Abstract
The analytical method for sweeteners in various food matrixes is very important for food quality control and regulation enforcement. A simple and rapid method for the simultaneous determination of 10 sweeteners [acesulfame potassium (ACS-K), aspartame (ASP), cyclamate (CYC), dulcin (DUL), glycyrrhizic acid (GA), neotame (NEO), neohesperidin dihydrochalcone (NHDC), saccharin (SAC), sucralose (SCL), and stevioside (STV)] in various foods by liquid chromatography/tandem mass chromatography (LC–MS/MS) was developed. The chromatographic separation was performed on a Phenomenex Luna Phenyl-Hexyl (5 μm, 4.6 mm × 150 mm) column with gradient elution of 10 mM ammonium acetate in water and 10 mM ammonium acetate in methanol. The recoveries of the 10 sweeteners were between 75% and 120%, and the coefficients of variation were less than 20%. The limits of quantification were 0.5 μg/kg for NHDC and SCL. For the other sweeteners, the limits of quantification were 0.1 μg/kg. Compared to the traditional high-performance liquid chromatography method, the LC–MS/MS method could provide better sensitivity, higher throughput, enhanced specificity, and more sweeteners analyzed in a single run. The samples included 27 beverages (16 alcoholic and 11 nonalcoholic beverages) and 15 pickled foods (1 pickled pepper, 3 candies, and 11 candied fruits). Two remanufactured wines were found to contain 7.2, 8.5 μg/g SAC and 126.5, 123 μg/g CYC, respectively. ACS-K, ASP, SCL, and NEO were detected in five beverages and drinks. The pickled peppers and candied fruits were found to contain SAC, GA, CYC, ASP, STV, NEO, and ACS-K. The wine with sweeteners detected was remanufactured wine, not naturally fermented wine. Therefore, the ingredient label for the sweeteners of remanufactured wine should be regulated by the proper authority for inspection of sweeteners.
Keywords: Beverages, Candied fruit, LC-MS/MS, Pickled peppers, Sweeteners
1. Introduction
Sweeteners are functional food additives that impart sweetness in food [1]. Sweeteners can be divided into two categories, natural and synthetic sweeteners. Synthetic sweeteners cannot be metabolized in the human body and provide no or little calories; therefore, they are also named nonnutritive sweeteners. Owing to the inherent low calories, nonnutritive sweeteners are beneficial for obesity, hypertension, diabetes, and dental caries control [2]. Demand for low-calorie foods other than soft drinks causes the increasing use of nonnutritive sweeteners; as a result, nonnutritive sweeteners have become ubiquitous in many foods. Increased consumption of nonnutritive sweeteners in recent years has become a global trend [3,4]. Occasionally, very high sweetener contents in food could happen. From the alert information of the Rapid Alert System for Food and Feed, the highest aspartame (ASP) concentration recorded in food was 6.98 g/kg [5] and the highest cyclamate (CYC) concentration recorded in food was 12.455 g/kg [6]. Because overconsumption of synthetic sweeteners can be harmful to health [7–9], regulatory monitoring of intense sweetener contents in food is necessary to protect consumers.
For the risk characterization of nonnutritive sweeteners, the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) had established an acceptable daily intake (ADI) of 15 mg/kg body weight for acesulfame potassium (ACS-K), 40 mg/kg body weight for ASP, 11 mg/kg body weight for sodium CYC, 5 mg/kg body weight for saccharin (SAC), 2 mg/kg body weight for neotame (NEO), 4 mg/kg body weight for steviol glycoside, and 15 mg/kg body weight for sucralose (SCL). The JECFA has given dulcin (DUL) an ADI of “not to be used”, which means that no DUL should be used in food. The JECFA did not establish an ADI for glycyrrhizic acid (GA), but the committee indicated that consumption of 100 mg/day would be unlikely to cause adverse effects in the majority of adults [10]. The Scientific Committee on Food in the European Union (EU) had established an ADI of 5 mg/kg body weight for neohesperidin dihydrochalcone (NHDC) [11].
Many countries around the world had different maximum usable dose regulations for synthetic sweeteners [11–13]. The regulatory status of the 10 sweeteners used in the present study in different countries is listed in Table 1. The EU had permitted seven artificial sweeteners—ACS-K, ASP, cyclamic acid and its salts, NHDC, NEO, SAC and its salts, and SCL—as food additives. In the recent Commission Regulation (EU) No. 1131/2011, the steviol glycosides level in food was regulated in the EU. The sweeteners ACS-K, advantame, alitame, ASP, CYC, NEO, SAC, steviol glycosides, SCL, and thaumatin were approved for use in Australia and New Zealand [14]. The US Food and Drug Administration (FDA) had only permitted five artificial sweeteners—ACS-K, ASP, NEO, SAC, and SCL—as food additives, and the use of CYC and NHDC were not permitted as food additives in the United States [15]. Even though purified steviol glycosides with rebaudioside A and STV as the principal components was considered GRAS (generally recognized as safe), crude extracts from Stevia plant were not permitted as food additives by the US FDA. There are five sweeteners—ACS-K, ASP, SAC, disodium glycyrrhizinate, and SCL—permitted for use in Japan [16]. Meanwhile, China has permitted ACS-K, alitame, ASP, CYC, GA, NEO, SAC, steviol glycosides, and SCL as food additives [17]. The sweeteners DUL and NHDC were not permitted in Taiwan, but ACS-K, ASP, CYC, GA, NEO, SAC, STV, and SCL were permitted as food additives.
Table 1.
Regulatory status for the 10 sweeteners in the present study in different countries.
| Compound | EU | US FDA | Japan | FSANZ | China | Taiwan |
|---|---|---|---|---|---|---|
| ACS-K | Yesa | Yes | Yes | Yes | Yes | Yes |
| ASP | Yes | Yes | Yes | Yes | Yes | Yes |
| CYC | Yes | No | No | Yes | Yes | Yes |
| DUL | Nob | No | No | No | No | No |
| GA | No | No | Yes | No | Yes | Yes |
| NEO | Yes | Yes | No | Yes | Yes | Yes |
| NHDC | Yes | No | No | No | No | No |
| SAC | Yes | Yes | Yes | Yes | Yes | Yes |
| STV | Yes | No | No | Yes | Yes | Yes |
| SCL | Yes | Yes | Yes | Yes | Yes | Yes |
ACS-K = acesulfame potassium; ASP = aspartame; CYC = cyclamate; DUL = dulcin; EU = European Union; FSANZ = Food Standards Australia New Zealand; GA = glycyrrhizic acid; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside; US FDA = US Food and Drug Administration.
