Abstract
Methamphetamine (METH) is a highly addictive psychostimulant. The continuous use of METH may lead to its abuse and neurotoxicity that have been associated with METH-induced increases in release of dopamine (DA) and glutamate in the brain. METH action in DA has been shown to be mediated by redistribution of DA from vesicles into cytoplasm via vesicular monoamine transporter 2 (VMAT2) and the subsequent reversal of membrane DA transporter (DAT), while little is known about the mechanisms underlying METH-induced glutamate release. Recent studies indicate that a subpopulation of midbrain DA neurons co-expresses VMAT2 and vesicular glutamate transporter 2 (VGLUT2). Therefore, we hypothesized that METH-induced glutamate release may in part originate from such a dual phenotype of DA neurons. To test this hypothesis, we used Cre-LoxP techniques to selectively delete VGLUT2 from midbrain DA neurons, and then examined nucleus accumbens (NAc) DA and glutamate responses to METH using in vivo brain microdialysis between DA-VGLUT2-KO mice and their VGLUT2-HET littermates. We found that selective deletion of VGLUT2 from DA neurons did not significantly alter basal levels of extracellular DA and glutamate, but attenuated METH-induced increases in extracellular levels of DA and glutamate. In addition, DA-VGLUT2-KO mice also displayed lower locomotor response to METH than VGLUT2-HET control mice. These findings, for the first time, suggest that cell-type specific VGLUT2 expression in DA neurons plays an important role in the behavioral and neurochemical effects of METH. Glutamate corelease from DA neurons may in part contributes to METH-induced increase in NAc glutamate release.
Keywords: Methamphetamine, Dopamine, Glutamate, Accumbent, VMAT2, VGLUT2
1. Introduction
Methamphetamine (METH) is a synthetic amphetamine-like psychostimulant. It is among the most widely used illicit substances next to cannabis, opiates, and other stimulants (UNODOC, 2019). METH abuse is especially prevalent in Southeast and East Asia, but is also of significant concern in other regions such as North America (Kuitunen-Paul et al., 2020), due to its simple process of synthesis, euphoric properties, low cost and availability. The highly addictive properties of METH are attributable to its effect on monoamine release, particularly dopamine (DA) release. METH is a substrate of the dopamine transporter (DAT) and the vesicular monoamine transporter 2 (VMAT2). METH is first concentrated in the cytoplasm via DAT and then enters vesicles via VMAT2. VMAT2 is a substrate/proton antiporter. Each molecule of METH that enters vesicle causes 2 protons extruded, which diminishes vesicular H+ concentration. The pH gradient is the main driving force for vesicular loading and retention of DA. In the absence of the pH gradient, the DA is rapidly accumulated in cytoplasma. This rapid increase in cytosolic DA causes the DAT to work in an opposite direction to release DA into the extracellular space (Baracz and Cornish, 2016; Elkashef et al., 2008; Freyberg et al., 2016). Unlike other drugs of abuse, METH also promotes glutamate release in multiple brain regions including the nucleus of accumbens (NAc), dorsal striatum, ventral tegmental area (VTA), prefrontal cortex (PFC), and hippocampus (Ernst and Chang, 2008; Mark et al., 2007; Raudensky and Yamamoto, 2007; Shoblock et al., 2003; Stephans and Yamamoto, 1994; Xue et al., 1996; Zhang et al., 2001; Szumlinski et al., 2017), but see (Lominac et al., 2016; Shoblock et al., 2003). Such increases in extracellular DA and glutamate have been associated with not only METH abuse and dependence (Baracz and Cornish, 2016; Sattler and Tymianski, 2001), but also DA neuron toxicity and neurodegeneration after excessive release by high doses of METH (Cadet and Krasnova, 2009; Sattler and Tymianski, 2001).
In contrast to DA response to METH, little is known about the mechanisms through which METH causes glutamate release. Several hypotheses have been proposed to explain this effect. A prevailing hypothesis is that the METH-induced increase in glutamate may be mediated indirectly by a GABAergic mechanism (Mark et al., 2007), i.e., METH may cause an increase in the striatonigral GABA release, which in turn decreases GABAergic nigrothalamic activity, disinhibit thalamocortical activity and increase corticostriatal glutamate release. However, this hypothesis does not explain how METH initially alters striatonigral GABA release (Mark et al., 2007). The second hypothesis is that METH may directly modulate presynaptic glutamate release (Zhang et al., 2014). However, it remains unknown how METH promotes presynaptic glutamate release.
Here we propose an alternative hypothesis that METH-enhanced glutamate release may be partially derived from a dual phenotype of DA-glutamate neurons. This is based on recent studies indicating that a subpopulation of midbrain DA neurons in the VTA and substantia nigra pars compacta (SNc) co-express tyrosine hydroxylase (TH) and VGLUT2 (Berube-Carriere et al., 2009; Chuhma et al., 2009; Shen et al., 2018) or co-express VMAT2 and VGLUT2 (Silm et al., 2019), and therefore, corelease DA and glutamate from DA terminals in the striatum in rats and mice (Morales and Margolis, 2017; Trudeau, 2004). In addition, VGLUT2 is also highly expressed in glutamatergic neurons in several subcortical brain regions such as the thalamus, VTA, and SNc (Kaneko and Fujiyama, 2002; Morales and Root, 2014). Selective deletion of the VGLUT2 gene in DA neurons decreased cocaine- or amphetamine-induced behavioral hyperactivity (Birgner et al., 2010; Hnasko et al., 2010) and altered cocaine and sucrose self-administration (Alsio et al., 2011), suggesting that glutamate release from DA neurons may play a role in psychostimulant abuse and addiction. In addition, selective deletion of VGLUT2 from DA neurons also increases vulnerability of DA neurons to neurotoxins (Fortin et al., 2012; Shen et al., 2018), suggesting that glutamate release from DA neurons is neuroprotective. However, it is unknown whether VGLUT2 in DA neurons plays a role in the behavioral and neurochemical effects of METH and whether glutamate release from DA neurons contribute to METH-induced increase in glutamate release.
VGLUT2 has been generally believed to promote glutamate loading into synaptic glutamate vesicles (Morales and Margolis, 2017; Zhang et al., 2015). However, recent studies indicate that VGLUT2 also promotes DA loading into vesicles (Aguilar et al., 2017; Hnasko et al., 2010), suggesting a possible interaction between VGLUT2 and VMAT2 in loading DA into vesicles. These findings raise such a possibility that METH may initially act at VMAT2 in the DA-glutamate neurons that causes co-release of DA and glutamate from this type of neurons. To test this hypothesis, we generated conditional VGLUT2-KO mice in midbrain DA neurons (DA-VGLUT2-KO) and examined DA and glutamate responses to METH using in vivo brain microdialysis. We found that selective deletion of VGLUT2 in DA neurons significantly attenuate the behavioral and neurochemical responses to METH, suggesting an important role of dopaminergic VGLUT2 in METH abuse and dependence.
