Summary
Cancer cell migration during metastasis is mediated by a highly polarized cytoskeleton. MARK2 and its invertebrate homologue Par1B are kinases that regulate the microtubule cytoskeleton to mediate polarization of neurons in mammals and embryos in invertebrates. However, the role of MARK2 in cancer cell migration is unclear. Using osteosarcoma cells, we found that in addition to its known localizations on microtubules and the plasma membrane, MARK2 also associates with the actomyosin cytoskeleton and focal adhesions and promotes stress fiber formation. Cells depleted of MARK proteins demonstrated that MARK2 promotes phosphorylation of both myosin II and the myosin phosphatase targeting subunit MYPT1 to synergistically drive myosin II contractility in cells. Studies with isolated proteins showed that MARK2 directly phosphorylates myosin II regulatory light chain, while its effects on MYPT1 phosphorylation are indirect. Using a mutant lacking the membrane binding domain, we found that membrane association is required for focal adhesion targeting of MARK2, where it specifically enhances cell protrusion by promoting FAK phosphorylation and formation of focal adhesions oriented in the direction of migration to mediate directionally persistent cell motility. Together, our results define MARK2 as a master regulator of the actomyosin and microtubule cytoskeletal systems and focal adhesions to mediate directional cancer cell migration.
Keywords: MARK2, Par1b, cell polarity, directed cell migration, Myosin II, MYPT1
eTOC Blurb
Pasapera et al. show that the microtubule regulatory and polarity protein MARK2 associates with actomyosin and promotes phosphorylation of myosin II regulatory light chain and MYPT1 to drive contractility. MARK2 also associates with focal adhesions (FAs) and promotes FAK activation and FA formation/orientation to drive directional cell migration.
Introduction
Directed cell migration mediates development, the immune response, and wound healing, and is mis-regulated during cancer metastasis1.To directionally migrate during metastasis, cancer cells sense environmental cues and transduce them to downstream effectors that polarize cytoskeletal organization and dynamics to steer cells toward cues. It is well established that the actomyosin and microtubule (MT) cytoskeletons are critical to directed cell movement2. Polymerization of actin at the leading edge drives lamellipodial protrusion, while contraction of actomyosin generates traction forces through integrin-based focal adhesions (FAs) and mediates retraction of the cell rear3. Actomyosin polarization is regulated by small GTPases that activate both actin nucleation and elongation factors at the leading edge1,4,5 and kinases including ROCK and MLCK that drive contractility by phosphorylating myosin II regulatory light chain (MRLC)6,7,8. The MT cytoskeleton also becomes polarized during migration, with MT growth in the leading edge9 and the MT organizing center (MTOC) orienting between the leading edge and the nucleus10,11. Like actin, MTs are also regulated by small GTPases that promote MT polymerization2,5,12 and MTOC orientation through MRCK-mediated MRLC phosphorylation to generate contraction in the dorsal cortex to move the nucleus rearward13. However, much remains unclear about the molecular mechanisms coordinating cytoskeletal polarization during metastatic cell migration.
The Par (partitioning defective) protein complex, first identified in Caenorhabditis elegans14, is an evolutionarily conserved regulator of cell polarity from yeast to mammals15,16. Par1b in C.elegans and Drosophila is required for establishing the body axis through cytoskeletal control of patterning cue position in the oocyte and early embryo17–20. Its mammalian homologue, known as MT-associated regulatory kinase 2 (MARK2), is a member of the AMPK kinase family that was discovered by its ability to phosphorylate neuronal MT-associated proteins (MAPs) such as tau21,22. In epithelial cells, MARK2 is required for apical-basal polarity23,24,25, mitotic spindle orientation26, and asymmetric cell division27, while in neurons it mediates neurite elongation28, dendrite/axon specification29,30, dendritic spine maturation31 and promotes the directional migration of cortical neurons32,33. In cancer, it is controversial as to whether MARK2 plays an inhibitory or promoting role34–36, and the mechanism of involvement is completely unknown. However, it is possible that MARK2 could play a role in metastasis by regulating cytoskeletal polarity during directed migration.
In migrating non-neuronal cells, MARK2 localizes to MTs and the MTOC, the plasma membrane where it concentrates in protruding cell edges, and was identified in the FA proteome27,37–40. MARK2 is thought to contribute to polarity in cell migration through phosphorylation of MAPs, promoting both MTOC orientation and leading edge MT growth 15,33,38,41,42,43. However, since polarization of the MTOC in migrating cells is mediated by actomyosin13,44,45, MARK2 could regulate contractility. Indeed, MARK2 can localize to the actomyosin cortex of dividing cells to mediate mitotic spindle orientation by integrating actin and astral MTs26,27. In addition, Par1b interacts with myosin II in C. elegans 46 and with myosin phosphatase in Drosophila oocytes41,47, however whether mammalian MARK2 functions similarly is not known. MARK2 localization at the plasma membrane, which is mediated by its C-terminal KA1 domain 48,49, also contributes to cell polarization50, although the mechanism is not known. Thus, how MARK2 regulates the cytoskeleton to mediate directed cancer cell migration remains to be elucidated.
Here we sought to test the hypothesis that MARK2 mediates polarization and directed migration of cancer cells through regulation of the actomyosin cytoskeleton. We found in osteosarcoma cells that in addition to its known localizations, MARK2 also associates with actomyosin stress fibers and FAs. We show that MARK2 directly phosphorylates MRLC and associates with the myosin phosphatase regulatory subunit MYPT1 and indirectly promotes its phosphorylation and inactivation, thus synergistically driving myosin II contractility. Furthermore, we found that MARK2’s membrane targeting domain is required for its localization to FAs where it promotes FA formation and orientation to drive directional cell migration. Together these results identify MARK2 as a major regulator of cell contractility and adhesion to mediate cancer cell motility.
Results
MARK2 localizes at the cell membrane, actomyosin cytoskeleton, and FAs
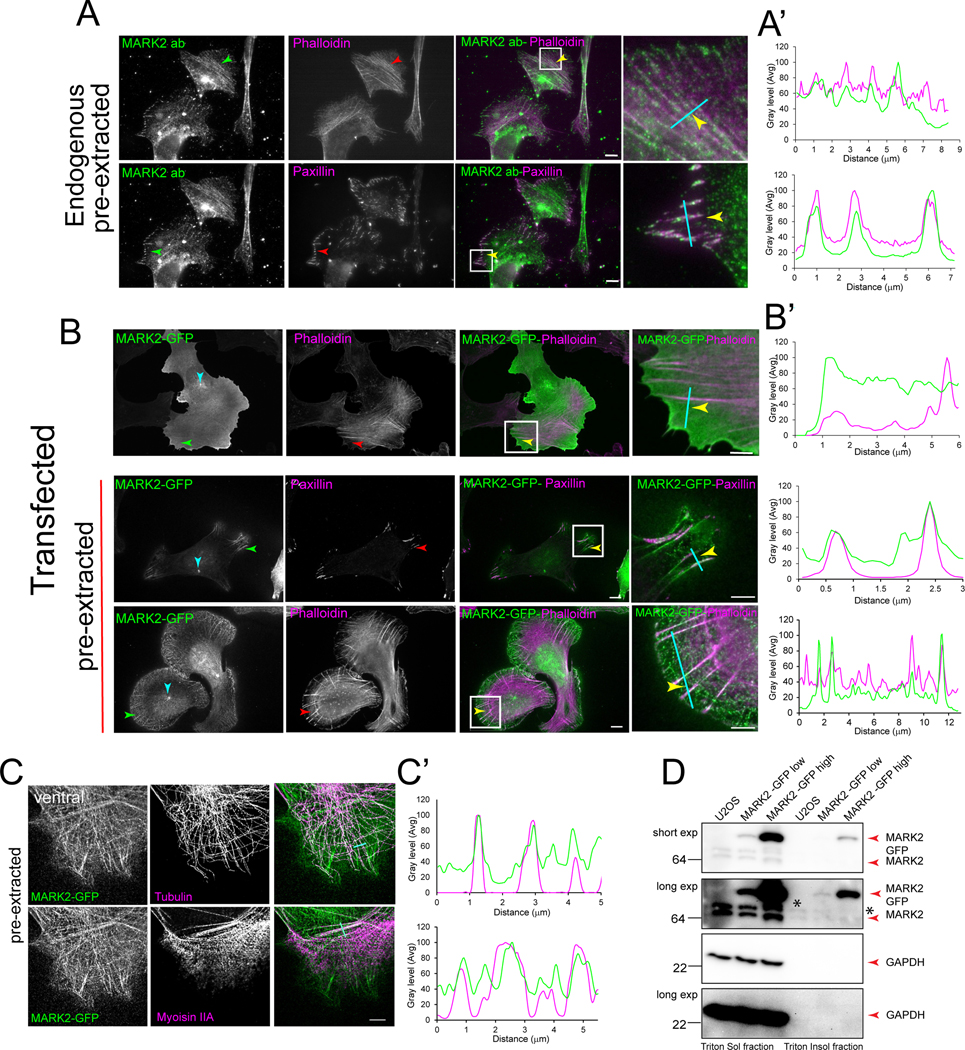
To define the role of MARK2 in cancer cell polarization and directed migration, we analyzed the localization of exogenously expressed tagged or endogenous MARK2 in U2OS human osteosarcoma cells. Following 8 hrs. of spreading on fibronectin, cells were either pre-extracted or not, fixed, stained, and imaged. This confirmed MARK2 localizations to the plasma membrane, MT cytoskeleton and MTOC33,38 in unextracted and extracted and immunostained cells, respectively (Figure 1B, C). Co-expression with soluble mCherry confirmed specific concentration of MARK2-GFP in cell protrusions (Figure S1A). Examination of MARK2-GFP in extracted cells revealed a filamentous localization reminiscent of the actomyosin cytoskeleton (Figure 1B, C, Figure S1B). Co-expression of MARK2-GFP and myosin IIA-TdTomato (Video S1) revealed a fraction of MARK2-GFP dynamically co-localized with myosin IIA filaments. Immunostaining of myosin IIA and tubulin or fluorescent phalloidin staining in cells expressing MARK2-GFP followed by line-scan analysis confirmed the co-localization of MARK2-GFP with stress fibers that were distinct from MTs (Figure 1B, B’, C, C’). 3D super-resolution imaging revealed preferential MARK2 accumulation on dorsal stress fibers (Figure S1B). Co-immunolocalization of MARK2 and the FA protein paxillin together with actin staining confirmed endogenous MARK2 on stress fibers and FA (Figure 1A, A’). Separation of triton-soluble (cytosolic) and -insoluble (actomyosin cytoskeleton) fractions of lysates of cells transfected with increasing MARK2-GFP demonstrated specific accumulation in the cytoskeletal fraction in an expression level-dependent manner (Figure 1D), with longer exposures of blots showing endogenous MARK2 in the cytoskeletal fraction (Figure 1D, asterisk). Thus, MARK2 not only associates with the plasma membrane at cell protrusions and the MT cytoskeleton as reported38,39, but also with the actomyosin cytoskeleton and FAs.
Figure 1. MARK2 localizes at the actomyosin cytoskeleton, MTs, and FAs.
Imaging (A-C) and western blot (D) of U2OS cells. (A) Pre-extracted cells were fixed and stained for F-actin (red), paxillin (green) and MARK2 (MARK2 ab, green). Arrows: stress fiber (upper row), FA (lower row). Cyan lines in (A-C) used for line scan analyses in (A’-C’). White boxes in (A,B) magnified at right. (B) Cells expressing MARK2-GFP (green) were pre-extracted (lower two rows) or not (upper row), fixed, and stained for paxillin (red) or F-actin (red). Arrows: cyan: centrosome, red: protrusion, green: FA, yellow: stress fiber. (C) Pre-extracted cells expressing MARK2-GFP (green) were fixed and stained for tubulin (red). Ventral (upper row) and dorsal (lower row) super-resolution confocal optical sections shown. Scale bars= 5μm (right center in A and B, right in C) and 2μm (right in A and B). (D) Western blot of triton soluble (cytosolic) and insoluble (actomyosin cytoskeleton) fractions from U2OS cell lysates transfected with different amounts (MARK2-GFP low or MARK2 GFP high) and probed for MARK2 (upper two panels) or GAPDH (lower two panels). Upper middle and lower panel, longer exposure (5 min). Asterisk: endogenous MARK2 in the triton insoluble fractions. See also Figure S1 and Video S1.