Yes: permitted food additive.
No: nonpermitted food additive.
There are several feasible analytical techniques for the analysis of sweeteners [18–24]. Many of the previous methods could analyze only one sweetener or simple sweetener mixtures. The synergistic use of sweeteners for cost reduction and taste quality improvement is often used, and the maximum permissible amount in food varies significantly [7,8,11,25,26]. An analytical method for the simultaneous determination of sweeteners in various food matrices is very important for food quality control and regulation enforcement.
Wasik et al [21] developed a high-performance liquid chromatography–evaporative light scattering detection (HPLC–ELSD) method for detecting six authorized sweeteners (ACS-K, ASP, CYC, NHDC, SAC, and SCL) and three sweeteners not authorized by current EU legislation (NEO, alitame, and DUL) in carbonated and noncarbonated soft drinks, and canned or bottled fruits and yogurt. The limits of detection (LODs) were below 15 mg/kg, and the limits of quantification (LOQs) were below 30 mg/kg for all sweeteners except DUL. For DUL, the LOD was 30 mg/kg and the LOQ 50 mg/kg. Buchgraber and Wasik [23] conducted an interlaboratory study with the HPLC–ELSD method described by Wasik et al [21] for detecting nine sweeteners in carbonated and noncarbonated soft drinks and canned or bottled fruits. Because HPLC was not as sensitive as liquid chromatography-mass chromatography (LC–MS), Koyama et al [27] first developed an LC–MS method for the simultaneous determination of nine types of sweeteners (ACS-K, SCL, SAC, CYC, ASP, DUL, GA, STV, and rebaudioside A) in various foods. Koyama et al [27] did not use internal standards in their work and the mass detection was performed by selected ion monitoring (SIM). The LOQs of ACS-K, SAC, CYC, ASP, and STV were 1 mg/kg, and those of SCL, DUL, GA, and REB were 5 mg/kg. Owing to the ion suppression effect in the dried plum sample, the analyte solution was diluted five times in order to obtain a satisfactory recovery for ACS-K. Yang and Chen [28] developed an LC–MS method with warfarin sodium as the internal standard to analyze eight sweeteners (ASP, SAC, ACS-K, NEO, SCL, CYC, alitame, and STV) in beverages, candied fruits, and cakes. In the study by Yang and Chen [28], the LODs were below 0.10 mg/mL, whereas the LOQs were below 0.30 mg/mL. Zygler et al [29] developed an LC–MS method to analyze nine EU-regulated sweeteners in beverages, dairy, and fish products. The internal standard was N-(2-methylcyclohexyl)sulfamate. The LODs were below 0.25 μg/g and the LOQs were 2.5 μg/g.
Most of the previous methods were based on SIM with a single quadruple mass spectrometer as the detector for chromatographic analysis. For confirmatory analysis in food safety regulation, the triple quadruple mass spectrometer could provide both qualitative and quantitative information with the multiple reaction monitoring (MRM) mode to increase the sensitivity and selectivity of the analysis. Therefore, liquid chromatography/tandem mass chromatography (LC–MS/MS) has become an increasingly popular tool for multiple sweeteners detection in foods and environment waters in recent years [25,30–34]. Scheurer et al [30] developed an LC–MS/MS method to analyze seven sweeteners in wastewater and surface water.
Previously, the official sweetener method adopted by the Taiwan FDA was based on HPLC–DAD (diode array detection) to detect four sweeteners (ACS-K, SAC, DUL, and CYC) [24]. Only four sweeteners could be analyzed simultaneously in a single run, and the sensitivity and selectivity of the official method were also not good compared to those of the LC–MS/MS method. Therefore, a recommended method for multiple sweeteners analyzed by LC–MS/MS with the MRM mode was recommended by the Taiwan FDA [35]. With polarity switching, 10 sweeteners could be analyzed in a single run. The sweeteners ACS-K, CYC, GA, NHDC, SAC, and STV were detected in the negative ion mode, and ASP, SCL, DUL, and NEO were detected in the positive ion mode. In the present study, an LC–MS/MS method to detect 10 sweeteners in wines, beverages, sports drinks, pickled peppers, and candied fruits has been developed. Because no previous studies were done for the matrix effect by LC–MS/MS, the present study evaluated the matrix effect by comparing the slope of matrix-matched standard curve with that of the standard calibration curve [36]. Compared to the traditional HPLC method [18,21,23,24], the LC–MS/MS method could provide better sensitivity, higher throughput, enhanced specificity, and more sweeteners analyzed in a single run.
2. Materials and methods
2.1. Samples, reagents, and chemicals
Food samples were purchased from the local markets for food safety inspection by the Public Health Bureau, Pingtung County Government, Pingtung, China from January 2012 to May 2012. The samples included 11 nonalcoholic beverages (i.e., 1 lemon tea, 2 sports drink, 2 soft drinks, and 6 juices), 16 alcoholic beverages (i.e., 1 cocktail, 3 plum flavored wine, 8 grape wines, and 4 flavored beers), and 15 preserved fruits and vegetables (i.e., 1 pickled pepper, 11 preserved fruits, and 3 candies).