2. Materials and methods
2.1. Animals
Male adult conditional VGLUT2-knockout (KO) mice (VGLUT2lox/lox;DATCre+/− or Slc17a6lox/lox;Slc6a3cre+/−, DA-VGLUT2-KO) and their heterozygous littermates (VGLUT2lox/+;DATCre+/−, VGLUT2-Het) (Backman et al., 2006; Hnasko et al., 2010), aged 8–12 weeks, were bred at the National Institute on Drug Abuse (NIDA), Intramural Research Program (IRP), and used in the behavioral and microdialysis experiments. DA-VGLUT2-KO mice were generated by crossing DAT-Cre (heterozygous) transgenic mice (Slc6a3IRESCre, 129/Sv/J background, Stock No. 006660, Jackson Laboratories) with homozygous VGLUT2lox/lox mice (Slc17a6IRESCre, C57BL/6 background, Stock No. 033638, Jackson Laboratories) carrying the exon 2 surrounded by loxP sites. Accordingly, 25% of the offspring from such mating were VGLUT2-Het control mice (i.e., DAT-Cre+/−;VGLUT2lox/+) and 25% lacked VGLUT2 in DA neurons (i.e., DAT-Cre+/−;VGLUT2lox/lox, i.e., DA-VGLUT2-KO mice). We chose VGLUT2-Het littermates as controls in this study since these mice also carried one copy of recombinase under the control of DAT (DAT-Cre+/−), similar as in DA-VGLUT2-KO mice. Thus, the only difference between the two groups of mice is that DA-VGLUT2-KO mice have 100% loss of VGLUT2 expression in DA neurons, while VGLUT2-Het control mice have only 50% VGLUT2 expression in DA neurons of normal wild-type mice (Hnasko et al., 2012). We didn’t choose WT mice as controls since it would require either to use non-littermate WT or to change the breeding scheme to cross with VGLUT2 heterozygotes instead of homozygotes. This is 50% less efficient in generating conditional VGLUT2-KO mice. These options present scientific and practical limitations, respectively. The mutant lines were bred for >10 generations on the background of C57BL/6 mice from Charles River Laboratories (Frederick, MD, USA). The genotype of the mice was determined by RT-PCR assays, conducted by Charles River Laboratories (Wilmington, MA, USA). The animals were maintained on a 12-h light/dark cycle with food and water available ad libitum. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Research Council and approved by the animal care committee of the National Institute on Drug Abuse of the National Institutes of Health.
2.2. RNAscope in situ hybridization (ISH)
RNAscope ISH was used to detect cell type-specific expression of VGLUT2 mRNA and TH mRNA. Mice were deeply anesthetized and the whole brain was removed and rapidly frozen on dry ice. Fresh-frozen tissue sections (14 μm thick) were mounted on positively charged microscopic glass slides (Fisher Scientific) and stored at −80 °C until RNAscope ISH assays were performed. Multiple target gene-specific RNAscope probes were used to observe the cellular distributions of VGLUT2 and TH mRNA in the VTA: VGLUT2 RNAscope probe (Mm-Slc17a6-C2- Vglut-2 probe, Cat #: 319171-C2, targeting 1986–2998 bp of the Mus musculus VGLUT2 mRNA sequence, NM_080853.3), and TH RNAscope probe (Mm-Th-C2, Cat #: 317621-C2, targeting 483–1603 bp of the Mus musculus TH mRNA sequence, NM_009377.1). Both probes were designed and provided by Advanced Cell Diagnostics (CA, USA). The RNAscope mRNA assays were performed following the manufacturer’s protocols. Stained slides were cover-slipped with fluorescent mounting medium (ProLong Gold anti-fade reagent P36930; Life Technologies) and scanned into digital images with an Olympus FluoView FV1000 confocal microscope at 40× or 60× magnification using manufacturer-provided software. Cells expressing TH mRNA, VGLUT2 mRNA, or both transcripts in the VTA were counted in 5–6 consecutive sections per brain with 64 μm spacing between sections and then normalized to the mean neuron counts per mm2 under 40 × magnification (Shen et al., 2018).
2.3. In vivo brain microdialysis
The general procedures for in vivo brain microdialysis are the same as we reported previously (Song et al., 2012; Xi et al., 2011). Naïve mice were anesthetized using a cocktail of ketamine hydrochloride (80 mg/ml) and xylazine hydrochloride (12 mg/ml) prior to insertion of intracranial guide cannulae (MAB 4.15.IC, SciPro Inc., Sanborn, NY, USA) into the NAc (stereotaxic coordinates: AP +1.4 mm, ML ±1.5 mm, DV −3.8 mm with an angle of 8° from vertical). Dental acrylic was applied to secure the guide cannulae to the skull. Standard aseptic surgical and stereotaxic procedures were followed. Subjects were given 7 days to recover preceding the experimental procedure. A probe (MAB 4.15.2. PES, SciPro Inc.) was inserted into the NAc 12 h before sample collection to reduce the occurrence of damage induced neurotransmitter release. A syringe pump (Bioanalytical Systems, West Lafayette, IN, USA) infused dialysate buffer at least 2 h prior to sample collection. Baseline micro-dialysate samples were taken in 20 min intervals for 1 h, followed by an i.p. METH injection (0.5 or 1 mg/kg) and further sampling for 2 h. Each mouse was implanted two guide-cannula bilaterally and carried out brain microdialysis experiments twice in randomized order – one side of the cannula for 0.5 mg/kg METH and another side of the cannula for 1 mg/kg METH (n = 13 VGLUT2-Het, 11 DA-VGLUT2-KO).
2.4. Quantification of DA with HPLC
Dialysate DA was measured with the ESA electrochemical detection system (ESA Inc., Chelmsford, MA, USA). The DA mobile phase contained 4.76 mM citric acid, 150 mM Na2HPO4, 3 mM sodium dodecyl sulfate, 50 mM EDTA, 10% methanol, and 15% acetylnitrile, pH 5.6. DA was separated using an ESA MD-150 × 3.2 mm reverse phase column and oxidized/reduced using an ESA Coulochem III detector. Three electrodes were used: a pre-injection port guard cell (+0.25 V) to oxidize the mobile phase, a reduction analytical electrode (E1, −0.1 V), and an oxidation analytical electrode (E2, 0.2 V). The area under curve of the DA peak was measured using an ‘EZChrom Elite’ ESA chromatography data system. DA values were quantified with an external standard curve (generated by three standard concentrations: 10, 100, 1000 pM). The limit of detection for DA was ~1 pM.
2.5. Quantification of glutamate with HPLC
Concentrations of glutamate in the dialysis samples were determined using HPLC with flourometric detection. The mobile phase consisted of 18% acetylnitrile (v/v), 100 mM Na2HPO4, 0.1 mM EDTA, pH 6.04. A reverse-phase column (RP-18, 10 cm × 3 μm ODS, Bioanalytical systems Inc.) was used to separate the amino acids, and pre-column derivatization of amino acids with o-phthalaldehyde was performed using an ESA Model 542 autosampler (ESA Inc.). Glutamate was detected by a fluorescence detector (L-2480, ESA Inc., Chelmsford, MA, USA). One set of excitation wavelengths (Exλ, 314 nm) and emission wavelengths (Emλ, 394 nm) was used to measure glutamate levels from the same samples. The area under curves of the glutamate peaks were measured using an ‘EZChrom Elite’ ESA chromatography data system. Glutamate values were quantified with external standard curves (generated by three standard concentrations: 0.1, 1.0, 5 μM). The limit of detection for glutamate was 10 nM.
