Abstract
Background
Alzheimer’s disease (AD) is one of the common neurodegenerative diseases, which often coexists with epilepsy. It is very significant to study the treatment options and the relationship between AD and epilepsy.
Aims
The purpose of this study was to analyze the top 100 cited papers about AD and epilepsy using bibliometrics, and to describe the current situation and predict research hot spots.
Methods
Top 100 papers were obtained from the Web of Science Core Collection (WoSCC). The WoSCC was used to analyze the author, institution, country, title, keywords, abstract, citation, subject category, publication year, impact factor (IF), and other functions. SPSS25 software was used for statistical analysis and CiteSpace V.5.7.R2 was used to visualize the information through collaborative networks.
Results
The number of publications gradually increased from 2000 to 2021. The total citation count for the top 100 papers ranged from 15 to 433(mean = 67.43). The largest number of papers were published in 2016 (n = 11). Meanwhile, USA (centrality: 0.93) and Columbia University (centrality: 0.06) were the most influential research country and institutions, respectively. The top contributing journals was Journal of Alzheimer’s Disease (8%). The IF for journals ranged from 1.819 to 53.44. A network analysis of the author’s keywords showed that “beta” (centrality: 0.39), “amyloid beta” (centrality: 0.29), “hyperexcitability” (centrality: 0.29) and “disease” (centrality: 0.29) had a high degree of centrality.
Conclusion
AD and epilepsy have been intensively studied in the past few years. The relationships, mechanisms and treatment of AD and epilepsy will be subjects of active research hotpots in future. These findings provide valuable information for clinicians and scientists to identify new perspectives with potential collaborators and cooperative countries.
Keywords: Alzheimer’s disease, epilepsy, bibliometric study, top-cited, citation
Introduction
Alzheimer’s disease (AD) and epilepsy are common neurological diseases. The number of patients with dementia and epilepsy in the global population is increasing, representing a growing problem for global health. The overall lifetime prevalence of epilepsy is 7.60 per 1,000 population [95% confidence interval (CI) 6.17–9.38] and the prevalence of epilepsy tends to peak in the elderly (Beghi, 2020). The prevalence of AD in Europe was estimated at 5.05% (95% CI, 4.73–5.39), and similar to epilepsy, AD prevalence increases with age (Niu et al., 2017). In fact, these two diseases are related. Epilepsy occurs more frequently in patients with AD than in those with non-Alzheimer’s disease (Samson et al., 1996). Recent findings showed that seizures could accelerate the decline of cognitive ability in patients with AD and that there might be an important bidirectional relationship between epilepsy and AD (Sen et al., 2018). AD and Epilepsy also share many pathological similarities (Lehmann et al., 2021), e.g., temporal lobe atrophy, neuronal death, gliosis, neuritic alterations, and neuroinflammation (Struble et al., 2010). In addition, AD plus epilepsy would lead to more serious clinical consequences, such as cognitive decline, weakness, anxiety, depression, social withdrawal, psychological and behavioral comorbidity, and poor treatment compliance (Cretin, 2021). Overtime, a large amount of literature has been published comprising a wide range of relevant research and clinical themes. However, the precise mechanisms leading to the development of seizures in the setting of AD are still under investigation and require further study. A meta-analysis showed that the quality of evidence on the treatment outcome of epilepsy in patients with AD was very low (Liu and Wang, 2021).
As a discipline emerging since its formal foundation, a bibliometric review of the literature was warranted to aid the synthesis and implementation of the evidence base. Despite citation analysis across a broad range of neurosciences (Yeung et al., 2017), there is limited information in the field of AD and epilepsy, with few published studies. Citation counting is an important metric to understand the significance of the contribution of research to a research field (Fox et al., 2021). Previous reviews only relied on individuals to study the research through literature summary and extraction, and thus cannot fully reflect the temporal and spatial distribution of researchers, institutions, and journals. Moreover, it is difficult to visualize the internal structure of the knowledge base and research focus, and systematic, comprehensive, and visual research are rarely found. Therefore, the present study aims to comprehensively analyze the current status, research hotpots, and development trends through a bibliometric analysis of the top 100 papers on AD and epilepsy published from 2000 to 2021. The findings may help follow-up researchers study the association between AD and epilepsy, identify journal publications and collaborators, and analyze keywords and research trends, which might promote research aiming to determine the cause, mechanism, and treatment of the disease.
Materials and methods
Data source
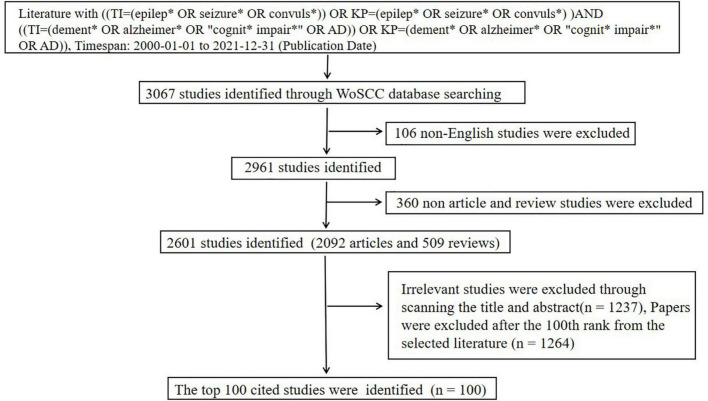
The retrieval data for measurement and statistical analysis were screened from the Web of Science Core Collection (WoSCC), which provided the citation search, giving access to multiple databases that reference cross-disciplinary research and allowing an in-depth exploration of specialized subfields (Wu et al., 2021). We conducted a literature search from the WoSCC on June 5th, 2022. In this study, the search criteria in the WoSCC database were as follows: ((TI = (epilep* OR seizure* OR convuls*)) OR KP = (epilep* OR seizure* OR convuls*)) AND ((TI = (dement* OR Alzheimer* OR “cognit* impair*” OR AD)) OR KP = (dement* OR Alzheimer* OR “cognit* impair*” OR AD)). Timespan: 2000-01-01 to 2021-12-31 (Publication Date). Document type: articles and reviews; Language: English. A total of 2601 records were retrieved. Then, two independent investigators reviewed the titles and abstracts and deleted studies that were not associated with AD and epilepsy, which excluded 1237 papers according to the criteria (Guo et al., 2021). And 1264 papers were excluded after the 100th rank from the selected literature. Finally, the top 100 studies were determined. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the WoSCC results is provided in Figure 1.
FIGURE 1.
Flow chart of literature collection.
Data analysis
In the present study, WoSCC was used to analyze the author, institution, country, title, keywords, abstract, citation, subject category, publication year, impact factor (IF), and other functions. The selected documents were imported into Excel (Microsoft Corporation, Redmond, WA, United States) and CiteSpace 5.8.R3 (64-bit) (Palop and Mucke, 2009). SPSS 25.0 statistical software (IBM Corp., Armonk, NY, United States) was used for statistical analysis. Continuous variables were expressed as the mean ± SD. Categorical variables were expressed as a percentage. CiteSpace, a bibliometric analysis tool, was created by Dr. Chaomei Chen (School of Information Science and Technology, Drexel University, Philadelphia, PA, United States) and his team. It visualizes countries/regions, institutions, authors and their cooperative relationships, co-cited references, and co-occurrence words through collaborative networks, which has been widely used in biomedical research fields.