MARK2 is required for cell polarization and directed cell migration
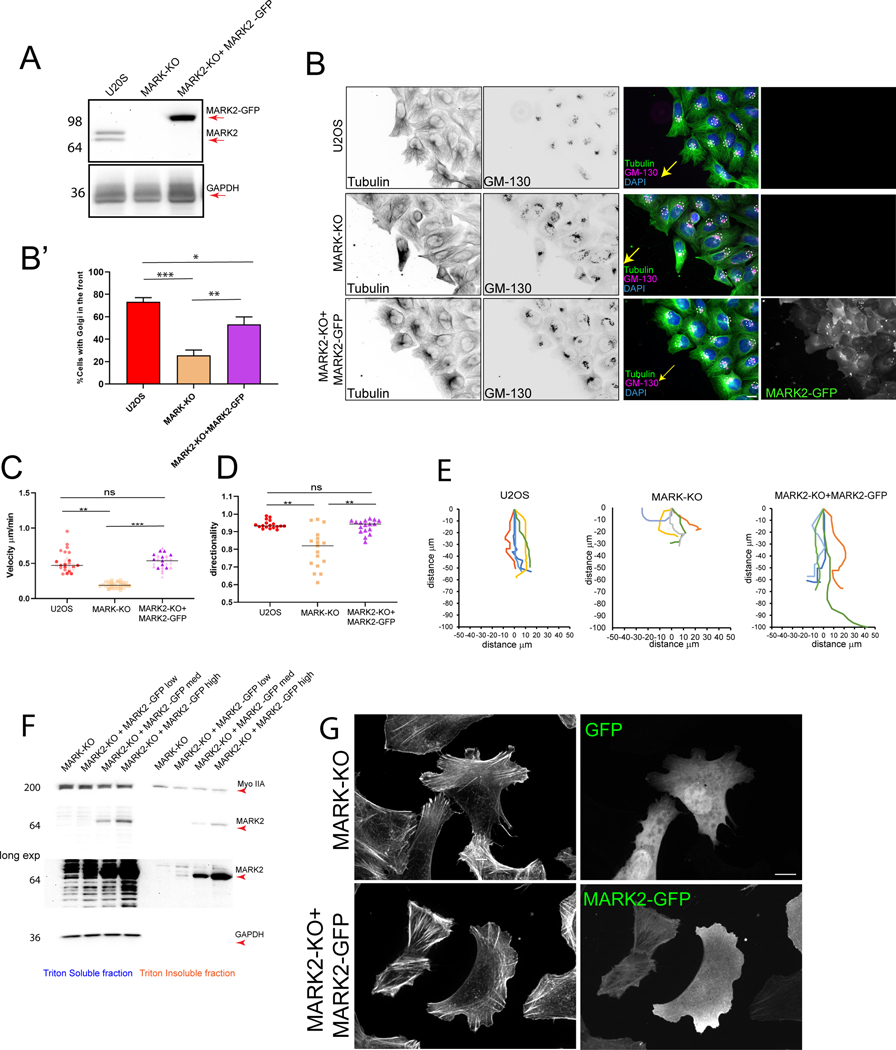
To determine how MARK2 contributes to cell polarization and migration, we used three different guide RNAs to generate several polyclonal U2OS cell lines with the MARK2 gene deleted by CRISPR-Cas9 (Figure S2A). One cell line lacking MARK2 expression was utilized for the remainder of the study (Figure 2A, Figure S2A; denoted MARK2-KO) and to make rescued cell lines re-expressing different levels of GFP- or mApple-MARK2 (Figure 2A, Figure S2B, B’, C, C’). Notably, re-expressed MARK2-GFP associated with similar structures as endogenous MARK2 in cells and cell lysates (Figure 2F, G). While deletion of MARK2 had no effect on MARK1 expression, it caused increased MARK3 expression (Figure S2D, E), in agreement with previous studies51–53. Thus, experiments using MARK2-KO cells were additionally depleted of MARK3 by siRNA (Figure S2F, G) and denoted as MARK-KO cells. Importantly, expression of MARK2-GFP in MARK2-KO caused MARK3 to return to low levels (Figure S2E, G), indicating that effects of rescue were not attributable to continued upregulation of MARK3, but likely due to the re-expression of MARK2, although the re-expression of low levels of MARK3 cannot be ruled out.
Figure 2. MARK2 is required for cell polarization and directional migration.
Imaging (B, C, D, E, G) and western blot (A, F) of U2OS cells (U2OS), U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing different levels of MARK2-GFP (low, medium or high). (A) Immunoblot of lysates probed for MARK2 (upper, red arrow) or GAPDH (Lower, red arrow). (B) Cell monolayer wound stained for DNA (DAPI, blue), tubulin (green), and GM-130 (Golgi, red)). Yellow arrow; direction of migration, white dotted circles: positions of Golgi. Scale bar= 10 μm. (B’) Fraction of wound-edge cells with Golgi positioned between nucleus and leading edge. n= 3 experiments, bars= SEM (C, D) Cell velocity (C) and directional persistence (D) during wound healing migration. Each point is one cell, different shades are different experiments n= 70 (U2OS), 42 (MARK-KO), 49 (MARK2-KO+MARK2-GFP) cells from 3 experiments, bar= mean (E) Cell trajectory plots over a 3 hour time-lapse movie. (F) Western blot of triton soluble (cytosolic) and insoluble (cytoskeleton) fractions from lysates probed for myosin IIA (MyoIIA, upper panel), MARK2 (middle two panels) or GAPDH (lower two panels). Lower middle: longer exposures (5 min). (G) Localization of GFP (upper right) or MARK2-GFP (lower right) and staining of F-actin (left). Scale bar= 5μm. In B’, C, D, *p<0.01, **p<0.005, ***p<0.0005 for unpaired T-test (B) or for Mann-Whitney test (C,D). See also Figure S2, Videos S2 and S3.
We validated known roles of MARK2 in cell polarization and migration38,54 for our genetically-modified U2OS cells. Analysis of Golgi orientation relative to the nucleus in cells migrating at the edge of wounded monolayers (Figure 2B, Figure S2H) showed that the majority of wild-type (WT) U2OS cells and MARK2-KO cells re-expressing MARK2-GFP oriented their Golgi between the nucleus and wound edge, while MARK-KO cells were significantly deficient (Figure 2B’). Time-lapse microscopy (Video S2) showed that depletion of MARK proteins caused a significant delay in wound closure due to a decrease in both cell migration speed and directional persistence when compared to WT U2OS and MARK2-KO cells re-expressing MARK2-GFP (Figure 2C, D, E). Similar defects were observed with a MARK2-KO cell line generated from a different guide RNA (Video S3) or after MARK2 knockdown by siRNA 38. These results confirm that MARK2 is required for cell polarization and rapid, directionally persistent migration, and establish the specificity of our genetically-modified cell line.
MARK2 regulates myosin II contractility by MYPT1 inactivation and direct phosphorylation of MRLC
We next sought to determine if MARK2 regulates actomyosin contractility. Fixation and immunostaining of cell is a wound-healing assay with phospho-specific antibodies to myosin II regulatory light chain phosphorylation on serine 19 (S19-pMRLC) as a proxy for myosin II activity (Figure 3A) showed that WT U2OS cells exhibited peripheral stress fibers and arcs behind the leading edge. In contrast, MARK-KO cells showed thin stress fibers with a reduced level of S19-pMRLC. Re-expression of GFP-MARK2 in MARK2-KO cells rescued thick peripheral actomyosin bundles. In addition, western blots showed that compared to WT U2OS cells, MARK-KO cells exhibited significantly lower S19-pMRLC, and re-expression of MARK2-GFP in MARK2-KO cells showed expression-level-dependent increases in S19-pMRLC (Figure 2B, B’; Figure S3B, B’). The effects of MARK2 depletion on S19-pMRLC levels observed in MARK-KO cells were validated by MARK2 siRNA (Figure S3A). Thus, MARK2 promotes stress fiber formation and activation of myosin II in migrating cancer cells.
Figure 3. MARK2 regulates myosin II contractility by direct phosphorylation of MRLC and indirect phosphorylation of MYPT1.
Imaging (A, G) and western blot (B, C, E, F, H, I) of U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing different levels of MARK2 GFP (low, medium or high). (A) Cell monolayer wound stained for F-actin (red) and myosin II regulatory light chain phosphorylated on serine 19 (S19-pMRLC). White boxes: magnified at right (ZOOM). Scale bars= 2μm. (B) Western blot of cell lysates probed for MYPT1 phosphorylated on threonine 696 (P-MYPT1 T696, upper panel), MARK2 (upper middle panel), S19-pMRLC, (lower middle panel), or tubulin (lower panel). (B’) Ratio of T696-pMYPT1 (upper panel) or S19-pMRLC (lower panel) to tubulin from n=4 experiments, bars = SEM *p<0.05, ***p<0.0005, ****p<0.0001, unpaired T-test. (C) Western blot of cell lysates (10% in put lysate) or anti-GFP immunoprecipitates (IP: GFP) from cells expressing GFP (GFP) or various levels of GFPMARK2, probed for GFP (WB: GFP) or myosin IIA (WB: MyoIIA). (D) Deconvoluted mass spectrum of MRLC peptide (no kinase, Control, top panel) and after phosphorylation with purified MLCK (middle panel) or MARK2 (bottom panel). Mass increase of 80Da indicates phosphorylation (red arrow, percent of total indicated). (E) Western blot of purified MRLC (no kinase, Control, left lane) or together with purified MLCK (middle lane) or MARK2 (right lane) and probed for S19-pMRLC. (F) Relative level of S19-pMRLC from dot blots of purified GST-tagged MRLC incubated with ATP and beads in untransfected Chinese Hamster ovary (CHO) cell lysates (BLK), or anti-GFP (GFP-Ab and MARK2-GFP-Ab) or IgG (GFP-Ctl and MARK2-GFP-Ctl) immunoprecipitates from CHO cells expressing either GFP (GFP-Ab and GFP-Ctl) or MARK2-GFP (MARK2-GFP-Ab or MARK2-GFP-Ctl) and probed for S19-pMRLC. n= 3 experiments, bars = SEM. (G) Localization of mApple-MARK2 (red) and staining of MYPT1 (green) and myosin IIA (red). White box: magnified (ZOOM) at right. Scale bars= 5 μm (upper right and lower left) and 2 μm (lower right). (H) Western blots of cell lysates (10% input lysate) or anti-GFP immunoprecipitates (IP: GFP) from cells expressing either GFP (GFP), mAppleMARK2, or GFP-MYPT1 and probed for MARK2 (WB: MARK2, upper and middle (longer exposure)) or MYPT1 (WB: MYPT1, lower panel). Asterisk: endogenous MARK2. (I) Relative level of T696-pMYPT1 from western blots of purified MYPT1 incubated with ATP and beads incubated lysates of untransfected Chinese Hamster ovary (CHO) cell lysates (BLK), or anti-GFP (GFP-Ab and MARK2-GFP-Ab) or (GFP-Ctl and MARK2-GFP-Ctl) immunoprecipitates from cells expressing either GFP (GFP-Ab and GFP-Ctl) or MARK2-GFP (MARK2-GFP-Ab or MARK2-GFP-Ctl) or incubated with purified ROCK and probed for T696-pMYPT1. n= 3 experiments, bars= SEM. See also Figure S3, Video S4.
We next sought to define the mechanism by which MARK2 promotes contractility. We first tested whether this occurred through MLCK or Rho-associated kinase (ROCK), which promotes contractility by phosphorylating both MRLC and the myosin phosphatase targeting subunit 1 (MYPT1) to inactivate myosin phosphatase 55–57. We found that serum starving U2OS cells caused a drop in S19-pMRLC which was further decreased by inhibition of ROCK with Y27632 and MLCK with ML-7. The substantial level (∼30%) of S19-pMRLC remaining after starvation and ROCK and MLCK inhibition was abolished by MARK-KO, and restored by re-expression of MARK2-GFP (Figure S3C, C’). Thus, MARK2 can promote S19S-pMRLC independently of ROCK and MLCK.
