI] [PubMed] [Google Scholar]; A review that provides significant evidence of crucial barriers, and successful models for community outreach to engage , and improve HTN control in African Americans.
- 92.Howard SC, Rothwell PM. Regression dilution of systolic and diastolic blood pressure in patients with established cerebrovascular disease. J Clin Epidemiol. 2003. Nov;56(11):1084–91. [DOI] [PubMed] [Google Scholar]
- 93.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. The Lancet. 2010. Mar;375(9718):895–905. [DOI] [PubMed] [Google Scholar]
- 94.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016. Aug 9;i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95*.Ma Y, Tully PJ, Hofman A, Tzourio C. Blood Pressure Variability and Dementia: A State-of-the-Art Review. Am J Hypertens. 2020. Dec 31;33(12):1059–66. [DOI] [PubMed] [Google Scholar]; A review that provides an overview of the relationship between Blood pressure variability as a risk factor for dementia as well as the potential mechanisms involved.
- 96**.Jia P, Lee HWY, Chan JYC, Yiu KKL, Tsoi KKF. Long-Term Blood Pressure Variability Increases Risks of Dementia and Cognitive Decline: A Meta-Analysis of Longitudinal Studies. Hypertension. 2021. Oct;78(4):996–1004. [DOI] [PubMed] [Google Scholar]; A meta-analysis that provides evidence that supports the linear relationship between higher blood pressure variability and the risk of dementia and cognitive impairment.
- 97.Jung H, Kim K. Blood Pressure Variability and Cognitive Function in the Elderly. Pulse. 2013;1(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, et al. Associations of Aortic Distensibility and Arterial Elasticity With Long-Term Visit-to-Visit Blood Pressure Variability: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2013. Jul 1;26(7):896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain. 2011. Nov;134(11):3398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tatasciore A, Zimarino M, Renda G, Zurro M, Soccio M, Prontera C, et al. Awake Blood Pressure Variability, Inflammatory Markers and Target Organ Damage in Newly Diagnosed Hypertension. Hypertens Res. 2008;31(12):2137–46. [DOI] [PubMed] [Google Scholar]
- 102.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol 2021 1710. 2021. Jun;17(10):639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of the pathophysiology of hypertension-induced cognitive impairment, and how pharmaceutical and lifestyle interventions that reduce blood pressure, in combination with treatments that promote microvascular health, can prevent or delay the pathogenesis of vascular cognitive impairment.
- 103.Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015. Dec;7(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hooghiemstra AM, Leeuwis AE, Bertens AS, Biessels GJ, Bots ML, Brunner-La Rocca H-P, et al. Frequent Cognitive Impairment in Patients With Disorders Along the Heart-Brain Axis. Stroke. 2019. Dec;50(12):3369–75. [DOI] [PubMed] [Google Scholar]
- 105.Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11:316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Libon DJ, Drabick DAG, Giovannetti T, Price CC, Bondi MW, Eppig J, et al. Neuropsychological Syndromes Associated with Alzheimer’s/Vascular Dementia: A Latent Class Analysis. J Alzheimers Dis. 2014. Sep 16;42(3):999–1014. [DOI] [PubMed] [Google Scholar]
- 107*.Emrani S, Lamar M, Price CC, Wasserman V, Matusz E, Au R, et al. Alzheimer’s/Vascular Spectrum Dementia: Classification in Addition to Diagnosis. J Alzheimers Dis. 2020. Jan 7;73(1):63–71. [DOI] [PubMed] [Google Scholar]; A hypothesis manuscript that proposed that in conjunction with current diagnostic criteria for Alzheimer’s disease (AD) and Vascular dementia (VaD), statistical modeling techniques based on neuropsychological testing can construct a system to classify individuals along the AD/VaD spectrum of disease.
- 108.Werry AE, Daniel M, Bergström B. Group differences in normal neuropsychological test performance for older non-Hispanic White and Black/African American adults. Neuropsychology. 2019. Nov;33(8):1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Norman MA, Moore DJ, Taylor M, Franklin D, Cysique L, Ake C, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test–Revised, Brief Visuospatial Memory Test–Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011. Aug;33(7):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manly JJ, Miller SW, Heaton RK, Byrd D, Reilly J, Velasquez RJ, et al. The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. J Int Neuropsychol Soc. 1998. May;4(3):291–302. [PubMed] [Google Scholar]


