Abstract
Streptomycetes are common soil inhabitants, yet few described species are plant pathogens. While the pathogenicity mechanisms remain unclear, previous work identified a gene, nec1, which encodes a putative pathogenicity or virulence factor. nec1 and a neighboring transposase pseudogene, ORFtnp, are conserved among unrelated plant pathogens and absent from nonpathogens. The atypical GC content of nec1 suggests that it was acquired through horizontal transfer events. Our investigation of the genetic organization of regions adjacent to the 3′ end of nec1 in Streptomyces scabies 84.34 identified a new insertion sequence (IS) element, IS1629, with homology to other IS elements from prokaryotic animal pathogens. IS1629 is 1,462 bp with 26-bp terminal inverted repeats and encodes a putative 431-amino-acid (aa) transposase. Transposition of IS1629 generates a 10-bp target site duplication. A 77-nucleotide (nt) sequence encompassing the start codon and upstream region of the transposase was identified which could function in the posttranscritpional regulation of transposase synthesis. A functional copy of IS1629 from S. turgidiscabies 94.09 (Hi-C-13) was selected in the transposon trap pCZA126, through its insertion into the λ cI857 repressor. IS1629 is present in multiple copies in some S. scabies strains and is present in all S. acidiscabies and S. turgidiscabies strains examined. A second copy of IS1629 was identified between ORFtnp and nec1 in S. acidiscabies strains. The diversity of IS1629 hybridization profiles was greatest within S. scabies. IS1629 was absent from the 27 nonpathogenic Streptomyces strains tested. The genetic organization and nucleotide sequence of the nec1-IS1629 region was conserved and identical among representatives of S. acidiscabies and S. turgidiscabies. These findings support our current model for the unidirectional transfer of the ORFtnp-nec1-IS1629 locus from IS1629-containing S. scabies (type II) to S. acidiscabies and S. turgidiscabies.
Insertion sequence (IS) elements constitute the simplest class of prokaryotic transposable genetic elements and are commonly found within prokaryotic genomes, plasmids, and phages. While they vary in size, typical IS elements generally share several properties, including (i) the presence of terminal inverted repeats, (ii) the absence of detectable IS-encoded activities other than those required for transposition or its regulation, and (iii) the generation of direct repeats of target nucleotide sequences upon insertion (5, 10). They have been found to effect a variety of genetic changes, vis-à-vis genetic organization (e.g., through deletion or duplication-inversion events) and gene expression (e.g., through insertional mutagenesis or the introduction of new cis-acting regulatory elements).
Gram-positive filamentous streptomycetes are cosmopolitan members of soil microbial communities, and close to 500 species have been described. They share a complex developmental program of growth and differentiation characterized by the elaboration of substrate mycelia followed by a phase of filamentous aerial growth, which culminates in the transformation of aerial filaments into chains of spores. Their soil-dwelling nature would suggest that many have evolved in close association with plants and derive nutrients from decaying organic matter. Four species have been described, however, which have developed the means to incite disease on subterranean parts of economically important tuber crops, e.g., the potato. These species—Streptomyces scabies, S. acidiscabies, S. turgidiscabies, and S. ipomoeae—are quite unrelated to one another based on several criteria, including 16S ribosomal DNA sequence and DNA-DNA relatedness data, as well as morphological and biochemical attributes (8, 11, 12, 17, 30). Despite this diversity, however, all of these species share the production of one or more of a family of phytotoxins, thaxtomins, which mimic disease symptomatology on host plants (1, 13). Since the production of this unusual phytotoxin is common to unrelated species, it has been suggested that pathogenicity factor(s) have been horizontally transferred among these diverse pathogens (8, 13).
Previous efforts aimed at elucidating other pathogenicity determinants in S. scabies 84.34 (ATCC 49173) identified a putative pathogenicity or virulence gene, nec1, which was sufficient to confer a necrogenic phenotype upon the nonpathogen S. lividans 66 TK24 (2). It was also found that nec1 is conserved in plant pathogens and is absent from nonpathogens. Subsequently, a strong correlation was shown to exist between thaxtomin production and the presence of nec1 in S. scabies, S. acidiscabies, and S. turgidiscabies strains (1).
Evidence for the transfer of pathogenicity genes among diverse pathogenic species has recently been presented. The codon bias and GC content (54%) of nec1 are atypical relative to high-GC coding regions characteristic of Streptomyces strains (32), suggesting that it was acquired from another taxon. Sequence analysis of the 5′ end of nec1 identified an IS256 transposase homolog, designated ORFtnp, which is apparently nonfunctional due to a frameshift mutation (2). It was subsequently found that the nucleotide sequence of the ORFtnp-nec1 region was identical among strains of S. scabies (three strains examined), S. acidiscabies (two strains examined), and S. turgidiscabies (two strains examined) (1). Taken together, these data strongly suggest that this region has recently been mobilized among these unrelated Streptomyces pathogens and may be involved in the evolution of plant pathogenicity within the genus.
