Abstract
A simple and sensitive electrochemical method for the simultaneous and quantitative detection of ranitidine (RT) and metronidazole (MT) was developed, based on a poly (chromotrope 2B) modified activated glassy carbon electrode (PCHAGCE). The PCHAGCE showed excellent electrocatalytic activity toward the reduction of both RT and MT in 0.1 mol/L phosphate buffer solution (pH 6.0). The peak-to-peak separations for the simultaneous detection of RT and MT between the two reduction waves in cyclic voltammetry were increased significantly from ~0.1 V at activated GCE, to ~0.55 V at PCHAGCE. By differential pulse voltammetry techniques, the reduction peak currents of RT and MT were both linear over the range of 1.0 × 10−5–4.0 × 10−4 mol/L. The detection limits (S/N = 3) were 5.4 × 10−7 mol/L and 3.3 × 10−7 mol/L for RT and MT, respectively. The modified electrode was successfully applied to the determination of RT and MT in pharmaceutical preparations and human serum as real samples with stable and reliable recovery data.
Keywords: Human serum, Metronidazole, Pharmaceutical formulations, Poly(chromotrope 2B), Ranitidine
1. Introduction
Ranitidine [RT; N-(2-{[(5-dimethylamino)methyl]2-furanyl}-methylthioethyl)-N′-methyl-2-nitro-1,1′-ethenodiamine] is an inhibitor of gastric acid secretion and is used in the treatment of gastric and duodenal ulcers. It competitively inhibits the action of histamine on the H2 receptors of parietal cells [1,2]. Metronidazole [MT; 2-(2-methyl-5-nitroimidazole-1-yl) ethanol] is a 5-nitronimidazole derivative with activity against anaerobic protozoa and anaerobic bacteria. MT is widely distributed and appears in most body tissues and fluids including bile, bone, breast milk, cerebral abscesses, and saliva, and generally achieves concentrations similar to those in plasma with the effective concentration in human serum ranging from 2 μg/mL to 8 μg/mL [3]. The combination of these two drugs, have been successfully used with antibiotics for the treatment of gastric Helicobacter pylori infections [4]. They have low plasma protein binding (20%), and their effective plasma concentrations are 100 mg/mL and 6 mg/mL, respectively [5].
Quantitative detection of RT and MT is very difficult and crucial for the bioanalysis of biological fluids and drug formulation because of the need to selectively remove the interferents such as proteins. There are several investigations concerning the determination of RT and/or MT in pharmaceutical preparations by spectrophotometry [6], ion selective electrodes [7], fluorimetric method [8], near-infrared reflectance spectrometry [9], and chromatography [5]. However, the lack of selectivity limits the application of these methods for pharmacokinetic studies in pharmaceutical and biological samples. Moreover, some of these methods are poorly sensitive and require expensive apparatuses, and most of them involve several manipulation steps before when the final result of the analysis can be obtained. Therefore, it is important to develop a simple instrumental method with high sensitivity and selectivity for the detection of these drugs. In this regard, electrochemical methods have several advantages: ease of operation, high sensitivity and selectivity to the specific analytes, fast and reagentless detection, etc. RT and MT contain an electrochemically active reducible nitro group that leads to the development of an electrochemical sensor for the simultaneous detection of both. The electrochemical detection of these two drugs at carbon fiber microdisk [10] has been reported, although the sensitivity was low. Single-wall carbon nanotube modified electrodes [11] were reported with high sensitivity; however, they may suffer from mechanical instability because the electrodes were constructed by adsorption of carbon material on substrates [12].
In the present work, we report that poly(chromotrope 2B) (PCH) modified activated glassy carbon electrode (GCE) (PCHAGCE) can be successfully used for the simultaneous determination of RT and MT in pharmaceutical preparations and human serum.
2. Materials and methods
2.1. Reagents
All reagents were of analytical grade and were used without further purification. RT, MT, chromotrope 2B, Na2HPO4, and NaH2PO4 were purchased from Sigma-Aldrich, St. Louis, MI, USA. Phosphate-buffered saline (PBS; 0.1 M) was prepared by mixing 0.1 mol/L Na2HPO4 and NaH2PO4. All solutions were prepared with deionized water (DI, 18MΩ/cm) obtained from a XF-D water purifying system, Xuefeng Water Treatment Equipment Co. Ltd, Zhengzhou, (China). All experiments were performed at room temperature.
