Abstract
In recent years, recreational use of Salvia divinorum (Lamiaceae), a herbal drug that contains a hallucinogenic ingredient, salvinorin A, has become a new phenomenon among young drug users. In Taiwan, as in many other countries, dry leaves of S. divinorum and its related concentrated extract products are available via the Internet. Besides S. divinorum, there are many endemic Salvia species whose salvinorin A content is yet unknown. To understand the abuse liability of these products, the aim of this study was to assess the concentration of salvinorin A in endemic Salvia species and Internet-available salvinorin A-related products. Samples of S. divinorum were purchased via the Internet and samples of eight endemic species of Salvia were collected in Taiwan, including S. arisanensis Hayata, S. coccinea Juss. ex Murr, S. hayatana Makino ex Hayata, S. japonica Thumb. ex Murr, S. nipponica Miq. Var. formosana (Hayata) Kudo, S. scapiformis Hance, S. tashiroi Hayata. Icon. PI. Formosan, and S. keitaoensis Hayata. The content of salvinorin A was determined by high performance liquid chromatography (HPLC). Salvinorin A was extracted from the dry leaves of S. divinorum and endemic species of Salvia with methanol and analyzed on a C-18 column by isocratic elution with a mobile phase of acetonitrile–water. Salvinorin A was detected in S. divinorum, but not in the endemic Salvia species of Taiwan. Therefore, endemic species of Salvia in Taiwan may not possess hallucinogenic potential. However, the potential harm from S. divinorum available via the Internet should be thoroughly assessed in Taiwan, and control measures similar to those implemented in many other countries should be considered.
Keywords: Drug abuse, Hallucinogenic herb, High performance liquid chromatography, Recreational drug, Salvia divinorum, Salvinorin A
1. Introduction
Salvia divinorum Epl. & Játiva-M. (Lamiaceae), which is endemic to the northeastern Sierra Mazateca mountain region of Mexico and traditionally used in spiritual rituals by the Mazatec Indians to produce hallucinatory effects, is used for traditional medicine purposes, including the treatment of diarrhea, headache, anemia, and rheumatism [1]. S. divinorum is ingested by chewing the fresh leaves, smoking the dry leaves, or drinking the extraction solution of fresh leaves to induce hallucinatory effects such as “entering another reality”, “dissociative sensations”, and “spatiotemporal dislocation” [2,3]. Salvinorin A, a potent hallucinogenic ingredient isolated from S. divinorum, is the primary non-nitrogenous neoclerodane diterpene selective agonist of naturally-occurring κ-opioid receptors [4]. The hallucinogenic mechanism of salvinorin A is different from those of classical hallucinogens, such as lysergic acid diethylamide (LSD), Δ9-tetrahydrocannabinol, and ketamine, because it does not interact with 5-hydroxytryptamine (5-HT) receptor (serotonin receptor), cannabinoid (CB) receptor, or N-methyl=d-aspartate (NMDA) receptor [5,6]. Previous studies have estimated that smoking approximately 200–500 μg of salvinorin A would be sufficient to cause intense hallucinogenic and psychotomimetic effects [7,8]. Salvinorin A is rapidly absorbed by inhalation, whether in dry leaves or from a “concentrated extract” product; the hallucinogenic effect can occur within about 30 seconds and can last up to 20–30 minutes [7]. Oral administration causes hallucination after about 5–10 minutes, and can last up to 1 hour [7]. Salvinorin A is rapidly degraded by blood esterase and several cytochrome P450 isoenzymes, including CYP2E1, CYP2C18, CYP1A1, and CYP2D6 [9,10]. The short psychoactive effects of salvinorin A are due to rapid hydrolysis to the inactive metabolite salvinorin B [9]. One study reported that the elimination half-life of salvinorin A was short, at 56.6 ± 24.8 minutes after intravenous administration [10]. In a previous study, salvinorin A was not detected in urine samples collected after 1.5 hours from two human volunteers who had smoked S. divinorum dry leaves [11].
In recent years, S. divinorum has become increasingly popular among adolescents and young adults. Babu et al [12] indicated that S. divinorum has several features attractive to young drug users and is a desirable hallucinogen substance: (1) owing to the legality of S. divinorum, its related products are easily purchased from various sources, including smart shops, head shops, and online vendors; (2) sellers always declare that evidence indicates that S. divinorum is safe and not addictive; (3) salvinorin A may not be detected in urine because it has a very short half-life [11].
According to the Substance Abuse and Mental Health Services Administration (SAMHSA) publication, the 2006 National Survey on Drug Use and Health (NSDUH) database, it is estimated that 1.8 million adolescents or young adults have used S. divinorum in their lifetime and 756,000 people have used S. divinorum in the past year [13]. A previous study, analyzing the 2006–2008 NSDUH public-use data (N = 166,453), indicated that the lifetime prevalence of S. divinorum use has increased by about 83% among individuals aged 12 years or older [14]. In 2010, the National Institute on Drug Abuse (NIDA) investigated 46,482 students in the 8th, 10th, and 12th grades from 396 public and private schools and 5.5% reported having used S. divinorum, which is greater than other recreational drugs, including heroin (0.9%), cocaine (2.9%), LSD (2.6%), methamphetamines (1.5%), ecstasy (4.5%), γ-hydroxybutyric acid (GHB) (1.4%), ketamine (1.6%), and oxycodone (5.1%) [15,16].