Three folders of the research were created, including input folder placing the data downloaded from WoSCC, a data folder containing the data after deleting duplicate documents, and a project folder containing the data processed by cite. We did not find duplicate documents that needed to be deleted. The overall selected time span was from January 2000 to December 2021. Then, the slice length was set as 2 years. The node type was selected according to the type of analysis performed. The link lines between the nodes indicated the collaborative relationships. The size of the circles represented the number of papers published by the country/region, institute, or author. Purple rings indicated that these countries/regions, institutes, or authors had greater centrality.
Results
The 100 most-cited publications
We retrieved the 100 most frequently cited papers related to AD and epilepsy. The results were ranked according to citation counts to represent the 100 most-cited publications (76 articles and 24 reviews). A comprehensive list of the 100 publications and a citation details are presented in the Table 1.
TABLE 1.
The 100 most-cited publications.
| Rank | Article title | Journal | IF (2020) | Times Cited | Times Cited, in 2021 |
Times Cited, per year |
| 1 | Epilepsy and Cognitive Impairments in Alzheimer Disease | ARCHIVES OF NEUROLOGY | 7.419 | 433 | 39 | 31 |
| 2 | Amyloid beta-Induced Neuronal Hyperexcitability Triggers Progressive Epilepsy | JOURNAL OF NEUROSCIENCE | 6.167 | 405 | 54 | 28.93 |
| 3 | Incidence and predictors of seizures in patients with Alzheimer’s disease | EPILEPSIA | 5.886 | 391 | 37 | 23 |
| 4 | Seizures and Epileptiform Activity in the Early Stages of Alzheimer’s Disease | JAMA NEUROLOGY | 18.302 | 357 | 56 | 35.7 |
| 5 | Epileptic activity in Alzheimer’s disease: causes and clinical relevance | LANCET NEUROLOGY | 44.182 | 213 | 64 | 35.5 |
| 6 | Incidence and Impact of Subclinical Epileptiform Activity in Alzheimer’s Disease | ANNALS OF NEUROLOGY | 10.442 | 201 | 43 | 28.71 |
| 7 | Antisense Reduction of Tau in Adult Mice Protects against Seizures | JOURNAL OF THE INTERNATIONAL NEUROPSYCHOLOGICAL SOCIETY | 6.167 | 192 | 31 | 19.2 |
| 8 | Seizures in Alzheimer Disease Who, When, and How Common? | ARCHIVES OF NEUROLOGY | 7.419 | 187 | 21 | 13.36 |
| 9 | Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease | NATURE MEDICINE | 53.44 | 172 | 54 | 28.67 |
| 10 | A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation | EPILEPSIA | 5.886 | 167 | 15 | 13.92 |
| 11 | Tau Loss Attenuates Neuronal Network Hyperexcitability in Mouse and Drosophila Genetic Models of Epilepsy | JOURNAL OF NEUROSCIENCE | 6.167 | 148 | 26 | 14.8 |
| 12 | Seizures and Epilepsy in Alzheimer’s Disease | CNS NEUROSCIENCE AND THERAPEUTICS | 5.243 | 135 | 23 | 12.27 |
| 13 | Seizures in elderly patients with dementia - Epidemiology and management | DRUGS AND AGING | 3.923 | 132 | 8 | 6.6 |
| 14 | Down syndrome, Alzheimer’s disease, and seizures | BRAIN AND DEVELOPMENT | 1.961 | 128 | 8 | 7.11 |
| 15 | Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections | BRAIN | 13.501 | 123 | 28 | 17.57 |
| 16 | Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease | EPILEPSY AND BEHAVIOR | 2.937 | 113 | 23 | 8.69 |
| 17 | Septal networks: relevance to theta rhythm, epilepsy and Alzheimer’s disease | JOURNAL OF NEUROCHEMISTRY | 5.372 | 105 | 9 | 6.18 |
| 18 | Prevalence and causes of seizures at the time of diagnosis of probable Alzheimer’s disease | DEMENTIA AND GERIATRIC COGNITIVE DISORDERS | 2.959 | 94 | 8 | 5.53 |
| 19 | Cognition and dementia in older patients with epilepsy | BRAIN | 7.419 | 93 | 31 | 18.6 |
| 20 | Seizures in patients with Alzheimer’s disease or vascular dementia: A population-based nested casecontrol analysis | EPILEPSIA | 5.886 | 89 | 14 | 8.9 |
| 21 | Incidence of New-Onset Seizures in Mild to Moderate Alzheimer Disease | ARCHIVES OF NEUROLOGY | 7.419 | 82 | 11 | 7.45 |
| 22 | Seizures in Alzheimer’s disease | NEUROSCIENCE | 3.59 | 81 | 13 | 10.13 |
| 23 | Shared cognitive and behavioral impairments in epilepsy and Alzheimer’s disease and potential underlying mechanisms | EPILEPSY AND BEHAVIOR | 2.937 | 80 | 9 | 8 |
| 24 | Epileptic Seizures in AD Patients | NEUROMOLECULAR MEDICINE | 3.843 | 78 | 3 | 6 |
| 25 | Seizures in Alzheimer’s Disease: Clinical and Epidemiological Data | EPILEPSY CURRENTS | 7.5 | 71 | 11 | 6.45 |
| 26 | Spontaneous epileptiform discharges in a mouse model of Alzheimer’s disease are suppressed by antiepileptic drugs that block sodium channels | EPILEPSY RESEARCH | 3.045 | 65 | 9 | 5.42 |
| 27 | Relative Incidence of Seizures and Myoclonus in Alzheimer’s Disease, Dementia with Lewy Bodies, and Frontotemporal Dementia | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 59 | 22 | 9.83 |
| 28 | Epileptic Seizures in Alzheimer Disease A Review | ALZHEIMER DISEASE AND ASSOCIATED DISORDERS | 2.703 | 59 | 11 | 8.43 |
| 29 | Interictal spikes during sleep are an early defect in the Tg2576 mouse model of beta-amyloid neuropathology | SCIENTIFIC REPORTS | 4.38 | 59 | 11 | 8.43 |
| 30 | Epileptic Prodromal Alzheimer’s Disease, a Retrospective Study of 13 New Cases: Expanding the Spectrum of Alzheimer’s Disease to an Epileptic Variant? | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 58 | 13 | 8.29 |
| 31 | Levetiracetam monotherapy in Alzheimer patients with late-onset seizures: a prospective observational study | EUROPEAN JOURNAL OF NEUROLOGY | 6.089 | 58 | 8 | 3.63 |
| 32 | Common factors among Alzheimer’s disease, Parkinson’s disease, and epilepsy: Possible role of the noradrenergic nervous system | EPILEPSIA | 5.886 | 57 | 3 | 5.18 |
| 33 | Dietary energy substrates reverse early neuronal hyperactivity in a mouse model of Alzheimer’s disease | JOURNAL OF NEUROCHEMISTRY | 5.372 | 56 | 5 | 5.6 |
| 34 | Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model | ALZHEIMER’S RESEARCH AND THERAPY | 6.982 | 54 | 12 | 6.75 |