2.2. Apparatus
Electrochemical experiments were conducted on a CHI430A electrochemical workstation (CH Instruments, USA). Electrochemical measurements were performed in a three-electrode cell system with a GCE (3 mm diameter) or PCHAGCE, a platinum wire, and a Ag/AgCl (3 mol/L KCl) electrode were used as working, counter, and reference electrodes, respectively. All potentials in this study referred to this reference electrode. Differential pulse voltammograms were obtained by scanning the potential at different potential window with the following pulse amplitude: 100 mV/s, pulse width; 2 milliseconds, and pulse period; 1000 milliseconds. All electrochemical experiments were performed in 0.1 M PBS, unless otherwise specified.
2.3. Preparation of modified electrode
GCE was first subjected to electrochemical activation in PBS (pH 7.0) by applying a constant potential of +1.8 V for 5 minutes. Prior to activation, GCE was polished with 0.05 μm alumina/water slurry on a polishing cloth (Buekler, Germany) to a mirror-like finish, followed by sonication, and then rinsed with water. The activated GCEs were then subjected to electropolymerization of chromotrope 2B by cyclic sweeping of potential for 20 times from −0.4 V to 0.6 V at 100 mV/s in 0.1 mol/L PBS (pH 7.0) containing 0.5 mmol/L of chromotrope 2B, and the resulting PCHAGCE was rinsed with and stored in PBS prior to use.
3. Results and discussion
3.1. Electrochemical behavior of RT and MT at PCHAGCE
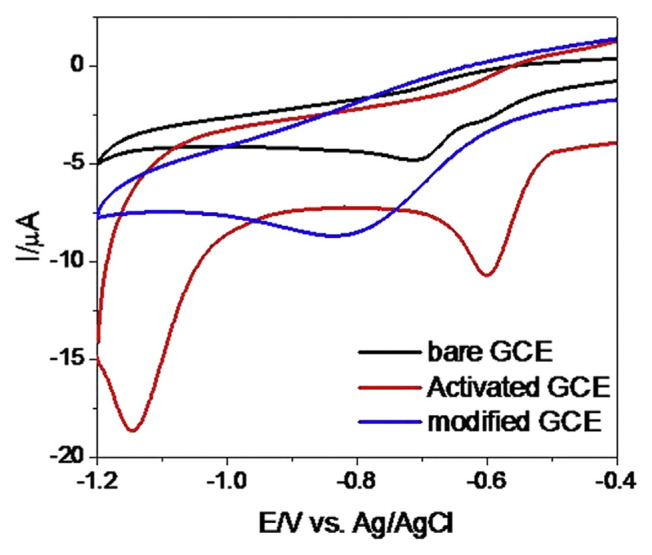
Fig. 1 shows the cyclic voltammogram (CVs) responses of a mixture of RT and MT (0.5 mmol/L of each) at bare GCE, AGCE, and PCHAGCE in PBS (pH 6.0). The CV obtained at bare GCE poses a rather broad oxidation peak and the indistinguishable peak potentials of these compounds. However, it was observed that RT is electrochemically active at AGCE giving a reduction peak at ca. −0.8 V, whereas the PCHAGCE gives a significantly distinguishable and increased reduction current with the negative shifting of reduction peak at ca. −1.15 V. MT gives a significantly distinguishable reduction peak and increased peak current at PCHAGCE compared to the AGCE with the reduction peak at ca. −0.58 V. These dramatic increases in the reduction peak current of RT and MT at PCHAGCE could be attributed to the strong catalytic ability of PCH. In conclusion, RT and MT give two clearly distinguishable reduction peaks at PCHAGCE with a significant increase in reduction peak current, whereas the reduction of these two compounds at AGCE does not give well-separated peak or increased peak current. It suggested a possible use of the modified electrode for the simultaneous determination of RT and MT.
Fig. 1.
Cyclic voltammograms of ranitidine and metronidazole (0.5 mmol/L of each) in phosphate-buffered saline at bare GCE, activated GCE, and poly(chromotrope 2B) modified activated GCE. GCE = glassy carbon electrode.