S. divinorum has not been placed on the list of the United Nations Drug Conventions, although it has become increasingly popular in recent years. In 2002, Australia became the first country to ban the possession and sale of S. divinorum [17]. In the USA, Missouri was the first state to restrict S. divinorum use in 2003. Two years later, Louisiana became the first state to pass a law regulating S. divinorum. In 2006, a Delaware teenager named Brett Chidester used S. divinorum, which led to suicide, and S. divinorum and salvinorin A were then placed on Schedule I of Delaware’s Controlled Substances Act. In 2007, the Drug Enforcement Administration (DEA) placed S. divinorum on the list of drugs of concern and into the preliminary stages of drug regulation [18]. In 2008, the DEA reported that nine states had passed a law controlling S. divinorum, four states banned its use and sale, and seven states proposed legislative bills, suggesting a tendency towards regulatory control in the USA [19]. In Europe, Denmark, Germany, Latvia, Italy, Sweden, Croatia, Romania, Lithuania, and Belgium have placed bans on under-the-table sale of S. divinorum and salvinorin A; Poland has banned the possession and sale of S. divinorum and its derivatives; Russia and Spain have prohibited the possession and sale of S. divinorum plants. However, in Norway, Iceland, Finland, and Estonia S. divinorum is legal for medical purposes that include treatment of cocaine and heroin addiction. In Asia, Japan and South Korea have also placed bans on S. divinorum and salvinorin A trafficking [17]. In 2012, in Hong Kong, S. divinorum was the subject of a proposed legislative bill that suggested regulatory control [20].
In recent years, dry leaves and related products of S. divinorum (Fig. 1), as well as information on methods and experience of use, have been available on the Internet in Taiwan. Because S. divinorum has become a popular recreational drug and various Salvia species may contain opioid receptor agonists [21], it would be of interest to learn if salvinorin A could be determined in various endemic Salvia species in Taiwan. The aim of this study was therefore to employ high performance liquid chromatography (HPLC) to detect the presence of salvinorin A in substantial amounts in S. divinorum and endemic species of Salvia in Taiwan.
Fig. 1.
Dried leaves and related products of Salvia divinorum purchased from the Internet in Taiwan. (A) Dry leaves of S. divinorum; (B) S. divinorum 100fx; (C) S. divinorum 30fx.
2. Materials and methods
2.1. Chemicals and samples
Salvinorin A (>97% purity) was purchased from ChromaDex (Irvine, CA, USA). 4-Bromonitrobenzene (TCI, Tokyo, Japan) was used as the internal standard (IS). Extraction solvents, methanol, acetonitrile, ethyl acetate, and ethanol, used to extract plant samples, were of analytical grade.
Dry leaves of S. divinorum and related concentrated extract products, including S. divinorum 100fx (source specified as Canada) and 30fx (source specified as Taiwan), were purchased through the Internet in Taiwan. Endemic species of Salvia, including S. arisanensis Hayata, S. coccinea Juss. Ex Murr, S. hayatana Makino ex Hayata, S. japonica Thumb. ex Murr, S. nipponica Miq. Var. formosana (Hayata) Kudo, S. scapiformis Hance, S. tashiroi Hayata. Icon. PI. Formosan, and S. keitaoensis Hayata, were collected from Xueshan, Aowanda, Yangmingshan Park, Pingxisiaotzushan, Shihbaluohanshan, and Qingshui Mountain and confirmed by methods of pharmacognosy in the laboratory of Dr Hsien-Chang Chang.
2.2. HPLC conditions
The HPLC system consisted of a Hitachi L-2130 pump (Schaumburg, IL, USA), a Waters 717 plus autosampler (Milford, MA, USA), and a Waters 486 UV detector (Milford, MA, USA). The HPLC column was a Phenomenex® Luna C-18 analytical column (250 4.6 mm I.D., 5 mm, Torrance, CA, USA), which was coupled with a Phenomenexi® analytical guard cartridge system (KJO-4282, Torrance, CA, USA) to protect the analytical column in all analysis processes. HPLC determinations were performed according to the method described previously with slight modifications [22]. The mobile phase of acetonitrile-water (35:65, v/v) was used for isocratic elution at a flow rate of 1.5 mL/min, and column eluate was detected by UV absorption at 208 nm.
2.3. Preparation of standard solutions
Salvinorin A was weighed and dissolved in methanol to a concentration of 1 mg/mL as the stock standard solution, and then working standard solutions of 0.5, 0.8, 1.0, 2.5, 4.0, 5.0, 8.0, and 10.0 μg/mL were prepared by dilution with methanol and stored at 4°C. The calibration curve of salvinorin A was made using the working standard solutions, as analyzed by HPLC.
2.4. Extraction of samples
Leaf samples of endemic Salvia species were dried under the shade before grinding to fine powder in a mortar. Dry leaves and concentrated extract products of S. divinorum 30fx and 100fx were directly ground to fine powder. In this study, a single extraction method as described by Gruber et al [22] was performed with modifications. Briefly, the fine powder samples (100 mg) of ground leaf or concentrated extract products were extracted in 100 mL extraction solution in a flask, followed by shaking for 1 minute, sonication for 1 minute, and steeping for 3 days. The purpose of sonication was to increase contact surface areas of the fine powder samples with methanol. Because longer sonication time (>2 minutes) resulted in a substantial temperature increase, sonication for 1 minute was adopted. The crude extracts were filtered with filter paper, and the extraction was repeatedly washed with appropriate amounts of methanol. The filtrates were evaporated to dryness in a rotary evaporator. The dried solid samples were resolubilized with methanol to make a concentration of 1 mg/mL.