| 35 | Increased Cortical and Thalamic Excitability in Freely Moving APPswe/PS1dE9 Mice Modeling Epileptic Activity Associated with Alzheimer’s Disease | CEREBRAL CORTEX | 5.357 | 51 | 8 | 5.1 |
| 36 | Down Syndrome and Dementia: Seizures and Cognitive Decline | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 51 | 8 | 4.64 |
| 37 | The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and Alzheimer’s disease/mild cognitive impairment: a review | NEURORADIOLOGY | 2.804 | 48 | 2 | 3.69 |
| 38 | CA1 hippocampal neuronal loss in familial Alzheimer’s disease – Presenilin-1 E280A mutation is related to epilepsy | EPILEPSIA | 5.886 | 48 | 3 | 2.53 |
| 39 | Bexarotene reduces network excitability in models of Alzheimer’s disease and epilepsy | NEUROBIOLOGY OF AGING | 4.673 | 46 | 4 | 5.11 |
| 40 | Seizure resistance without parkinsonism in aged mice after tau reduction | NEUROBIOLOGY OF AGING | 4.673 | 45 | 5 | 5 |
| 41 | Early-Onset Network Hyperexcitability in Presymptomatic Alzheimer’s Disease Transgenic Mice Is Suppressed by Passive Immunization with Anti-Human APP/A beta Antibody and by mGluR5 Blockade | FRONTIERS IN AGING NEUROSCIENCE | 5.75 | 43 | 11 | 7.17 |
| 42 | Epigenetic suppression of hippocampal calbindin-D28k by Delta FosB drives seizure-related cognitive deficits | NATURE MEDICINE | 53.44 | 42 | 17 | 7 |
| 43 | Seizures in dominantly inherited Alzheimer disease | NEUROLOGY | 9.91 | 42 | 9 | 6 |
| 44 | Seizures in Alzheimer’s disease: a retrospective study of a cohort of outpatients | EPILEPTIC DISORDERS | 1.819 | 41 | 7 | 3.15 |
| 45 | Epilepsy presenting as AD: Neuroimaging, electroclinical features, and response to treatment | NEUROLOGY | 9.91 | 41 | 0 | 1.95 |
| 46 | Ectopic white matter neurons, a developmental abnormality that may be caused by the PSEN1 S169L mutation in a case of familial AD with myoclonus and seizures | JOURNAL OF NEUROPATHOLOGY AND EXPERIMENTAL NEUROLOGY | 3.685 | 41 | 0 | 1.86 |
| 47 | Alzheimer’s disease and late-onset epilepsy of unknown origin: two faces of beta amyloid pathology | NEUROBIOLOGY OF AGING | 4.673 | 40 | 16 | 10 |
| 48 | From here to epilepsy: the risk of seizure in patients with Alzheimer’s disease | EPILEPTIC DISORDERS | 1.819 | 40 | 6 | 5.71 |
| 49 | Alzheimer’s disease underlies some cases of complex partial status epilepticus - Clinical, radiologic, EEG, and pathologic correlations | JOURNAL OF CLINICAL NEUROPHYSIOLOGY | 2.177 | 40 | 2 | 1.74 |
| 50 | Early Onset of Hypersynchronous Network Activity and Expression of a Marker of Chronic Seizures in the Tg2576 Mouse Model of Alzheimer’s Disease | PLOS ONE | 3.24 | 39 | 10 | 4.88 |
| 51 | Chronic Temporal Lobe Epilepsy Is Associated with Enhanced Alzheimer-Like Neuropathology in 36 x Tg-AD Mice | PLOS ONE | 3.24 | 39 | 6 | 3.55 |
| 52 | Overview of cannabidiol (CBD) and its analogs: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease | EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY | 6.514 | 38 | 24 | 12.67 |
| 53 | Adult-Onset Epilepsy in Presymptomatic Alzheimer’s Disease: A Retrospective Study | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 38 | 9 | 6.33 |
| 54 | Seizures and dementia in the elderly: Nationwide Inpatient Sample 1999–2008 | EPILEPSY AND BEHAVIOR | 2.937 | 38 | 4 | 4.22 |
| 55 | Senile myoclonic epilepsy: Delineation of a common condition associated with Alzheimer’s disease in Down syndrome | SEIZURE-EUROPEAN JOURNAL OF EPILEPSY | 3.184 | 38 | 2 | 2.92 |
| 56 | Presenilin-1 mutation Alzheimer’s disease: A genetic epilepsy syndrome? | EPILEPSY AND BEHAVIOR | 2.937 | 37 | 4 | 3.08 |
| 57 | Sleep EEG Detects Epileptiform Activity in Alzheimer’s Disease with High Sensitivity | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 36 | 9 | 6 |
| 58 | Incidence and risk of seizures in Alzheimer’s disease: A nationwide population-based cohort study | EPILEPSY RESEARCH | 3.045 | 33 | 11 | 4.13 |
| 59 | Low brain ascorbic acid increases susceptibility to seizures in mouse models of decreased brain ascorbic acid transport and Alzheimer’s disease | EPILEPSY RESEARCH | 3.045 | 33 | 2 | 4.13 |
| 60 | Alzheimer’s Disease and Down Syndrome Rodent Models Exhibit Audiogenic Seizures | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 33 | 3 | 2.54 |
| 61 | Paroxysmal slow cortical activity in Alzheimer’s disease and epilepsy is associated with blood-brain barrier dysfunction | SCIENCE TRANSLATIONAL MEDICINE | 17.992 | 31 | 14 | 7.75 |
| 62 | Prevalence, Semiology, and Risk Factors of Epilepsy in Alzheimer’s Disease: An Ambulatory EEG Study | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 30 | 12 | 6 |
| 63 | Incidence of stroke and seizure in Alzheimer’s disease dementia | AGE AND AGEING | 10.668 | 30 | 7 | 3.75 |
| 64 | A presenilin 1 mutation (L420R) in a family with early onset Alzheimer disease, seizures and cotton wool plaques, but not spastic paraparesis | NEUROPATHOLOGY | 1.906 | 30 | 1 | 1.88 |
| 65 | Association of epileptiform abnormalities and seizures in Alzheimer disease | NEUROLOGY | 9.91 | 29 | 16 | 9.67 |
| 66 | Alzheimer-like amyloid and tau alterations associated with cognitive deficit in temporal lobe epilepsy | BRAIN | 13.501 | 28 | 15 | 9.33 |
| 67 | Tau-Induced Pathology in Epilepsy and Dementia: Notions from Patients and Animal Models | INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES | 5.924 | 28 | 10 | 5.6 |
| 68 | A mouse model of Alzheimer’s disease displays increased susceptibility to kindling and seizure-associated death | EPILEPSIA | 5.886 | 28 | 3 | 3.5 |
| 69 | Early Seizure Activity Accelerates Depletion of Hippocampal Neural Stem Cells and Impairs Spatial Discrimination in an Alzheimer’s Disease Model | CELL REPORTS | 9.423 | 27 | 14 | 6.75 |
| 70 | Traumatic Brain Injury Increases the Expression of Nos1, A beta Clearance, and Epileptogenesis in APP/PS1 Mouse Model of Alzheimer’s Disease | MOLECULAR NEUROBIOLOGY | 5.59 | 27 | 9 | 3.86 |