3.2. Effect of pH
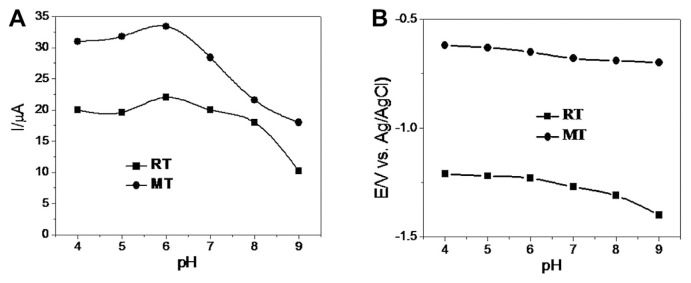
The effect of pH on the electrochemical response of RT and MT at PCHAGCE was evaluated by CV (as shown in Fig. 2) in the pH range 4.0–9.0. It was observed that with the increase of the pH of the cosolution, both reduction peaks for the two compounds were sifted negatively, whereas the reduction peak current is highest at pH 6.0 for both RT and MT. Therefore, pH 6.0 PBS was used as the supporting electrolyte in the following measurements.
Fig. 2.
Effect of pH on (A) peak currents and (B) peak potentials of RT and MT (0.5 mmol/L of each). MT = metronidazole; RT = ranitidine.
3.3. Effect of scan rate
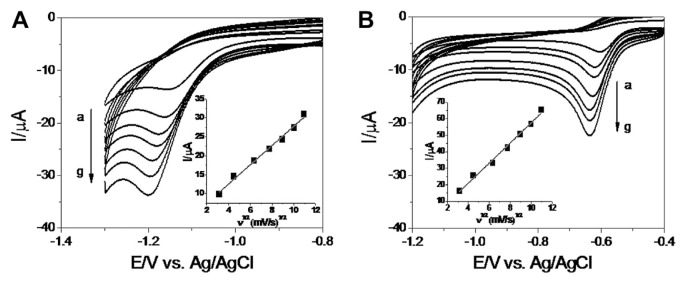
Fig. 3 shows the typical CVs of the electroreduction of RT(a) and MT(b) at different scan rates. The reduction peak potential for both RT and MT were observed to shift negatively with the increase in scan rate. The Ipc for both RT and MT were directly proportional to the square root of the scan rate over the range of 10–120 mV/s with the regression coefficient of 0.99 for both RT and MT. This strongly suggested that the reduction of RT and MT are diffusion controlled [13].
Fig. 3.
Cyclic voltammograms of (A) RT and (B) MT (0.5 mmol/L of each) at poly(chromotrope 2B) modified activated glassy carbon electrode in pH 6.0 phosphate-buffered saline at different scan rates (a–g: 10 mV/s, 20 mV/s, 40 mV/s, 60 mV/s, 80 mV/s, 100 mV/s, and 120 mV/s). Inset of each plots show the plot of peak current density versus square root of scan rates for RT and MT. MT = metronidazole; RT = ranitidine.
3.4. Simultaneous determination of RT and MT using differential pulse voltammetry
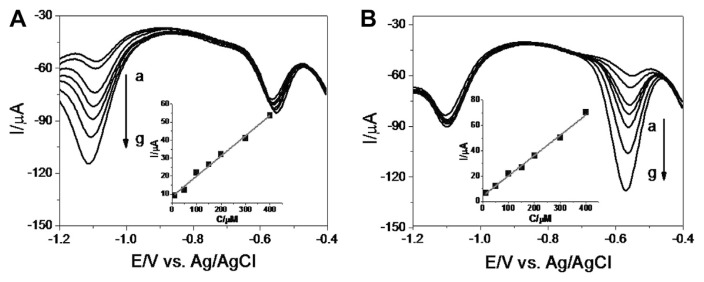
The result described in the previous section clearly indicated that both RT and MT can be detected independently and simultaneously on the PCHAGCE using CV. We further studied the quantitative detection of these compounds by differential pulse voltammetry (DPV) techniques. Fig. 4 shows the DPVs recorded for various concentrations (in the range of 1.0 × 10−5–4.0 × 10−4 mol/L) of RT and MT, respectively, in the presence of a constant concentration of counterpart (2.0 × 10−4 mol/L MT and 1.5 × 10−4 mol/L RT). The cathodic current of RT, which peaked at ca. −1.10 V, increased linearly as the concentration of RT increased, whereas the peak current of MT, at ~−0.55 V, remained almost unchanged. Similar patterns were observed for MT with the cathodic peak at ~−0.55 V with respect to RT without any mutual interference. The insets of both panels show the calibration plots constructed from the DPV responses for RT and MT. This yielded a regression equation of:
Fig. 4.