2.5. Quantitative analysis of salvinorin A in S. divinorum, concentrated extract products, and endemic Salvia species of Taiwan
Before evaporation to dryness, the crude extracts of S. divinorum dry leaves and S. divinorum 100fx were diluted 10-fold, and that of S. divinorum 30fx was diluted 20-fold with methanol. The dry solids were spiked with known levels of salvinorin A, i.e., 0, 0.5, 1.0, 2.5, or 5.0 μg/mL with IS 4-bromonitrobenzene (10.0 μg/mL) to make a redissolved solution of 1.0 mg/mL. Aliquots of 20 μL redissolved solutions were injected into the HPLC system. Recovery of salvinorin A during the filtration and evaporation processes was investigated by adding 10.0 μg/ mL of salvinorin A to the endemic Salvia species of Taiwan. The amount of salvinorin A present in the plant samples was calculated from the peak area ratio of salvinorin A to the IS.
3. Results
3.1. Separation conditions of the mobile phase
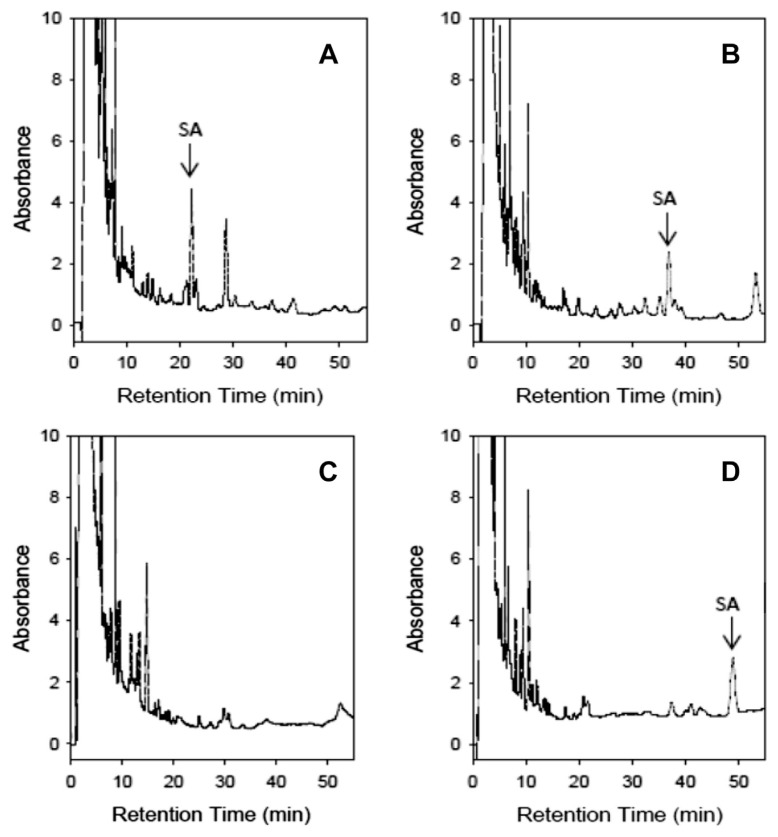
The mobile phase composition, modified by Gruber et al [22], consisted of a mixed extraction of six endemic Salvia species in Taiwan spiked with 10 μg/mL salvinorin A. Fig. 2A and B showed that in the initial mobile phase of acetonitrile–water (45:55, v/v) with a flow rate of 1.0 mL/min and the modification of the acetonitrile–water (40:60, v/v) with a flow rate of 1.0 mL/min, the salvinorin A peak was interfered with by the endogenous components of the crude extraction of the mixed six endemic Salvia species. In the modified mobile phase of acetonitrile–water (35:65, v/v) at a flow rate of 1.0 mL/min, the salvinorin A peak was not eluted within 55 minutes (Fig. 2C). The modified mobile phase of acetonitrile–water (35:65, v/v) with a flow rate of 1.5 mL/min under isocratic elution had a better separation efficiency in the mixed extraction, leading to salvinorin A being eluted at approximately 48.85 minutes (Fig. 2D). Theoretically, a shorter retention time could be achieved with shorter analytical columns, such as 150 × 4.6 × 5 μm. In this study, the separation condition was not further approached because the analytical evaluation of salvinorin A content in Salvia species for the administrative purpose had been fulfilled.
Fig. 2.
Effect of the mobile phase of acetonitrile–water on the retention of six Salvia species spiked with 10 μg/mL salvinorin A. (A) Acetonitrile–water (45:55, v/v) at a flow rate of 1.0 mL/min; (B) acetonitrile–water (40:60, v/v) at a flow rate of 1.0 mL/min; (C) acetonitrile–water (35:65, v/v) at a flow rate of 1.0 mL/min; (D) acetonitrile–water (35:65, v/v) at a flow rate of 1.5 mL/min.