| 71 | Alternative ion channel splicing in mesial temporal lobe epilepsy and Alzheimer’s disease | GENOME BIOLOGY | 2.763 | 27 | 2 | 1.69 |
| 72 | Hyperpolarization-activated cyclic nucleotide gated channels: a potential molecular link between epileptic seizures and A beta generation in Alzheimer’s disease | MOLECULAR NEURODEGENERATION | 14.195 | 26 | 4 | 2.36 |
| 73 | Untangling Alzheimer’s Disease and Epilepsy | EPILEPSY CURRENTS | 7.5 | 26 | 2 | 2.36 |
| 74 | Increased Epileptiform EEG Activity and Decreased Seizure Threshold in Arctic APP Transgenic Mouse Model of Alzheimer’s Disease | CURRENT ALZHEIMER’S RESEARCH | 3.498 | 25 | 7 | 3.57 |
| 75 | GSK3 beta and Tau Protein in Alzheimer’s Disease and Epilepsy | FRONTIERS IN CELLULAR NEUROSCIENCE | 5.505 | 24 | 12 | 8 |
| 76 | Alterations of Coherent Theta and Gamma Network Oscillations as an Early Biomarker of Temporal Lobe Epilepsy and Alzheimer’s Disease | FRONTIERS IN INTEGRATIVE NEUROSCIENCE | 2.763 | 24 | 11 | 4.8 |
| 77 | Seizure susceptibility in the APP/PS1 mouse’ model of Alzheimer’s disease and relationship with amyloid beta plaques | BRAIN RESEARCH | 3.252 | 24 | 9 | 4 |
| 78 | A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy | BIOMEDICINE AND PHARMACOTHERAPY | 6.53 | 24 | 2 | 3.43 |
| 79 | Early onset familial Alzheimer disease with spastic paraparesis, dysarthria, and seizures and N135S mutation in PSEN1 | ALZHEIMER DISEASE AND ASSOCIATED DISORDERS | 2.703 | 22 | 5 | 1.47 |
| 80 | Epilepsy and antiepileptic drug use in elderly people as risk factors for dementia | JOURNAL OF THE NEUROLOGICAL SCIENCES | 3.181 | 22 | 4 | 1.38 |
| 81 | Do we know how to diagnose epilepsy early in Alzheimer’s disease? | REVUE NEUROLOGIQUE | 2.607 | 21 | 3 | 3.5 |
| 82 | Increased risk of epilepsy in patients registered in the Swedish Dementia Registry | EUROPEAN JOURNAL OF NEUROLOGY | 6.089 | 20 | 8 | 5 |
| 83 | Seizures as an early symptom of autosomal dominant Alzheimer’s disease | NEUROBIOLOGY OF AGING | 4.673 | 20 | 7 | 5 |
| 84 | Inflammasome-derived cytokine IL18 suppresses amyloid-induced seizures in Alzheimer-prone mice | PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA | 11.205 | 20 | 7 | 4 |
| 85 | Epileptic Amnesic Syndrome revealing Alzheimer’s disease | EPILEPSY RESEARCH | 3.045 | 20 | 0 | 1.82 |
| 86 | In Silico Analyses for Key Genes and Molecular Genetic Mechanism in Epilepsy and Alzheimer’s Disease | CNS AND NEUROLOGICAL DISORDERS-DRUG TARGETS | 4.388 | 19 | 4 | 3.8 |
| 87 | Seizures Can Precede Cognitive Symptoms in Late-Onset Alzheimer’s Disease | JOURNAL OF ALZHEIMER’S DISEASE | 4.472 | 19 | 0 | 1.58 |
| 88 | Dementia, delusions and seizures: storage disease or genetic AD? | EUROPEAN JOURNAL OF NEUROLOGY | 6.089 | 19 | 1 | 1.19 |
| 89 | A Case Series of Epilepsy-derived Memory Impairment Resembling Alzheimer Disease | ALZHEIMER DISEASE AND ASSOCIATED DISORDERS | 2.703 | 18 | 2 | 1.29 |
| 90 | A novel mutation (L250V) in the presenilin 1 gene in a Japanese familial Alzheimer’s disease with myoclonus and generalized convulsion | JOURNAL OF THE NEUROLOGICAL SCIENCES | 3.181 | 18 | 0 | 0.9 |
| 91 | Epilepsy and Alzheimer’s Disease: Potential mechanisms for an association | BRAIN RESEARCH BULLETIN | 4.079 | 17 | 7 | 5.67 |
| 92 | Subclinical epileptiform activity during sleep in Alzheimer’s disease and mild cognitive impairment | CLINICAL NEUROPHYSIOLOGY | 3.708 | 17 | 7 | 5.67 |
| 93 | A Longitudinal Study of Epileptic Seizures in Alzheimer’s Disease | FRONTIERS IN NEUROLOGY | 4.003 | 17 | 7 | 4.25 |
| 94 | Seizures in Alzheimer’s disease are highly recurrent and associated with a poor disease course | JOURNAL OF NEUROLOGY | 4.849 | 16 | 9 | 5.33 |
| 95 | Pharmacotherapeutic strategies for treating epilepsy in patients with Alzheimer’s disease | EXPERT OPINION ON PHARMACOTHERAPY | 3.889 | 16 | 5 | 3.2 |
| 96 | From Molecular Circuit Dysfunction to Disease: Case Studies in Epilepsy, Traumatic Brain Injury, and Alzheimer’s Disease | NEUROSCIENTIST | 7.519 | 16 | 1 | 2.29 |
| 97 | Alzheimer beta-amyloid blocks epileptiform activity in hippocampal neurons | MOLECULAR AND CELLULAR NEUROSCIENCE | 4.314 | 16 | 0 | 1.14 |
| 98 | Memory disturbances and temporal lobe epilepsy simulating Alzheimer’s disease: a case report | FUNCTIONAL NEUROLOGY | 1.855 | 16 | 0 | 0.8 |
| 99 | Epileptic seizures in autosomal dominant forms of Alzheimer’s disease | SEIZURE-EUROPEAN JOURNAL OF EPILEPSY | 3.184 | 15 | 3 | 3 |
| 100 | DBA/2J Genetic Background Exacerbates Spontaneous Lethal Seizures but Lessens Amyloid Deposition in a Mouse Model of Alzheimer’s Disease | PLOS ONE | 4.24 | 15 | 0 | 1.88 |
As shown in Table 1, The 100 most-cited articles received a total of 6743 citations (according to Web of science, WOS). The median number citations was 39, with a range of 15–433. For annual citations, the mean value was 7.41 with a range of 0.8–35.70. Seventeen papers were cited more than 100 times, and 36 were cited more than 50 times. The review entitled “Epilepsy and Cognitive Impairments in Alzheimer Disease” from Palop and Mucke (2009) was the most-cited publication (n = 433).
Publication years
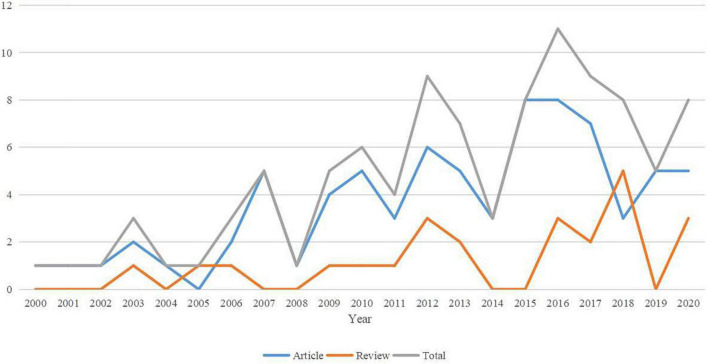
As shown in Figure 2, the top 100 most-cited papers were published from 2000 to 2020. Overall, publications showed a fluctuating upward trend. The largest number of studies was published in 2016 (n = 11), including eight articles and three reviews. The number of papers published in 2011 was higher than the number of papers published between 2000 and 2010.
FIGURE 2.
The number of publications among different types of papers according to publication year.
Contributions of countries
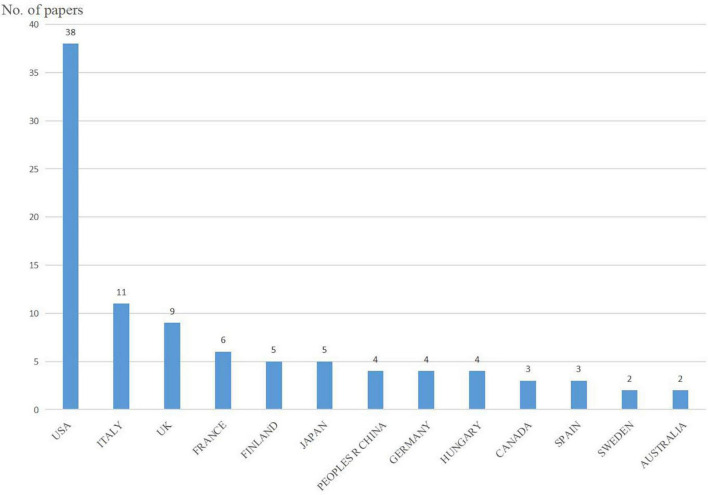
Overall, 22 countries contributed to the included studies, with nine countries publishing only one study. The United States was the largest contributor of studies (38%), followed by Italy (11%) and the United Kingdom (9%). The contributing countries are shown in Figure 3.