It was therefore of interest to explore the genetic organization of regions adjacent to the 3′ end of nec1 in S. scabies 84.34, reasoning that other novel pathogenicity factors or elements that may have played a role in the mobilization and horizontal transfer of pathogenicity genes could be identified in this region. We report here the identification and characterization of a functional pathogenicity-associated IS element, designated IS1629, from our analysis of the 3′ flanking region of nec1. We present evidence for the unidirectional transfer of an element that contains IS1629, from IS1629-containing (type II) S. scabies isolates to S. acidiscabies and S. turgidiscabies. This region may constitute a portion of an as-yet-uncharacterized pathogenicity island in filamentous gram-positive plant pathogens.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The pathogenic (Thax+ nec1+) and nonpathogenic (Thax−, do not have nec1) Streptomyces strains included in this study are listed in Table 1. These strains were all grown in tryptic soy broth (TSB; Difco) at 28°C, and total DNA was extracted according to the method of Rao et al. (20). Plasmid pCZA126 (28) propagated in E. coli ET12567 (F− dam-13::Tn9 dcm-6 hsdM hsdR recF14 zjj::Tn10 galK2 galT22 ara14 lacY1 xyl-5 leuB6 thi-1 tonA31 rpsL136 hisG tsx78 mtl-1 glnV44) (15) was used to transform protoplasts of S. turgidiscabies 94.09 (Hi-C-13) by established procedures (9). Transformants were selected on R2YE medium (9) overlaid with thiostrepton (Sigma Chemical Co.) (50 μg/ml, final concentration). S. turgidiscabies 94.09(pCZA126) was grown in TSB medium supplemented with 50 μg of thiostrepton per ml. Plasmid pools recovered from S. turgidiscabies carrying pCZA126 were used to transform Escherichia coli DH5α. E. coli DH5α(pCZA126) transformants were selected on Luria-Bertani agar at 30°C supplemented with 100-μg/ml concentrations of both apramycin sulfate and ampicillin (both from Sigma Chemical Co.) or with ampicillin alone where indicated.
TABLE 1.
Streptomyces strains used in this study
| Streptomyces strain | Source or origin |
|---|---|
| Pathogenic strains | |
| S. scabies | |
| 84.34 (ATCC 49173) | N.Y. |
| 84.42 | N.Y. |
| 84.70 | N.Y. |
| 84.232 | W.Va. |
| 85.08 | Maine |
| 85.30 | Ohio |
| 87.22 | Wis. |
| 87.67 | Wis. |
| 92.07 | Minn. |
| 94.17 | Japan |
| 94.34 | N.Y. |
| 95.01 | South Africa |
| 95.03 | South Africa |
| 95.14 | Japan |
| 96.15 | Germany |
| S. acidiscabies | |
| 84.104 | N.Y. |
| 84.110 (ATCC 49003) | Maine |
| 85.06 | W.Va. |
| 90.25 | Maine |
| S. turgidiscabies | |
| 94.08 (CAR 8) | Japan |
| 94.09 (Hi-C-13) | Japan |
| 94.10 (Hi-C-16) | Japan |
| 94.11 (Hi-C-19) | Japan |
| 94.12 (Hi-C-20) | Japan |
| 94.13 (Hi-C-36) | Japan |
| Nonpathogenic strains | |
| 83.40 | Maine |
| 84.05 | N.Y. |
| 84.29 | N.Y. |
| 84.130 | N.Y. |
| 84.222 | Pa. |
| 86.31 | N.Y. |
| 87.80 | Wis. |
| 88.24 | Maine |
| 88.25 | Maine |
| 89.05 | Alaska |
| 89.08 | Alaska |
| 89.18 | N.Y. |
| 92.01 | N.Y. |
| 92.03 | N.Y. |
| S. griseus | ATCC 10246 |
| S. diastatochromogenes | ATCC 12309 |
| S. eurythermus | ATCC 14975 |
| S. griseoviridis | ATCC 23920 |
| S. bottropensis | ATCC 25435 |
| S. setonii | ATCC 25497 |
| S. neyagawensis | ATCC 27449 |
| S. albidoflavus | IMRU 3018 |
| S. griseus IFO 13350 | S. Horinouchi |
| S. clavuligerus NRRL 3585 | J. Piret |
| S. lividans 66 TK24 | K. F. Chater |
| S. coelicolor M146 | K. F. Chater |
| S. griseofuscus C581 | P. Solenberg |
Genomic library construction, DNA sequencing, amplification, and hybridization conditions.