We next tested if MARK2 promotes contractility by phosphorylating MYPT147,58. Immunolocalization of MYPT1 in U2OS cells expressing mApple MARK2 (Figure 3G) or live imaging of MARK2-mApple and MYPT1-GFP (Video S4) showed that MYPT1 and MARK2 co-associated with actomyosin stress fibers. Immunoprecipitation with anti-GFP antibodies from lysates of MARK2-KO cells co-expressing mApple-MARK2 and GFP-MYPT1 revealed an association of GFP-MYPT1 with both mApple-MARK2 and endogenous MARK2 (Figure 3H). Western blot showed that MARK-KO cells displayed a significant decrease in MYPT1 phosphorylation at its regulatory threonine 696 (T696-pMYPT1) compared to WT U2OS, which was restored by re-expression of MARK2-GFP (Figure 3B, B’). Thus, MARK2 associates with and promotes MYPT1 phosphorylation to mediate inactivation of myosin phosphatase.
We then aimed to determine if MARK2-mediated phosphorylation of MYPT1 was direct or indirect. A previous report suggested that isolated MARK2 promotes MYPT1 phosphorylation59. We isolated MARK2-GFP from transfected COS1 cells by immunoprecipitation and tested its ability to phosphorylate MYPT1 in vitro. Because full-length recombinant MYPT1 is refractory to purification, we used a GST-fusion peptide consisting of amino acids 654-880 (termed MYPT1 654-880) that includes two critical phosphorylation sites (T696 and T853) and which adopts a conformation capable of inhibiting MLCP activity 60,61. However, phospho-dot blot analysis showed that immuno-isolated MARK2-GFP was unable to phosphorylate MYPT1 654-880 (Figure 3I). Similarly, we failed to detect phosphorylation of MYPT1 654-880 by purified MARK2 or MARK3 by both western blot and mass spectrometry (Figure S3D, D’, E, F). Further experiments showed that MARK2 isolated in a potassium ion buffer mimicking cytoplasmic conditions (Figure S3G, “K-Ms”) promoted T696 and T853 phosphorylation of MYPT1 654-880, while MARK2 isolated in stringent NaCl buffer did not, suggesting that MARK2 may associate by ionic interactions with a kinase that directly phosphorylates MYPT1 (Figure S3G). Importantly, our purified MARK proteins phosphorylated both ROCK and a peptide derived from the MARK phosphorylation sequence in cdc25, and our purified MYPT1 654-880 fragment was readily phosphorylated on T696 by ROCK (Figure 3I, Figure S3F). Thus, MARK2 interacts with MYPT1 and promotes its phosphorylation indirectly to mediate inactivation of myosin phosphatase.
Because PAR1b associates with myosin II in C. elegans46, we next tested whether its homologue MARK2 could interact with myosin II and phosphorylate MRLC to promote contractility. Immunoprecipitation from cell lysates showed that myosin IIA co-precipitated with MARK2-GFP (Figure 3C). Phospho-western blot and mass spectrometry confirmed that purified or immunoprecipitated MARK2 phosphorylated S19 of either MRLC or an MRLC peptide, although to a lesser extent than MLCK (Figure 3D, E, F). Together, these results show that MARK2 interacts with MYPT1 and myosin II and promotes myosin II contractility by indirect phosphorylation and inactivation of myosin phosphatase and direct phosphorylation of MRLC.
MARK2 localization at the plasma membrane regulates lamellipodium dynamics and promotes efficient leading edge protrusion
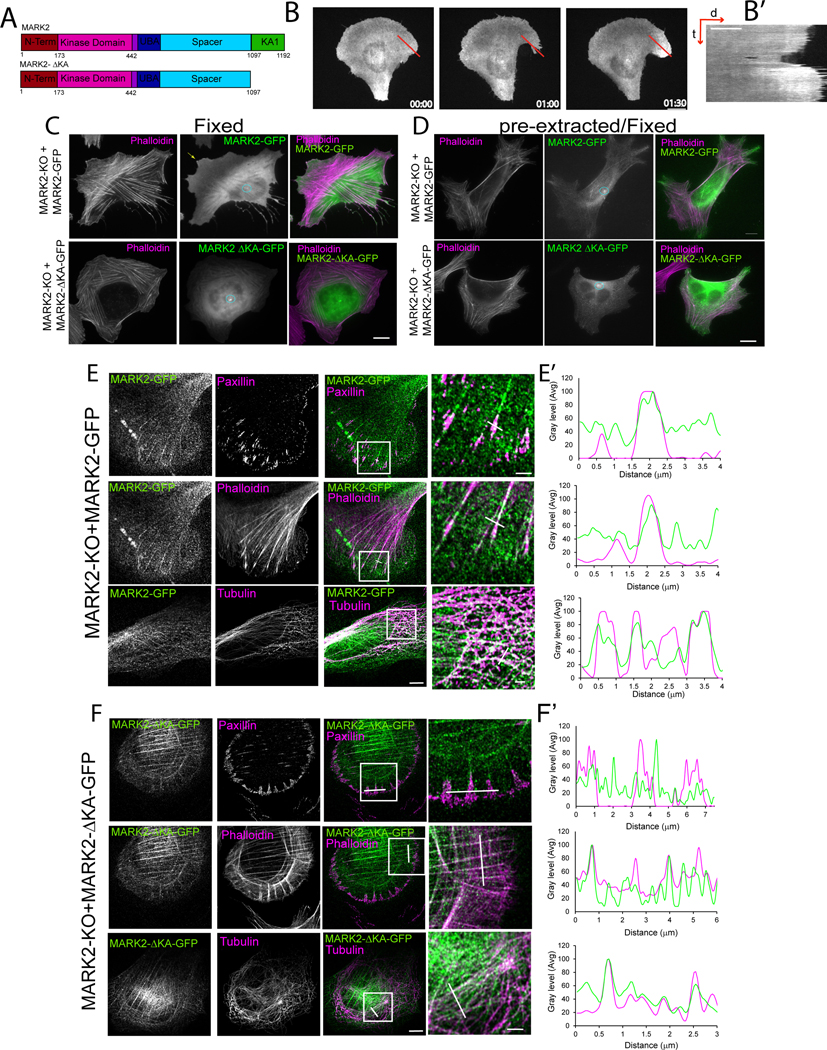
We next sought to determine the role of MARK2 localization to membrane protrusions in cell migration. We plated cells expressing MARK2-GFP on “crossbow” shaped fibronectin islands approximating the polarized shape of a migrating cell62 (Figure 4B, Figure S4). Time-lapse microscopy (Video S5) allowed analysis of MARK2 localization during cell edge protrusion-retraction cycles independent of leading edge advance or cell movement. Kymograph analysis showed that MARK2 was specifically concentrated in the protrusion that extended beyond the ECM island relative to the level in the bulk plasma membrane (Figure 4B,B’). This suggests that MARK2 in the plasma membrane may regulate lamellipodial protrusion.
Figure 4: MARK2 association with the plasma membrane is required for leading edge advance and FA localization.
Imaging and analyses of U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2-GFP lacking amino acids 1098-1192 (MARK2-ΔKAGFP). (A) Diagram of domains for MARK2 and MARK2-ΔKA lacking the KA1 membrane-targeting domain. (B) Time-lapse image series (time in min) and kymograph (B’) taken along the red line (right panel distance (d) and time (t)) of a cell expressing MARK2-GFP plated on a “crossbow”-shaped fibronectin island surrounded by non-adhesive substrate. Bar=10μm. (C-E) Localization of MARK2-GFP (C, D top, E) or MARK2-ΔKA-GFP (C, D, bottom, F) and staining of F-actin (C-F), paxillin (E, F) or tubulin (E, F) in fixed (C) or pre-extracted and fixed cells (D, E, F). White boxes: regions magnified at right (zoom), white lines: regions used for line scan analyses in (E’, F’). Circle and arrows in (C, D: MARK2 at the centrosome and a protrusion, respectively. Bars in (C, D, and E (left)= 5μm, (E, right)= 2μm. See also Figure S4, Video S5.
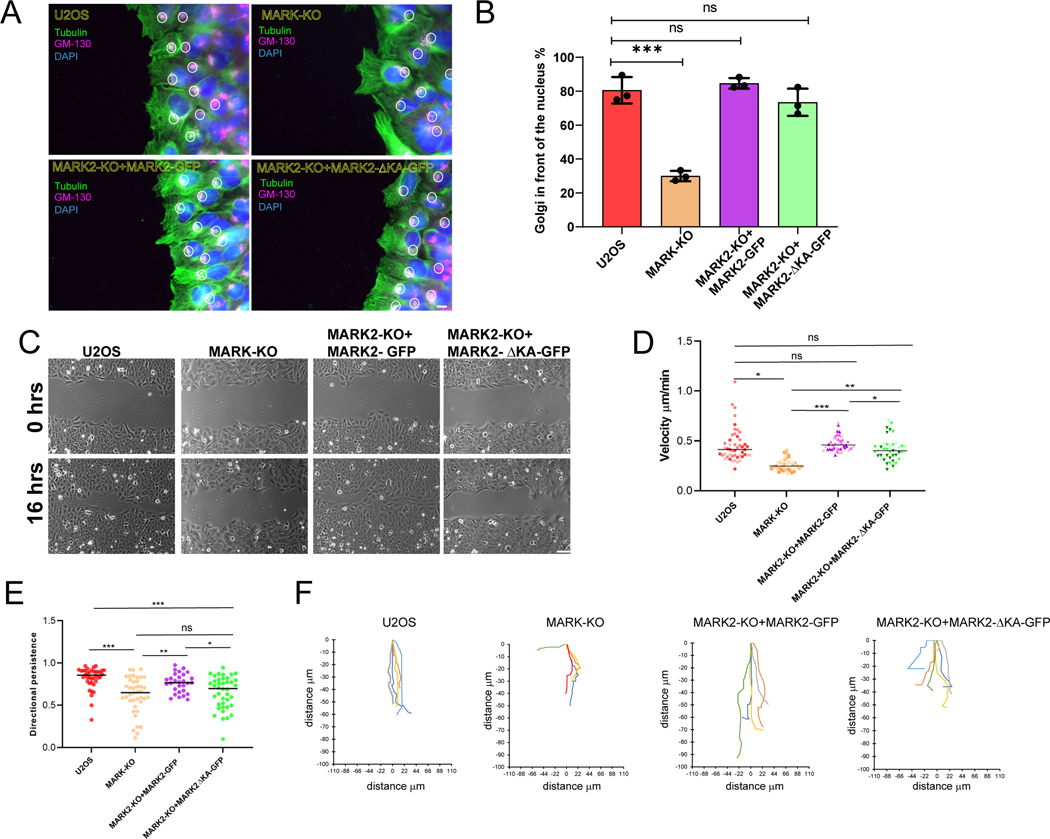
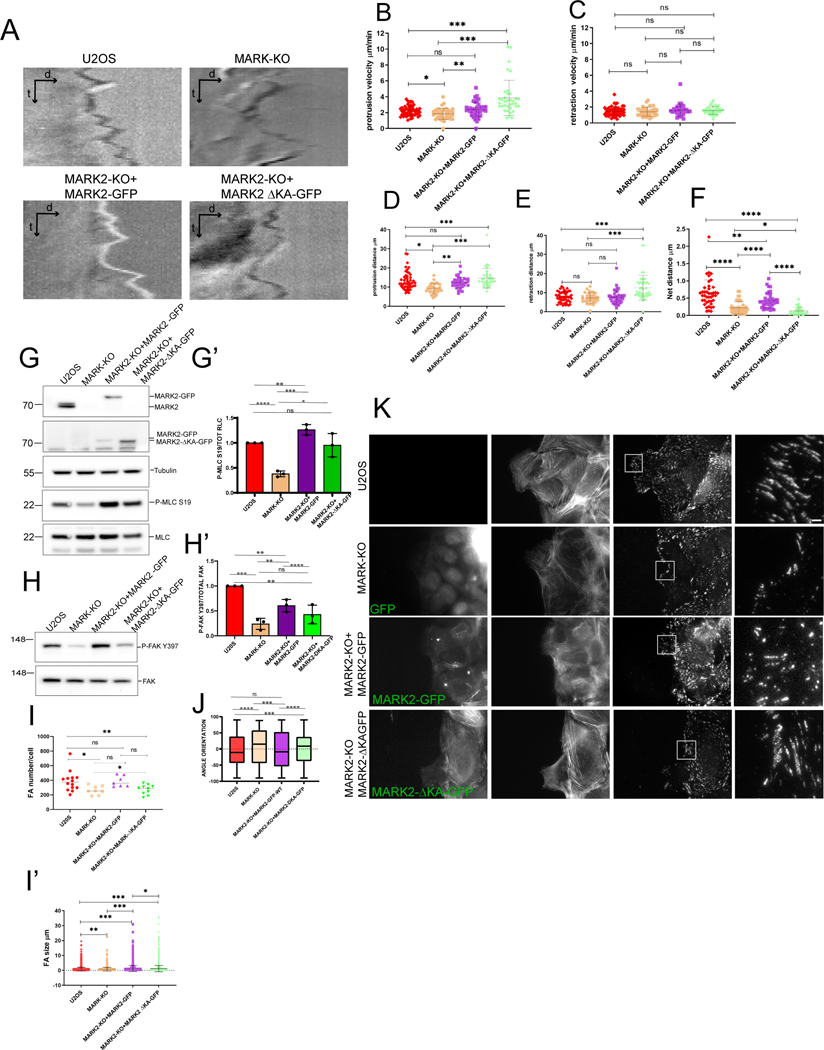
To determine the function of plasma membrane-association, we utilized a MARK2-GFP construct lacking the C-terminal amino acids 1097-1192 that encode the membrane-targetting KA1 domain48 (Figure 4A, termed MARK2ΔKA-GFP). When expressed in MARK2-KO cells, MARK2ΔKA-GFP associated with stress fibers, the centrosome, and MTs, but was strongly reduced in the plasma membrane, protrusions and FA compared to MARK2-GFP (Figure 4C-F). Reconstitution of MARK2-KO cells with either MARK2-GFP or MARK2ΔKA-GFP showed that both could restore polarization of the Golgi towards the leading edge that was lost upon MARK-KO (Figure 5A, B). However, time lapse microscopy showed that, unlike MARK2-GFP that restored deficits in wound closure and directional persistence caused by loss of MARK proteins, MARK2ΔKA-GFP was unable to rescue these defects (Figure 5 C-F, Video S6). Time-lapse DIC microscopy (Video S7) and kymograph analysis of cell edge dynamics in wound healing assays showed that WT U2OS and MARK2-KO cells re-expressing MARK2-GFP exhibited protrusion-retraction cycles that, over time, resulted in net advance of the cell edge (Figure 6A,F). In contrast, MARK-KO and MARK2-KO cells re-expressing MARK2ΔKA-GFP were unable to advance their leading edges (Figure 6A, F). Similarly, when MARK2-KO cells were plated on crossbow shaped fibronectin islands, protrusion of the cell beyond the island was inhibited (Video S5). Quantitative analysis showed that while protrusion distance was significantly farther than retraction distance in WT U2OS and MARK2-KO cells re-expressing MARK2-GFP, protrusion distance was reduced in MARK-KO cells, and reconstitution with MARK2ΔKA-GFP only partially restored protrusion and actually enhanced retraction (Figure 6 B-E). Therefore, MARK2 in the membrane and FA is not required for polarization of the Golgi relative to the nucleus, but promotes directionally persistent migration by enhancing cell protrusion to advance the leading edge.