2.6. Open-field locomotion
This experiment was designed to study the effects of METH on open-field locomotor behavior between the DA-VGLUT2-KO mice and their Het littermate control mice. During the week before locomotor testing, mice were habituated to locomotor detection chambers (Accuscan Instruments, Columbus, OH, USA) during 3 h daily sessions. On the test day, mice were placed in the chamber for 1 h of habituation (baseline), after which data collection was paused and METH (0.5, 1 mg/kg, i.p.) was injected. Next, mice were placed back in the open-field apparatus and locomotor activity was measured over a 2 h period. Each METH dose (0, 0.5, 1 mg/kg, i.p.) was tested in a balanced manner in 2 genotype groups of mice (n = 8 each). The time intervals between the drug tests were 2–4 days. The distance traveled before and after injections was collected in 10-min intervals using the VersaMax data analysis system (Accuscan Instruments).
2.7. Statistical analysis
Data are expressed as means ± SEM. A one-way ANOVA was used to evaluate the difference in basal levels of extracellular DA and glutamate between two genotypes of mice. Two-way ANOVAs for repeated measures over time were used to compare the DA/glutamate or locomotor responses to METH between two genotypes of mice. Significant interactions were followed by post-hoc tests using Fishers Least Significant Difference. Statistical significance was set to p < 0.05.
3. Results
3.1. Absence of VGLUT2 mRNA from DA neurons in DA-VGLUT2-KO mice
The DA-VGLUT2-KO mice were generated by crossing DAT-Cre+/− mice with VGLUT2-floxed mice. To confirm the loss of VGLUT2 in DA neurons in the presently-used DA-VGLUT2-KO mice, we used RNAscope in situ hybridization (ISH) assay to detect VGLUT2 and TH mRNA expression in midbrain. Fig. 1-A/B shows VGLUT2 mRNA (red) and TH mRNA (green) staining in the VTA, illustrating co-localization of VGLUT2 and TH in a subset of TH-positive neurons in VGLUT2-Het control mice (upper panel), but not in DA-VGLUT2-KO mice (lower panel). Fig. 1-C/D shows cell counts of three types of neurons observed in the VTA, illustrating that ~18% TH-positive neurons co-express VGLUT2 in the VGLUT2-het control mice, which number is significantly higher than ~5% observed in DA-VGLUT2-KO mice (Fig. 1C, one-way ANOVA, F1,8 = 56.75, p < 0.001). The small population of double-labeled cells in KO mice likely reflect neurons expressing TH but not DAT (Lammel et al., 2015; Stamatakis et al., 2013). In contrast, no significant differences were observed between two genotypes of mice in number of TH-positive only neurons.
Fig. 1.
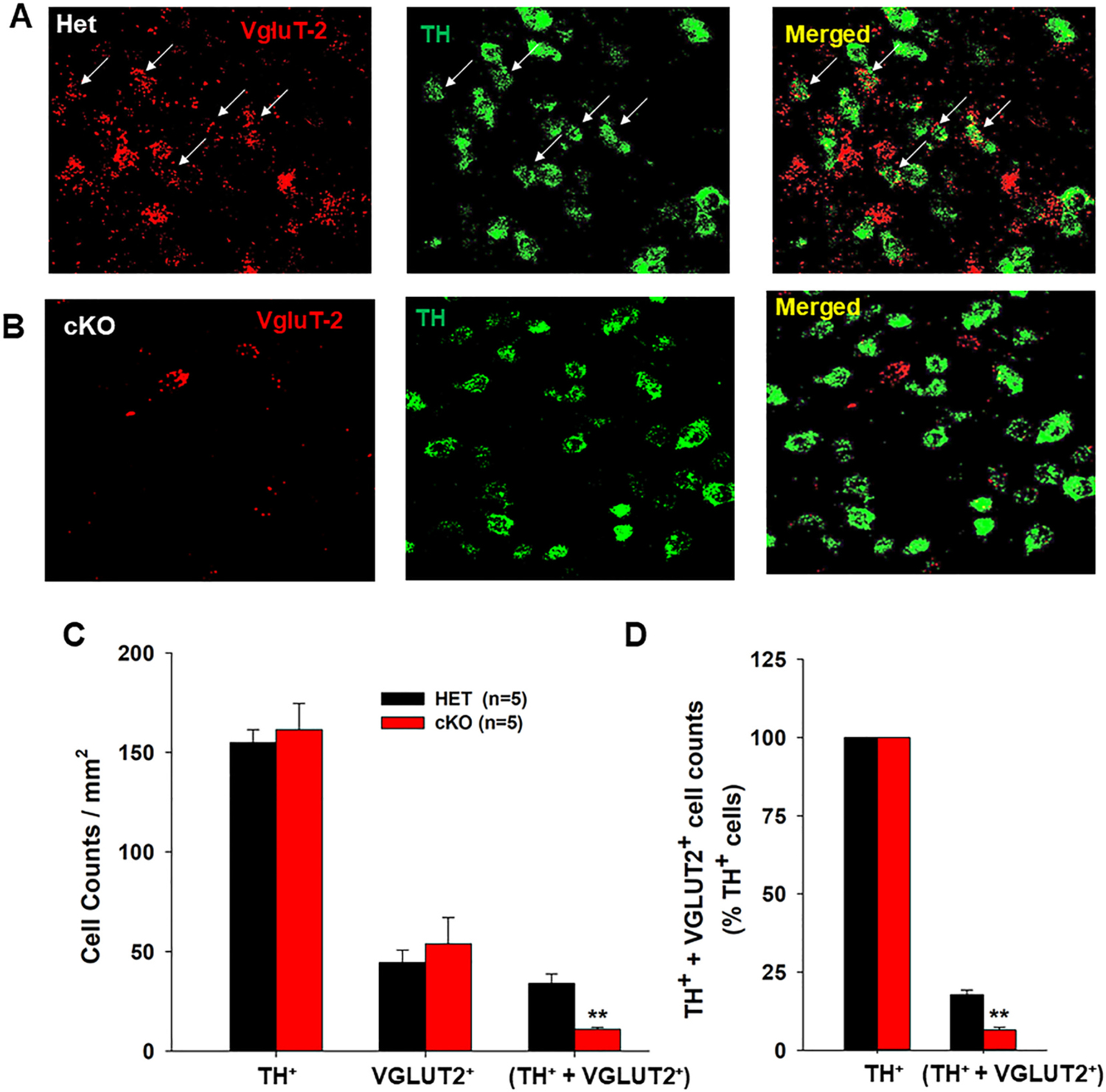
Identification of VGLUT2 expression in midbrain DA neurons. A-B: Representative confocal images, illustrating VGLUT2 mRNA (red)- and TH mRNA (green)-staining in the VTA of both VGLUT2-Het (Het) control mice and DA-VGLUT2-KO (cKO) mice. No VGLUT2 mRNA was detected in TH+ neurons in the VTA of the cKO mice. C: Averaged cell counts of TH+ only, VGLUT2+ only, and dual TH+-VGLUT2+ neurons in each section under 20× magnification in Het (n = 5) and cKO (n = 5) mice. D: cKO mice display a significant (~75%) reduction in dual TH+-VGLUT2+ neurons compared to Het control mice. **p < 0.01, compared to Het control mice. Data indicate means ± SEM.