Genomic libraries of S. scabies 84.34 were constructed and amplified by using genome-walking methodologies (Genome Walker kit; Clontech) according to manufacturer’s recommendations, except that restriction endonucleases which cleave high-GC recognition sequences were substituted in some instances. Briefly, genomic DNA was digested with restriction endonucleases that leave blunt ends. Double-stranded “adaptors” were then ligated to blunt-ended restriction digestion products. Genomic library amplification reactions were then done by using nested adapter oligonucleotide primers (Genome Walker kit; Clontech) and nested species-specific (nec1) oligonucleotide primers. The nested species-specific oligonucleotide primers, based on the sequence of nec1 (GenBank-EMBL accession number AFO31232), were 5′-GCTGCGTTCGCCAATTCCACCTTCACTGCT (ORF3.1) and 5′-TTTTATCGAGACAATGGCGGGCAGGTG (ORF3.2).
The nec1-IS1629 region was amplified from genomic DNA of representatives of S. acidiscabies (84.104, 84.110, and 90.25) and S. turgidiscabies (94.08, 94.09, 94.10, 94.11, and 94.12) by using primers ORF3.1 and ORF2R.3 (IS1629-specific) (5′-TCTCGCTGGACCACTTCTTC) with the following thermal cycling parameters: 95°C for 2.5 min (1 cycle) and 95°C for 30 s, 60°C for 45 s, and 72°C for 1.5 min (35 cycles). Genomic DNA was amplified in 50-μl reaction volumes containing 5 μl of 10× PCR reaction buffer (Perkin-Elmer), 2.5 mM MgCl2, 20 μM concentrations of each deoxynucleoside triphosphate, 1 μM concentration of each oligonucleotide primer, 2% dimethyl sulfoxide, 0.3 U of Taq DNA polymerase, and 10 ng of genomic DNA. Genomic library PCR amplification products were sequenced directly after purification from agarose gels (Qiaex II gel extraction kit; Qiagen) by using an ABI 377XL Automated DNA sequencer (BioResource Center, Cornell University).
Oligonucleotide primers flanking the insertion of IS1629 in pCZA126, based on the cI857 sequence (GenBank accession number E00770), were 5′-GTTCAGGCAGGGATGTTCTCACC (cI857F) and 5′-CTCAAGCCAGAATGCAGAATCACT (cI857R). Hybridization conditions for Southern and dot blot analyses were similar to those previously described (2).
Amplification of IS1629 on the 5′ end of nec1 from S. acidiscabies 84.104 and 84.110 (ATCC 49003) genomic DNA was done with primers Ir and If (1) and Accurase DNA polymerase (1.25 U; Tetra Link International, Lewiston, N.Y.) under the following conditions: 95°C for 30 s, 60°C for 1 min, and 68°C for 2 min (35 cycles). The nucleotide sequence of the entire product from S. acidiscabies 84.104 DNA amplification was obtained with If and Ir primers as well as with internal primers (set 1 [IS-f, 5′-CGCTGCAAGATCCACGAAGG; IS-r: 5′-GAGGGTGCGTTCGGTGTGG]; set 2 [IS-ff, 5′-GCGTGGGGAAAGAGCAGGC; IS-rr, 5′-GCTACCGGATCCCGAGCG]. Nucleotide sequencing of the product from S. acidiscabies 84.110 was done with primers If and Ir.
Nucleotide sequence accession number.
The nucleotide sequence of IS1629 reported here has been deposited in the GenBank database under accession number AF109404.
RESULTS
An IS element lies on the 3′ end of nec1 in S. scabies.
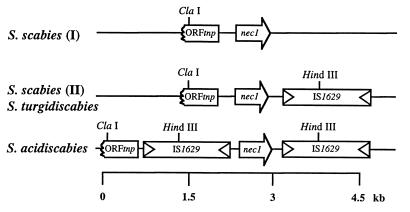
The identification of the unusual nec1 gene and the sequence identity of the region spanning the ORFtnp pseudogene and nec1 among three described species of Streptomyces plant pathogens prompted an investigation of the genetic region flanking the 3′ end of nec1. S. scabies 84.34 genomic libraries were constructed and amplified with nested adapter primers and nested species-specific oligonucleotide primers based on the nec1 sequence. This strategy allowed us to specifically amplify the repetitive region adjacent to nec1. This region, including nec1 and ORFtnp, is shown in Fig. 1. Amplification of genomic libraries yielded a 3-kb PCR product. A portion of the sequence obtained is given in Fig. 2. Sequence analysis of this 70% GC 1,462-bp region revealed the presence of an open reading frame, the putative 431-aa translation product of which exhibits homology with previously described transposases and putative transposases of IS elements from various prokaryotic animal pathogens. The putative transposase was found to be 31% identical (45% similar) over 340 aa to an IS element from pathogenic strains of Mycobacterium ulcerans (23), 30% identical (45% similar) over 244 aa to PGIS2 from Porphyromonas gingivalis (31), 30% identical (48% similar) over 244 aa to AsIS1 from Aeromonas salmonicida (6), 23% identical (40% similar) over 384 aa to IS1358 from Vibrio cholerae (29), and 24% identical (39% similar) over 369 aa to H-rpt sequences from E. coli (33) (Fig. 3). Further analysis of the sequence revealed the presence of 26-bp imperfect terminal inverted repeats (Fig. 2) and 10-bp direct repeats flanking the inverted repeat sequences (see below and Fig. 5B). This newly described IS element has been designated IS1629 by the Plasmid Reference Center, Department of Microbiology and Immunology, Stanford University School of Medicine.