Figure 5: MARK2 association with the plasma membrane is required for directional migration.
Imaging and analyses of U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2 GFP or MARK2 GFP lacking amino acids 1097-1192 (MARK2-ΔKAGFP). (A) Cells monolayer wound stained for DNA (DAPI, blue), tubulin (green) and GM-130 (Golgi, red)). Scale bar= 10μm. B) Fraction of wound-edge cells with Golgi positioned between nucleus and leading edge. n = 60 (U2OS), 50 (MARK-KO), 52 (MARK2-KO+MARK2-GFP), 67 (MARK2-KO+MARK2ΔKA-GFP) cells from 3 experiments, bars= SEM ***p<0.0005, unpaired T test. (C) Images taken immediately (0 hrs, upper row) and 16 hours after (16 hrs, lower row) monolayer wounding. Scale bar= 20μm (D, E) Cell velocity (D) or directional persistence (E) during wound healing migration. Each point is one cell, different shades are different experiments. n = 38 (U2OS), 40 (MARK-KO), 29 (MARK2-KO+MARK2-GFP), 40 (MARK2KO+MARK2ΔKA-GFP) cells from 3 experiments, bars= means, *p<0.01, **p<0.005, ***p<0.0005 Mann-Whitney (F) Cell trajectory plots over a 3 hour time-lapse movie. See also Video S6.
Figure 6: MARK2 association with the plasma membrane promotes FA density and orientation to mediate leading edge advance.
Imaging (A-F, I-K) and western blot (G-H’) of U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2-GFP lacking amino acids 1097-1192 (MARK2-ΔKA-GFP). (A) Kymographs generated from time-lapse DIC image series of the leading edges of cells migrating at the edge of a monolayer wound. (B-F) Leading edge dynamics from kymographs. Velocity of protrusion (B) or retraction (C) excursions; distance of protrusion (D) or retraction (E) excursion; (F) Net protrusion distance in a one minute interval. n= 2 kymographs per cell from n= 51 (U2OS), 37 (MARK-KO), 42 (MARK2-KO+MARK2-GFP), 36 (MARK2-KO+MARK2ΔKA-GFP) cells in 2 experiments, bars= means *p<0.01, **p<0.005, ***p<0.0005 ****p<0.0001, Mann-Whitney. (G, H) Western blot of cell lysates probed for MARK2 (G, top), GFP (G, top middle), tubulin (G, middle), myosin regulatory light chain phosphorylated on serine 19 (G, P-MRLC S19, bottom middle) myosin regulatory light chain (G, MRLC, bottom), FAK phosphorylated on tyrosine 397 (H, P-FAK Y397, top) or FAK (H, bottom). (G’, H’) Ratio of P-MRLC S19 to MRLC (G’) or P-FAK Y397 to FAK (H’). n=3 experiments, bars= SEM. (I) Number of FAs per cell (K) for cells migrating at the edge of a monolayer wound. n = 16 (U20S), 9 (MARK-KO), 9 (MARK2-KO+MARK2-GFP) or 10 (MARK2-KO+MARK2ΔKA-GFP) cells in 2 experiments, bars= SD. White box (K) is magnified (ZOOM) at right. Scale bars= 10 μm (left) and 2μm (right). (J) FA orientation relative to the direction of migration in a monolayer wound. n = 5422 (U20S), 2401 (MARK-KO), 3723 (MARK2-KO+MARK2-GFP) or 4041 (MARK2-KO+MARK2ΔKA-GFP) FAs in 2 experiments, box= interquartile range, bar= median, whiskers= upper and lower quartiles. See also Video S7.
MARK2 localization at the plasma membrane regulates FA number and orientation
We next sought to determine whether MARK2 in the plasma membrane regulates FA formation or organization. FAs in wound-edge U2OS and MARK2-KO cells expressing MARK2-GFP and mCherry-paxillin showed a range of sizes, with nascent FA at the cell edge and mature FA that were largely oriented parallel to the presumed direction of migration (perpendicular to the wound) (Figure 6I, J, K). In contrast, in MARK-KO cells and MARK2-KO cells expressing MARK2ΔKA-GFP, FAs were less numerous and spanned a broader range of orientations relative to the direction of migration (Figure 6I, J, K). Although FA maturation and orientation are controlled by myosin II contractility63–65, western blot analysis showed that the KA domain of MARK2 was not required for restoring the deficit in pS19-MRLC caused by MARK-KO, indicating MARK2’s role in FA organization was independent of its effects on contractility (Figure 6G, G’). In contrast, analysis of FAK phosphorylation on Y397 (pFAK) as a measure of FA signaling showed that depletion of MARK proteins caused a reduction in pFAK that was not restored by expression of MARK2ΔKA-GFP (Figure 6H, H’). Thus, MARK2 in the plasma membrane promotes FAK phosphorylation to enhance FA number and orientation, and thereby mediate stable advance of the leading edge to drive directionally persistent migration.
DISCUSSION
Here we discovered new roles for the MT regulator and polarity protein MARK2 in actomyosin contractility and FA organization in migrating human cancer cells. MARK2 and its homologues have well-characterized roles in polarization of neurons24,33,66,67, epithelial and immune cells68 this is thought to be mediated by its effects on microtubules. Although previous studies suggested that MARK2 homologs in invertebrates may regulate contractility to drive embryonic polarity 69,70 and oogenesis47, it is generally thought that ROCK, MRCK and MLCK are the major regulators of contractility in migrating cancer cells. We found in human osteosarcoma cells that in addition to the MT cytoskeleton and plasma membrane, MARK2 also associates with the actomyosin cytoskeleton and promotes myosin II activation and stress fiber formation independently of ROCK and MLCK. We showed that MARK2 associates with and mediates phosphorylation of both myosin II and MYPT1 to synergistically activate contractility in cells. Our in vitro studies revealed that MARK2 directly phosphorylates MRLC, while its effects on MYPT1 phosphorylation are indirect, likely by scaffolding an associated kinase. We additionally discovered that membrane targeting of MARK2 is not required for Golgi orientation relative to the nucleus, yet is necessary for directional migration. This upends the notion that Golgi orientation is required for directed migration71,72. Instead, membrane-associated MARK2 specifically enhances cell protrusion by targeting MARK2 to FA where it promotes FAK phosphorylation and formation of FAs oriented in the direction of migration. This shows that even if the MT cytoskeleton and secretory apparatus are oriented, the cell cannot migrate directionally without getting it’s FAs pointed in the right direction, and MARK2 in the plasma membrane is critical for this function. Together, our results define MARK2 as a master regulator of the MT and actomyosin cytoskeletons and FAs to mediate directional migration of cancer cells.
It is well documented that MARK2 is membrane-associated, localizing basolaterally in polarized epithelia and concentrating in protrusions of migrating cells38,50,66,73. We showed here that MARK2 localization at the plasma membrane is required to stabilize lamellipodia protrusion. One possibilty is that MARK2 in membrane protrusions could phosphorylate and inactivate GEF-H174, resulting in RhoA inactivation, as RhoA is required for the protrusion phase of leading edge protrusion-retraction cycles75,76. However, we also found that membrane-association targets MARK2 to FAs and promotes FAK activation and FA formation and alignment with the axis of migration to steer motility77. Since FAK activation is mediated by tyrosine phosphorylation and MARK2 is a serine-threonine kinase, MARK-mediated activation of FAK must be indirect. One possibility is that MARK2 may function to locally initiate myosin II contractility in membrane-associated nascent FA78,79, which would serve both to mechanically activate FAK downstream of integrin engagement and to promote FA maturation. MARK2 promotion of local contractility would also serve to orient FAs, since the direction of myosin IIdriven actin retrograde flow is what defines the axis of FA growth during FA maturation64,80,81. These possible mechanisms for MARK2 functions at the plasma membrane will be interesting to test in the future.
Our identification of MARK2 as a regulator of the contractile and adhesive machinery in guiding directional osteosarcoma cell migration suggests that MARK2 could be a critical regulator of metastasis. In cancer models, metastatic cells often migrate out of the tumor as clusters undergoing collective migration 82–86, similar to the wound-healing assays we used in this study. Collective migration is thought to be driven by high actomyosin contractility at cell-matrix contacts combined with less activity at the cell-cell junctions87,88. Indeed, border cell migration, a model of collective migration in the Drosophila egg chamber, is mediated by polarized activation of myosin II within the cell cluster which is regulated by Par141 emphasizing the role of MARK kinases in asymmetrical activation of myosin II as one of the main contributors to collective cell behavior. Whether MARK2 is required for metastasis of single or clusters of cells remains to be determined.
STAR★METHODS
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Clare Waterman (watermancm@nhlbi.nih.gov).
Materials Availability
U2OS-MARK2-KO, U2OS-MARK2-KO-MARK2, U2OS-MARK2-KO-MARK2-Δ-KA-GFP and U2OS-MARK-KO-MARK2-mApple-add-back cell lines generated in this study as well as plasmids corresponding to MARK2-GFP, MARK2-mApple, MARK2-Δ-KA-GFP are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary files. Raw data will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Subject details
U2OS cells (ATCC, HTB-96, Female) obtained from (American Type Culture Collection, Manassas, VA) were cultured in McCoy’s 5A media supplemented with 10% FBS (Atlanta Biological, Norcross, GA) and 1x penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37 C in 5% CO2 and grown in 10 cm cell culture Petri dishes. MARK2-KO polyclonal cell lines were cultured in McCoy’s 5A media supplemented with 10% FBS and 1x penicillin-streptomycin at 37 C in 5% CO2 and grown in 10 cm cell culture Petri dishes. Stable transfected cell lines GFP, and MARK2-GFP, GFP-MARK2-ΔKA-GFP or MARK2-mApple, add add backs were cultured in McCoy’s 5A media supplemented with 10% FBS and 1x penicillin-streptomycin at 37 C in 5% CO2 and grown in 10 cm cell culture Petri dishes. All cells were frozen in 10% DMSO in FBS at −80 C for a week, and then transferred to liquid Nitrogen for preservation. For live imaging, the McCoy’s 5A medium no phenol red, was supplemented with 25 mM HEPES pH=7.2 and oxyrase 300 μm/ml media (Oxyrase Inc., Mansfield, OH).