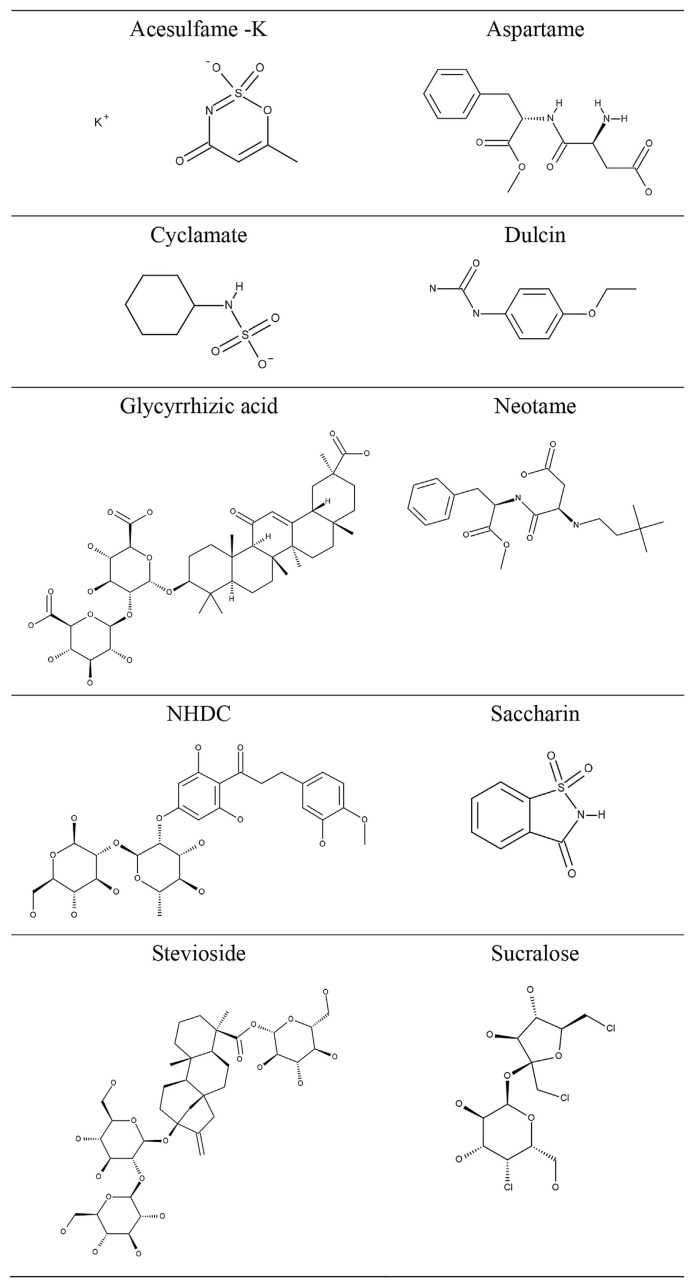
ASP (99.9%), sodium CYC (99.9%), and sodium SAC (99.9%) were purchased from Supelco Co. (Bellefonte, PA, USA), whereas ACS-K (99%) and SCL (98%) were obtained from Fluka Chemie (Buchs, Switzerland). Stevioside (STV; 98.4%) and GA (98.9%) were purchased from ChromaDex Inc. (Irvine, CA, USA). NEO (99%) and NHDC (99.9%) were obtained from Sigma-Aldrich (Munich, Germany). DUL (99.8%) was obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). All chemicals were of analytical grade. Methanol (HPLC-grade) was obtained from Biosolve (Valkenswaard, The Netherlands), whereas purified deionized water (R = 18 MΩ cm) was produced by a MilliQ unit (Millipore, Billerica, MA, USA). The chemical structures of all 10 sweeteners are shown in Fig. 1. Formic acid and acetonitrile were of HPLC grade and purchased from J.T. Baker Co., Ltd. (Philipsburg, NJ, USA). All other reagents were of analytical grade.
Fig. 1.
Structures of the 10 sweeteners in the present study.
2.2. Instruments
The homogenizer (Retsch Knife Mill Grindomix GM200) was purchased from Thermo Fisher Scientific Co. (Haan, Germany) and the centrifuge (model Himac CF 16RX) was purchased from Hitachi Koki Co (Ibaraki, Japan). The ultrasonicator was purchased from Ney Dental Inc. (Yucaipa, CA, USA). The LC system was performed using Dionex Co. (Sunnyvale, CA, USA) with an autosampler and 30 μL sample loop. The LC-electrospray ionization (ESI)–MS/MS detection of sweeteners was achieved using an AB SCIEX QTRAP 4000 (Framingham, MA, USA) mass spectrometer. Data acquisition was conducted using the Analyst 6.1 software (Framingham, MA, USA). The sweeteners were separated on an analytical column, Luna Phenyl-Hexyl (5 μm, 4.6 × 150 mm) from Phenomenex (Torrance, CA, USA).
2.3. Preparation of standard solutions
The stock solution of GA was prepared by dissolving 0.05 g of the pure sweetener in 40 mL of deionized water at 80°C and diluting to 50 mL with deionized water. Stock solutions for other sweeteners were prepared by dissolving 0.05 g of pure sweeteners and making up the volume to 50 mL with deionized water. A series of working standard solutions were prepared with a concentration range of 0.05–10 μg/mL by diluting the stock solutions with an appropriate amount of deionized water. All stock solutions and working solutions were stored at 4°C and brought to room temperature prior to use.
2.4. Sample preparation
Because all the sweeteners in the present study have good solubility in water, the food samples are extracted by deionized water as in the official method of the Taiwan FDA for sweetener analysis. For the beverage samples, a 5-g sample was dissolved in 30 mL of deionized water and degassed in an ultrasonic bath for 10 minutes. After cooling down to room temperature, deionized water was added volumetrically to 50 mL. The sample solution was filtered through a 0.22-μm syringe filter prior to being injected into HPLC. For the solid food samples, a 5-g homogenized sample was dissolved in 30 mL of deionized water and ultrasonicated in an ultrasonic bath for 10 minutes, and followed by centrifugation at 2100g for 10 minutes. The supernatant was transferred into a 50-mL volumetric flask. The precipitate was washed with 10 mL of deionized water and repeated extraction. The supernatants were pooled into the same 50-mL volumetric flask, and deionized water was added volumetrically to 50 mL level. The sample solution was filtered through a 0.22-μm syringe filter prior to being injected into HPLC.
2.5. Chromatographic and mass spectrometric analysis
The sweeteners were separated on a 4.6 × 150 mm, 5 μm particle Phenomenex Luna Phenyl-Hexyl column. The mobile phase consisted of deionized water containing 10 mM ammonium acetate in channel A, and methanol containing 10 mM ammonium acetate in channel B. The column temperature was set at 35°C. The mobile phase flow rate was set at 1.0 mL/minute and the injection volume was 30 μL. The mobile phase gradient is listed in Table 2. Mass spectrum analysis was carried out using the ESI method with polarity switching on the AB SCIEX QTRAP 4000 triple quadrupole tandem mass spectrometer. The mass operation parameters were set as follows: curtain gas (CUR), 10 psi; collision gas (CAD), high; ionspray voltage (IS), 4500 V; temperature (TEM), 500°C; nebulizer gas (GS1), 50 psi; turbo gas 2 (GS2), 50 psi; dwell time, 50 milliseconds; scan type, MRM mode. The mass transition parameters for MRM are listed in Table 3.
Table 2.
Gradient program of the mobile phase for HPLC separation of sweeteners in the present study.
| Time (min) | Flow rate (mL/min) | Mobile phase | |
|---|---|---|---|
|
| |||
| Aa (%) | Bb (%) | ||
| 0 | 1.0 | 80 | 20 |
| 3 | 1.0 | 80 | 20 |
| 6 | 1.0 | 50 | 50 |
| 12 | 1.0 | 0 | 100 |
| 15 | 1.0 | 0 | 100 |
| 15.5 | 1.0 | 80 | 20 |
| 18 | 1.0 | 80 | 20 |
HPLC = high performance liquid chromatography.