FIGURE 3.
Countries among the top 100 most-cited papers.
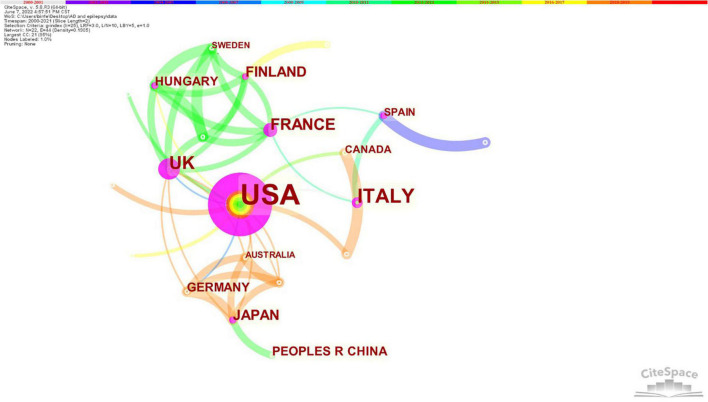
Generating a country map using CiteSpace resulted in 22 nodes and 44 links (Figure 4). The 100 papers were published by research groups in 22 countries. The top five countries were the United States, Italy, the United Kingdom, France, and Finland. The top three countries in terms of centrality were the United States (0.93), the United Kingdom (0.44), and France (0.21). An analysis in terms of publication and centrality indicated that the United States, the United Kingdom, Italy, and France were the main research powers in this research. United States has established cooperation with 12 countries, and the strongest collaborations were identified between United States, Canada, Israel, Greece, India, and Switzerland.
FIGURE 4.
Contributions of countries. Purple rings indicated that these countries/regions had greater centrality (no less than 0.1). The size of the circle represents the number of papers published by the country. The shorter the distance between two circles, the greater the cooperation between the two countries. United States was the most influential research country.
Contributions of institutions
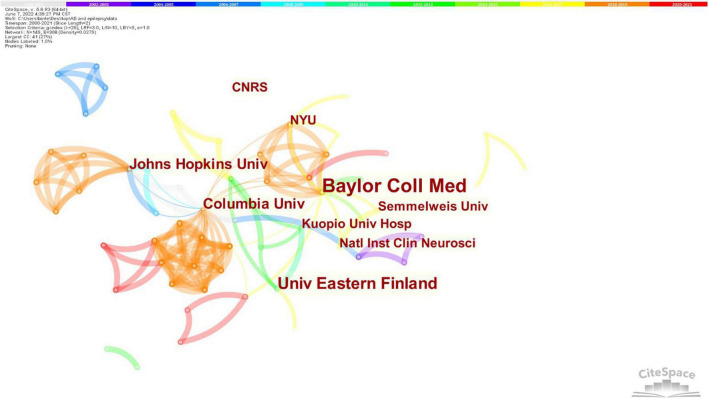
A total of 178 institutions published at least one top-cited paper, the distribution of institutions was very scattered, with 21 (11.80%) institutions publishing two papers and 148 (83.15%) institutions publishing only one paper. A small number of institutions accounted for a high proportion of the highest cited papers. Table 2 shows that the top nine institutions collectively published at least three papers. Baylor College of Medicine from United States topped the list with eight papers.
TABLE 2.
Top nine institutions with at least three papers in the top 100 most-cited papers.
| Rank | Institution | No. of papers | Centrality |
| 1 | Baylor College of Medicine | 8 | 0.04 |
| 2 | University of Eastern Finland | 5 | 0 |
| 3 | Columbia University | 4 | 0.06 |
| 4 | Johns Hopkins University | 4 | 0.04 |
| 5 | Kuopio University Hospital | 3 | 0 |
| 6 | Semmelweis University | 3 | 0 |
| 7 | National Institute of Clinical Neurosciences | 3 | 0 |
| 8 | New York University | 3 | 0.01 |
| 9 | Centre National de la Recherche Scientifique | 3 | 0 |
Figure 5 shows that generating an institution map resulted in 149 nodes and 308 links. The top 100 publications were distributed among 149 research institutions. The top five institutions were Baylor College Medicine, University of Eastern Finland, Columbia University, and Johns Hopkins University and Kuopio University Hospital. The top four institutions in terms of centrality were Columbia University (0.06), Baylor College of Medicine (0.04), Johns Hopkins University (0.04) and Indiana University (0.03). Analysis in terms of centrality indicated that Columbia University was the most influential research institution.
FIGURE 5.
Contributions of institutions. Nodes represent institutes, and the size of each node corresponds to the co-occurrence frequency of the institutes. The size of the circle represents the number of papers published by the institute. The shorter the distance between two circles, the greater the cooperation between the two institutes. Columbia University was the most influential research institutions.
Distribution of journals
The 100 most-cited articles were published in 59 journals; 18 journals published more than one study, which were distributed in the four partitions of Journal Citation Reports (JCR) (McVeigh, 2008). The JCR data provide both detailed journal information and flexible context, and category information to allow people to understand the way each journal functions in the literature. The major contributing journals are presented in Table 3. The top five journals that published the 100 most-cited AD and epilepsy studies included Journal of Alzheimer’s Disease (n = 8), Epilepsia (n = 6), Epilepsy and Behavior (n = 4), Neurobiology of Aging (n = 4) and Epilepsy Research (n = 4). With regard to the average citation number per paper, Journal of Neuroscience ranked first with a mean of 20.98 citations per paper, followed by Nature Medicine, with a mean of 17.84.
TABLE 3.
Journals contributed ≥ 2 papers in the top 100 most-cited papers.