3.2. Deletion of VGLUT2 in DA neurons blunts DA responses to METH
We then examined whether selective deletion of VGLUT2 in DA neurons altered NAc DA response to METH. In vivo brain microdialysis with HPLC assays showed a trend to reduction from basal levels of extracellular DA (from 1.10 ± 0.24 nM in VGLUT2-Het mice to 0.72 ± 0.15 nM in DA-VGLUT2-KO mice). However, this reduction was not statistically significant (Fig. 2A, one-way ANOVA, F1,22 = 3.01, p > 0.05). Selective deletion of VGLUT2 also failed to alter a low dose (0.5 mg/kg, i.p.) of METH-induced increase in extracellular DA (Fig. 2B). A two-way ANOVA for repeated measures over time revealed a genotype main effect (F1,22 = 0.56, p > 0.05), time main effect (F8, 176 = 59.04, p < 0.001), and genotype × time interaction (F8, 176 = 1.58, p > 0.05). Post-hoc individual group comparisons indicated a significant increase in DA after 0.5 mg/kg METH administration compared to the baseline (p < 0.001). There is no difference in DA response to 0.5 mg/kg METH at any time point between VGLUT2-Het and DA-VGLUT2-KO mice (Fig. 2B). We then increased METH dose to 1 mg/kg and found that DA-VGLUT2-KO mice displayed a significantly lower DA response to 1.0 mg/kg of METH than the control mice (Fig. 2C). A two-way ANOVA for repeated measures over time did not reveal a significant genotype main effect (Fig. 2C, F1,22 = 0.041, p > 0.05), but revealed a significant time main effect (F8,176 = 12.09, p < 0.001) and genotype × time interaction (F8, 176 = 2.94, p < 0.05). Post-hoc individual group comparisons revealed a significant reduction in 1 mg/kg METH-enhanced DA in DA-VGLUT2-KO mice compared to VGLUT2-Het mice at the time point of 80 min (i.e., 20 min after METH, p < 0.05).
Fig. 2.
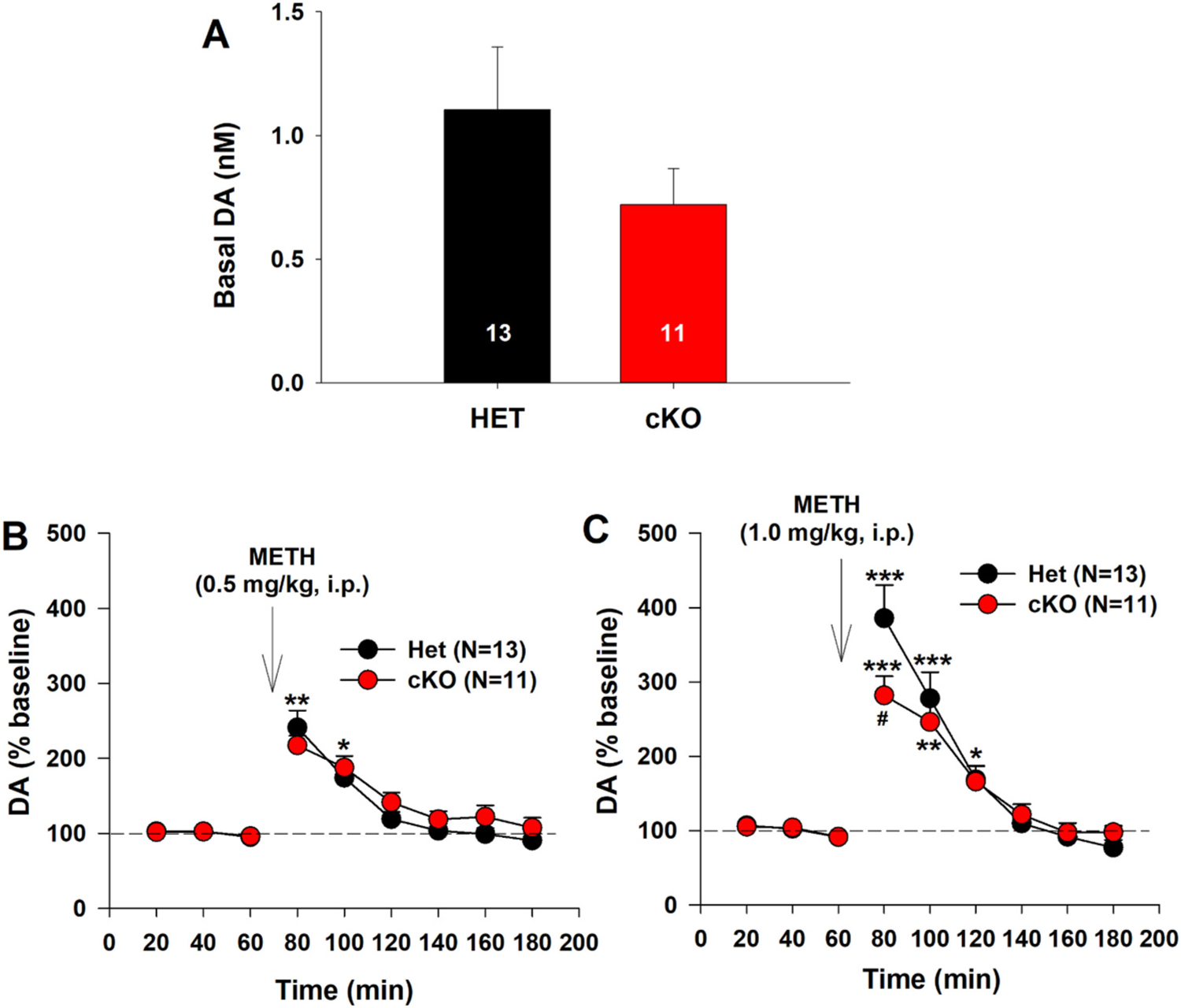
Selective deletion of VGLUT2 from DA neurons blunted DA response to METH. A: Basal level of extracellular DA in the NAc in HET and cKO mice. B: NAc DA response to 0.5 mg/kg METH. There is no significant difference observed between two genotypes of mice. C: NAc DA response to a higher dose (1 mg/kg, i.p.) of METH. The cKO mice displayed a blunted DA response to METH compared to the HET control mice. *p < 0.05, **p < 0.01, ***p < 0.001, compared to baseline before METH injection. #p < 0.05, compared to HET.
3.3. Deletion of VGLUT2 in DA neurons blunts glutamate responses to METH
We also measured extracellular glutamate levels in the same microdialysis samples and found that selective deletion of VGLUT2 in DA neurons failed to alter basal levels of extracellular glutamate in the NAc (Fig. 3A, F1,20 = 1.09, p > 0.05), but significantly lowered 0.5 mg/kg METH-induced increase in extracellular glutamate (Fig. 3-B, C). A two-way ANOVA for repeated measures over time revealed genotype main effect (Fig. 3B, F1,20 = 4.01, p = 0.059), time main effect (F8,160 = 14.81, p < 0.001), and genotype × time interaction (F8,160 = 3.02, p < 0.01). Post-hoc individual group comparisons revealed a significant reduction in extracellular glutamate (p < 0.05) at the time points of 160 min and 180 min in DA-VGLUT2-KO mice compared to VGLUT2-Het control mice. Fig. 3C shows the time course of the glutamate response to a higher dose (1 mg/kg) of METH. A two-way ANOVA for repeated measures over time revealed a genotype main effect (Fig. 3C, F1,20 = 5.16, p < 0.05), time main effect (F8,160 = 9.86, p < 0.001), and genotype × time interaction (F8,160 = 2.35, p < 0.01). Post-hoc group comparisons revealed a significant reduction in METH-enhanced glutamate in DA-VGLUT2-KO mice compared to the VGLUT2-Het control mice.