A: 10 mM ammonium acetate in deionized water.
B: 10 mM ammonium acetate in methanol.
Table 3.
MRM parameters for the sweeteners in the present study by LC–MS/MS.
| Compound | Precursorion (m/z) | Production (m/z) | Cone voltage (V) | Collision energy (eV) |
|---|---|---|---|---|
| ACS-K | 162 | 82a | −34 | −18 |
| 162 | 78 | −34 | −41 | |
| CYC | 178 | 80a | −74 | −35 |
| 178 | 96 | −74 | −31 | |
| SAC | 182 | 42a | −61 | −48 |
| 182 | 106 | −61 | −27 | |
| ASP | 293 | 261a | −40 | −14 |
| 293 | 200 | −40 | −20 | |
| SCL | 395 | 359a | −103 | −18 |
| 397 | 361 | −91 | −16 | |
| NHDC | 611 | 303a | −130 | −50 |
| 611 | 125 | −130 | −70 | |
| STV | 641.2 | 479a | −183 | −61 |
| 641.2 | 317 | −183 | −69 | |
| GA | 821 | 113a | −124 | −80 |
| 821 | 351 | −124 | −63 | |
| DUL | 181 | 108a | 55 | 32 |
| 181 | 136 | 55 | 22 | |
| NEO | 379 | 172a | 63 | 35 |
| 379 | 319 | 63 | 27 |
ACS-K = acesulfame potassium; ASP = aspartame; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; LC–MS/MS = liquid chromatography–tandem mass chromatography; MRM = multiple reaction monitoring; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
Ion for quantification.
2.6. Method validation and matrix effect evaluation
Method validation was done on a sweetener-free flavored beer and a sweetener-free dried guava as the representative matrix for beverage samples and solid food samples, respectively. For beverage samples, 5 g of the sweetener-free flavored beer in a 50-mL volumetric flask was spiked with the sweetener standard solution at the level of 0.5 μg/g, 1.0 μg/g, and 2.5 μg/g for the recovery test. The sample preparation procedure was the same as the beverage samples. For solid food samples, 5 g of the sweetener-free dried guava in a 50-mL volumetric flask was spiked with the sweetener standard solution at the level of 1.0 μg/g, 2.5 μg/g, and 5.0 μg/g for the recovery test. The sample preparation procedure was the same as the solid food samples.
The matrix effect was evaluated according to the procedure described by Chang et al [36]. Two calibration curves were constructed to evaluate the matrix effect. The standard calibration curve was obtained by four sweetener concentrations of 0.1 μg/mL, 0.25 μg/mL, 0.5 μg/mL, and 1.0 μg/mL. The matrix-matched calibration curve was obtained by spiking four sweetener concentrations of 0.1 μg/mL, 0.25 μg/mL, 0.5 μg/mL, and 1.0 μg/mL into the sweetener-free dried guava samples.
3. Results and discussion
3.1. Mass spectrometric detection
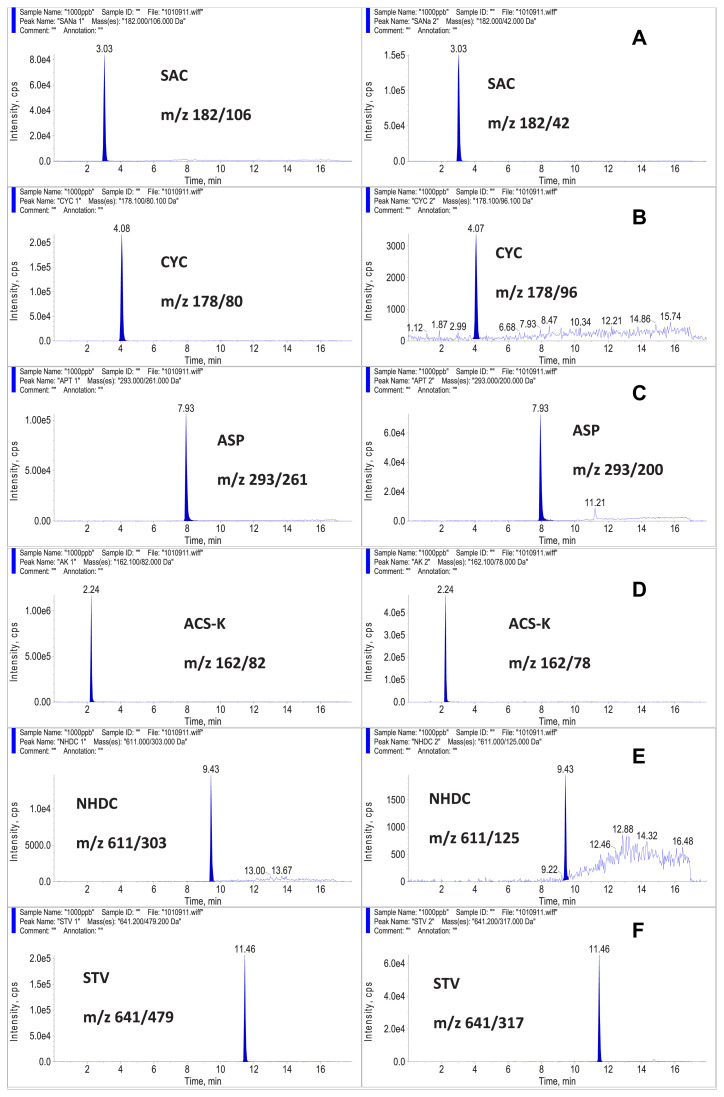
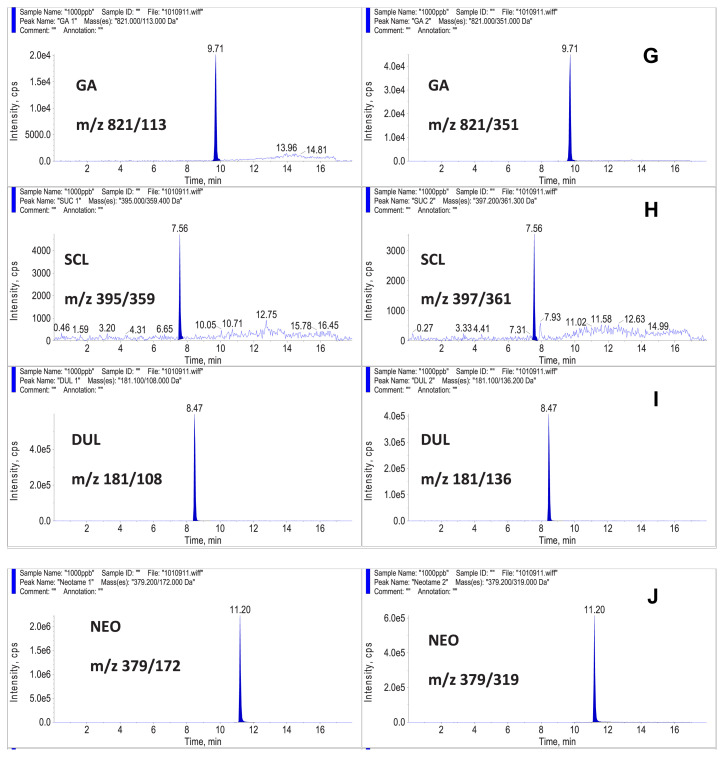
Structures of the 10 sweeteners in the present study are shown in Fig. 1. The HPLC was connected to an ESI probe of the AB Sciex 4000Q triple quadruple mass spectrometer that was operated under the MRM mode. The product ion analysis of sweeteners was performed in the polarity-switching mode, which allowed the use of positive and negative ionization during the same run. The sweeteners ACS-K, SCL, SAC, CYC, ASP, GA, STV, and NHDC were detected in the negative ion mode. The [M-H]− ions were used as precursor ions for these eight sweeteners. DUL and NEO were detected in the positive ion mode. The [M+H]+ ions were the precursor ions for these two sweeteners. Two MRM transitions were selected to confirm the identity of each sweetener. The product ion with a stronger signal was selected as the ion for quantification, and the product ion with a weaker signal was selected as the ion for identification. The MRM transitions for each sweetener are shown in Table 3. The MRM chromatograms for 1.0 μg/mL sweeteners in the present study are shown in Fig. 2. In previous LC–MS studies by Koyama et al [27], Yang and Chen [28], and Zygler et al [26,29], only nine sweeteners could be detected under the SIM mode. The present study used the MRM mode to detect 10 sweeteners. Compared to the previous SIM mode, the MRM mode was more sensitive and had less matrix interference.
Fig. 2.
Multiple reaction monitoring chromatograms for 10 sweeteners at the level of 1.0 μg/mL in the present study.