3.2. Selection of extraction solvent and extraction time
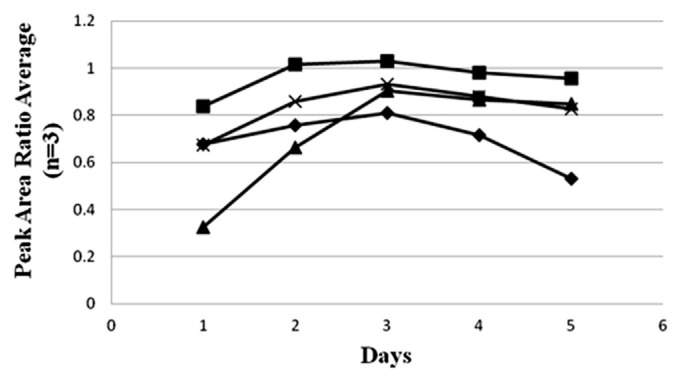
Previous studies have used chloroform and acetonitrile to extract salvinorin A from S. divinorum, but chloroform is more toxic and extraction with acetonitrile probably requires a relatively long time [8,22]. In this study, we tried methanol, ethanol, acetonitrile, and ethyl acetate as extraction solvents. The results shown in Fig. 3 demonstrate the extraction conditions that were selected: the use of methanol as the extraction solvent and steeping for 3 days; methanol extracted the contents of salvinorin A in S. divinorum at a greater efficiency than ethanol, acetonitrile, or ethyl acetate. The extraction time was optimized with steeping for 3 days, because this condition extracted the maximum amount of salvinorin A from S. divinorum, and the salvinorin A content decreased with steeping for 4 days or more, probably due to the degradation or other biological causes of the compounds in the crude extract of plant samples.
Fig. 3.
Effect of solvent and extraction days on the extraction of salvinorin A (n = 3). ACN (◆) = acetonitrile; EtOH (▲) = ethanol; EA (×) = ethyl acetate; MeOH (■) = methanol.
3.3. Method validation
Because salvinorin A is an endogenous component in the plant samples, the accuracy of the method cannot be estimated using a known concentration of salvinorin A in the crude extraction of plant samples. The validation of the method for determination of salvinorin A can be used as standard addition for analysis of salvinorin A in Salvia species. To evaluate the quantitative application of the method, five different concentrations over the range of 0.5–10.0 μg/mL salvinorin A spiked in Salvia species and a fixed concentration of IS were analyzed. The linear regression equation was y = (0.0288 ± 0.0005) x + (0.0065 ± 0.0002), with the correlation coefficient (r) above 0.999 (n = 5). These data demonstrate the high linearity of this method. The precision and accuracy of the proposed method was evaluated on spiked salvinorin A in Salvia species and estimated by relative standard deviation (RSD) and relative error (RE). As shown in Table 1, the RSD and RE values of different concentrations from high, medium, to low were all below 2.6% and 1.4%, respectively. The limit of detection (LOD) was determined by a signal-to-noise ratio of 3 (S/N = 3) and the limit of quantification (LOQ) was determined by an S/N ratio of 10. The LOD and LOQ were 0.1 μg/mL and 0.5 μg/mL, respectively. The LOD and LOQ were obtained from a mixed solution of six endemic Salvia species spiked with salvinorin A. The precision of the slope and intercept of the regression equations were estimated by standard addition analysis for salvinorin A in the dry leaves of S. divinorum, the concentrated extract products, and eight endemic Salvia species of Taiwan, and all were less than 4.2% and 3.9%, respectively (Table 2). Salvinorin A at 10.0 μg/mL was spiked in endemic Salvia species for the recovery test. The peak area ratio of the tested chemical standard was 10.0 μg/mL and the absolute recovery was 74.1 ± 3.2% (n = 3). From these results, better extractability of Salvinorin A in endemic Salvia species was observed.
Table 1.
Determination of accuracy and precision of reference salvinorin A.
| Concentration known (μg/mL) | Concentration found (μg/mL) | RSD (%) | RE (%) |
|---|---|---|---|
| Intra-day (n = 5)a | |||
| 0.8 | 0.811 ± 0.008 | 0.98 | 1.37 |
| 4.0 | 3.979 ± 0.067 | 1.68 | −0.52 |
| 8.0 | 8.036 ± 0.194 | 2.41 | 0.45 |
| Inter-day (n = 5)b | |||
| 0.8 | 0.805 ± 0.020 | 2.48 | 0.62 |
| 4.0 | 4.018 ± 0.104 | 2.58 | 0.45 |
| 8.0 | 7.960 ± 0.126 | 1.58 | −0.50 |
RE = relative error; RSD = relative standard deviation.
Intra-day assay variance was validated with three known levels of salvinorin A at five intervals in 1 day.
Inter-day assay variance was validated with three levels of the analyte on 5 successive days.
Table 2.
Precision of the slope and intercept of the regression equations by the standard addition method in the analysis of salvinorin A in Salvia.