Fig. 3.
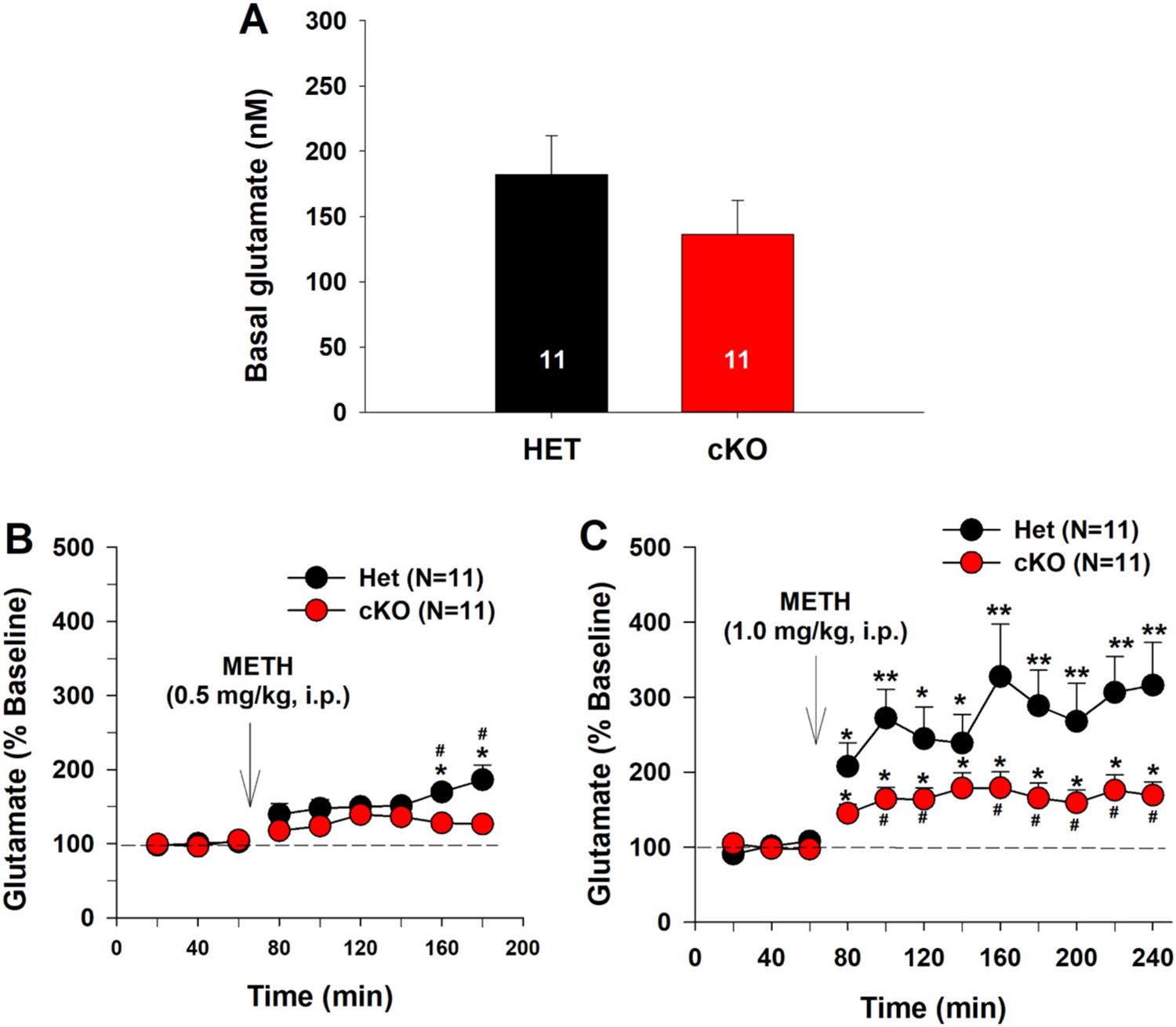
Selective deletion of VGLUT2 from DA neurons blunted NAc glutamate response to METH. A: Basal level of extracellular glutamate in the NAc in HET and cKO mice. B: NAc glutamate response to 0.5 mg/kg METH. C: NAc glutamate response to 1 mg/kg METH. cKO mice displayed an attenuated glutamate response to METH compared to HET control mice. *p < 0.05, **p < 0.01, compared to baseline before METH injection. #p < 0.05, compared to HET.
3.4. Deletion of VGLUT2 in DA neurons blunts locomotor responses to METH
Given an important role of DA and glutamate in locomotion, we next observed the locomotor response to METH. Fig. 4 shows that the dose-dependent effects of METH on open-field locomotion on VGLUT2-HET (Fig. 4A) and DA-VGLUT2-KO (Fig. 4B) mice. Given the basal levels of locomotion varied across different dose groups, we normalized METH-induced changes in locomotion over baseline (% baseline, Fig. 4-C, D). We found that 0.5 mg/kg METH did not significantly alter locomotor activity in both genotypes of mice (Fig. 4C), while 1 mg/kg METH produced a significant increase in locomotion in VGLUT2-HET mice, but not in DA-VGLUT2-KO mice (Fig. 4D). A two-way ANOVA for repeated measures over time revealed a genotype main effect (Fig. 4D, F1,14 = 4.03, p < 0.05), time main effect (F14,196 = 8.60, p < 0.001), and genotype × time interaction (F14,196 = 1.91, p < 0.05). Post-hoc group comparisons revealed a significant reduction in DA-VGLUT2-KO mice compared to the control mice at the time points of 50–70 min (i.e., 10–30 min after METH as shown in Fig. 4D).
Fig. 4.