The LC–MS/MS method, recommended by the Taiwan FDA, suggested using the positive ion mode to detect ASP and SCL [35]. The MRM transitions for ASP were m/z 295 > 120 and m/z 295 > 180. The MRM transitions for SCL were m/z 414 > 199 and m/z 414 > 216. Under the positive ion mode, the sweeteners ASP and SCL could not be detected below the level of 0.1 μg/mL. Therefore, we used the negative ion mode to detect ASP and SCL in the present study.
Yang and Chen [28] had selected warfarin sodium as the internal standard for ESI negative ion detection of sweeteners. In the present study, both positive and negative ionization modes were used for sweetener detection. Warfarin sodium was not suitable for the positive ion detection mode, so no internal standard was used in the present study.
In previous study by Koyama et al [27], the mobile phase consisted of 5 mM dibutylammonium acetate (an ion pair reagent) as mobile phase A and acetonitrile/deionized water (8:2, v/v) as mobile phase B [27]. In the study of Yang and Chen [28], the mobile phase included formic acid and triethylamine as the buffer solution. The addition of 0.1% formic acid in the mobile phase did not only cause ACS-K, SAC, and CYC to have longer retention times, but also decreased their sensitivity. For the current work, triethylamine should not be used, because the triethylamine signal would persist for positive ion mode detection and the memory effect of triethylamine would interfere with the LC–MS analysis [37]. In the study of Chen et al [32], trifluoroacetic acid was added to the mobile phase. Similarly, trifluoroacetic acid could not be used if the negative ion work is planned on the same day, because it would induce memory effects and ion suppression, especially in the negative-ion mode [38]. Therefore, only 10 mM ammonium acetate was added to the mobile phase and the gradient program in Table 1 was used for chromatographic separation. The flow rate was set at 1.0 mL/minute and the injection volume was 30 μL. The analysis could be completed in 18 minutes for the 10 sweeteners. The MRM chromatograms of the 10 sweeteners in the present study are shown in Fig. 2.
3.2. Linearity, LOQ, and matrix effect
The calibration curves in the concentration range of 0.01–0.5 μg/L for the 10 sweeteners are shown in Table 4. In Table 4, the correlation coefficients for all 10 sweeteners were higher than 0.99, which revealed a good linearity in the concentration range for each sweetener. For the LOQ, a signal-to-noise ratio of 10 was evaluated. The LOQs of NHDC and SCL were 0.5 μg/g, and those of the other sweeteners were 0.1 μg/g, which were all less than the maximum regulatory limits.
Table 4.
Linearity, limits of quantification, and matrix effects of the sweeteners in the present study.
| Sweeteners | y = ax + ba | R 2 | LOQ (μg/g) | Matrix effect (%) |
|---|---|---|---|---|
| SAC | y = 491x + 14,600 | 0.9912 | 0.1 | 1.2 |
| CYC | y = 1500x + 13,400 | 0.9995 | 0.1 | 0.5 |
| ASP | y = 670x + 4950 | 0.9995 | 0.1 | −0.8 |
| ACS-K | y = 6120x + 71,700 | 0.9980 | 0.1 | 2.1 |
| NHDC | y = 72.6x + 2240 | 0.9956 | 0.5 | −12.5 |
| STV | y = 1140x + 386 | 0.9992 | 0.1 | 1.3 |
| GA | y = 133x + 1470 | 0.9990 | 0.1 | −2.3 |
| SCL | y = 21.2x + 874 | 0.9965 | 0.5 | −3.8 |
| DUL | y = 3520x + 48,400 | 0.9990 | 0.1 | 0.0 |
| NEO | y = 11,200x + 129,000 | 0.9985 | 0.1 | −1.0 |
ACS-K = acesulfame potassium; ASP = aspartame; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; LOQ = limit of quantification; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
Linear range: 0.01–0.5 μg/mL.
The matrix effects of the 10 sweeteners are listed in Table 4. For sweetener contents from 0.1 μg/g to 5 μg/g and from 1 μg/g to 100 μg/g, the calibration curves at the concentration range of 0.01 μg/mL to 0.5 μg/mL and 0.1 μg/mL to 10 μg/mL were selected to evaluate the matrix effects, respectively. In the dried guava, the matrix effect for NHDC is −12.5%. The other nine sweeteners have matrix effects of less than 5%.