| Journal | JCR category | Frequency | JCR partition | IF (2020) | IF (5 year) | Citations WOS Mean ± SD | Citations in 2021 Mean ± SD | Citations per year Mean ± SD |
| JOURNAL OF ALZHEIMER’S DISEASE | Neurosciences | 8 | Q2 | 4.472 | 4.851 | 40.50 ± 14.21 | 9.5 ± 6.66 | 5.65 ± 2.74 |
| EPILEPSIA | Clinical Neurology | 6 | Q1 | 5.886 | 7.1211 | 130 ± 136.84 | 12.5 ± 13.26 | 9.51 ± 7.82 |
| EPILEPSY AND BEHAVIOR | Behavioral Sciences; Clinical Neurology; Psychiatry | 4 | Q2, Q3, Q3 | 2.937 | 3.224 | 67 ± 36.63 | 10 ± 8.98 | 6.0 ± 2.76 |
| EPILEPSY RESEARCH | Clinical Neurology | 4 | Q3 | 3.045 | 3.143 | 37.75 ± 19.17 | 5.5 ± 5.32 | 3.88 ± 1.50 |
| NEUROBIOLOGY OF AGING | Geriatrics and Gerontology; Neurosciences | 4 | Q2, Q2 | 4.673 | 5.164 | 37.75 ± 12.12 | 8 ± 5.48 | 6.28 ± 2.48 |
| ALZHEIMER DISEASE AND ASSOCIATED DISORDERS | Clinical Neurology; Pathology | 3 | Q3, Q3 | 2.703 | 3.007 | 33 ± 22.61 | 6 ± 4.58 | 3.73 ± 4.07 |
| ARCHIVES OF NEUROLOGY | Clinical Neurology | 3 | Q1 | 7.419 | 7.249 | 234 ± 180.16 | 23.67 ± 14.19 | 17.27 ± 12.25 |
| BRAIN | Clinical Neurology; Neurosciences | 3 | Q1, Q1 | 13.501 | 14.25 | 81.33 ± 48.56 | 24.67 ± 8.51 | 15.17 ± 5.08 |
| EUROPEAN JOURNAL OF NEUROLOGY | Clinical Neurology; Neurosciences | 3 | Q1, Q1 | 6.089 | 5.308 | 32.33 ± 22.23 | 5.67 ± 4.04 | 3.27 ± 1.93 |
| JOURNAL OF NEUROSCIENCE | Neurosciences | 3 | Q1 | 6.167 | 6.993 | 248.33 ± 137.45 | 37 ± 14.93 | 20.98 ± 7.23 |
| NEUROLOGY | Clinical Neurology | 3 | Q1 | 9.91 | 10.664 | 37.33 ± 12.12 | 8.33 ± 8.02 | 5.87 ± 3.86 |
| PLOS ONE | Multidisciplinary Sciences | 3 | Q3 | 3.24 | 3.788 | 31 ± 13.86 | 5.33 ± 5.03 | 3.44 ± 1.50 |
| EPILEPSY CURRENTS | Clinical Neurology | 2 | Q1 | 7.5 | 8.772 | 48.50 ± 31.82 | 6.50 ± 6.36 | 4.41 ± 2.89 |
| EPILEPTIC DISORDERS | Clinical Neurology | 2 | Q4 | 1.819 | 2.352 | 40.50 ± 0.71 | 6.50 ± 0.71 | 4.43 ± 1.81 |
| JOURNAL OF NEUROCHEMISTRY | Biochemistry and Molecular Biology; Neurosciences | 2 | Q2, Q1 | 5.372 | 5.69 | 80.50 ± 34.65 | 7 ± 2.83 | 5.89 ± 0.41 |
| JOURNAL OF THE NEUROLOGICAL SCIENCES | Clinical Neurology; Neurosciences | 2 | Q3, Q3 | 3.181 | 3.403 | 20 ± 2.83 | 2 ± 2.83 | 1.14 ± 0.34 |
| NATURE MEDICINE | Biochemistry and Molecular Biology; Cell Biology; Research and Experimental Medicine | 2 | Q1, Q1, Q1 | 53.44 | 49.248 | 107.00 ± 91.92 | 35.50 ± 26.16 | 17.84 ± 15.32 |
| SEIZURE-EUROPEAN JOURNAL OF EPILEPSY | Clinical Neurology; Neurosciences | 2 | Q3, Q3 | 3.184 | 3.729 | 26.50 ± 16.26 | 2.50 ± 0.71 | 2.96 ± 0.06 |
The IF for journals in the top 100 most-cited papers ranged from 1.819 to 53.44, among which 37 journals had an IF between 3 and 5, 34 journals had an IF between 5 and 10, and 3 journals had an IF above 20 (Figure 6).
FIGURE 6.
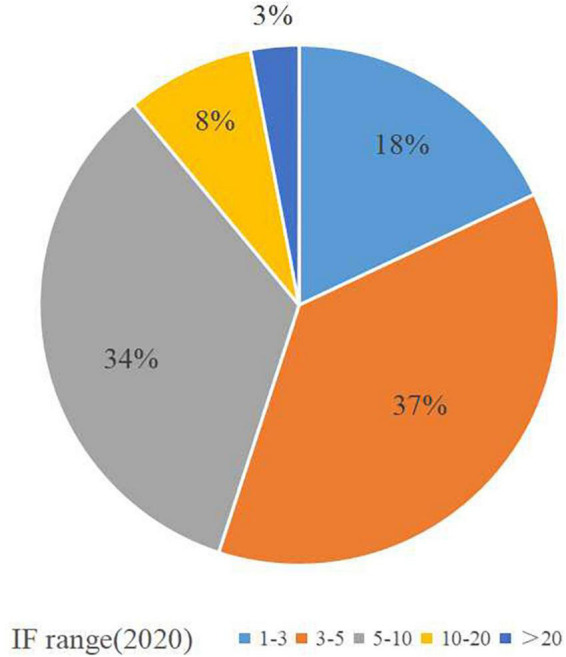
The number of articles corresponding to different IF in the top 100 most-cited papers.
In Table 4, the top 100 papers were classified into different study fields on the basis of WOS categories. The leading WOS category was “Clinical Neurology” (n = 54), following by “Neurosciences” (n = 49) and “Behavioral Sciences” (n = 10).
TABLE 4.
The number of study fields on the basis of WOS categories.
| WoS Categories | Frequency |
| Clinical Neurology | 54 |
| Neurosciences | 49 |
| Behavioral Sciences | 10 |
| Geriatrics and Gerontology | 8 |
| Multidisciplinary Sciences | 5 |
| Psychiatry | 5 |
| Pathology | 5 |
| Biochemistry and Molecular Biology | 5 |
Major contributing authors
Overall, a total of 606 authors contributed to the 100 studies. There was wide, disparate authorship of first authors, with 90 different first authors represented in the 100 included publications. A total of four contributors published the most articles, namely Tanila Heikki, Noebels Jeffrey, Pitkänen, Asla and Scharfman, Helen E, who all published five publications, the total number of citations for the papers were 629, 592, 578, and 234 respectively. Only three authors have published three studies as a first author. Keith A. Vossel from the University of California, as a first author and corresponding author, had the largest number of total citations in 2021 (n = 185). Table 5 presents results for authors who contributed three or more of the 100 most-cited papers.
TABLE 5.
Major contributing authors in the top 100 most-cited list.