| Sample (intra-day, n = 5, 0.5–10 μg/mL) | Regression equation | Correlation coefficient (r) | Slopec | Interceptc |
|---|---|---|---|---|
| S. divinorum | y = (0.0302 ± 0.0009)x + (0.0225 ± 0.0004) | 0.9997 | 0.0302 ± 0.0009 (2.9) | 0.0225 ± 0.0004 (1.6) |
| S. divinorum 100fxa | y = (0.0306 ± 0.0004)x + (0.0132 ± 0.0004) | 0.9999 | 0.0306 ± 0.0004 (1.3) | 0.0132 ± 0.0004 (3.1) |
| S. divinorum 30fxa | y = (0.0325 ± 0.0006)x + (0.0225 ± 0.0008) | 0.9998 | 0.0325 ± 0.0006 (1.8) | 0.0225 ± 0.0008 (3.9) |
| S. arisanensis b | y = (0.0311 ± 0.0013)x + (0.0014 ± 0.0004) | 0.9997 | 0.0311 ± 0.0010 (4.2) | — |
| S. coccinea b | y = (0.0288 ± 0.0007)x + (0.0016 ± 0.0007) | 0.9998 | 0.0288 ± 0.0007 (2.5) | — |
| S. hayatana b | y = (0.0286 ± 0.0012)x + (0.0009 ± 0.0006) | 0.9999 | 0.0286 ± 0.0012 (4.1) | — |
| S. scapiformis b | y = (0.0298 ± 0.0008)x + (0.0005 ± 0.0006) | 0.9998 | 0.0297 ± 0.0008 (2.7) | — |
| S. tashiroi b | y = (0.0285 ± 0.0011)x + (0.0008 ± 0.0006) | 0.9999 | 0.0284 ± 0.0011 (3.9) | — |
| S. nipponica b | y = (0.0289 ± 0.0007)x + (0.0005 ± 0.0004) | 0.9999 | 0.0289 ± 0.0007 (2.5) | — |
| S. japonica b | y = (0.0300 ± 0.0006)x + (0.0005 ± 0.0003) | 0.9999 | 0.0300 ± 0.0006 (2.1) | — |
| S. keitaoensis b | y = (0.0304 ± 0.0009)x + (0.0001 ± 0.0005) | 0.9999 | 0.0303 ± 0.0009 (3.2) | — |
RSD = relative standard deviation; SD = standard deviation.
“Concentrated extract” products.
Endemic species of Salvia in Taiwan.
Mean ± SD (n = 5) with the % RSD values in parentheses.
3.4. Qualitative and quantitative analyses of salvinorin A in S. divinorum, concentrated extract products, and endemic Salvia species of Taiwan
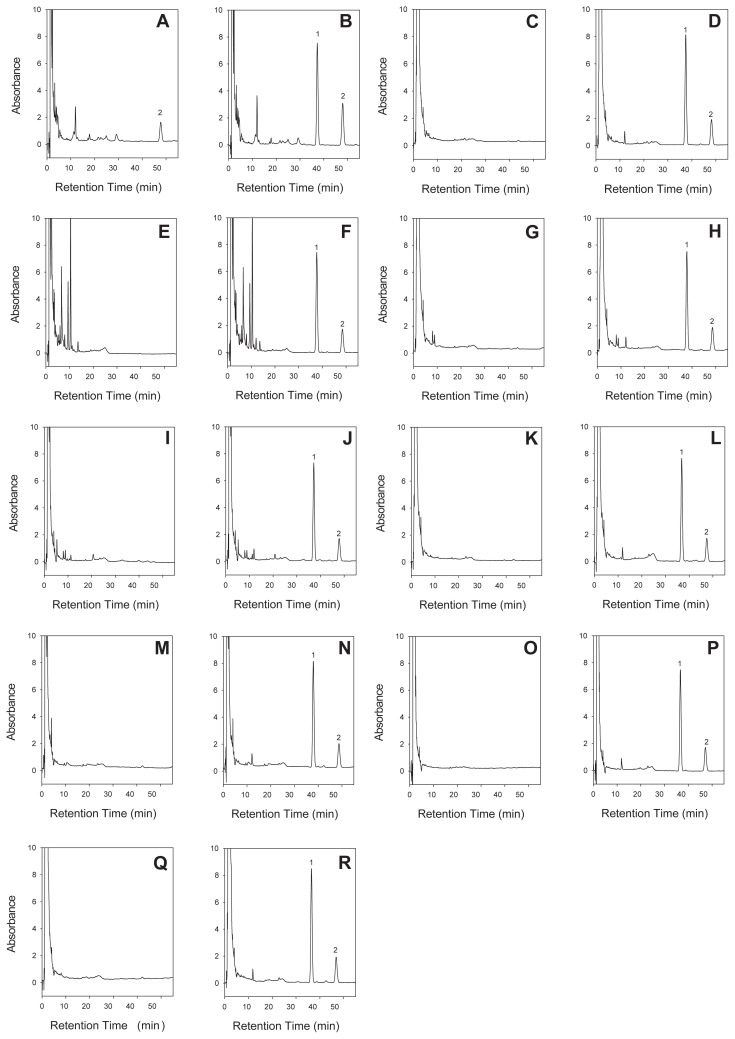
A qualitative study of salvinorin A from dry leaves of S. divinorum, related products, and endemic Salvia species involved spiking with 10 μg/mL salvinorin A to compare with blank samples. The results are shown in Fig. 4. Salvinorin A was not found in endemic Salvia species under the collection conditions employed, but was only found in S. divinorum purchased from the Internet in Taiwan. The salvinorin A contents in endemic Salvia species were probably affected by the various sources and collection conditions. The content of the hallucinogen salvinorin A was analyzed by the standard addition method for various plant samples spiked with salvinorin A. The results of the quantitative analysis of salvinorin A are shown in Table 3. The salvinorin A content in the dry leaves of S. divinorum was 84.10 ± 3.06 μg/100 mg. A previous study indicated that dry leaf samples of S. divinorum contained 63–370 μg/100 mg [22]. As salvinorin A is an endogenous substance, the various contents in S. divinorum were probably subject to the influences of different sources, collection conditions, or cultivation methods. The concentrated extract products of S. divinorum 30fx (source specified as Taiwan) and 100fx (source specified as Canada) contained 163.97 ± 6.48 μg/ 100 mg and 55.75 ± 1.73 μg/100 mg, respectively. The salvinorin A contents in the concentrated extract products were claimed by the vendors to be standardized and concentrated. According to previous studies, Tsujikawa et al [8] indicated that a concentrated extract product labeled as being of a potency of 2 × −25 × contained 410–3890 μg/100 mg, and Wolowich et al [23] reported that a concentrated extract with a labeled potency of 5 × −20 × contained 12.6–113.7 μg/100 mg. The results indicated that the standardized contents of salvinorin A in the concentrated extract products were lower than that of S. divinorum dry leaves, which is similar to the results reported by Tsujikawa et al [8].