Differential pulse voltammograms for poly(chromotrope 2B) modified activated glassy carbon electrode in co-solution of (A) different concentrations (a–g: 1.0×10−5 mol/L, 5.0×10−5 mol/L, 1.0×10−4 mol/L, 1.5×10−4 mol/L, 2.0×10−4 mol/L, 3.0×10−4 mol/L, and 4.0×10−4 mol/L) of RT containing 1.5×10−4 mol/L MT and (B) different concentrations (a–g: 1.0×10−5 mol/L, 5.0×10−5 mol/L, 1.0×10−4 mol/L, 1.5×10−4 mol/L, 2.0×10−4 mol/L, 3.0×10−4 mol/L, and 4.0×10−4 mol/L) of MT containing 2.0×10−4 mol/L RT. Insets show the calibration plots of RT and MT. MT = metronidazole; RT = ranitidine.
and
with the regression coefficient of 0.99 for both RT and MT. The detection limits (S/N = 3) of 5.4 × 10−7 mol/L and 3.3 × 10−7 mol/L were obtained for RT and MT, respectively.
3.5. Reproducibility and stability
For evaluating the reproducibility of the response, the electrochemical determinations were performed 10 times repeatedly with an identical electrode in the solution containing RT and MT (0.5 mmol/L of each) at pH 6.0. The relative standard deviations were 2.54% and 2.26% for the 10-time measurements of RT and MT, respectively, showing a satisfactory reproducibility of the present protocol. The storage stability of the modified electrode was also evaluated by measuring DPV responses after storing the modified electrode for 2 weeks. The peak currents for RT and MT slightly decreased ca. 3% of the original response, showing an acceptable stability of the modified electrode.
3.6. Interference studies
In order to assess the possible use of the electrodes for the analysis of real samples, the interference of contaminants on the response signals was studied. The effect of possible interfering compounds on the CV response was verified using 0.5 mmol/L of RT and MT solutions containing excess amounts of interfering compounds. It was found that interference from metal ions and organic compounds was rather low. Interfering current originating from Na+, K+, dextrin (500 × higher concentration than RT and MT), Ca2+, Mg2+, Cu2+, Fe3 +, Zn2 + (200×), sucrose, glycerol, lactose, gelatin (50×), and uric acid, ascorbic acid (equal amount) was less than 5% of the oxidation current for 0.5 mmol/L of RT and MT. Thus, PCHAGCE was found to be rather insensitive to foreign substances, suggesting the usefulness of the electrode in the analysis of real samples.
3.7. Application to real sample analysis
To investigate the applicability of the proposed method for the simultaneous determination of RT and MT, pharmaceutical preparations (RT: Jiangxi Huiren, 150 mg/tablet; MT: Shanghai Hengshan, 150 mg/tablet) were used for quantitative analysis. The spike and recovery experiments were performed by measuring DPV responses for 100 mL dissolved tablet solutions in which known amounts of RT and MT were added. Their amounts were then determined by calibration and are summarized in Table 1. The recoveries for RT and MT were 97.0–105.0% and 99.0–103.0%, respectively. In addition, we measured these analytes in the human serum sample (male AB, from Xinxiang First People’s Hospital), Xinxiang, China which was diluted five times with the buffer solution prior to the measurements to prevent the matrix effect and then spiked with a certain amount of RT and MT. The slopes of peak current versus concentration were virtually close to those obtained in Fig. 4, and the recovery rates were 99.9% and 99.3% for RT and MT, respectively, in three independent measurements (100 μM of each). The above results indicate the applicability and reliability of the proposed method in real sample analysis.
Table 1.
The determination of RT and MT in pharmaceutical preparations (n = 3).
| Analyte sample | Nominal (mg/100 mL) | Added (mg/100 mL) | Founda (mg/100 mL) | Recovery (%) |
|---|---|---|---|---|
| RT | 150 | 10 | 159.7 | 97.0 |
| 20 | 171.0 | 105.0 | ||
| 30 | 179.6 | 98.7 | ||
| MT | 150 | 10 | 160.3 | 103.0 |
| 20 | 169.8 | 99.0 | ||
| 30 | 180.5 | 101.7 |
Average of six measurements.