FIG. 1.
Genetic organization of ORFtnp-nec1-IS1629 region in S. scabies (type I and II), S. acidiscabies, and S. turgidiscabies strains.
FIG. 2.
Nucleotide sequence of IS1629, with putative transposase sequence given below. Terminal inverted repeats are double underlined; the nucleotide sequence involved in the proposed RNA secondary structure is underlined once (also see Fig. 6). The probable Shine-Dalgarno sequence is boxed.
FIG. 3.
Clustal alignment of putative IS1629 transposase with transposase homologs. M.u., putative transposase from Mycobacterium ulcerans; P.g. PGIS2, Porphyromonas gingivalis IS2; A.s. AsIS1, Aeromonas salmonicida IS1; V.c. IS1358, V. cholerae IS1358.
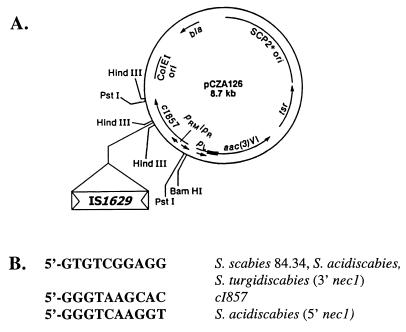
FIG. 5.
IS1629 selection in pCZA126 (adapted from Solenberg and Burgett [28]) (A) and nucleotide sequences of duplicated IS1629 target insertion sites from the following: the 3′ region of nec1 in S. scabies 84.34, S. turgidiscabies, and S. acidiscabies; λ cI857; and S. acidiscabies 84.104 and 84.110 (5′ of nec1) (B).
IS1629 is present in representatives of three described Streptomyces plant pathogens and is absent from all nonpathogenic Streptomyces species examined.
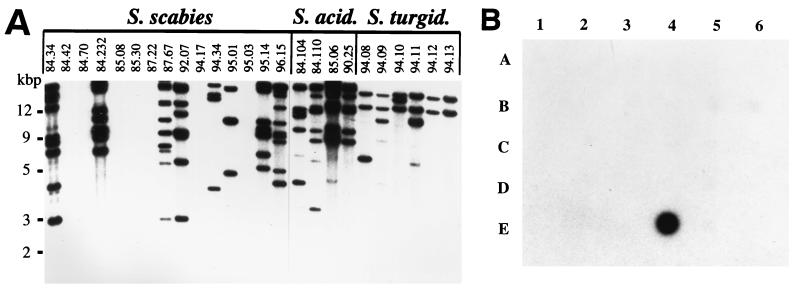
Since the ORFtnp-nec1 region had been shown to be conserved among diverse Streptomyces plant pathogens and was not found in any nonpathogenic species tested, we were interested in evaluating plant pathogens other than S. scabies 84.34, as well as nonpathogens, for the presence of IS1629. Southern hybridization and dot blot analyses were performed with an ∼1.4-kb ApaI fragment of IS1629 as the probe against total DNA extracted from the strains listed in Table 1. As illustrated in Fig. 4A, IS1629 was present in representatives of all of the plant-pathogenic species tested. The copy numbers ranged from 0 to ≥10, ≥6, and ≥2 in S. scabies, S. acidiscabies, and S. turgidiscabies, respectively. While it was absent from 7 of the 15 S. scabies strains (type I) tested, IS1629 was present in all of the S. acidiscabies and S. turgidiscabies strains examined. Total DNA from 27 nonpathogenic (Thax−, do not have nec1) Streptomyces strains (Table 1) was immobilized on membrane filters and probed with the 1.4-kb fragment of IS1629. As shown in Fig. 4B, IS1629 probe did not hybridize to DNA from any of these strains.
FIG. 4.
Southern hybridization of 1.4-kb [α-32P]dCTP-labeled ApaI fragment of IS1629 with KpnI-digested total DNA from the indicated Streptomyces pathogens (IS1629 does not have a KpnI site) (A) and dot blot analysis with the same fragment with the total DNA from nonpathogenic strains. (B) Panel B is arranged as follows: row A1 to A6, 83.40, 84.05, 84.29, 84.130, 84.222, and 86.31; row B1 to B6, 87.80, 88.24, 88.25, 89.05, 89.08, and 89.18; row C1 to C6, 92.01, 92.03, ATCC 10246, ATCC 12309, ATCC 14975, and ATCC 23920; row D1 to D6, ATCC 25435, ATCC 25497, ATCC 27449, IMRU 3018, IFO 13350, and NRRL 3585; row E1 to E5, TK24, M146, C581, 84.34, and calf thymus DNA.