Fig. 4.
Liquid chromatograms for (A) Salvia divinorum, (B) S. divinorum spiked with internal standard (IS) (10.0 μg) and salvinorin A (10.0 μg), (C) S. arisanensis, (D) S. arisanensis spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (E) S. coccinea, (F) S. coccinea spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (G) S. hayatana, (H) S. hayatana spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (I) S. tashiroi, (J) S. tashiroi spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (K) S. japonica, (L) S. japonica spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (M) S. nipponica, (N) S. nipponica spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (O) S. scapiformis, (P) S. scapiformis spiked with IS (10.0 μg) and salvinorin A (10.0 μg), (Q) S. keitaoensis, (R) S. keitaoensis spiked with IS (10.0 μg) and salvinorin A (10.0 μg). Peak 1 = 4-bromonitrobenzene (IS); peak 2 = salvinorin A. High performance liquid chromatography (HPLC) conditions with a stationary phase of C-18 column (250 × 4.6 mm I.D., 5 μm) and a mobile phase of acetonitrile–water (35:65, v/v) at a flow rate of 1.5 mL/min and UV detection at 208 nm.
Table 3.
Contents of salvinorin A in plant samples.
| Salvia species | Source | Collection/purchase date | Concentration (μg/100 mg)c |
|---|---|---|---|
| S. divinorum | Internet in Taiwan | 2011.07.06 | 84.10 ± 3.06 |
| S. divinorum 100fxa | Internet in Taiwan | 2011.07.06 | 55.75 ± 1.73 |
| S. divinorum 30fxa | Internet in Taiwan | 2012.04.12 | 163.97 ± 6.48 |
| S. arisanensis b | Xueshan | 2011.06.16 | — |
| S. coccinea b | Aowanda | 2011.06.18 | — |
| S. hayatana b | Xueshan | 2011.07.06 | — |
| S. scapiformis b | Pingxisiaotzushan | 2011.08.14 | — |
| S. tashiroi b | Shihbaluohanshan | 2011.07.22 | — |
| S. nipponica b | Yangmingshan Park | 2011.06.26 | — |
| S. japonica b | Yangmingshan Park | 2011.06.26 | — |
| S. keitaoensis b | Qingshui Mountain | 2012.02.11 | — |
“Concentrated extract” products.
Endemic species of Salvia in Taiwan.
Mean ± standard deviation (SD) (n = 5).
4. Discussion
The results of this study demonstrated that salvinorin A could be detected in dry leaves of S. divinorum and its related concentrated extract products, but not in the dry leaves of endemic Salvia species of Taiwan, including S. arisanensis, S. coccinea, S. hayatana, S. japonica, S. nipponica, S. scapiformis, S. tashiroi, and S. keitaoensis. Siebert [7] reported that smoking 40–70 mg of the S. divinorum plant (equivalent to 200–500 μg of salvinorin A) produced strong hallucinogenic effects that lasted for about 20–30 minutes. The salvinorin A contents in the dry leaves, 30fx (source specified as Taiwan), and 100fx (source specified as Canada) of S. divinorum were found to be 84.10 ± 3.06 μg/100 mg, 163.97 ± 6.48 μg/100 mg, and 55.75 ± 1.73 μg/100 mg, respectively. These results indicated that the contents of salvinorin A in the related concentrated extract products were significantly lower than that in dry leaves of S. divinorum, and the concentration levels found through content analysis were not of the labeled potency [8].