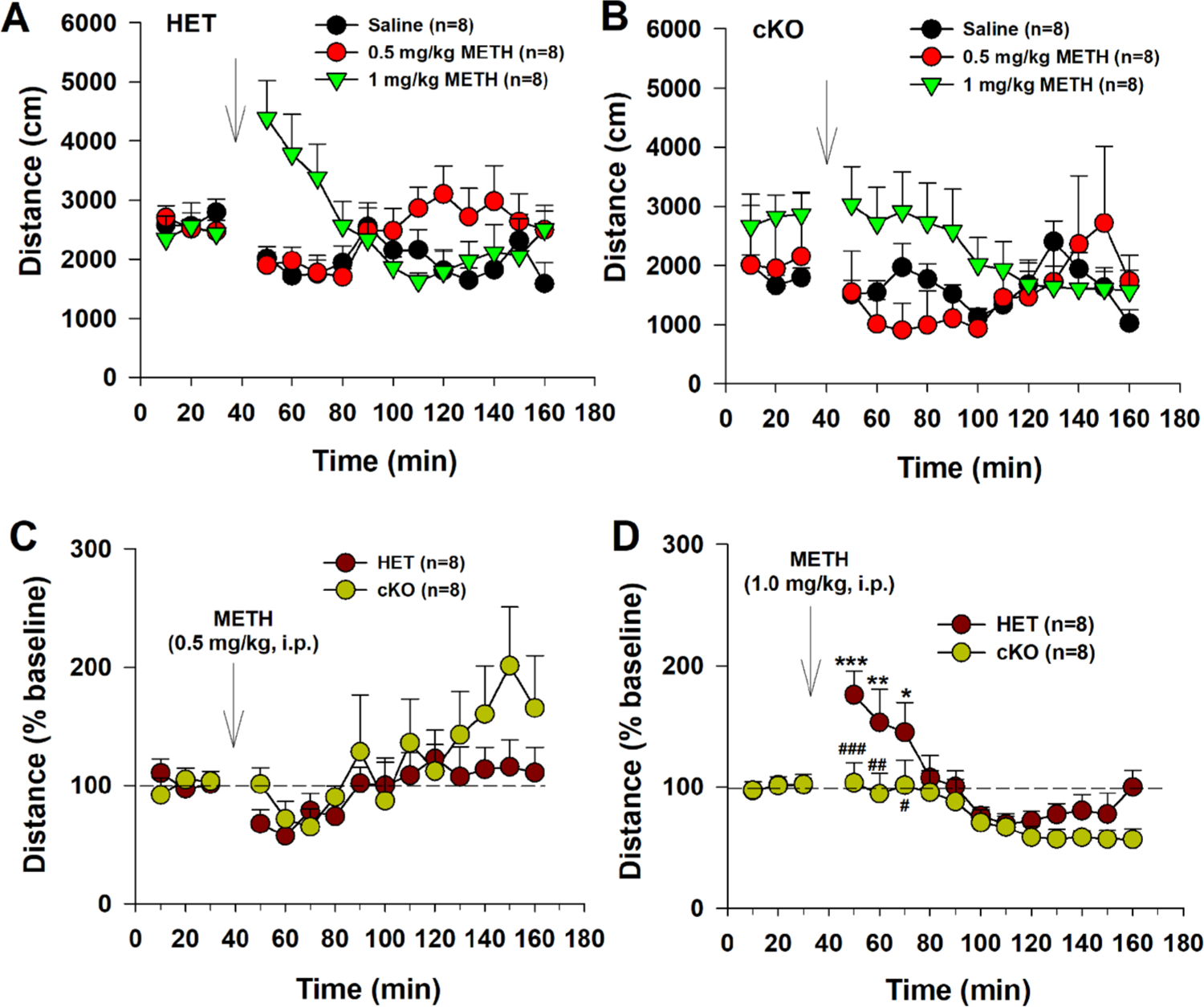
Selective deletion of VGLUT2 from DA neurons blunted locomotor response to METH. A, B: Dose-dependent effects of METH in HET and cKO mice. C: Locomotor response (% baseline) to 0.5 mg/kg METH. D: Locomotor response (% baseline) to 1 mg/kg METH. METH, at 1 mg/kg, increased open-field locomotion in HET, but not in cKO mice. *p < 0.05, **p < 0.01, ***p < 0.001, compared to baseline before METH injection. #p < 0.05, ##p < 0.01, ###p < 0.001, compared to HET group.
4. Discussion
The major finding in the present study is that genetic deletion of VGLUT2 expression in midbrain DA neurons significantly reduced the behavioral and neurochemical responses to METH, particularly in NAc glutamate response. These results suggest that, first, VGLUT2 expression in DA neurons plays an important role in METH action, and second, glutamate released from DA neurons contributes to METH-induced increases in extracellular glutamate levels. Thus, cell type specific VGLUT2 expression in DA neurons may constitute a new therapeutic target in medication development for the treatment of METH abuse and dependence and also of METH-induced neurodegeneration.
There are three types of vesicular glutamate transporters (VGLUT1–3) identified in the brain (Takamori, 2006). VGLUT1 and VGLUT2 are specific markers for glutamatergic neurons, while VGLUT3 is present mainly in cholinergic, serotoninergic, or GABAergic neurons (Fremeau Jr. et al., 2004). VTA glutamatergic neurons mainly express VGLUT2 (Fremeau Jr. et al., 2004; Herzog et al., 2006). Within the VTA and SNc, there are two types of VGLUT2+ neurons – VGLUT2+ glutamatergic neurons and dual VGLUT2+-TH+ neurons (Morales and Margolis, 2017). The proportion of the DA neurons expressing VGLUT2 varies between studies. Some studies show that VGLUT2 is expressed in early developing DA neurons and then decreased during the late embryonic and the postnatal periods (Berube-Carriere et al., 2009; Dal Bo et al., 2008; Yamaguchi et al., 2007). In contrast to these reports, other studies reported that as much as 15%–50% TH+ DA neurons in the VTA and as much as 25% TH+ DA neurons in the SNc express VGLUT2 in adult rats and mice (Fortin et al., 2012; Kawano et al., 2006; Mendez et al., 2008). In cell cultures, over 80% DA neurons have been shown to express VGLUT2 (Dal Bo et al., 2008). Furthermore, VGLUT2 expression in DA neurons appears to be dynamic and inducible. For example, exposure to the neurotoxin 6-hydroxydopamine can increase VGLUT2 expression in mesencephalic DA neurons (Dal Bo et al., 2008). These large differences in proportion remain to be explained but could reflect differences in technical approaches and experimental conditions. In the present study, we found that ~20% DA neurons in the VTA co-express VGLUT2 in adult VGLUT2-het control mice (2–3 months old), while this proportion was decreased to ~5% DA neurons in DA-VGLUT2-KO mice. In theory, DA-VGLUT2-KO mice should not show any VGLUT2+-TH+ co-localization. The residual VGLUT2+-TH+ “co-localization” observed in VGLUT2-cKO mice could be false colocalization due to overlays of VGLUT2+ neurons and TH+ DA neurons. This is supported by electrophysiological evidence indicating that VGLUT2 deletion from DA neurons not only eliminates glutamate release, but also reduces DA release in our recent report (Shen et al., 2018) and others (Alsio et al., 2011; Fortin et al., 2012; Hnasko et al., 2010). Alternatively, the apparent colocalization could represent a population of VTA neurons expressing TH but not DAT, thus being spared recombination.