Selection for functional copy of IS1629.
In order to demonstrate that functional copies of IS1629 were present in Streptomyces plant pathogens, we employed plasmid pCZA126 to select for the transposition of mobile genetic elements into the temperature-sensitive λ cI857 repressor (28), resulting in the derepression of aac(3)VI (apramycin resistance gene) expression. Since strains of S. scabies are recalcitrant to plasmid transformation, protoplasts of S. turgidiscabies 94.09 were transformed with pCZA126 after propagation of the vector in E. coli ET12567. Plasmid pools recovered from thiostrepton-resistant S. turgidiscabies 94.09(pCZA126) transformants were used to transform E. coli DH5α to apramycin resistance. Five such transformants were identified, and one of these was found to contain an ∼1.5-kb insertion in cI857, as determined by restriction analysis (Fig. 5A). The remaining four apramycin-resistant transformants did not appear to harbor any inserts. The frequency of insertion of this element into pCZA126 was estimated to be ≥1 × 10−4 (i.e., the number of apramycin- and ampicillin-resistant transformants containing plasmids with insertions per the number of ampicillin-resistant transformants).
Restriction analysis of the region of insertion into pCZA126 allowed the design of oligonucleotide primers flanking the insertion; these were used to obtain the nucleotide sequence of the insertion. The sequence was found to be identical to the IS1629 sequence adjacent to nec1 in S. scabies 84.34.
Transposition of IS1629 to the 5′ end of nec1 in S. acidiscabies strains.
Previous Southern hybridization data (not shown) revealed the presence of an ∼1.5-kb insertion between ORFtnp and nec1 in all of the strains of S. acidiscabies examined. This region was amplified from S. acidiscabies 84.104 and 84.110 (ATCC 49003) genomic DNA. The entire nucleotide sequence of this region was obtained for S. acidiscabies 84.104, and the insertion was found to be identical to IS1629. A 10-bp target duplication site was also identified and is given in Fig. 5B. Partial sequence analysis of the insertion from S. acidiscabies 84.110 DNA confirmed that the insertion was IS1629 and that the insertion target site was identical to that found in S. acidiscabies 84.104 (nucleotide sequence data not shown).
nec1-IS1629 nucleotide sequence is conserved among diverse Streptomyces plant pathogens.
Previous Southern hybridization data (not shown) had suggested that IS1629 was present on the 3′ end of nec1 in all strains of S. acidiscabies and S. turgidiscabies examined. By using nec1- and IS1629-specific primers, we amplified an expected ∼1.9-kb product from three representatives of S. acidiscabies and five representatives of S. turgidiscabies. The nucleotide sequences of the nec1-IS1629 intergenic region from all strains were identical to the same region in S. scabies 84.34. The 10-bp insertion sequence target sites, as well as the 5′ end of IS1629, were also identical to that of S. scabies 84.34 (data not shown).
DISCUSSION
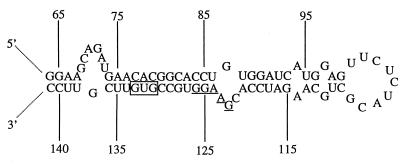
Relatively little is known regarding the mechanism(s) of pathogenicity employed by plant-pathogenic Streptomyces spp. The search for pathogenicity or virulence factors involved in disease development led to the identification of nec1, which is found exclusively in genetically diverse Streptomyces plant pathogens (2). Further, the GC content and codon usage of nec1 strongly suggests that it was introduced into these organisms via horizontal gene transfer from an unrelated taxon. Analysis of the 5′ flanking sequence of nec1 revealed the presence of the pseudogene ORFtnp, which belongs to the IS256 transposase family. Interestingly, the ORFtnp-nec1 region was found to be conserved (100% nucleotide sequence identity) among genetically diverse plant pathogens (1). These findings prompted us to explore the immediate genetic region bordering the 3′ end of nec1, resulting in the identification of the novel IS element, IS1629. In addition to its aforementioned properties, a region was also identified within IS1629, from nt 64 to 141, which encompasses the putative start codon, a probable Shine-Dalgarno sequence, and 67 nt upstream of the transposase start codon. This region would be predicted to form a stable RNA secondary structure (Fig. 6). Similar intramolecular pairings within IS10 and IS50 have previously been shown to function in the regulation of transposase synthesis by inhibiting translation of transcripts whose expression was initiated from external promoters (4, 14, 26). This method of posttranscriptional regulation has been shown to efficiently reduce the potentially deleterious production of transposase in the event that IS elements transpose into the vicinity of sequences that could serve as strong promoters of transposase expression.