The results indicated that, under the collection time and conditions, Salvia species endemic to Taiwan may not become recreational drugs in Taiwan. However, S. divinorum dry leaves and related concentrated extract products have been available via the Internet in Taiwan. Because they have various features that are attractive to young drug users [12], S. divinorum will probably become a new recreational drug. The abuse potential of S. divinorum has been examined, and it has been reported that the salvinorin A can produce a rewarding effect in the conditioned place preference test, as well as inducing an increase in dopamine levels in the nucleus accumbens shell, which is considered to be associated with addiction behavior [24–26]. Some reports have indicated that patients using S. divinorum can experience psychotic symptoms and schizophrenia, and one study reported that S. divinorum users had a higher incidence rate of depression than that of nonusers [14]. An animal model demonstrated that attention and cognitive deficits were induced by salvinorin A [14,16,27–29]. Violence and suicidal behavior can take place after using S. divinorum [30,31]. Concern has been increasing regarding the potential harm caused by S. divinorum. Currently, S. divinorum and its hallucinogenic ingredient salvinorin A are regulated in many countries. The first country to control S. divinorum was Australia, in which S. divinorum and salvinorin A were placed on Schedule 9, which is defined as a prohibited substance—substances that may be abused or misused, the manufacture, possession, sale, or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching, or training purposes with the approval of Commonwealth and/or State or Territory Health Authorities [32]. In the USA, many states, including Florida, Virginia, Hawaii, Kentucky, Michigan, Minnesota, Connecticut, Missouri, Illinois, Oklahoma, Kansas, Mississippi, Ohio, South Dakota, Georgia, Wyoming, Delaware, Alabama, North Dakota, Indiana, Pennsylvania, Nebraska, and Colorado, have placed S. divinorum and/or salvinorin A in Schedule I, which includes substances that have a high potential for abuse and no currently accepted medical use in the United States, and for which there is a lack of accepted safety evidence for use of the drug or other substance under medical supervision [31,33]. Other states in the USA, including Maryland, Tennessee, North Carolina, Louisiana, California, West Virginia, Wisconsin, California, and Maryland, have enacted specific age restrictions, particular regulation or scheduling in order to control S. divinorum and/or salvinorin A [31]. In Canada, S. divinorum and its main active ingredient salvinorin A have been suggested to be listed on Schedule III of the Controlled Drugs and Substances Act (CDSA) [34]. Some countries in Europe, such as Belgium, Croatia, Denmark, Germany, Italy, Latvia, Lithuania, Romania, Sweden, Poland, Russia, and Spain, have strictly regulated or banned S. divinorum and/or salvinorin A trafficking, possession, and/or sale. In Norway, Finland, Estonia and Iceland, S. divinorum is legal only for medicinal purposes [17]. In Asia, Japan and South Korea also banned S. divinorum and salvinorin A trafficking, and Hong Kong has proposed regulatory controls for S. divinorum and salvinorin A, because these substances have a high potential for harm, and have unknown or are unapproved for medical purposes [17,20]. However, S. divinorum and its related concentrated extract products have not been regulated in Taiwan. The hallucinogenic substance salvinorin A in S. divinorum has subsequently been reported to have an addiction potential, risk of harm through intoxication, and tendency to increase drug abuse in several countries. According to Nutt et al [35], the potential harm of new recreational drugs and their related schedule level can be assessed based on fact and scientific knowledge. Therefore, regulation of S. divinorum/salvinorin A should be evaluated and the legal status considered in Taiwan.
5. Conclusions
In this study, we developed a method that improved the extraction efficiency of salvinorin A in S. divinorum, and established a method modified from a previous study to detect salvinorin A in various Salvia species. The results indicated that the hallucinogenic substance salvinorin A was only found in S. divinorum and its related concentrated extract products but not in eight species of Salvia endemic to Taiwan under the conditions of collection. S. divinorum-related products are controlled in many countries because they could lead to several health problems, such as addiction (dependence) potential, abuse potential, and the potential to cause social harm, and these products have become popular recreational drugs among young drug users. Currently, definite medical purposes of salvinorin A have not yet been proven. Therefore, we suggest that S. divinorum and its related concentrated extract products may need close scrutiny and should be further assessed in Taiwan.
Acknowledgments
This study was supported in part by grants from the Committee on Chinese Medicine and Pharmacy (CCMP100-CP-006 and CCMP101-CP-002) and the Food and Drug Administration (DOH102-FDA-61102), Department of Health, Taiwan.
Funding Statement
This study was supported in part by grants from the Committee on Chinese Medicine and Pharmacy (CCMP100-CP-006 and CCMP101-CP-002) and the Food and Drug Administration (DOH102-FDA-61102), Department of Health, Taiwan.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Valdes LJ, 3rd, Diaz JL, Paul AG. Ethnopharmacology of ska Maria Pastora (Salvia divinorum, Epling and Jativa-M.) J Ethnopharmacol. 1983;7:287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez D, Riba J, Bouso JC, et al. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–62. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 3. Yan F, Roth BL. Salvinorin A: a novel and highly selective kappa-opioid receptor agonist. Life Sci. 2004;75:2615–9. doi: 10.1016/j.lfs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4. Roth BL, Baner K, Westkaemper R, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–9. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walentiny DM, Vann RE, Warner JA, et al. Kappa opioid mediation of cannabinoid effects of the potent hallucinogen, salvinorin A, in rodents. Psychopharmacology. 