| Author | Frequency | Total citations WOS | Total citation in 2021 | First author | Correspond author | Co-author | Constitution | Country |
| Tanila, Heikki | 5 | 629 | 92 | 0 | 2 | 4 | Kuopio University Hospital | Finland |
| Noebels, Jeffrey | 5 | 592 | 110 | 1 | 2 | 3 | Baylor Coll Medicine | United States |
| Pitkänen, Asla | 5 | 578 | 84 | 0 | 2 | 3 | Kuopio University Hospital | Finland |
| Scharfman, Helen E. | 5 | 234 | 33 | 1 | 2 | 3 | New York University Langone Medical Center | United States |
| Vossel, Keith A. | 4 | 830 | 185 | 3 | 4 | 0 | University of California | United States |
| Beagle, Alexander J. | 3 | 617 | 121 | 1 | 0 | 2 | University of California | United States |
| Miller, Bruce L. | 3 | 701 | 163 | 0 | 0 | 3 | University of California | United States |
| Mucke, Lennart | 3 | 991 | 138 | 0 | 0 | 3 | University of California | United States |
| Scarmeas, Nikolaos | 3 | 393 | 55 | 1 | 2 | 1 | University of California | United States |
| Szûcs, Anna | 3 | 128 | 31 | 0 | 0 | 3 | Semmelweis University | Hungary |
| Barcs, Gábor | 3 | 128 | 31 | 0 | 0 | 3 | Semmelweis University | Hungary |
| Horváth, András | 3 | 128 | 31 | 3 | 3 | 0 | Semmelweis University | Hungary |
| Kamondi, Anita | 3 | 128 | 31 | 0 | 0 | 3 | Semmelweis University | Hungary |
| Blanc, Frédéric | 3 | 120 | 22 | 0 | 0 | 3 | University of Strasbourg | France |
| Cretin, Benjamin | 3 | 94 | 18 | 3 | 3 | 0 | University of Strasbourg | France |
| Sellal, Francois | 3 | 120 | 22 | 0 | 0 | 3 | University of Strasbourg | France |
| Gurevicius, Kestutis | 3 | 172 | 22 | 1 | 1 | 2 | Kuopio University Hospital | Finland |
| Ziyatdinova, Sofya | 3 | 146 | 21 | 2 | 1 | 1 | University of Eastern Finland | Finland |
| Larner, A. J. | 3 | 214 | 15 | 2 | 3 | 0 | Walton Centre for Neurology and Neurosurgery | United Kingdom |
Analysis of co-occurring keywords
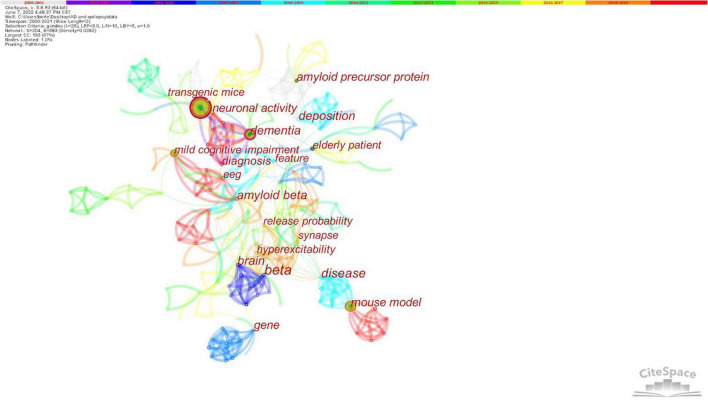
CiteSpace was used to extract the keywords from the top 100 papers on AD and epilepsy. A network analysis of the author’s keywords or subject words was carried out during the publication time of the articles, namely, 2000–2021. Table 6 shows that the top five keywords are epilepsy (n = 41), dementia (n = 21), mouse model (n = 21), mild cognitive impairment (n = 13), and Alzheimer’s disease (n = 11). The greater the centrality value, the more cooperation between the node and other nodes. Figure 7 shows that “beta” (centrality: 0.39), “amyloid beta” (centrality: 0.29), “hyperexcitability” (centrality: 0.29) and “disease” (centrality: 0.29) had a high degree of centrality during this period. A comprehensive analysis of centrality showed that “beta” (n = 7, centrality: 0.39), “disease” (n = 3, centrality: 0.29), “dementia” (n = 21, centrality: 0.25) and “brain” (n = 4, centrality: 0.25) are the most influential keywords in this field.
TABLE 6.
Frequency of co-occurring keywords.
| Keyword | Frequency | Centrality |
| Epilepsy | 41 | 0.13 |
| Dementia | 21 | 0.25 |
| Mouse model | 21 | 0.11 |
| Mild cognitive impairment | 13 | 0.14 |
| Alzheimer’s disease | 11 | 0.17 |
| Amyloid precursor protein | 10 | 0.13 |
| Diagnosis | 8 | 0.23 |
| Elderly patient | 8 | 0.2 |
| Neuronal activity | 7 | 0.23 |
| Beta | 7 | 0.39 |
| EEG | 6 | 0.09 |
| Transgenic mice | 6 | 0.12 |
| Amyloid beta | 6 | 0.29 |
FIGURE 7.
Analysis of co-occurrence Keywords. Nodes represent keywords, and the size of each node corresponds to the co-occurring frequency of the keywords. The color of the lines that appear together between keywords indicates chronological order: grey represents the oldest, and orange the newest. “beta” had a highest degree of centrality.
Discussion
The aim of this paper was to perform a bibliometric study of the top 100 most-cited publications on AD and epilepsy over the last 22 years. To the best of our knowledge, this was the first study to provide an overview the current main status of development, hot spots of study, and the future trends in AD and epilepsy. Our study is of great significance for students, researchers, and clinicians working in the field. Here, we summarize several characteristics of these papers to help understand the history and professional prospects comprehensively.
The average number of citations for the 100 most-cited clinical trials in AD and epilepsy was 67.43 (range: 15–433), well below the number of citations observed in general neuroscience articles (3,087) (Yeung et al., 2017). However, citation counts are not a direct measure of scientific quality and importance. The number of citations can be regarded as a relatively reasonable index to evaluate the quality of papers, which varies in different sub disciplines and depends on the size of the scientific community. Citation count analysis might be related to journal IF, publication frequency, and publication year. In general, an article with 100 or more citations is considered a “classic” in the research field and might even be a seminal paper (Xiong et al., 2021). However, our study showed that only 17 papers were cited more than 100 times, this can possibly come from the fact that comorbid epilepsy in AD may not be an interesting topic for many clinicians. In fact, cognitivists dealing with AD patients are not highly aware of epilepsy and, inversely, epileptologists may be poorly informed of dementing diseases. Therefore, the AD-epilepsy topic may have interest only for a small research community, which can result in a relatively low citation index.
The most-cited publication was “Epilepsy and Cognitive Impairments in Alzheimer’s Disease” (Palop and Mucke, 2009), wrote by Palop and Mucke (2009), with 433 total citations, 31 annual citations, and 39 citations in 2021. It may be relevant to current research and have far-reaching implications. However, in general, consensus and position papers, guides, and systematic reviews receive more citations than original articles, which is a bias that must be considered in citation analysis. The article that ranked second, which was based on a “Amyloid beta-Induced Neuronal Hyperexcitability Triggers Progressive Epilepsy” (Minkeviciene et al., 2009) had a total of 405 citations, 28.93 annual citations and 54 citations in 2021; it was published in Journal of Neuroscience in 2009 and written by Minkeviciene et al. (2009). The team performed video-EEG recordings in mice carrying mutant human APPswe and PS1dE9 genes (APdE9 mice) and their wild-type littermates, identifying fibrillar Abeta may be the cause of epileptiform activity.
The number of papers published has increased in recent years. Although the most cited publications tend to be from the first few years (2000–2010), the number of highly cited articles published from 2011–2020 is nearly three times that published from 2000–2010. In 2016, the number of publications was the largest, reaching 11, which showed that a recently published article is likely to gradually improve the quality of research in recent years and have a potential academic importance in the future.
The majority of research originated from the United States (38%). A bibliometric analysis of the most cited articles in neurocritical care research showed that United States was the country with most articles (60, 35 primary research) and citations (6115) among the top 100 (Ramos et al., 2019). Baylor College of Medicine from United States topped the list with eight papers. The distribution of country and institutions was very scattered. The United States and its institutions have played a leading role in AD and epilepsy research. The United States was also ranked first in other fields of neurology (Xiong et al., 2021), and it is also a research leader in terms of quality and quantity. However, compared with other studies, our study showed that the cooperation between countries and institutions was not close (Yin et al., 2019). In fact, just like the study of Parkinson’s disease (Li et al., 2008), more cooperation between different countries and institutions might be an effective way to promote the development of AD and epilepsy research worldwide. Contrastingly, distribution analysis showed that research was widespread all over the world and the diseases had research value.
Baylor College of Medicine and Columbia University played important roles in in the field of AD and epilepsy research. Baylor College of Medicine, as one of the top colleges and universities in Texas, United States, focused on cooperative research programs, discovered basic insights into human health and diseases through extensive interdisciplinary and interdisciplinary cooperation, and applied their findings to develop new diagnostic tools and treatments. Columbia University is a world-class private research university located in Manhattan, NY, United States. It carried out extensive research in the field of neuroscience.