3.3. Precision and recovery
The results of the precision and recovery test for the 10 sweeteners in beverage and solid food samples are listed in Tables 5 and 6, respectively. For beverage samples, the recoveries were 85–120% and the coefficients of variation (CVs) were 1.8–15.1%. For solid food samples, the recoveries were 75–118% and the CVs were 2.9–15.3%.
Table 5.
Recoveries and CVs of the sweeteners in the flavored beer sample.
| Sweetener | Spiked level (μg/g) | Recovery (%) | CV (%) |
|---|---|---|---|
| SAC | 0.5 | 98 | 7.1 |
| 1.0 | 120 | 5.3 | |
| 2.5 | 118 | 5.9 | |
| CYC | 0.5 | 112 | 5.1 |
| 1.0 | 118 | 3.8 | |
| 2.5 | 109 | 2.5 | |
| ASP | 0.5 | 110 | 5.6 |
| 1.0 | 115 | 3.2 | |
| 2.5 | 114 | 1.8 | |
| ACS-K | 0.5 | 85 | 12.5 |
| 1.0 | 100 | 5.3 | |
| 2.5 | 97 | 4.1 | |
| NHDC | 0.5 | 98 | 6.7 |
| 1.0 | 95 | 5.4 | |
| 2.5 | 115 | 3.1 | |
| STV | 0.5 | 116 | 13.5 |
| 1.0 | 120 | 6.1 | |
| 2.5 | 107 | 5.2 | |
| GA | 0.5 | 101 | 12.1 |
| 1.0 | 119 | 8.9 | |
| 2.5 | 109 | 7.2 | |
| SCL | 0.5 | 95 | 15.1 |
| 1.0 | 107 | 10.6 | |
| 2.5 | 110 | 11.2 | |
| DUL | 0.5 | 102 | 5.1 |
| 1.0 | 116 | 3.8 | |
| 2.5 | 115 | 1.7 | |
| NEO | 0.5 | 107 | 4.2 |
| 1.0 | 120 | 3.1 | |
| 2.5 | 115 | 2.1 |
ACS-K = acesulfame potassium; ASP = aspartame; CV = coefficient of variation; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
Table 6.
Recoveries and CVs of the sweeteners in the dried guava sample.
| Sweetener | Spiked level (μg/g) | Recovery (%) | CV (%) |
|---|---|---|---|
| SAC | 1.0 | 104 | 5.1 |
| 2.5 | 91 | 4.5 | |
| 5.0 | 100 | 3.9 | |
| CYC | 1.0 | 75 | 5.9 |
| 2.5 | 83 | 4.7 | |
| 5.0 | 95 | 2.9 | |
| ASP | 1.0 | 79 | 5.1 |
| 2.5 | 96 | 4.2 | |
| 5.0 | 105 | 3.8 | |
| ACS-K | 1.0 | 116 | 10.8 |
| 2.5 | 82 | 8.4 | |
| 5.0 | 118 | 4.1 | |
| NHDC | 1.0 | 75 | 15.3 |
| 2.5 | 86 | 10.8 | |
| 5.0 | 77 | 11.8 | |
| STV | 1.0 | 75 | 12.8 |
| 2.5 | 90 | 8.9 | |
| 5.0 | 105 | 6.8 | |
| GA | 1.0 | 82 | 13.1 |
| 2.5 | 86 | 10.2 | |
| 5.0 | 90 | 9.1 | |
| SCL | 1.0 | 78 | 13.1 |
| 2.5 | 105 | 10.6 | |
| 5.0 | 86 | 8.7 | |
| DUL | 1.0 | 79 | 8.1 |
| 2.5 | 88 | 6.7 | |
| 5.0 | 89 | 3.9 | |
| NEO | 1.0 | 80 | 6.9 |
| 2.5 | 93 | 5.4 | |
| 5.0 | 96 | 3.8 |
ACS-K = acesulfame potassium; ASP = aspartame; CV = coefficient of variation; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
3.4. Analytical results of sweeteners in foods on the market
Because the maximum usable dose for sweeteners could be as high as 1000 mg/kg in Taiwan, two calibration curves at the concentration range of 0.1–1.0 μg/mL and 1.0–10 μg/mL were recommended for real sample analysis. It is necessary to dilute the extracted sample solutions from 10 to 100 times to fit the concentration range of the above calibration curves. The detected sweetener contents of 27 beverage samples are shown in Table 7, and those of 15 solid food samples are shown in Table 8. Two wine samples with SAC and CYC were remanufactured red wine and not naturally fermented wines. Remanufactured wine is often made by blending edible alcohol with sweeteners and fruit juice. Therefore, the label for the sweetener in remanufactured wine should be regulated. Although NHDC was not permitted as a food additive in Taiwan, it was detected in two citrus lemonade extract samples. The maximum usable dose of NHDC in beverages was 30 ppm in the EU. The levels of NHDC found in the two citrus lemonade extract samples were lower than the EU regulatory level. The US FDA had regulated NHDC as a flavoring agent and not as a sweetener. Using the developed method, four solid food products were mislabeled in terms of the sweeteners used. The ingredient label for one pickled pepper was NEO, but SAC was detected instead. SAC was forbidden as a food additive for pickled peppers in Taiwan. One honey mongo was labeled SAC and CYC, but a blend of 71.5 μg/kg CYC, 11.4 μg/kg ACS-K, 25.4 μg/kg STV, and 18.5 μg/kg NEO were detected instead. One pickled kumquat and one dried plum were found to contain CYC, which was not specified in the ingredient label.
Table 7.