Method Details
Plasmids
Plasmids generated in this study are available from the lead contact upon request. The cDNA constructs used in this study were as follows. MARK2-GFP and MARK2-mApple were described in38. Myosin IIA-TdTomato was the kind gift of Michael Davidson (Florida State). GFP-MYPT1 vector was constructed using pRK5-GFP vector (provided by Ian Macara at University of Virginia) and human MYPT1 full length cDNA, described previously 56. Deletion of the MARK2 KA1 domain was done by site-directed mutagenesis of MARK-GFP by introducing a stop codon in place of T642, using QuikChange II XL (200521; Agilent Technologies, Wilmington, DE). GFP and mCherry were obtained from CloneTek. Myc-tagged MYPT1 was generated by amplifying the MYPT1 fragment from a HeLa cell cDNA library by PCR ligation into a pKmyc vector (provided by Ian Macara, Vanderbilt University). All sequences were confirmed.
The cDNA constructs used in this study were as follows. MARK2-GFP and MARK2-mApple were described in38. Myosin IIA-TdTomato was the kind gift of the late Michael Davidson (Florida State). GFP-MYPT1 vector was constructed using pRK5-GFP vector (provided by Ian Macara at University of Virginia) and human MYPT1 full length cDNA, described previously 56. Deletion of the MARK2 KA1 domain was done by site-directed mutagenesis of MARK-GFP by introducing a stop codon in place of T642, using QuikChange II XL (200521; Agilent Technologies, Wilmington, DE). GFP and mCherry were obtained from CloneTek. Myc-tagged MYPT1 was generated by amplifying the MYPT1 fragment from a HeLa cell cDNA library by PCR ligation into a pKmyc vector (provided by Ian Macara, Vanderbilt University). All sequences were confirmed.
Crossbow micro-patterns
Cross-bow (60 μm2) patterned ECM substrates were created as previously described89 using the Primo system (Alveole, Paris). Alexa 647-labeled fibronectin was created by combining 1 mg Alexa Fluor 647 NHS Succinimidyl Ester (Cat #A20006, ThermoFisher) with 2 mg human plasma fibronectin (FC010; Millipore) in 1 ml PBS with stirring at room temperature for 2 hours. Unincorporated Alexa label was removed by extensive dialysis against PBS at 4C. Labeled fibronectin was used to adsorb to cleared micropatterns at 10 mg/ml for 5 minutes.
CRISPR-Cas9 knock-out
Stable polyclonal MARK2 knock-out cell lines were generated using CRISPR-cas9 technology. In brief, U2OS cells were transfected by electroporation using Solution V from Amaxa nucleofection (Lonza, Walkersville, MD) with a vector (pSpCas9 (BB)-2A-Puro (PX459) V2.0; 62988; Addgene, Cambridge, MA)90 containing an sgRNA guides targeting the second exon of the human MARK2 gene (https://zlab.bio/guide-design-resources), either MARK2-KO Guide 1 (GGAGCCGGTAGTTTCCAATG ) MARK2-KO Guide2 (GTGTCGGGCCAACTTCACCT) or MARK2-KO Guide 3(AGCCCCACATTGGAAACTAC). Transfected cells were subjected to puromycin selection (48h in 2 μg/ml puromycin (Life Technologies, Carlsbad, CA). Surviving cells were expanded and pools of cells that had been transfected with each sgRNA were collected, analyzed for the level of MARK2 by western blot, and propagated as polyclonal lines. A line generated from MARK2-KO Guide1 was used for the bulk of the experiments in this study, although the effects of MARK2 knockout on monolayer wound healing were also examined in a line generated from MARK2-KO Guide 2. Since MARK2-KO resulted in compensatory MARK3 overexpression, for experiments, MARK3 expression was inhibited in MARK2-KO by transfecting cells with 500 nM MARK3 smart pool siRNA (Dharmacon/horizon) for 48 hrs. Briefly, 1 ×10 6 U2OS cells were transfected with 500 nm of MARK3 on target smart pool SiRNA, for 24 and 48 hrs. We found MARK3 protein expression was reduced more than 80% by 48 hrs and we named this MARK-KO cells.
MARK2 rescued cell lines
For stable expression of MARK2-GFP constructs in MARK2-KO cells, cells were transfected with 0.8 mg of MARK2-GFP, MARK2-mApple, or GFP-MARK2-ΔKA-GFP using Amaxa nucleofector Solution V and 24 hrs later transfected cells were incubated in selection media (McCoy’s 5A media supplemented with 10% FBS, 1x Penicillin/streptomycin, and 1 mg/ml G-418 (geneticin, Thermo Fischer). Cell death was monitored under the microscope until cell colonies started growing again, in approximately seven days. Polyclonal cell lines expressing specific ranges of fluorescently-tagged MARK2 or its ΔKA variant were obtained by FACS gating and sorting for low, medium and high levels of expression of the fluorescent protein, with untransfected U2OS cells used as a control.
Wound healing and migration
For cell migration experiments in a scratch wound, cells were plated on 15 μg/ml fibronectin-coated plates (Millipore FC010-10MG) at 80% confluency. The next day, cells already grown to confluency were wounded by generating a longitudinal scratch through the monolayer using a 10 μl pipette tip and washed 2 times with McCoy 5A media to remove any cell debris and returned to the incubator at 37°C in 5% CO2 for 3h to recover. After recovery, time-lapse images were acquired by phase-contrast microscopy every 10 min for 18 hrs (Figure 5). For immunofluorescence, cells were fixed 4 hrs after wounding in (4% PFA in Cytoskeleton Buffer (CB; 10 mM MES 6.1, 138 mM KCl, 3mM MgCl, 2 mM EGTA).
Immunofluorescence
U2OS cells plated on fibronectin-coated (15μg/ml) coverslip were either pre-extracted in 0.25% paraformaldehyde, PFA (16% stock solution Electron Microscopy Science) and 0.05% triton in cytoskeleton buffer (CB; 10 mM MES 6.1, 138 mM KCl, 3mM MgCl, 2 mM EGTA) for 1 min at 37°C, and fixed in 4% PFA in CB for 20 min, or fixed in 4% PFA in CB for 20 min at 37°C. After fixation, coverslips were permeabilized in 0.5% Triton X-100 in CB for 5 min, free aldehydes were reacted with 100 mM glycine, washed in TBS, and blocked in blocking solution (2% BSA TBS-T) for 1 hr. Cells were incubated with primary antibodies (anti-MARK2 (1:250, Abcam), anti-tubulin DM1A (1:500, Sigma), anti-GM130 (1:500 Cell Signaling), anti-S19-PMRLC (1:200, Cell Signaling), anti-myosin IIA (1:400; Sigma Aldrich,), anti-paxillin (1:500; BD Biosciences, San Jose, CA) or anti-MYPT1 (1:250, Abcam) diluted in blocking solution, washed 3 times 10 min each in TBS-T and then incubated with fluorophore-conjugated secondary antibodies (1:400; Jackson ImmunoResearch Laboratories) and Alexa Fluor 488 or 568 phalloidin (1:400; Invitrogen), washed again and mounted on slides with mounting media (Dako, Pathology Products, Carpinteria, CA), or in TBS supplemented with n-propylgalate for TIRF imaging.
Western Blot
Cells lysates were obtained by rinsing the cells with PBS and then scraping them into 2X Laemmli sample buffer ( 65.8 mM Tris-HCl, pH 6.8, 26.3% (w/v) glycerol,2.1% SDS 0.01% and 355mM 2-mercaptoethanol), separated on 4–12% or 4–20% SDS-PAGE gels (Invitrogen) in running buffer (250 mM Tris-Base, 192.4 mM Glycine, 0.1% SDS) at 100 V and then electro-transferred to immobilon-P membranes at 25 V for 2 hrs for proteins smaller than 150 kDa, and overnight at 15 V at 4° C for proteins bigger than 150 kDa . After transfer, membranes were blocked with 5% (w/v) nonfat dry milk in TBS (50mM Tris-HCl, 150 mM NaCl) Tween 0.1% (v/v)Tween-20 (TBS-T) for 1 hr and incubated with respective primary and secondary antibodies labeled with HRP in blocking buffer (TBS-T+ 5% nonfat milk). When HRP-conjugated secondary antibodies visualized by chemiluminescence using Radiance ECL as an HRP substrate (Azure Biosystems, Dublin, CA,). The antibodies and dilutions used for western blotting in this study were: anti-MARK1 (1:1000 Cell Signaling), anti-MARK3 (1:1000 Cell Signaling), anti-MARK2 tail domain (1:1000, Abcam), anti-GFP (1:2000 Abcam), anti-mCherry (1:1000, Abcam), anti-MRLC (1:1000, Sigma), anti-FAK (1:1000, Millipore), anti-Y397-PFAK (1:1000 Thermo Fisher), anti-chicken T850 PMYPT1 (1:500, clone sa19, Millipore), anti-human T853 pMYPT1 (1:1000 Cell Signaling), anti-tubulin DM1A clone (1:3,000, Sigma), anti-GAPDH (1:5,000, Cell Signaling) anti-Myosin IIA ( 1:500, Sigma), anti S19-pMRLC (1:500 Cell Signaling), anti-T696-pMYPT1 (1:1000 Millipore), and anti-MYPT1 (1:500, Millipore). Secondary antibodies: HRP-conjugated Donkey anti-mouse or anti-rabbit were used at a dilution 1:10 000, in blocking buffer (Jackson ImmunoResearch). Quantification of western blots was performed from digital images of with local background subtraction adjacent to bands of interest using Metamorph software (Molecular Devices).
Immunoprecipitation
Immunoprecipitation assays were performed either by GFP-Trap (Chromotek.com) or using antibodies to myc tag (Cell signaling) or myosin IIA (Sigma). GFP-Trap precipitations were done according to manufacturer’s instructions. Briefly, for each immunoprecipitation reaction, we used 106 to 107 U2OS cells expressing GFP-tagged proteins (GFP-MYPT1 or GFP-MARK2). To obtain cell lysates, culture media was aspirated and cells were rinsed twice in cold PBS, cells were then scraped into 500 μl of cold PBS and transferred to a precooled 1.7 ml tube, spun at 500g for 3 min at 4°C. The pellet was washed twice with 500 ml of ice-cold PBS and gently resuspended in 200 μl ice-cold lysis buffer (10 mM Tris/Cl pH 7.5, 150 mM NaCl; 0.5mM EDTA; 0.5% NP-40, 0.02% Thimerosal) by extensive pipetting (30 min on ice) or using a syringe. Cell lysates (supernatant) were obtained by centrifugation at 20,000x G for 10 min at 4°C and supplemented with 1 mM PMSF and phosphatase (PhosStOP) and protease inhibitors (cOmplete). For myosin IIA immonoprecipitates, 500 μg of antibodies were incubated with cell lysates overnight with rotational agitation at 4°C. Magnetic GFP-TRAP or protein A beads were then added and incubated for 1 hr at 4°C. Beads were then magnetically separated until supernatant was clear, washed with cold-PBS. Then beads were resuspended in 100 μl 2X sample buffer, boiled for 10 min at 95°C to dissociate immunocomplexes. Samples were analyzed by Western blotting using the appropriate antibodies.
Fractionation of Triton soluble and insoluble cytoskeleton.