FIG. 6.
Proposed RNA secondary structure adopted by IS1629 transposase transcribed from exogenous promoter sequences. Nucleotides are numbered from the start of the IS1629 sequence. The probable ribosome binding site is underlined, and the putative start codon is boxed.
We were able to demonstrate that functional copies of IS1629 were present in plant pathogenic streptomycetes by using a Streptomyces-E. coli shuttle vector originally described by Solenberg and Burgett (28). This vector allows for the direct selection and cloning of transposable genetic elements through their insertion into the temperature-sensitive bacteriophage lambda cI857 allele. Disruption of the repressor or its operator results in the derepression of apramycin resistance gene expression. Of five apramycin-resistant E. coli transformants recovered, one was found to contain an ∼1.5-kb insertion within the repressor gene. The nucleotide sequence of this insertion element was identical to the IS1629 sequence from S. scabies 84.34. It is presumed that the four plasmids recovered from the remaining apramycin- and ampicillin-resistant transformants that did not harbor insertions were the result of other mutations in the cI857 operator region and/or structural gene (e.g., frameshift mutations, point mutations, deletions, etc.) or of chromosomally derived apramycin resistance.
Southern hybridization analysis of DNA representing three pathogenic species probed with IS1629 revealed that while IS1629 was present in all of the S. acidiscabies and S. turgidiscabies strains tested (four and six strains, respectively), it was found in only about half of the S. scabies strains tested (type II; 8 of 15). Among those S. scabies strains tested, there did not appear to be any relationship between the presence or copy number of IS1629 and their geographic origin. Thus, half of the strains originally isolated in New York, Wisconsin, Japan, and South Africa contained ≥3 copies of IS1629. One strain each from Maine and Ohio did not contain any copies of IS1629. Conversely, based on IS1629 hybridization profiles, the S. acidiscabies and S. turgidiscabies strains exhibited less diversity than did those S. scabies strains that did carry copies of IS1629, with S. turgidiscabies strains exhibiting the lowest diversity. These findings are consistent with intraspecies DNA-DNA relatedness data among S. scabies, S. acidiscabies, and S. turgidiscabies (8, 17).
The IS1629 and DNA-DNA hybridization data suggest that the divergence of S. scabies strains occurred earlier than that of S. acidiscabies and S. turgidiscabies. Indeed, S. acidiscabies and S. turgidiscabies have only recently been reported as pathogens (16, 17). Further, both S. turgidiscabies and S. acidiscabies appear to have a very limited geographic range; they have only been reported to occur in Japan and the northeastern United States, respectively. Perhaps these two species have only recently acquired the ability to parasitize plants. Dot blot analyses demonstrated that IS1629 was not present in any of 27 nonpathogenic Streptomyces species tested, including those which have otherwise been shown to be taxonomically related to pathogenic species and those which were isolated from host lesion tissue in pathogen-infested soils. Likewise, nec1 has not been identified from nonpathogenic Streptomyces spp.; although ORFtnp homologs, which may be functional, exist in some nonpathogens (1, 2). At this point, we have not examined the host range of IS1629 outside of the genus Streptomyces.
Several lines of evidence suggest that the ORFtnp-nec1-IS1629 region has been transferred horizontally and in a unidirectional manner through the dissemination of a “pathogenicity island” from IS1629-containing (type II) S. scabies isolates to S. acidiscabies and S. turgidiscabies based on our current understanding of pathogenicity islands (7). These lines of evidence are as follows: (i) the greater intraspecies diversity of S. scabies compared with S. acidiscabies and S. turgidiscabies (8, 30); (ii) the absence of IS1629 from roughly half of the S. scabies isolates tested, suggesting that S. scabies acquired IS1629 after the introduction of ORFtnp-nec1 (IS1629-containing S. scabies could then have served as a donor of the pathogenicity island to nonpathogenic “ancestral” S. acidiscabies and S. turgidiscabies isolates); (iii) the sequence identity of the ORFtnp-nec1-IS1629 region among the three diverse Streptomyces species examined; (iv) the atypical GC content of nec1 relative to every other previously characterized gene within the genus Streptomyces; and (v) the association of IS1629 with otherwise unrelated plant pathogens, as well as its absence from 27 nonpathogenic isolates. One of the predictions of this model of horizontal transfer is the occurrence of Streptomyces isolates that are nonpathogenic and yet are otherwise related to described pathogenic species. Indeed, previous taxonomic studies have identified Streptomyces cluster groups into which S. acidiscabies and S. turgidiscabies could be rationally placed, yet no other plant pathogens have been described within these groups (11, 17).