2010;210:275–84. doi: 10.1007/s00213-010-1827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Mol Interv. 2006;6:257–65. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- 7. Siebert DJ. Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–6. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 8. Tsujikawa K, Kuwayama K, Miyaguchi H, et al. Determination of salvinorin A and salvinorin B in Salvia divinorum-related products circulated in Japan. Forensic Sci Int. 2008;180:105–9. doi: 10.1016/j.forsciint.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9. Tsujikawa K, Kuwayama K, Miyaguchi H, et al. In vitro stability and metabolism of salvinorin A in rat plasma. Xenobiotica. 2009;39:391–8. doi: 10.1080/00498250902769967. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt MD, Schmidt MS, Butelman ER, et al. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse. 2005;58:208–10. doi: 10.1002/syn.20191. [DOI] [PubMed] [Google Scholar]
- 11. Pichini S, Abanades S, Farre M, et al. Quantification of the plant-derived hallucinogen Salvinorin A in conventional and non-conventional biological fluids by gas chromatography/ mass spectrometry after Salvia divinorum smoking. Rapid Commun Mass Spectrom. 2005;19:1649–56. doi: 10.1002/rcm.1970. [DOI] [PubMed] [Google Scholar]
- 12. Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: Salvia divinorum and Kratom. Clin Toxicol. 2008;46:146–52. doi: 10.1080/15563650701241795. [DOI] [PubMed] [Google Scholar]
- 13.Drug Enforcement Administration (DEA) Salvia divinorum and salvinorin A. Washington: DEA, Office of Diversion Control, Drug and Chemical Evaluation Section; 2010. [Google Scholar]
- 14. Wu LT, Woody GE, Yang C, et al. Recent national trends in Salvia divinorum use and substance-use disorders among recent and former Salvia divinorum users compared with nonusers. Subst Abuse Rehabil. 2011;2011:53–68. doi: 10.2147/SAR.S17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute on Drug Abuse (NIDA) InfoFacts. Rockville: NIDA; 2011. [Google Scholar]
- 16. Braida D, Donzelli A, Martucci R, et al. Learning and memory impairment induced by salvinorin A, the principal ingredient of Salvia divinorum, in Wistar rats. Int J Toxicol. 2011;30:650–61. doi: 10.1177/1091581811418538. [DOI] [PubMed] [Google Scholar]
- 17. Listos J, Merska A, Fidecka S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol Rep. 2011;63:1305–9. doi: 10.1016/s1734-1140(11)70694-6. [DOI] [PubMed] [Google Scholar]
- 18. Khey DN, Miller BL, Griffin OH. Salvia divinorum use among a college student sample. J Drug Educ. 2008;38:297–306. doi: 10.2190/DE.38.3.g. [DOI] [PubMed] [Google Scholar]
- 19.Drug Enforcement Administration (DEA) Sage of the Seers, Diviner’s Sage, Salvia, Sally-D, Magic Mint) Washington: DEA, Office of Diversion Control, Drug and Chemical Evaluation Section; 2008. Salvia divinorum and salvinorin A (street names: Maria Pastora. [Google Scholar]
- 20.Legislative Council of the Hong Kong Special Administrative Region of the People’s Republic of China. Dangerous drugs ordinance (amendment of first and third schedules) order 2012. Central, Hong Kong: Legislative Council of Hong Kong; 2012. [Google Scholar]
- 21. Fontana G, Savona G, Rodriguez B, et al. Synthetic studies of neoclerodane diterpenoids from Salvia splendens and evaluation of opioid receptor affinity. Tetrahedron. 2008;64:10041–8. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruber JW, Siebert DJ, Der Marderosian AH, et al. High performance liquid chromatographic quantification of salvinorin A from tissues of Salvia divinorum Epling & Jativa-M. Phytochem Anal. 1999;10:22–5. [Google Scholar]
- 23. Wolowich WR, Perkins AM, Cienki JJ. Analysis of the psychoactive terpenoid salvinorin A content in five Salvia divinorum herbal products. Pharmacotherapy. 2006;26:1268–72. doi: 10.1592/phco.26.9.1268. [DOI] [PubMed] [Google Scholar]
- 24. Braida D, Limonta V, Capurro V, et al. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry. 2008;63:286–92. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 25. Braida D, Iosue S, Pegorini S, et al. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–9. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 26. Braida D, Sala M. Role of the endocannabinoid system in MDMA intracerebral self-administration in rats. Br J Pharmacol. 2002;136:1089–92. doi: 10.1038/sj.bjp.0704825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer EG, Writer BW. Salvia divinorum. Psychosomatics. 2012;53:277–9. doi: 10.1016/j.psym.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28. Paulzen M, Grunder G. Toxic psychosis after intake of the hallucinogen salvinorin A. J Clin Psychiatry. 2008;69:1501–2. doi: 10.4088/jcp.v69n0919c. [DOI] [PubMed] [Google Scholar]
- 29. Przekop P, Lee T. Persistent psychosis associated with Salvia divinorum use. Am J Psychiatry. 2009;166:832. doi: 10.1176/appi.ajp.2009.08121759. [DOI] [PubMed] [Google Scholar]
- 30. Griffin OH, Miller BL, Khey DN. Legally high? Legal considerations of Salvia divinorum. J Psychoactive Drugs. 2008;40:183–91. doi: 10.1080/02791072.2008.10400629. [DOI] [PubMed] [Google Scholar]
- 31. Stogner J, Khey DN, Griffin OH, et al. Regulating a novel drug: an evaluation of changes in use of Salvia divinorum in the first year of Florida’s ban. Int J Drug Policy. 2012;23:512–21. doi: 10.1016/j.drugpo.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health and Ageing (DHA), Australian Government. Poisons standard 2011. Woden: DHA; 2011. [accessed 31, 03,13]. Available at, http://www.comlaw.gov.au/Details/F2011L01612. [Google Scholar]
- 33.Drug Enforcement Administration (DEA), Office of Diversion Control. Definition of controlled substance schedules. Reno: DEA; 2012. [accessed 18, 01, 13]. Available at, http://www.deadiversion.usdoj.gov/schedules/index.html#list. [Google Scholar]
- 34.Department of Justice (DOJ) Canada. Health Canada’s agenda for Salvia divinorum and salvinorin A. Ottawa: DOJ; 2011. [accessed 18, 01, 13]. Available at, http://isomerdesign.com/Cdsa/newsHC.php. [Google Scholar]
- 35. Nutt D, King LA, Saulsbury W, et al. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–53. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]