VGLUT2 functionally promotes glutamate loading into glutamate vesicles (Zhang et al., 2015), while VMAT2 promotes DA loading into DA vesicles (Nickell et al., 2014). METH reverses VMAT2 and promotes DA exit from vesicles into cytoplasm possibly by collapsing the vesicular proton gradient in part via VMAT2’s function as a substrate/proton antiporter (Hnasko et al., 2010; Aguilar et al., 2017; Freyberg et al., 2016), causing massive DA release into extracellular space by reversal of membrane DAT (Baracz and Cornish, 2016; Elkashef et al., 2008; Freyberg et al., 2016). However, the functional role of glutamate released from DA neurons are not fully understood (Hnasko et al., 2010; Shen et al., 2018). Given the important role of DA and glutamate in locomotion and motivated behavior (Beninger, 1983; Galaj et al., 2020), in the present study, we examined whether VGLUT2 deletion in DA neurons alters NAc DA and glutamate response to the psychostimulant METH. We studied METH because METH abuse is a serious public health problem without approved medications for its treatment. Therefore, understanding the neural mechanisms underlying METH action are critical in developing new pharmacotherapy for the treatment of METH abuse and dependence. We found that METH produced robust increases in extracellular DA and glutamate in a dose-dependent manner, which was reduced significantly by genetic deletion of VGLUT2 from DA neurons, suggesting an important role of dopaminergic VGLUT2 in METH action. We note that not all evidence shows an increase in NAc glutamate after acute METH administration (Lominac et al., 2016; Shoblock et al., 2003). This may be related to the differences in drug doses, species (rats vs. mice), target brain regions (NAc-core vs. NAc-shell), and the experimental methods (conventional vs. no net-flux microdialysis) experimental conditions. Whatever the reasons, our findings in the present study in mice are consistent with the majority of reports indicating that acute administration of METH increases extracellular DA and glutamate in the NAc as stated in the introduction above. However, the precise mechanisms through which VGLUT2 deletion attenuates METH action are still unclear.
Functional assays show that DA and glutamate may be present in the same pool of vesicles where VGLUT2 may promote loading of glutamate and DA into the same vesicles in a subpopulation of DA neurons (Birgner et al., 2010; Hnasko et al., 2010; Hnasko and Edwards, 2012; Aguilar et al., 2017). In addition, METH exposure alters expression of glutamate receptors and VGLUTs, including VGLUT2, in the striatum (Mark et al., 2007; Furlong et al., 2018). Blockade of VGLUTs attenuated METH-induced hyperactivity and locomotor sensitization (He et al., 2013). Thus, one possibility is that VMAT2 and VGLUT2 may form functional complex and regulate uptake of both DA and glutamate into presynaptic vesicles in DA neurons. Thus, deletion of VGLUT2 in DA neurons would decrease vesicular DA/glutamate contents, attenuating METH-induced co-release of DA and glutamate from the same vesicles in DA neurons. In contrast to this hypothesis, morphological evidence indicates that VGLUT2 and VMAT2 are not always co-localized in the same vesicles and there is only a subpopulation of vesicles that co-express VGLUT2 and VMAT2 (Silm et al., 2019). In contrast, most DA and glutamate vesicles appear to be segregated (Zhang et al., 2015; Silm et al., 2019). In theory, if VGLUT2 and VMAT2 are not colocalized in the same vesicles, deletion of VGLUT2 from glutamate vesicles should not affect METH action on VMAT2 in DA vesicles. As stated above, VGLUT2 not only promotes vesicular glutamate update but also promotes vesicular DA uptake, and METH not only blocks VMAT2 but also affects VGLUT expression (Mark et al., 2007; Furlong et al., 2018). Thus, an interaction may exist between METH and VGLUT2 or between VGLUT2 and VMAT2. This may in part explain why deletion of VGLUT2 attenuates METH action in release of DA and glutamate in the NAc. Clearly, more studies are required to further address this issue.
Another interesting finding in this study is that deletion of VGLUT2 from DA neurons also produced a trend toward reduction in basal levels of extracellular DA and glutamate levels, suggesting that VGLUT2 is involved basal vesicular DA and glutamate release. This small decrease in basal neurotransmitter release can be explained by the fact that VGLUT2 deletion in DA neurons affected only a minority (~20%) of DA-glutamate neurons in the midbrain. In addition, it is well known that the conventional microdialysis techniques used in the present study have limitations of poor time and spatial resolutions (Szumlinski et al., 2017; Lominac et al., 2016), and therefore, may not be sensitive enough to detect such a subtle reduction in basal levels of extracellular DA and glutamate in mice after VGLUT2 deletion. Therefore, more studies are required by using a more sensitive procedure such as no net-flux microdialysis to further address this issue (Szumlinski et al., 2017; Lominac et al., 2016). Nevertheless, our finding is in general congruent with other reports demonstrating that DA-VGLUT2-KO mice did not show significant changes in motor function, basal locomotor activity, and learning and memory function (Fortin et al., 2012; Hnasko et al., 2010; Shen et al., 2018).
A third important finding is that DA-VGLUT2-KO mice also displayed blunted locomotor response to METH, an effect that is similar to NAc DA/glutamate responses to METH. These findings are consistent with previous reports demonstrating that DA-VGLUT2-KO mice displayed a significant reduction in locomotor response to cocaine or amphetamine (Birgner et al., 2010; Fortin et al., 2012; Hnasko et al., 2010), in NAc DA response to single pulse electrical stimulation (Fortin et al., 2012), or in cocaine-induced reduction in glutamate transmission in the NAc (Adrover et al., 2014). We note that DA-VGLUT2-KO mice displayed an enhancement in cocaine self-administration (Alsio et al., 2011), which may be interpreted as a compensatory response in drug intake due to blunted DA and glutamate response to cocaine.
We note that selective deletion of VGLUT2 in DA neurons had little effect on acquisition of intracranial self-stimulation (ICSS) and place preference reinforced by optogenetic stimulation of VTA DA neurons in TH-Cre mice (Wang et al., 2017), since only a minority (~20%) of DA neurons expresses VGLUT2 in the VTA and optical stimulation of the remaining majority of DA neurons alone are sufficient to maintain optical ICSS and place preference. Interestingly, it is likely that optical stimulation of VTA glutamate neurons or their terminals in the NAc is sufficient to maintain ICSS and promotes reinforcement in a DA-independent mechanism (Zell et al., 2020). This new finding suggests an important role of midbrain glutamate neurons in brain reward function.
It is important to note that the VGLUT2-Het littermate control mice used in these experiments had only 50% VGLUT2 expression in DA neurons in wild-type mice. Electrophysiological evidence indicates that the mean evoked EPSC amplitude in NAc neurons from these HET control mice was ~50% that of age-matched wild-type mice (Hnasko et al., 2010). Thus, the difference in DA/glutamate response to METH observed in the present study between VGLUT2-Het and DA-VGLUT2-KO mice actually reveals only a half of the difference presence between wild-type and DA-VGLUT2-KO mice, which further support an important role of dopaminergic VGLUT2 in METH action.
In conclusion, the present study for the first time demonstrates that selective deletion of VGLUT2 expression in dopaminergic neurons significantly reduced the behavioral and neurochemical response to METH, suggesting that VGLUT2 in DA neurons could be a new acting target of METH. Accordingly, new medication strategies that decrease VGLUT2 expression or inhibit VGLUT2 function in DA neurons would produce therapeutic effects against METH abuse and dependence as well as METH-induced neurotoxicity.
Acknowledgments
We thank the NIDA breeding staff for breeding the transgenic animals. This research was supported by NIDA IRP (Z1A DA000633-01).