The 100 included articles were published in 59 journals, among which 18 journals published more than one study. The papers were distributed in four partitions of JCR. The IF of the top five journals was less than six. The top journal among the list of the 100 most-cited studies was Journal of Alzheimer’s Disease (IF = 4.472). The leading WOS categories focused on “Clinical Neurology” and “Neurosciences,” with few interdisciplinary studies. Resulting from its interdisciplinary nature, Psycho-Oncology has been subject to extensive interdisciplinary research, which has provided great help for the development of the discipline (Fox et al., 2021). Therefore, we look forward to more interdisciplinary research.
Author analysis revealed a network of core author collaborations in the field of AD and epilepsy research. This information might be relevant to clinical researchers and research institutions who are searching for a network of research leaders in the field to explore potential collaborations. Our authorship analysis does not show as many authors as other areas in research. The most contributing author was Tanila, Heikki from Kuopio University Hospital from Finland. Related research focuses on the pathogenesis of epilepsy in mouse models of AD, which is a research hotspot in this field.
Keyword analysis showed that “beta,” “amyloid beta,” “hyperexcitability,” “disease,” “dementia,” and “brain” had high influential. indicating that these research directions are very significant. This result showed that researchers have begun to extend their research to the pathogenesis. These results also show that AD and epilepsy is still a disease that requires to be solved urgently. We must explore the deficiencies and innovations in this field, such as treatment, pathological mechanism, and disease management on improve the quality of life of patients.
Limitations
The present study has some limitations. First, there are several intrinsic limitations of using citation analysis to evaluate the academic importance of a specific article, author, or journal. There is a certain bias in citation analysis, such as the fact that papers written in English, papers that can be accessed through open access, and papers published in journals with high IFs are more likely to be cited. In addition, through a “snowball effect,” people tended to cite publications that are already highly cited (Yeung et al., 2017). We selected the top 100 papers, but citation searches are “time-dependent,” older articles are likely to be cited more often, and the newest list of highly cited articles may be dominated by some older articles. Furthermore, citation analysis might severely underestimate the impact of clinical research as compared to basic research (van Eck et al., 2013). Second, the search was limited to the WOS database. It did not record citations by textbooks and other databases. Our study only selected papers written in English, which might have yielded an incomplete search.
Conclusion
We identified the 100 most cited papers in the field of AD and epilepsy. By reviewing these top cited papers, researchers can immediately understand the hot topics and research collaborations on AD and epilepsy, and improve their work. This study shows that the relationship, mechanism, and treatment of AD and epilepsy have been widely studied, and in recent years, this field has shown new vitality; however, there is a general lack of cooperation between countries, and the mechanism of epilepsy and AD is unclear, which deserves further study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
G-FZ and YG designed the study. G-FZ drafted and edited the manuscript. G-FZ and W-XG analyzed the data. G-FZ, Z-Y-RX, YG, and W-XG revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81871010).
References
- Beghi E. (2020). The Epidemiology of Epilepsy. Neuroepidemiology 54 185–191. 10.1159/000503831 [DOI] [PubMed] [Google Scholar]
- Cretin B. (2021). Treatment of Seizures in Older Patients with Dementia. Drugs Aging 38 181–192. 10.1007/s40266-020-00826-2 [DOI] [PubMed] [Google Scholar]
- Fox S., Lynch J., D’Alton P., Carr A. (2021). Psycho-Oncology: a Bibliometric Review of the 100 Most-Cited Articles. Healthcare 9:1008. 10.3390/healthcare9081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Xu Z. Y. R., Cai M. T., Gong W. X., Shen C. H. (2021). Epilepsy With Suicide: a Bibliometrics Study and Visualization Analysis via CiteSpace. Front. Neurol. 12:823474. 10.3389/fneur.2021.823474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann L., Lo A., Knox K. M., Barker-Haliski M. (2021). Alzheimer’s Disease and Epilepsy: a Perspective on the Opportunities for Overlapping Therapeutic Innovation. Neurochem. Res. 46 1895–1912. 10.1007/s11064-021-03332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Ho Y. S., Li C. Y. (2008). Bibliometric analysis on global Parkinson’s disease research trends during 1991-2006. Neurosci. Lett. 441 248–252. 10.1016/j.neulet.2008.06.044 [DOI] [PubMed] [Google Scholar]
- Liu J., Wang L. N. (2021). Treatment of epilepsy for people with Alzheimer’s disease. Cochrane Database Syst. Rev. 12:CD011922. 10.1002/14651858.CD011922.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh M. E. (2008). Neurology in the journal citation reports. Neurology 71 1848–1849. 10.1212/01.wnl.0000338902.81462.88 [DOI] [PubMed] [Google Scholar]
- Minkeviciene R., Rheims S., Dobszay M. B., Zilberter M., Hartikainen J., Fülöp L. (2009). Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29 3453–3462. 10.1523/JNEUROSCI.5215-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H., Álvarez-Álvarez I., Guillén-Grima F., Aguinaga-Ontoso I. (2017). Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia 32 523–532. 10.1016/j.nrl.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Palop J. J., Mucke L. (2009). Epilepsy and Cognitive Impairments in Alzheimer Disease. Arch. Neurol. 66 435–440. 10.1001/archneurol.2009.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M. B., Koterba E., Rosi Júnior J., Teixeira M. J., Figueiredo E. G. A. (2019). Bibliometric Analysis of the Most Cited Articles in Neurocritical Care Research. Neurocrit. Care 31 365–372. 10.1007/s12028-019-00731-6 [DOI] [PubMed] [Google Scholar]
- Samson W. N., van Duijn C. M., Hop W. C., Hofman A. (1996). Clinical features and mortality in patients with early-onset Alzheimer’s disease. Eur. Neurol. 36 103–106. 10.1159/000117218 [DOI] [PubMed] [Google Scholar]
- Sen A., Capelli V., Husain M. (2018). Cognition and dementia in older patients with epilepsy. Brain 141 1592–1608. 10.1093/brain/awy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble R. G., Ala T., Patrylo P. R., Brewer G. J., Yan X. X. (2010). Is brain amyloid production a cause or a result of dementia of the Alzheimer’s type? J. Alzheimers Dis. 22 393–399. 10.3233/JAD-2010-100846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck N. J., Waltman L., van Raan A. F., Klautz R. J., Peul W. C. (2013). Citation analysis may severely underestimate the impact of clinical research as compared to basic research. PLoS One. 8:e62395. 10.1371/journal.pone.0062395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. Q., Wu D. Q., Hu C. P., Iao L. S. (2021). Studies on Children With Developmental Coordination Disorder in the Past 20 Years: a Bibliometric Analysis via CiteSpace. Front Psychiatry 12:776883. 10.3389/fpsyt.2021.776883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H. Y., Liu H., Wang X. Q. (2021). Top 100 Most-Cited Papers in Neuropathic Pain From 2000 to 2020: a Bibliometric Study. Front. Neurol. 12:765193. 10.3389/fneur.2021.765193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. W. K., Goto T. K., Leung W. K. (2017). At the Leading Front of Neuroscience: a Bibliometric Study of the 100 Most-Cited Articles. Front. Hum. Neurosci. 11:363. 10.3389/fnhum.2017.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Cheng F., Wang X., Mu J., Ma C., Zhai C., et al. (2019). Top 100 cited articles on rheumatoid arthritis: a bibliometric analysis. Medicine 98:e14523. 10.1097/md.0000000000014523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.