The biological role of the nec1 gene product is currently not understood, although the expression of nec1 in Streptomyces lividans, a nonpathogen, is necessary and sufficient for the colonization and sporulation of S. lividans on plant tissue. This observation suggests that nec1 may provide some fitness advantage, allowing the utilization of plant cell nutrients which are otherwise unavailable to soilborne streptomycetes. The transmission of adaptive fitness traits among other gram-positive pathogens has been shown to involve various mobilizable elements, such as conjugative transposons or, less frequently, mobilizable transposons (18, 25, 27). These types of elements, which frequently carry insertion sequences, have not been reported in filamentous streptomycetes but rather are associated with clinically important Staphylococcus, Enterococcus, and Listeria species. The chromosomally linked conjugative transposon Tn5385 of Enterococcus faecalis, which encodes multidrug as well as heavy metal resistance determinants, was recently shown to be flanked by direct repeats of the insertion sequence IS1216 (22). Raze et al. (21) have recently reported a plasmid-borne multidrug resistance locus which was bordered by IS1216 in E. hirae. Interestingly, the frameshift-corrected ORFtnp exhibits 32% amino acid identity with the amino terminus of IS256, a component of Tn5385, as well as other composite transposons of enterococcal or staphylococcal origin (3, 19, 24), suggesting that the ORFtnp-nec1-IS1629 may be, or may have been, part of a conjugative element. Based on pulsed-field gel electrophoresis data (not shown), nec1 resides on the chromosome in S. scabies 84.34.
The data presented here represent the first report of an IS element, functional or otherwise, which is associated with plant-pathogenic Streptomyces spp. Several fundamental questions, however, remain unanswered. If the ORFtnp-nec1-IS1629 region constitutes part of a mobilizable pathogenicity island, what are its structural characteristics, e.g., how large is it, what are its borders, and what other pathogenicity factors may be found within it? The correlation between the presence of nec1 and the production of thaxtomin phytotoxins by plant pathogens (1) invites the speculation that these loci are perhaps linked within the boundaries of an as-yet-uncharacterized pathogenicity island. If this region has been mobilized among diverse plant pathogens, how has this occurred? What genetic or physiological factors would predispose certain streptomycetes to become plant pathogens, and what role, if any, has IS1629 played in the evolution of pathogen diversity, especially among S. scabies isolates? Physical analyses of the genetic context of these pathogenicity-associated loci, as well as experimental demonstration of genetic transfer among Streptomyces species, may aid in answering these questions.
ACKNOWLEDGMENTS
We would like to thank Keith Chater, Sueharu Horinouchi, Jacqueline Piret, Pat Solenberg, and Toru Takeuchi for generously providing Streptomyces strains and plasmids used in this study. We thank Merck for generously providing E. coli ET12567. We would also like to thank Steve Winans for critical review of this manuscript.
This work was supported by USDA NRI grant 97-35303-4487.
REFERENCES
- 1.Bukhalid R A, Chung S Y, Loria R. nec1, a gene conferring a necrogenic phenotype, is conserved in plant pathogenic Streptomyces species and linked to a transposase pseudogene. Mol Plant-Microbe Interact. 1998;11:960–967. doi: 10.1094/MPMI.1998.11.10.960. [DOI] [PubMed] [Google Scholar]
- 2.Bukhalid R A, Loria R. Cloning and expression of a gene from Streptomyces scabies encoding a putative pathogenicity factor. J Bacteriol. 1997;179:7776–7783. doi: 10.1128/jb.179.24.7776-7783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne M E. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989;81:361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- 4.Davis M A, Simons R W, Kleckner N. Tn10 protects itself at two levels from fortuitous activation by external promoters. Cell. 1985;43:379–387. doi: 10.1016/0092-8674(85)90043-1. [DOI] [PubMed] [Google Scholar]
- 5.Grindley N D F, Reed R R. Transpositional recombination in prokaryotes. Annu Rev Biochem. 1985;54:863–896. doi: 10.1146/annurev.bi.54.070185.004243. [DOI] [PubMed] [Google Scholar]
- 6.Gustafson C E, Chu S, Trust T J. Mutagenesis of the paracrystalline surface protein array of Aeromonas salmonicida by endogenous insertion elements. J Mol Biol. 1994;237:452–463. doi: 10.1006/jmbi.1994.1247. [DOI] [PubMed] [Google Scholar]
- 7.Hacker J, Blum Oehler G, Muehldorfer I, Tschaepe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 8.Healy F G, Lambert D H. Relationships among Streptomyces spp. causing potato scab. Int J Syst Bacteriol. 1991;41:479–482. [Google Scholar]