Actomyosin cytoskeleton and soluble fractions were obtained as previously described91. U2OS or MARK2-GFP transfected cells were plated on fibronectin-coated cell culture dishes (15 μg/ml), cultured for 18 hours, and lysed in TIF buffer (50 mM Tris-HCl, pH 7.4, 5 mM NaCl, 140 mM potassium-acetate, 0.6% Triton X-100, 1 mM EDTA, 5 mM EGTA, 5 mM DTT) supplemented with phosphatase inhibitor and complete protease inhibitor cocktails (Roche, Indianapolis, IN). Lysates were incubated at 4°C to depolymerize microtubules and then soluble and insoluble cytoskeleton fractions were separated by centrifugation at 8,000×g for 15 min at 4°C. The supernatant (Triton X-100 soluble fraction) and pellet (Triton X-100 insoluble fraction) were resuspended in equal volumes 2X Laemmli sample buffer and analyzed by western blot.
Immuno-kinase assay
COS1 cells were purchased from ATCC and grown in DMEM (high glucose, Gibco) supplemented with 5% FBS (Atlanta Biologicals). COS1 cells in a 10 cm dish were transiently transfected for 24-36 h with 5 μg of DNA vector using XtremeGene 9 (Roche) transfection reagent. For the immuno-kinase assay with GFP-tagged MARK2 proteins, transient transfection was conducted using the expression vector for GFP alone or the GFP-MARK2-WT protein. Cells were lysed with a “high-stringency lysis buffer” including 0.2 M NaCl, 50 mM Tris-HCl (pH 8.0), 1.0 mM EGTA, 1 % Triton-X100, 0.4 mM Pefabloc™ and 0.5mM TCEP, and then the lysates were subjected to immunoprecipitation using anti-GFP antibody and PrecipHen beads (Aves Lab, #GFP-1020 and P-1010). The beads were washed 3 times with the high stringency lysis buffer and the once with the kinase assay buffer. Phospho-dot blotting with each immunocomplex was conducted using isolated MRLC or GST-MYPT1(654-880) as described above. Ab and Ctl indicate immunoprecipitation with and without anti-GFP, respectively. BLK indicates blank beads treated with cell lysates without transfection (Figure S3E) Immuno-kinase assay was conducted using immune-complexes of recombinant H6S-MARK2 prepared under two different conditions. The H6S-MARK2 WT were transiently expressed for 36 h in COS1 cells. Cells were lysed for 15 min on ice with two different lysis buffer indicated as “K-Ms” and ”NaCl”. The K-Ms buffer includes 73.4mM potassium methane sulfonic acid (K-Ms), 30 mM PIPES (pH7.1), 7.35mM Mg-Ms, 1mM EGTA and pH 7.1. The K-Ms buffer mimics cytoplasmic conditions and was optimized for myography of skinned smooth muscle strips. The conventional NaCl buffer includes 100mM NaCl, 50mM MOPS-NaOH (pH7.0), 1mM EGTA, and 5% glycerol. Both buffers were supplemented with 0.1% Tween20, 1μM microcystin-LR, 0.3mM sodium vanadate (NaVi), 0.5mM TCEP and 4mM Pefabloc™. The suspensions were clarified by centrifugation and the extracts were subjected to pulldown using S-protein agarose beads (30μL of 50% slurry). After washing three times with each lysis buffer, and then once with the kinase assay buffer (10mM Mg(OAc)2, 25mM MOPS-NaOH (pH7.0), 1mM TCEP, 4mM Pefabloc, and 1μM MCLR. The kinase assay buffer (50μL) including 0.4mg/mL native myosin (chicken gizzard) and 0.1mg/mL GST-MYPT1(654-880) was added to the beads. Phosphorylation was initiated by adding 5mM ATP. The mixture was incubated for 30 min at 30°C and the reaction was terminated by adding 2x Laemmli buffer. Western blotting was conducted using antibodies indicated. H6S-MARK2 was detected using anti-6xHis (Qiagen #34650) and GST-MYPT1 was detected using anti-GST92.
In vitro phosphorylation assay
In vitro phosphorylation assays with purified MARK2 or MARK3 were carried out in buffer containing 10 mM MOPS pH 7.2, 50 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 0.2 mM CaCl2, 1xPhosStop (Roche), 0.2 mM ATP for 1.5h at room temperature. In vitro phosphorylation assays with purified MLCK were carried out in buffer containing 10 mM MOPS pH 7.2, 50 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 1xPhosStop (Roche), 0.2 mM ATP, and 0.1 μM calmodulin for 1.5h at room temperature. Control reactions lacked the kinase. Final protein and peptide concentrations in the assays were as follows: MYPT1: (fragment 654-880, Millipore): 1 μM; His-MRLC: 2 μM (prepared as described in 93; MARK2 (SignalChem): 0.4-10 μg/ml; MARK3 (SignalChem): 0.4-10 μg/ml; MLCK (SignalChem): 4 μg/ml; ROCK2 (SignalChem): 0.4-4 μg/ml; CHKtide (SignalChem): 0.1 mg/ml; MRLC peptide (SignalChem): 0.4 mg/ml. The phosphorylation reaction was stopped by either flash freezing the samples in liquid nitrogen followed by the addition of SDS loading dye and boiling of the sample or the addition of 5% acetonitrile and 0.05% trifluoroacetic acid. The extent of phosphorylation was determined by western blot analysis or intact protein HPLC-mass spectrometry as described before94.
Microscopy and Image Analysis
Living and fixed and immunostained cells plated on coverslips were imaged by epifluorescence or DIC microscopy on a Nikon TE-2000 microscope system using a 60X 1.4 NA Plan Apo DIC objective lens. For superreresolution confocal imaging of immunostained samples, Zeiss Airyscan imaging was performed with a 63× 1.4 NA objective. Raw data was processed using default Airyscan processing settings in Zen software. For wound healing cell migration experiments, time-lapse microscopy was performed at 37°C using an inverted microscope (model Eclipse Ti-E Nikon), and phase-contrast images were acquired every 10 min for 20 hrs using 10X 0.3 NA Plan Fluor objective lens and a 0.52 NA LWD condenser using MetaMorph’s Multi-dimensional Acquisition (MDA) software module. Fluorescent live-cell imaging was performed at 37 °C using McCoy media without phenol red and supplemented with 10% FBS, 25 mM HEPES. Images were acquired with CoolSnap HQ2 cooled CCDs (Photometrics) and all microscope functions were controlled using Elements software. Stage temperature was controlled using an airstream incubator.
Line Scan analysis
Line scan analysis was performed by averaging over a 10 pixel wide line and normalizing to the maximal value using Metamorph software (Molecular Devices).
Cell polarity analysis
For cell polarity analysis in wound healing experiments, images of fixed cells stained for MTs with DM1A antibody (anti-α-tubulin ), anti GM-130 antibody ( anti-Golgi marker) and DNA (DAPI) were aligned with the wound parallel to the image x axis and lines were plotted parallel to the x axis to bisect each nucleus of cells at the wound edge38. GM-130 + staining at 90 +/− 45 degrees perpendicular to the front of the nucleus was considered “oriented” to the front.
Cell migration assays
Cell migration in wound healing assays was quantified by hand-tracking the nucleus in time-lapse phase-contrast image series using the “track points” function in MetaMorph. The instantaneous velocity was calculated as the total displacement (μm) summed over consecutive frames divided by the elapsed time, directional persistence was calculated using the DiPer cell migration macro for Excel 95.
Kymographs
Kymographs from time-lapse DIC or fluorescence image series were generated and analyzed as described75. Measurements describing leading edge dynamics velocities and distances were obtained from kymographs of DIC movies by calculating the slope and length of each protrusion and retraction excursion. To obtain the data, kymographs were obtained from at least 5 cells per position in at least 10 positions per wound per condition. Each experiment was repeated 2 times. Net edge advance was calculated by measuring the distance between the start of the first protrusion at the beginning of the kymograph to the end of the last retraction in the kymograph.
Focal adhesion Analysis
For imaging of FA in a wound edge, cells were fixed 4 hrs. after wounding and FA were immunostained for paxillin as a FA marker. FAs were imaged using a 60× 1.49 NA Apo TIRF, FAs were segmented by subtracting the background and thresholding as previously described79. To analyze FA number and orientation, images were cropped to obtain only the first row of cells using phase contrast images to obtain the mask, and focal adhesion were segmented by background subtraction. FA angle orientation was obtained from image analysis software using the Integrated Morphometry Analysis package in MetaMorph software.
Quantification and Statistical Analysis
Statistical analyses were performed in Prism 9.0.2 (GraphPad). Data were analyzed with one-way analysis of variance (Tukey-Kramer test) , Non parametric t-test (Mann-Whitney) unless otherwise stated big data was ploted as superplot 96. A p value < 0.05 was considered statistically significant. *** signifies significance at p < 0.001; ** signifies significance at p value of 0.001 to 0.01, and * signifies significance at p value of 0.01 to 0.05. Data are presented as the mean ± S.D
Supplementary Material
Video S1: MARK2 colocalizes with Myosin IIA, related to Figure 1.
U2OS cell expressing MARK2-GFP and myosin IIA-TdTomato imaged by spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S2: CRSPR-mediate knockout of MARK2 demonstrates that it is required for efficient directional migration, related to Figure 2.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S3: CRSPR-mediated knockout or siRNA knockdown of MARK2 demonstrates that it is required for efficient directional migration, related to Figure 2.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (using a different guide RNA than that used in Video S2) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S4: MARK2 colocalizes with MYPT1, related to Figure 3.
U2OS cell expressing MYPT1-GFP and myosin IIA-TdTomato imaged by spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S5: MARK2 promotes protrusion, related to Figure 4.
U2OS cells with MARK2 knocked out by CRSPR-cas9 and MARK3 knocked down by siRNA (bottom row) or MARK2-KO cells expressing MARK2-GFP (top row) plated on a “crossbow”-shaped island of fibronectin (green) surrounded by non-adhesive surface and imaged by phase contrast (middle panels) and spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S6: MARK2 association with the plasma membrane is required for directional migration, related to Figure 5.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2 GFP lacking amino acids 1097-1192 (MARK2-ΔKA-GFP) undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S7: MARK2 association with the plasma membrane is required for leading edge advance, related to Figure 6.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2 GFP lacking amino acids 1097-1192 (MARK2-ΔKA-GFP) undergoing monolayer wound healing imaged by DIC microscopy. Time shown in min:sec, bar= 50μm.