Detected sweetener contents in 27 beverage samples.
| Variety | Sweetener content (μg/g) | Ingredient label |
|---|---|---|
| Plum wine | N.D. | |
| Sour (サワー)a | N.D. | |
| Red wine | N.D. | |
| Red wine | N.D. | |
| Plum grape wine | SAC 7.2, CYC 126.5 | |
| Plum grape wine | SAC 8.5, CYC 123 | |
| Grape wine | N.D. | |
| Red wine | N.D. | |
| Red wine | N.D. | |
| Sports drink | NEO 0.6 | NEO |
| Melon dew | ACS-K 41.4, SCL 8.3 | SCL |
| Melon dew | N.D. | |
| Sports drink | N.D. | |
| Fruit juice with yukisio salt | N.D. | |
| Iced tea with lemon flavor | N.D. | |
| Mango flavored beer | N.D. | |
| Pineapple flavored beer | N.D. | |
| Passion fruit flavored beer | N.D. | |
| Peach flavored beer | N.D. | |
| Rose wine | N.D. | |
| Red yeast rice flavored grapewine | N.D. | |
| Red wine | N.D. | |
| Carbonated soft drink | ACS-K 174, ASP 55.1 | ACS-K, ASP |
| Cola Zero | ACS-K 175, ASP 98.4, SCL 59.8 | ACS-K, ASP, SCL |
| Peach flavored water | SCL 73.5 | SCL |
| Citrus lemonades extract | NHDC 23.4 | |
| Citrus lemonades extract | NHDC 19.1 |
ACS-K = acesulfame potassium; ASP = aspartame; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; N.D. = not detected; NEO = neotame; NHDC = neohesperidin dihy-drochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
Sour (サワー) is a Japanese cocktail wine made by mixing distilled citrus wine and soda.
Table 8.
Detected sweetener contents in 15 solid food samples
| Variety | Sweetener content (μg/g) | Ingredient label |
|---|---|---|
| Pickled pepper | SAC 228 | NEO |
| Liquorice root marinated olives | NEO 6.9 | NEO, ACS-K, ASP, GA |
| ACS-K 9 3.8 | ||
| ASP 87.1 | ||
| GA 28.9 | ||
| Hibiscus sabdariffa flower | SAC 106.5 | SAC, CYC |
| CYC 1365 | ||
| Dried plum | CYC 87 | SAC, CYC |
| Honey mango | CYC 71.5 | SAC, CYC |
| ACS-K 11.4 | ||
| STV 25.4 | ||
| NEO 18.5 | ||
| Dried guava | N.D.a | |
| Dried mango | N.D. | |
| Dried guava | N.D. | |
| Kumquat flavored candy | N.D. | |
| Fruit flavored lollipop | N.D. | |
| Ginseng flavored candy | N.D. | |
| Dried cranberries | N.D. | |
| Pickled kumquats | CYC 621 | |
| Dried plums | CYC 859 | |
| Raisins | N.D. |
ACS-K = acesulfame potassium; ASP = aspartame; CYC = cyclamate; DUL = dulcin; GA = glycyrrhizic acid; N.D. = not detected; NEO = neotame; NHDC = neohesperidin dihydrochalcone; SAC = saccharin; SCL = sucralose; STV = stevioside.
The present method was able to detect 10 popular and regulated sweeteners in foods. In the United States, thaumatin is a GRAS flavoring agent and also approved as a sweetener in the EU and Taiwan. The molecular weight of thaumatin is 2000 kDa, and it could not be detected directly by the current method without enzyme hydrolysis pretreatment. Therefore, thaumatin was not included in the current study.
4. Conclusion
A simple and rapid method for the simultaneous determination of 10 kinds of sweeteners (ACS-K, ASP, CYC, DUL, GA, NEO, NHDC, SAC, SCL, and STV) in various foods using LC–MS/MS was developed. In the official HPLC method for ACS-K, SAC, DUL, and CYC, the sample was directly subjected to HPLC analysis after the extraction by deionized water. Owing to interference from coeluents, the analysis was not confirmatory. Because CYC does not have a chromophore that absorbs UV or visible light, it needs to be derivatized prior to HPLC analysis. The developed LC-MS/MS method could analyze CYC without the derivatization step. The sample preparation step was quick and simple, and the recoveries and CVs were also very good with this method. Therefore, the method could be used routinely for regulation inspection.
Footnotes
Conflicts of interest
The authors have certified that they have no conflicts of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1. DuBois GE, Prakash I. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers. Annu Rev Food Sci Technol. 2012;3:353–80. doi: 10.1146/annurev-food-022811-101236. [DOI] [PubMed] [Google Scholar]
- 2. Hendriksen M, Tijhuis M, Fransen H, et al. Impact of substituting added sugar in carbonated soft drinks by intense sweeteners in young adults in the Netherlands: example of a benefit–risk approach. Eur J Nutr. 2011;50:41–51. doi: 10.1007/s00394-010-0113-z. [DOI] [PubMed] [Google Scholar]
- 3. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640–6. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kokotou MG, Asimakopoulos AG, Thomaidis NS. Artificial sweeteners as emerging pollutants in the environment: analytical methodologies and environmental impact. Anal Methods. 2012;4:3057–70. [Google Scholar]
- 5.Rapid Alert System for Food and Feed (RASFF) BBO too high content of sweetener E 951 — aspartame (6.98 g/kg) in sugar free chewing gum from Russia. 2007. [accessed 05, 06, 13]. Available at: https://webgate.ec.europa.eu/rasff-window/portal/index.cfm?event=notificationDetail&NOTIF_REFERENCE=2007.BBO.
- 6.Rapid Alert System for Food and Feed (RASFF) ASU sweetener E 950 – acesulfame k (3002.9 mg/kg ppm), sweetener E 951 – aspartame (1416.8 mg/kg ppm) and sweetener E 952 – cyclamate (12455 mg/kg ppm) unauthorised in candy from China. 2011. [accessed 05, 06, 13]. Available at: https://webgate.ec.europa.eu/rasff-window/portal/index.cfm?event=notificationDetail&NOTIF_REFERENCE=2011.ASU.
- 7. Renwick AG. The intake of intense sweeteners – an update review. Food Addit Contam. 2006;23:327–38. doi: 10.1080/02652030500442532. [DOI] [PubMed] [Google Scholar]
- 8. Leth T, Jensen U, Fagt S, et al. Estimated intake of intense sweeteners from non-alcoholic beverages in Denmark, 2005. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:662–8. doi: 10.1080/02652030701765749. [DOI] [PubMed] [Google Scholar]
- 9. Kroger M, Meister K, Kava R. Low-calorie sweeteners and other sugar substitutes: a review of the safety issues. Compr Rev Food Sci Food Saf. 2006;5:35–47. [Google Scholar]
- 10. Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46:167–92. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11. Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scand J Food Nutr. 2006;50:104–16. [Google Scholar]
- 12. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. Diabetes Care. 2012;35:1798–808. doi: 10.2337/dc12-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay S, Raychaudhuri U, Chakraborty R.Artificial sweeteners – a review. J Food Sci Technol. 2011. [DOI] [PMC free article] [PubMed]
- 14.Food Standards Australia New Zealand (FSANZ) Australia New Zealand Food Standards Code — Standard 1.3.1 — Food Additives. [accessed 05, 06, 13]. Available at: http://www.comlaw.gov.au/Series/F2008B00614.