- 9.Hopwood D A, Bibb M J, Chater K F, Kieser T, Burton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: The John Innes Foundation; 1985. [Google Scholar]
- 10.Iida S, Meyer J, Arber W. Prokaryotic IS elements. In: Shapiro J A, editor. Mobile genetic elements. New York, N.Y: Academic Press, Inc.; 1983. pp. 159–221. [Google Scholar]
- 11.Lambert D H, Loria R. Streptomyces acidiscabies sp. nov. Int J Syst Bacteriol. 1989;39:393–396. [Google Scholar]
- 12.Lambert D H, Loria R. Streptomyces scabies sp. nov., nom. rev. Int J Syst Bacteriol. 1989;39:387–392. [Google Scholar]
- 13.Loria R, Bukhalid R A, Fry B A, King R R. Plant pathogenicity in the genus Streptomyces. Plant Dis. 1997;81:836–846. doi: 10.1094/PDIS.1997.81.8.836. [DOI] [PubMed] [Google Scholar]
- 14.Ma C K, Kolesnikow T, Rayner J C, Simons E L, Yim H, Simons R W. Control of translation by mRNA secondary structure: the importance of the kinetics of structure formation. Mol Microbiol. 1994;14:1033–1047. doi: 10.1111/j.1365-2958.1994.tb01337.x. [DOI] [PubMed] [Google Scholar]
- 15.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 16.Manzer F E, McIntyre G A, Merriam D C. A new potato scab problem in Maine. Univ Maine Orono Life Sci Agric Exp Stn Tech Bull. 1977;85:3–24. [Google Scholar]
- 17.Miyajima K, Tanaka F, Takeuchi T, Kuninaga S. Streptomyces turgidiscabies sp. nov. Int J Syst Bacteriol. 1998;48:495–502. doi: 10.1099/00207713-48-2-495. [DOI] [PubMed] [Google Scholar]
- 18.Murphy E. Transposable elements in gram-positive bacteria. In: Berg D E, editor. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 269–288. [Google Scholar]
- 19.Quintiliani R J, Courvalin P. Characterization of Tn1547, a composite transposon flanked by the IS16 and IS256-like elements, that confers vancomycin resistance in Enterococcus faecalis BM4281. Gene. 1996;172:1–8. doi: 10.1016/0378-1119(96)00110-2. [DOI] [PubMed] [Google Scholar]
- 20.Rao R N, Richardson M A, Kuhstoss S. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 1987;153:166–198. doi: 10.1016/0076-6879(87)53053-1. [DOI] [PubMed] [Google Scholar]
- 21.Raze D, Dardenne O, Hallut S, Martinez B-M, Coyette J, Ghuysen J M. The gene encoding the low-affinity penicillin-binding protein 3r in Enterococcus hirae S185R is borne on a plasmid carrying other antibiotic resistance determinants. Antimicrob Agents Chemother. 1998;42:534–539. doi: 10.1128/aac.42.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice L B, Carias L L. Transfer of Tn5385, a composite multiresistance chromosomal element from Enterococcus faecalis. J Bacteriol. 1998;180:714–721. doi: 10.1128/jb.180.3.714-721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross B C, Marino L, Oppedisano F, Edwards R, Robins-Browne R M, Johnson P D R. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol. 1997;35:1696–1700. doi: 10.1128/jcm.35.7.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouch D A, Byrne M E, Kong Y C, Skurray R A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987;133:3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- 25.Salyers A A, Shoemaker N B, Stevens A M, Li L Y. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev. 1995;59:579–590. doi: 10.1128/mr.59.4.579-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz V P, Reznikoff W S. Translation initiation of IS50R read-through transcripts. J Mol Biol. 1991;221:65–80. doi: 10.1016/0022-2836(91)80205-9. [DOI] [PubMed] [Google Scholar]
- 27.Scott J R, Churchward G G. Conjugative transposition. Annu Rev Microbiol. 1995;49:367–397. doi: 10.1146/annurev.mi.49.100195.002055. [DOI] [PubMed] [Google Scholar]
- 28.Solenberg P J, Burgett S G. Method for selection of transposable DNA and characterization of a new insertion sequence IS493 from Streptomyces lividans. J Bacteriol. 1989;171:4807–4813. doi: 10.1128/jb.171.9.4807-4813.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L K, Albert M J, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae O1 and O139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi T, Sawada H, Tanaka F, Matsuda I. Phylogenetic analysis of Streptomyces spp. causing potato scab based on 16S rRNA sequences. Int J Syst Bacteriol. 1996;46:476–479. doi: 10.1099/00207713-46-2-476. [DOI] [PubMed] [Google Scholar]
- 31.Wang C Y, Bond V C, Genco C A. Identification of a second endogenous Porphyromonas gingivalis insertion element. J Bacteriol. 1997;179:3808–3812. doi: 10.1128/jb.179.11.3808-3812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright F, Bibb M J. Codon usage in the G+C-rich Streptomyces genome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Sandt C H, Feulner G, Vlazny D A, Gray J A, Hill C W. Rhs elements of Escherichia coli K-12 complex composites of shared and unique components that have different evolutionary histories. J Bacteriol. 1993;175:2799–2808. doi: 10.1128/jb.175.10.2799-2808.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]