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
References
- Adrover MF, Shin JH, Alvarez VA, 2014. Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J. Neurosci 34 (9), 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar JI, Dunn M, Mingote S, Karam CS, Farino ZJ, Sonders MS, Choi SJ, Grygoruk A, Zhang Y, Cela C, Choi BJ, Flores J, Freyberg RJ, McCabe BD, Mosharov EV, Krantz DE, Javitch JA, Sulzer D, Sames D, Rayport S, Freyberg Z, 2017. Neuronal depolarization drives increased dopamine synaptic vesicle loading via VGLUT. Neuron 95 (5), 1074–1088 (e1077). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsio J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LE, Kullander K, Levesque D, Wallen-Mackenzie A, 2011. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J. Neurosci 31 (35), 12593–12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC, 2006. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis 44 (8), 383–390. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Cornish JL, 2016. The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Front. Neuroendocrinol 43, 1–18. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, 1983. The role of dopamine in locomotor activity and learning. Brain Res. 287 (2), 173–196. [DOI] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L, 2009. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J. Comp. Neurol 517 (6), 873–891. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A, 2010. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc. Natl. Acad. Sci. U. S. A 107 (1), 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, 2009. Molecular bases of methamphetamine-induced neurodegeneration. Int. Rev. Neurobiol 88, 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Choi WY, Mingote S, Rayport S, 2009. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience 164 (3), 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bo G, Berube-Carriere N, Mendez JA, Leo D, Riad M, Descarries L, Levesque D, Trudeau LE, 2008. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience 156 (1), 59–70. [DOI] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J, 2008. Pharmacotherapy of methamphetamine addiction: an update. Subst. Abus 29 (3), 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, 2008. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J. NeuroImmune Pharmacol 3 (3), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Berube-Carriere N, Claveau AM, Descarries L, Sadikot AF, Wallen-Mackenzie A, Trudeau LE, 2012. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J. Neurosci 32 (48), 17477–17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT Jr., Voglmaier S, Seal RP, Edwards RH, 2004. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27 (2), 98–103. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Sonders Mark S., Aguilar Jenny I., Hiranita Takato, Karam Caline S., Flores Jorge, Pizzo Andrea B., Zhang Yuchao, Farino Zachary J., Chen Audrey, Martin Ciara A., Kopajtic Theresa A., Fei Hao, Hu Gang, Lin Yi-Ying, Mosharov Eugene V., McCabe Brian D., Freyberg Robin, Wimalasena Kandatege, Hsin Ling-Wei, Sames Dalibor, Krantz David E., Katz Jonathan L., Sulzer David, Javitch Jonathan A., 2016. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat. Commun 7 (10652. doi: 10.1038/ncomms10652). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Corbit LH, Brown RA, Balleine BW, 2018. Methamphetamine promotes habitual action and alters the density of striatal glutamate receptor and vesicular proteins in dorsal striatum. Addict. Biol 23, 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Newman AH, Xi ZX, 2020. Dopamine D3 receptor-based medication development for the treatment of opioid use disorder: rationale, progress, and challenges. Neurosci. Biobehav. Rev 114, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Yan L, Yong Z, Dong Z, Dong H, Gong Z, 2013. Chicago sky blue 6B, a vesicular glutamate transporters inhibitor, attenuates methamphetamine-induced hyperactivity and behavioral sensitization in mice. Behav. Brain Res 239, 172–176. [DOI] [PubMed] [Google Scholar]
- Herzog E, Takamori S, Jahn R, Brose N, Wojcik SM, 2006. Synaptic and vesicular co-localization of the glutamate transporters VGLUT1 and VGLUT2 in the mouse hippocampus. J. Neurochem 99 (3), 1011–1018. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH, 2012. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol 74, 225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH, 2010. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65 (5), 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH, 2012. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J. Neurosci 32 (43), 15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, 2002. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci. Res 42 (4), 243–250. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, Hisano S, 2006. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol 498 (5), 581–592. [DOI] [PubMed] [Google Scholar]
- Kuitunen-Paul S, Roessner V, Basedow LA, Golub Y, 2020. Beyond the tip of the iceberg: a narrative review to identify research gaps on comorbid psychiatric disorders in adolescents with methamphetamine use disorder or chronic methamphetamine use. Subst. Abus 1–20. [DOI] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L, Malenka RC, 2015. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85 (2), 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK, 2016. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur. J. Neurosci 43, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ, Yamamoto BK, 2007. Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J. Neurosci 27 (25), 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE, 2008. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J. Neurosci 28 (25), 6309–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Margolis EB, 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci 18 (2), 73–85. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH, 2014. Glutamate neurons within the midbrain dopamine regions. Neuroscience 282, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell JR, Siripurapu KB, Vartak A, Crooks PA, Dwoskin LP, 2014. The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse. Adv. Pharmacol 69, 71–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK, 2007. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 1135 (1), 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Tymianski M, 2001. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol 24 (1–3), 107–129. [DOI] [PubMed] [Google Scholar]
- Shen H, Marino RAM, McDevitt RA, Bi GH, Chen K, Madeo G, Lee PT, Liang Y, De Biase LM, Su TP, Xi ZX, Bonci A, 2018. Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice. Proc. Natl. Acad. Sci. U. S. A 115 (49), E11532–E11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD, 2003. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology 165 (4), 359–369. [DOI] [PubMed] [Google Scholar]
- Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, Newman AH, Ford CP, Edwards RH, 2019. Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties. Neuron 102 (4), 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Li X, Bi GH, Gardner EL, Xi ZX, 2012. Increased vulnerability to cocaine in mice lacking dopamine D3 receptors. Proc. Natl. Acad. Sci. U. S. A 109 (43), 17675–17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD, 2013. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80 (4), 1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK, 1994. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse 17 (3), 203–209. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugmann M, Phillips TJ, Kippin TE, 2017. Methamphetamine addiction vulnerability: the glutamate, the bad, and the ugly. Biol. Psychiatry 81, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, 2006. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci. Res 55 (4), 343–351. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, 2004. Glutamate co-transmission as an emerging concept in monoamine neuron function. J. Psychiatry Neurosci 29 (4), 296–310. [PMC free article] [PubMed] [Google Scholar]
- UNODOC, 2019. 2019. World Drug Report 2019 (United Nations Publication, Sales No. E.19.XI.9). Vienna, Austria, United Nations Office on Drugs and Crime. [Google Scholar]
- Wang DV, Viereckel T, Zell V, Konradsson-Geuken A, Broker CJ, Talishinsky A, Yoo JH, Galinato MH, Arvidsson E, Kesner AJ, Hnasko TS, Wallen-Mackenzie A, Ikemoto S, 2017. Disrupting glutamate co-transmission does not affect acquisition of conditioned behavior reinforced by dopamine neuron activation. Cell Rep. 18 (11), 2584–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL, 2011. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat. Neurosci 14 (9), 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME, 1996. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. J. Neurochem 67 (1), 352–363. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M, 2007. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci 25 (1), 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell V, Steinkellner T, Hollon NG, Warlow SM, Souter E, Faget L, Hunker AC, Jin X, Zweifel LS, Hnasko TS, 2020. VTA glutamate neuron activity drives positive reinforcement absent dopamine co-release. Neuron 107 (5), 864–873 (e864). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA, 2001. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann. N. Y. Acad. Sci 937, 93–120. [DOI] [PubMed] [Google Scholar]
- Zhang S, Jin Y, Liu X, Yang L, Ge Z, Wang H, Li J, Zheng J, 2014. Methamphetamine modulates glutamatergic synaptic transmission in rat primary cultured hippocampal neurons. Brain Res. 1582, 1–11. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M, 2015. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci 18 (3), 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]