4. Conclusions
In conclusion, sensitive and simple electrochemical method for the simultaneous and quantitative detection of RT and MT was developed based on a PCHAGCE. The PCHAGCE showed two well-defined reduction waves for RT and MT in both CV and DPV with a peak potential separation of ca. 0.55 V, which was large enough for simultaneous detection. Excellent catalytic activity rates for the electrochemical reduction of RT and MT were achieved at the PCHAGCE. A linear range of 1.0 × 10−5–4.0 × 10−4 mol/L for both RT and MT with detection limits of 5.4 × 10−7 mol/L and 3.3 × 10−7 mol/L for RT and MT, respectively, were obtained. The present protocol offers the advantages of accuracy, sensitivity, and time saving as well as simplicity of reagents and apparatus. In addition, the results obtained in the analysis of RT and MT in pharmaceutical preparations and human serum demonstrate the applicability of this method for real sample analysis.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Richter P, Toral MI, Vargas F. Polarographic behaviour and determination of ranitidine in pharmaceutical formulations and urine. Analyst. 1994;119:1371–4. doi: 10.1039/an9941901371. [DOI] [PubMed] [Google Scholar]
- 2. Zamarreno MD, Méndez JH, Pérez AS. Electrochemical study and polarographic determination of ranitidine. Anal Chim Acta. 1985;176:279–84. [Google Scholar]
- 3. Lamp KC, Freeman CD, Klutmam NE, et al. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacol. 1999;36:353–73. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 4. Farup PG, Tholfsen J, Wetternus S, et al. Comparison of three triple regimens with omeprazole or ranitidine bismuth citrate for Helicobacter pylori eradication. Scand J Gastroenterol. 2002;37:1374–9. doi: 10.1080/003655202762671233. [DOI] [PubMed] [Google Scholar]
- 5. Nascimento TG, Oliveira EJ, Macêdo RO. Simultaneous determination of ranitidine and metronidazole in human plasma using high performance liquid chromatography with diode array detection. J Pharmaceut Biomed Anal. 2005;37:777–83. doi: 10.1016/j.jpba.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 6. Vega E, Sola N. Quantitative analysis of metronidazole in intravenous admixture with ciprofloxacin by first derivative spectrophotometry. J Pharm Biomed Anal. 2001;25:523–30. doi: 10.1016/s0731-7085(00)00596-3. [DOI] [PubMed] [Google Scholar]
- 7. Issa YM, Badawy SB, Mutair AA. Ion-selective electrodes for potentiometric determination of ranitidine hydrochloride, applying batch and flow injection analysis techniques. Anal Sci. 2005;21:1443–8. doi: 10.2116/analsci.21.1443. [DOI] [PubMed] [Google Scholar]
- 8. Erroz CL, Vinas P, Campillo N, et al. Flow injection fluorimetric method for the determination of ranitidine in pharmaceutical preparations using o-phthaladehyde. Analyst. 1996;121:1043–6. [Google Scholar]
- 9. Dreassi E, Ceramelli G, Corti P, et al. Application of near-infrared reflectance spectrometry to the analytical control of pharmaceuticals: ranitidine hydrochloride tablet production. Analyst. 1996;121:219–22. doi: 10.1039/an9962100219. [DOI] [PubMed] [Google Scholar]
- 10. Bartlett PN, Ghoneim E, El-Hefnawy G, et al. Voltammetry and determination of metronidazole at a carbon fiber microdisk electrode. Talanta. 2005;66:869–74. doi: 10.1016/j.talanta.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 11. Salimi A, Izadi M, Hallaj R, et al. Simultaneous determination of ranitidine and metronidazole at glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis. 2007;19:1668–76. [Google Scholar]
- 12. Yu JJ, Du W, Zhao FQ, et al. High sensitive simultaneous determination of catechol and hydroquinone at mesoporous carbon CMK-3 electrode in comparison with multi-walled carbon nanotubes and Vulcan XC-72 carbon electrodes. Electrochim Acta. 2009;54:984–8. [Google Scholar]
- 13. Roy PR, Okajima T, Ohsaka T. Simultaneous electrochemical detection of uric acid and ascorbic acid at poly(N,N-dimethylaniline) film-coated GC electrode. J Electroanal Chem. 2004;561:75–82. [Google Scholar]