Key Resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-GFP | ABCAM | Cat# ab290 |
| Anti GAPDH | Cell Signaling | Cat# 3675 S |
| Anti-6xHis | Qiagen | Cat# 34650 |
| Anti-GST | Cell Signaling | Car# 2622S |
| Anti-GM-130 | Cell Signaling | Cat# 12480S |
| Anti-P-FAK-Y397 | ThermoFisher | Cat# 44-624G |
| Anti-FAK Antibody, clone 4.47 | Millipore | Cat# 05-537 |
| Anti- MARK2 C-terminal | ABCAM | Cat# ab136872 |
| Anti-MARK1 | Cell Signaling | Cat# 3319S |
| Anti-MARK3 | ABCAM | Cat# Ab 52626 |
| Anti p-MLC S19 | Cell Signaling | Cat# 3675S |
| Anti-Myc tag | Cell Signaling | Cat# 2276 |
| Anti-MYPT1 | Millipore | Cat# 07-672-1 |
| Anti P-MYPT T696 | Millipore | Cat# AB545 |
| Anti-P-MYPT T853 | Cell Signaling | Cat# 4563S |
| Anti-Myosin IIA (MYH9) | Sigma | Cat# M8064 |
| Anti-alpha tubulin clone DM1A | Sigma | Cat# 05-829 |
| HRP-conjugated goat anti-mouse | Jackson ImmunoResearch | Cat# 115-035-174 |
| HRP-conjugated Donkey anti-mouse | Jackson ImmunoResearch | Cat# 112-035-175 |
| Bacterial and virus strains | ||
| MAX Efficiency™ DH5α Competent Cells | ThermoFisher | Cat# 18258012 |
| Chemicals, peptides, and recombinant proteins | ||
| MARK2, Active | SignalChem | Cat# M44-10G |
| MARK3, Active | SignalChem | Cat# M45-10G |
| ROCK2, Active | SignalChem | Cat# R11-RH-10 |
| MLCK, Active | 89 | N/A |
| His-MRLC | 90 | N/A |
| Calmodulin | .89 | N/A |
| CHKtide | SignalChem | Cat# C10-58 |
| MRLC peptide | SignalChem | Cat# C09-58 |
| MYPT1, (fragment 654-880) | Millipore Sigma | Cat# 12-457 |
| PhosSTOP | Roche | Cat#04906845001 |
| PrecipHen | Aves Lab | Cat# P-1010 |
| Native myosin (chicken gizzard) | 91 | N/A |
| Purified MRLC (chicken gizzard) | 91 | N/A |
| S-protein agarose | Merck Millipore | Cat# 69704 |
| PhosSTOP Phosphatase inhibitors (Roche) | Millipore/sigma | Cat# 4906845001 |
| cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail (Roche) | Millipore/Sigma | Cat# 11836170001 |
| Tween-20 | Sigma | Cat# P1379 |
| Fetal Bovine Serum | Atlanta Biologicals-Biotechme | Cat# S11150 |
| McCoy’s 5A | ThermoFisher | Cat# 36600021 |
| EC-Oxyrase | OXYRASE | Cat# EC-0005 |
| Radiance ECL | Azure Biosystems |
Cat# AC2204 |
| Alexa Fluor 647 NHS esther | ThermoFisher | Cat# A20006 |
| Human Plasma Fibronectin | Millipore | Cat# FC010 |
| Alexa Fluor 488 Phalloidin | ThermoFisher | Cat# A12379 |
| Alexa 568 Phalloidin | ThermoFisher | Cat# A12380 |
| Critical commercial assays | ||
| EndoFree Plasmid Maxi Kit | Qiagen | Cat# 12362 |
| Primo system (micropattering) | Alveole (Paris) | N/A |
| SE Cell Line 4D-Nucleofector X Kit V | Lonza | Cat #: VACA-1003 |
| GFP-Trap magnetic Agarose | Chromotek | Cat# gtma-20 |
| XtremeGene9 | Roche | Cat# 6365779001 |
| QuikChange II XL | Agilent Technologies | Cat# 200521 |
| Experimental models: Cell lines | ||
| U2OS | ATCC | Cat# HTB-96 |
| U2OS MARK2-KO | This study | N/A |
| U2OS MARK2-KO-MARK2-EGFP | This study | N/A |
| U2OS MARK2-KO-MARK2-DKA-EGFP | This study | N/A |
| U2OS MARK2-KO-MARK2-mApple | This study | N/A |
| Cos1 | ATCC | Cat# CRL-1650 |
| Oligonucleotides | ||
| On Target plus Human MARK3 SiRNA | Dharmacon (Horizon) | Cat# L-00357-00-0020 |
| On Target plus Human MARK2 SiRNA | Dharmacon (Horizon) | Cat# L-004260-00-0020 |
| MARK2-KO Guide 1 (GGAGCCGGTAGTTTCCAATG ) |
This paper | N/A |
| MARK2-KO Guide2 (GTGTCGGGCCAACTTCACCT) | This paper | N/A |
| MARK2-KO Guide 3(AGCCCCACATTGGAAACTAC) | This paper | N/A |
| Recombinant DNA | ||
| MARK2-EGFP | 38 | N/A |
| MARK2-mApple | 38 | N/A |
| MARK2-DKA-EGFP | This paper | N/A |
| pGFP-MYPT1 | 92 | N/A |
| pTriEX4-MARK2 | This project | N/A |
| pTriEX4 | MERK Sigma | 70824 |
| Myosin IIA-GFP | Michael Davidson Lab Florida State University | N/A |
| EGFP-C1 | Michael Davidson Lab Florida State University | N/A |
| m-Apple-C1 | Michael Davidson Lab Florida State University | N/A |
| Paxillin-GFP | Michael Davidson Lab Florida State University | N/A |
| pSpCas9n(BB)-2A-Puro (PX462) V2.0 | Addgene | Cat# 62987 |
| Software and algorithms | ||
| Metamorph 7.8.0.0 ID:32415 | 1992-2013 | https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/me |
| GraphPad Prism 9.0.2 | 2021 | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Novex Wedgewell 4–12 % tris-Glycine gel Invitrogen | ThermoFisher | Cat# XP04120BOX |
| Novex Wedgewell 4–20 % tris-Glycine gel Invitrogen | ThermoFisher | Cat# XP04202BOX |
HIGHLIGHTS.
MARK2 associates with the actomyosin cytoskeleton
MARK2 promotes phosphorylation of MRLC and MYPT1 to drive contractility
MARK2 associates with focal adhesions via its membrane-binding domain
MARK2 promotes FAK activation and focal adhesion formation and orientation
Acknowledgements:
We thank the NHLBI Flow Cytometry, Light Microscopy and Biochemistry core facilities, and William Shin, James Sellers, Duck-Yeon Lee, Magdalena Preciado-Lopez and Ankita Jha for their support and discussions. Supported by the Intramural Research Program of the NHLBI (AMP, SMH, YN, RSF, HRT, and CMW), R01GM143414 to SMH, and Pennsylvania CURE to ME
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, and Horwitz AR. (2003). Cell Migration: Integrating Signals from Front to Back. Science (80-. ). 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, and Waterman-Storer CM. (2003). Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5, 599–609. [DOI] [PubMed] [Google Scholar]
- 3.Lauffenburger DA, and Horwitz AF. (1996). Cell migration: A physically integrated molecular process. Cell 84, 359–369. [DOI] [PubMed] [Google Scholar]
- 4.Small JV, Stradal T, Vignal E, and Rottner K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120. [DOI] [PubMed] [Google Scholar]
- 5.Wittmann T, and Waterman-Storer CM. (2001). Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–803. [DOI] [PubMed] [Google Scholar]
- 6.Young LE, and Higgs HN. (2018). Focal Adhesions Undergo Longitudinal Splitting into Fixed-Width Units. Curr. Biol. 28, 2033–2045.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrzanowska-Wodnicka M, and Burridge K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura F, Ono S, Yamakita Y, Totsukawa G, and Yamashiro S. (1998). Specific Localization of Serine 19 Phosphorylated Myosin II during Cell Locomotion and Mitosis of Cultured Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterman-Storer CM., and Salmon ED. (1997). Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139, 417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schliwa M, Euteneuer U, Gräf R, and Ueda M. (1999). Centrosomes, microtubules and cell migration. Biochem. Soc. Symp. 65, 223–31. [PubMed] [Google Scholar]
- 11.Luxton GWG, and Gundersen GG. (2011). Orientation and function of the nuclear-centrosomal axis during cell migration. Curr. Opin. Cell Biol. 23, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zegers MM, and Friedl P. (2014). Rho GTPases in collective cell migration. Small GTPases 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes ER, Jani S, and Gundersen GG. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463. [DOI] [PubMed] [Google Scholar]
- 14.Munro EM. (2006). PAR proteins and the cytoskeleton: A marriage of equals. Curr. Opin. Cell Biol. 18, 86–94. [DOI] [PubMed] [Google Scholar]
- 15.Marx A, Nugoor C, Panneerselvam S, and Mandelkow E. (2010). Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1related kinases. FASEB J. 24, 1637–1648. [DOI] [PubMed] [Google Scholar]
- 16.McDonald JA. (2014). Canonical and Noncanonical Roles of Par-1/MARK Kinases in Cell Migration. In International Review of Cell and Molecular Biology (Elsevier Inc.), pp. 169–199. [DOI] [PubMed] [Google Scholar]
- 17.Kemphues KJ, Priess JR, Morton DG, and Cheng N. (1988). Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311–320. [DOI] [PubMed] [Google Scholar]
- 18.Kemphues K. (2000). PARsing Embryonic Polarity Minireview embryonic polarity. The polarity genes with the strong. [Google Scholar]
- 19.Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, and Ephrussi A. (2000). A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat. Cell Biol. 2, 458–460. [DOI] [PubMed] [Google Scholar]
- 20.Shulman JM, Benton R, and St Johnston D. (2000). The Drosophila Homolog of C. elegans PAR-1 Organizes the Oocyte Cytoskeleton and Directs oskar mRNA Localization to the Posterior Pole. Cell 101, 377–388. [DOI] [PubMed] [Google Scholar]
- 21.Drewes G, Ebneth A, and Preuss U. (1997). MARK, a Novel Family of Protein Kinases That Phosphorylate Microtubule-Associated Proteins and Trigger Microtubule Disruption. [DOI] [PubMed] [Google Scholar]
- 22.Schwalbe M, Biernat J, Bibow S, Ozenne V, Jensen MR, Kadavath H, Blackledge M, Mandelkow E, and Zweckstetter M. (2013). Phosphorylation of human tau protein by microtubule affinity-regulating kinase 2. Biochemistry 52, 9068–9079. [DOI] [PubMed] [Google Scholar]
- 23.Cohen D, Brennwald PJ, Rodriguez-Boulan E, and Müsch A. (2004). Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 164, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akchurin O, Du Z, Ramkellawan N, Dalal V, Han SH, Pullman J, Müsch A, Susztak K, and Reidy KJ. (2016). Partitioning-defective 1a/b depletion impairs glomerular and proximal tubule development. J. Am. Soc. Nephrol. 27, 3725–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D, Tian Y, and Müsch A. (2007). Par1b Promotes Hepatic-type Lumen Polarity in Madin Darby Canine Kidney Cells via Myosin II- and E-Cadherin–dependent Signaling. Mol. Biol. Cell 18, 2203–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zulkipli I, Clark J, Hart M, Shrestha RL, Gul P, Dang D, Kasichiwin T, Kujawiak I, Sastry N, and Draviam VM. (2018). Spindle rotation in human cells is reliant on a MARK2-mediated equatorial spindle-centering mechanism. J. Cell Biol. 217, 3057–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart M, Zulkipli I, Shrestha RL, Dang D, Conti D, Gul P, Kujawiak I, and Draviam VM. (2019). MARK2/Par1b kinase present at centrosomes and retraction fibres corrects spindle off-centring induced by actin disassembly. Open Biol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejia-Gervacio S, Murray K, Sapir T, Belvindrah R, Reiner O, and Lledo PM. (2012). MARK2/Par-1 guides the directionality of neuroblasts migrating to the olfactory bulb. Mol. Cell. Neurosci. 49, 97–103. [DOI] [PubMed] [Google Scholar]
- 29.Chen YM., Wang QJ., Hu HS., Yu PC., Zhu J., Drewes G., Piwnica-Worms H., and Luo ZG. (2006). Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl. Acad. Sci. U. S. A. 103, 8534–8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura Y, Terabayashi T, and Miki H. (2010). Par1b/MARK2 Phosphorylates Kinesin-Like Motor Protein GAKIN/KIF13B To Regulate Axon Formation. Mol. Cell. Biol. 30, 2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terabayashi T, Itoh TJ, Yamaguchi H, Yoshimura Y, Funato Y, Ohno S, and Miki H. (2007). Polarity-regulating kinase partitioning-defective 1/microtubule affinity-regulating kinase 2 negatively regulates development of dendrites on hippocampal neurons. J. Neurosci. 27, 13098–13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapir T, Shmueli A, Levy T, Timm T, Elbaum M, Mandelkow EM, and Reiner O. (2008). Antagonistic effects of doublecortin and MARK2/Par-1 in the developing cerebral cortex. J. Neurosci. 28, 13008–13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapir T, Sapoznik S, Levy T, Finkelshtein D, Shmueli A, Timm T, Mandelkow EM, and Reiner O. (2008). Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. J. Neurosci. 28, 5710–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteverde T, Muthalagu N, Port J, and Murphy DJ. (2015). Evidence of cancer-promoting roles for AMPK and related kinases. FEBS J. 282, 4658–4671. [DOI] [PubMed] [Google Scholar]
- 35.Beghini A, Magnani I, Roversi G, Piepoli T, Di Terlizzi S, Moroni RF, Pollo B, Fuhrman Conti AM, Cowell JK, Finocchiaro G, et al. (2003). The neural progenitor-restricted isoform of the MARK4 gene in 19q13.2 is upregulated in human gliomas and overexpressed in a subset of glioblastoma cell lines. Oncogene 22, 2581–2591. [DOI] [PubMed] [Google Scholar]
- 36.Marshall EA, Ng KW, Anderson C, Hubaux R, Thu KL, Lam WL, and Martinez VD. (2015). Gene expression analysis of microtubule affinity-regulating kinase 2 in non-small cell lung cancer. Genomics Data 6, 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald JA. (2014). Canonical and Noncanonical Roles of Par-1/MARK Kinases in Cell Migration 1st ed. (Elsevier Inc.). [DOI] [PubMed] [Google Scholar]
- 38.Nishimura Y, Applegate K, Davidson MW, Danuser G, and Waterman CM. (2012). Automated screening of microtubule growth dynamics identifies MARK2 as a regulator of leading edge microtubules downstream of RAC1 in migrating cells. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng HW, Hsiao C. Te, Chen YQ., Huang CM, Chan SI, Chiou A, and Kuo JC. (2019). Centrosome guides spatial activation of Rac to control cell polarization and directed cell migration. Life Sci. Alliance 2, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo JC, Han X, Hsiao C. Te, Yates JR, and Waterman CM. (2011). Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald JA, Khodyakova A, Aranjuez G, Dudley C, and Montell DJ. (2008). PAR-1 Kinase Regulates Epithelial Detachment and Directional Protrusion of Migrating Border Cells. Curr. Biol. 18, 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desai A, and Mitchison TJ. (1997). Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117. [DOI] [PubMed] [Google Scholar]