- 15.US FDA. PART 172—Food additives permitted for direct addition to food for human consumption. [accessed 05, 06, 13]. Available at: http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=ce943c512a658069d08eafb5802dc6cb&ty=HTML&h=L&n=21y3.0.1.1.3&r=PART. [PubMed]
- 16.FFCR Japan. Standards for Use, according to Use Categories. The Japan Food Chemical Research Foundation; [accessed 20, 02, 14]. Available at: http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/7bd44c20b0dc562649256502001b65e9/8a4352b95978b195492569990007fbaa/$FILE/Standards%20for%20Use%2013Dec04.pdf. [Google Scholar]
- 17.Ministry of Health of the People’s Republic of China. GB2760 2011 Food Safety National Standards — Standards for use of food additives. 2011. [accessed 05, 06, 13]]. Available at: http://www.moh.gov.cn/cmsresources/mohwsjdj/cmsrsdocument/doc11938.pdf. [in Chinese]
- 18. Lawrence JF, Charbonneau CF. Determination of seven artificial sweeteners in diet food preparations by reverse-phase liquid chromatography with absorbance detection. J AOAC. 1988;71:934–7. [PubMed] [Google Scholar]
- 19. Zhu Y, Guo Y, Ye M, et al. Separation and simultaneous determination of four artificial sweeteners in food and beverages by ion chromatography. J Chromatogr A. 2005;1085:143–6. doi: 10.1016/j.chroma.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 20. Herrmannová M, Křivánková L, Bartoš M, et al. Direct simultaneous determination of eight sweeteners in foods by capillary isotachophoresis. J Sep Sci. 2006;29:1132–7. doi: 10.1002/jssc.200500441. [DOI] [PubMed] [Google Scholar]
- 21. Wasik A, McCourt J, Buchgraber M. Simultaneous determination of nine intense sweeteners in foodstuffs by high performance liquid chromatography and evaporative light scattering detection — development and single-laboratory validation. J Chromatogr A. 2007;1157:187–96. doi: 10.1016/j.chroma.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 22. Yang D, Chen B. Determination of neotame in beverages, cakes and preserved fruits by column-switching high-performance liquid chromatography. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27:1221–5. doi: 10.1080/19440049.2010.487875. [DOI] [PubMed] [Google Scholar]
- 23. Buchgraber M, Wasik A. Determination of nine intense sweeteners in foodstuffs by high-performance liquid chromatography and evaporative light-scattering detection: interlaboratory study. J AOAC Int. 2009;92:208–22. [PubMed] [Google Scholar]
- 24.FDA Taiwan. Method of test for sweeteners in foods test of acesulfame potassium, saccharin, dulcin and cyclamate. [accessed 05, 06,13]]. Available at: http://www.fda.gov.tw/upload/133/Content/2013030111250898714.pdf. [in Chinese]
- 25. Huvaere K, Vandevijvere S, Hasni M, et al. Dietary intake of artificial sweeteners by the Belgian population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:54–65. doi: 10.1080/19440049.2011.627572. [DOI] [PubMed] [Google Scholar]
- 26. Zygler A, Wasik A, Kot-Wasik A, et al. The content of high-intensity sweeteners in different categories of foods available on the Polish market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:1391–401. doi: 10.1080/19440049.2012.699475. [DOI] [PubMed] [Google Scholar]
- 27. Koyama M, Yoshida K, Uchibori N, et al. Analysis of nine kinds of sweeteners in foods by LC/MS. Shokuhin Eiseigaku Zasshi. 2005;46:72–8. doi: 10.3358/shokueishi.46.72. [In Japanese, English abstract] [DOI] [PubMed] [Google Scholar]
- 28. Yang DJ, Chen B. Simultaneous determination of nonnutritive sweeteners in foods by HPLC/ESI-MS. J Agric Food Chem. 2009;57:3022–7. doi: 10.1021/jf803988u. [DOI] [PubMed] [Google Scholar]
- 29. Zygler A, Wasik A, Kot-Wasik A, et al. Determination of nine high-intensity sweeteners in various foods by high-performance liquid chromatography with mass spectrometric detection. Anal Bioanal Chem. 2011;400:2159–72. doi: 10.1007/s00216-011-4937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheurer M, Brauch HJ, Lange FT. Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT) Anal Bioanal Chem. 2009;394:1585–94. doi: 10.1007/s00216-009-2881-y. [DOI] [PubMed] [Google Scholar]
- 31. Ordóñez EY, Quintana JB, Rodil R, et al. Determination of artificial sweeteners in water samples by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2012;1256:197–205. doi: 10.1016/j.chroma.2012.07.073. [DOI] [PubMed] [Google Scholar]
- 32. Chen XH, Zhao YG, Shen HY, et al. Application of dispersive solid-phase extraction and ultra-fast liquid chromatography–tandem quadrupole mass spectrometry in food additive residue analysis of red wine. J Chromatogr A. 2012;1263:34–42. doi: 10.1016/j.chroma.2012.09.074. [DOI] [PubMed] [Google Scholar]
- 33. Lim HS, Park SK, Kwak IS, et al. HPLC-MS/MS analysis of 9 artificial sweeteners in imported foods. Food Sci Biotechnol. 2013;22:233–40. [Google Scholar]
- 34. Gan Z, Sun H, Wang R, et al. A novel solid-phase extraction for the concentration of sweeteners in water and analysis by ion-pair liquid chromatography–triple quadrupole mass spectrometry. J Chromatogr A. 2013;1274:87–96. doi: 10.1016/j.chroma.2012.11.081. [DOI] [PubMed] [Google Scholar]
- 35.FDA Taiwan. Method of test for sweeteners in foods. [accessed 05, 06, 13]]. Available at: http://www.fda.gov.tw/upload/133/Content/2013040309475498163.pdf. [in Chinese]
- 36. Chang CS, Wang WH, Tsai CE. Simultaneous determination of 18 quinolone residues in marine and livestock products by liquid chromatography/tandem mass spectrometry. JFDA. 2010;18:87–97. [Google Scholar]
- 37. Rütters H, Möhring T, Rullkötter J, et al. The persistent memory effect of triethylamine in the analysis of phospholipids by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:122–3. doi: 10.1002/(SICI)1097-0231(20000130)14:2<122::AID-RCM844>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Garofolo F. LC-MS instrument calibration. In: Chan CC, Lee YC, Lam H, et al., editors. Analytical method validation and instrument performance verification. Hoboken, NJ: Wiley-Interscience; 2004. pp. 197–220. [Google Scholar]