- 43.Goedert M, Baur CP, Ahringer J, Jakes R, Hasegawa M, Spillantini MG, Smith MJ, and Hill F. (1996). PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. J. Cell Sci. 109, 2661–2672. [DOI] [PubMed] [Google Scholar]
- 44.Chang W, Folker ES, Worman HJ, and Gundersen GG. (2013). Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol. Biol. Cell 24, 3869–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouthas F., Girard P., Lecaudey V., Ly TBN., Gilmour D., Boulin C., Pepperkok R., and Reynaud EG. (2008). In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. J. Cell Sci. 121, 2406–2414. [DOI] [PubMed] [Google Scholar]
- 46.Guo S, and Kemphues KJ. (1996). A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature 382, 455–458. [DOI] [PubMed] [Google Scholar]
- 47.Majumder P, Aranjuez G, Amick J, and McDonald JA. (2012). Par-1 controls myosin-II activity through myosin phosphatase to regulate border cell migration. Curr. Biol. 22, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moravcevic K, Mendrola JM, Schmitz KR, Wang YH, Slochower D, Janmey PA, and Lemmon MA. (2010). Kinase associated-1 domains drive MARK/PAR1 kinases to membrane targets by binding acidic phospholipids. Cell 143, 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folkmann AW, and Seydoux G. (2019). Spatial regulation of the polarity kinase PAR-1 by parallel inhibitory mechanisms. Dev. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurov JB, Watkins JL, and Piwnica-Worms H. (2004). Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14, 736–41. [DOI] [PubMed] [Google Scholar]
- 51.Lennerz JK, Hurov JB, White LS, Lewandowski KT, Prior JL, Planer GJ, Gereau RW, Piwnica-Worms D, Schmidt RE, and Piwnica-Worms H. (2010). Loss of Par-1a/MARK3/C-TAK1 Kinase Leads to Reduced Adiposity, Resistance to Hepatic Steatosis, and Defective Gluconeogenesis. Mol. Cell. Biol. 30, 5043–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davezac N, Baldin V, Blot J, Ducommun B, and Tassan JP. (2002). Human pEg3 kinase associates with and phosphorylates CDC25B phosphatase: A potential role for pEg3 in cell cycle regulation. Oncogene 21, 7630–7641. [DOI] [PubMed] [Google Scholar]
- 53.Matenia D, and Mandelkow EM. (2009). The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem. Sci. 34, 332–342. [DOI] [PubMed] [Google Scholar]
- 54.Totsukawa G, Wu Y, Sasaki Y, Hartshorne DJ, Yamakita Y, Yamashiro S, and Matsumura F. (2004). Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts. J. Cell Biol. 164, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumura F, and Hartshorne DJ. (2008). Myosin phosphatase target subunit: Many roles in cell function. Biochem. Biophys. Res. Commun. 369, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eto M, Kirkbride JA, and Brautigan DL. (2005). Assembly of MYPT1 with protein phosphatase-1 in fibroblasts redirects localization and reorganizes the actin cytoskeleton. Cell Motil. Cytoskeleton 62, 100–109. [DOI] [PubMed] [Google Scholar]
- 57.Woodsome TP, Polzin A, Kitazawa K, Eto M, and Kitazawa T. (2006). Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J. Cell Sci. 119, 1769–1780. [DOI] [PubMed] [Google Scholar]
- 58.Martin AC. (2010). Pulsation and stabilization: Contractile forces that underlie morphogenesis. Dev. Biol. 341, 114–125. [DOI] [PubMed] [Google Scholar]
- 59.Zagórska A, Deak M, Campbell DG, Banerjee S, Hirano M, Aizawa S, Prescott AR, and Alessi DR. (2010). New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci. Signal. 3. [DOI] [PubMed] [Google Scholar]
- 60.Khromov A, Choudhury N, Stevenson AS, Somiyo AV, and Eto M. (2009). Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J. Biol. Chem. 284, 21569– 21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mori S, Iwaoka R, Eto M, and Ohki SY. (2009). Solution structure of the inhibitory phosphorylation domain of myosin phosphatase targeting subunit 1. Proteins Struct. Funct. Bioinforma. 77, 732–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Théry M, Racine V, Piel M, Pépin A, Dimitrov A, Chen Y, Sibarita J-B, and Bornens M. (2006). Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl. Acad. Sci. U. S. A. 103, 19771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, and Bershadsky AD. (2011). Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 13, 1457–1465. [DOI] [PubMed] [Google Scholar]
- 64.Swaminathan V., Kalappurakkal JM., Mehta SB., Nordenfelt P., Moore TI., Koga N., Baker DA., Oldenbourg R., Tani T., Mayor S., et al. (2017). Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc. Natl. Acad. Sci. 114, 10648–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer RS, Sun X, Baird MA, Hourwitz MJ, Seo BR, Pasapera AM, Mehta SB, Losert W, Fischbach C, Fourkas JT, et al. (2021). Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates. Proc. Natl. Acad. Sci. U. S. A. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. (2004). aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14, 1425–35. [DOI] [PubMed] [Google Scholar]
- 67.Illenberger S, Zheng-Fischhöfer Q, Preuss U, Stamer K, Baumann K, Trinczek B, Biernat J, Godemann R, Mandelkow EM, and Mandelkow E. (1998). The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol. Biol. Cell 9, 1495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin J, Hou KK, Piwnica-Worms H, and Shaw AS. (2009). The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J. Immunol. 183, 1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munro E, Nance J, and Priess JR. (2004). Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424. [DOI] [PubMed] [Google Scholar]
- 70.Motegi F, and Seydoux G. (2013). The PAR network: Redundancy and robustness in a symmetry-breaking system. Philos. Trans. R. Soc. B Biol. Sci. 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hao H, Niu J, Xue B, Su QP, Liu M, Yang J, Qin J, Zhao S, Wu C, and Sun Y. (2020). Golgi-associated microtubules are fast cargo tracks and required for persistent cell migration. EMBO Rep. 21, e48385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yadav S, and Linstedt AD. (2011). Golgi positioning. Cold Spring Harb. Perspect. Biol. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baas AF, Kuipers J, Van Der Wel NN, Batlle E, Koerten HK, Peters PJ, and Clevers HC. (2004). Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466. [DOI] [PubMed] [Google Scholar]
- 74.Yamahashi Y, Saito Y, Murata-Kamiya N, and Hatakeyama M. (2011). Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J. Biol. Chem. 286, 44576–44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giannone G, Dubin-Thaler BJ, Döbereiner HG, Kieffer N, Bresnick AR, and Sheetz MP. (2004). Periodic lamellipodial contractions correlate with rearward actin waves. Cell. [DOI] [PubMed] [Google Scholar]
- 76.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, and Danuser G. (2009). Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Pasapera AM, Koretsky AP, and Waterman CM. (2013). Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc. Natl. Acad. Sci. U. S. A. 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shutova M, Yang C, Vasiliev JM, and Svitkina T. (2012). Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS One 7, e40814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasapera AM, Plotnikov SV, Fischer RS, Case LB, Egelhoff TT, and Waterman CM. (2015). Rac1-dependent phosphorylation and focal adhesion recruitment of myosin IIA regulates migration and mechanosensing. Curr. Biol. 25, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamashiro S, and Watanabe N. (2014). A new link between the retrograde actin flow and focal adhesions. J. Biochem. 156, 239–48. [DOI] [PubMed] [Google Scholar]
- 81.Alexandrova AY., Arnold K., Schaub S., Vasiliev JM., Meister J-J., Bershadsky AD., and Verkhovsky AB. (2008). Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One 3, e3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shamir ER, and Ewald AJ. (2015). Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration. Curr. Top. Dev. Biol. 112, 353–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haeger A, Wolf K, Zegers MM, and Friedl P. (2015). Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 25, 556–66. [DOI] [PubMed] [Google Scholar]
- 84.van Helvert S, Storm C, and Friedl P. (2018). Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedl P, and Mayor R. (2017). Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, and Auer M. (2012). Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J. Cell Sci. 125, 2638–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bui J, Conway DE, Heise RL, and Weinberg SH. (2019). Mechanochemical Coupling and Junctional Forces during Collective Cell Migration. Biophys. J. 117, 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandya P, Orgaz JL, and Sanz-Moreno V. (2017). Actomyosin contractility and collective migration: may the force be with you. Curr. Opin. Cell Biol. 48, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melero C, Kolmogorova A, Atherton P, Derby B, Reid A, Jansen K, and Ballestrem C. (2019). Light-Induced Molecular Adsorption of Proteins Using the PRIMO System for Micro-Patterning to Study Cell Responses to Extracellular Matrix Proteins. J. Vis. Exp. [DOI] [PubMed] [Google Scholar]
- 90.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beach JR, Licate LS, Crish JF, and Egelhoff TT. (2011). Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells. BMC Cell Biol. 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khasnis M, Nakatomi A, Gumpper K, and Eto M. (2014). Reconstituted human myosin light chain phosphatase reveals distinct roles of two inhibitory phosphorylation sites of the regulatory subunit, MYPT1. Biochemistry 53, 2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguilar-Cuenca R, Llorente-González C, Chapman JR, Talayero VC, Garrido-Casado M, Delgado-Arévalo C, Millán-Salanova M, Shabanowitz J, Hunt DF, Sellers JR, et al. (2020). Tyrosine Phosphorylation of the Myosin Regulatory Light Chain Controls Non-muscle Myosin II Assembly and Function in Migrating Cells. Curr. Biol. 30, 2446–2458.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasquez CG, Heissler SM, Billington N, Sellers JR, and Martin AC. (2016). Drosophila non-muscle myosin II motor activity determines the rate of tissue folding. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorelik R, and Gautreau A. (2014). Quantitative and unbiased analysis of directional persistence in cell migration. Nat. Protoc. 9, 1931–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: MARK2 colocalizes with Myosin IIA, related to Figure 1.
U2OS cell expressing MARK2-GFP and myosin IIA-TdTomato imaged by spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S2: CRSPR-mediate knockout of MARK2 demonstrates that it is required for efficient directional migration, related to Figure 2.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S3: CRSPR-mediated knockout or siRNA knockdown of MARK2 demonstrates that it is required for efficient directional migration, related to Figure 2.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (using a different guide RNA than that used in Video S2) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S4: MARK2 colocalizes with MYPT1, related to Figure 3.
U2OS cell expressing MYPT1-GFP and myosin IIA-TdTomato imaged by spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S5: MARK2 promotes protrusion, related to Figure 4.
U2OS cells with MARK2 knocked out by CRSPR-cas9 and MARK3 knocked down by siRNA (bottom row) or MARK2-KO cells expressing MARK2-GFP (top row) plated on a “crossbow”-shaped island of fibronectin (green) surrounded by non-adhesive surface and imaged by phase contrast (middle panels) and spinning disk confocal microscopy. Time shown in min:sec, bar= 10μm.
Video S6: MARK2 association with the plasma membrane is required for directional migration, related to Figure 5.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2 GFP lacking amino acids 1097-1192 (MARK2-ΔKA-GFP) undergoing monolayer wound healing imaged by phase contrast microscopy. Time shown in hr:min, bar= 50μm.
Video S7: MARK2 association with the plasma membrane is required for leading edge advance, related to Figure 6.
U2OS cells (U2OS), or U2OS cells with MARK2 knocked out by CRSPR-cas9 (MARK2-KO) and MARK3 knocked down by siRNA (MARK-KO) or MARK2-KO cells expressing MARK2-GFP or MARK2 GFP lacking amino acids 1097-1192 (MARK2-ΔKA-GFP) undergoing monolayer wound healing imaged by DIC microscopy. Time shown in min:sec, bar= 50μm.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the manuscript and its supplementary files. Raw data will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.