Abstract
Calamondin has been demonstrated to exhibit antioxidant function and tyrosinase inhibitory activity, which might be attributed to its flavonoid compounds. To improve their application, the flavonoid compositions and antioxidant activity of calamondin extracts, prepared by different solvents, were investigated. The results showed that total phenolic and flavonoid contents of extracts from peel of calamondin were higher than that from pulp, except the flavonoid content in hot water extract. The flavonoids found in extracts of calamondin were 3′, 5′-di-C-β-glucopyranosylphloretin (DGPP), naringin, hesperidin, nobiletin, tangeretin, and diosmin. DGPP exhibited the highest quantity, while naringin and hesperidin were the other two major flavonoids. The content of DGPP in hot water extract of peel was higher than in extracts of organic solvents, however, the contents of nobiletin and tangeretin were found only in extracts of organic solvents. The highest levels of total flavonoids and DGPP were obtained in hot water extract from peel at 90°C. The extracts of hot water and ethyl acetate showed higher 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging potency than that of ethanol and methanol. A positive relationship existed between total phenolic contents and DPPH scavenging potency (p < 0.01), while total flavonoid compositions also showed correlation (p < 0.05). Thus, DGPP, naringin, and hesperidin might contribute to antioxidant activity. Collectively, the hot water extract of calamondin peel might have potential for health food and cosmetic applications due to its good antioxidant activity and high level of DGPP.
Keywords: Antioxidant activity, Calamondin, Flavonoid compositions, Solvent extraction
1. Introduction
Calamondin (Citrus mitis Blanco), a cultivar related to citrus, is a hybrid between Citrus reticulate Blanco and Fortunella spp. [1,2]. It bears small-sized fruits and has been used to make a hot drink in Taiwan for many years due in part to its potential health beneficial properties. It may be attributed to the presence of bioactive compounds such as flavonoids in citrus peel [3]. The main production area of calamondin in Taiwan is Pingtung County with 68.8% production in 2010. The flavonoid composition of Fortunella species differs from those of the Citrus species [4,5]. Calamondin contains a large quantity of 3′,5′-di-C-β-glucopyranosylphloretin (DGPP) in its peel, juice sac, and leaf [4]. In another study, eight polymethoxyflavones were isolated and identified from the peel of calamondin [6]. Flavonoids, such as poncirin, dydimin, neohesperidin, hesperidin, narirutin, diosmin, and isorhoifolin, have also been extracted by 80% methanol from the dried calamondin pulp powder [7]. In our previous study, DGPP was identified as a major compound along with naringin and hesperidin in the hot water extract of immature calamondin peel [8]. More DGPP, naringin, and hesperidin were found in the water extract of immature calamondin peel, while nobiletin and tangeretin were only observed in the ethyl acetate extract [9]. It is, therefore, suggested that different solvents used for extraction could lead to different compositions of phenolic compounds in extracts, because the solubility of each phenolic compound in a given solvent would be quite different. Consequently, the bioactivity of an extract might also be affected. The antioxidant activities of naringin and hesperidin have previously been reported [10,11]. DGPP contained good tyrosinase inhibitory activity [8]. Polymethoxy-flavones such as nobiletin and tangeretin exhibit a broad spectrum of biological activity, including anti-inflammatory, anticarcinogenic, antiviral, antithrombogenic, and antiatherogenic properties [12,13]. Therefore, it is desirable to study the flavonoid compositions of different extracts for the potential application of calamondin.
In order to characterize the major phenolic compounds and antioxidant activity in calamondin, extractions of calamondin peel and pulp with different solvents, including hot water at different temperatures, ethanol of various concentrations, methanol, and ethyl acetate, were carried out and subjected to the analysis of phenolic compositions and antioxidant activity. The relationship between the content of phenolic compounds and antioxidant activity was also evaluated.
2. Methods
2.1. Materials
Calamondin (Citrus mitis Blanco) was collected from a calamondin estate in the Jao-Si region, Ilan, Taiwan in the winter of 2008. The collected calamondin with whole yellow appearance was identified as mature calamondin in the laboratory and its average weight was 18.44 ± 3.46 g. After manual peeling, the separated peels and pulps were lyophilized for 48 hours. Prior to extraction, the peels and pulps were pulverized in a blender and passed through a 40 mesh sieve. The obtained powders of calamondin peel and pulp were stored at −18°C in brown bottles with screw caps.
2.2. Chemicals
Methanol, ethanol, ethyl acetate, and acetonitrile were liquid chromatography (LC) grade and were obtained from Merck Chemical Co. (Darmstadt, Germany). Acetic acid, Na2CO3, AlCl3·6H2O, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and Folin-Ciocalteu’s phenol reagent were analytical grade. Gallic acid, quercetin, 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid, 97% (Trolox), and standards of flavonoids, including diosmin, diosmetin, hesperetin, hesperidin, kaempferol, luteolin, naringin, naringenin, neohesperidin, nobiletin, rutin, tangeretin, sinensetin, and neoeriocitrin, were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DGPP was obtained from a hot water extract of immature calamondin peel in our laboratory. The extract was separated by a semipreparative high performance liquid chromatography (HPLC) and the compound was collected. After lyophilization, the residue was redissolved in deionized water and stored at −18°C until further use.
2.3. Extraction procedure
Three grams of dried and powdered calamondin peels and pulps were extracted with (1) 50 mL deionized hot water (80°C, 90°C, and 100°C) for 1 hour in a shaking water bath at the same temperature, or extracted with (2) 30 mL ethanol (50%, 60%, 70%, 80%, and 95%), or (3) 30 mL methanol, or (4) 30 mL ethyl acetate in a shaker at room temperature for 1 hour. The shaking rate was 100 revolutions per minute (rpm). The extract was filtered with a Whatman No. 1 filter paper. The obtained residue was extracted by the same procedure two more times. Three resulting filtrates were transferred into a 250 mL flask and dried using a rotary vacuum evaporator at 50°C. To dissolve the filtrate, a suitable volume of deionized water, ethanol, methanol, and ethyl acetate was added to the flask for each extract. The obtained solutions were poured into brown bottles with screw caps and stored at −18°C until further use. Triplicate determinations (n = 3) were carried out during the study.
2.4. Determination of total phenolic content
Two hundred and fifty microliters of calamondin extract or standard solution was mixed with 250 μL of Folin-Ciocalteu’s phenol reagent for 3 minutes [14]. The mixture was added to 2.5 mL of 20% Na2CO3 solution and then incubated in the dark for 30 minutes at room temperature. After incubation, the absorbance was measured at 750 nm against the blank. The standard curve was constructed with gallic acid, and the total phenolic content was expressed as mg gallic acid equivalent (GAE) per 100 g dry extract using the standard curve. All samples were analyzed in triplicate.
2.5. Determination of total flavonoid content
Five hundred microliters of calamondin extract or standard solution was mixed with five hundred microliters of 2% methanolic AlCl3·6H2O [15]. The mixture was then incubated for 10 minutes at room temperature. After incubation, the absorbance of the mixture was measured at 430 nm. Six calibration solutions of quercetin (2, 4, 6, 8, 10, and 20 ppm final concentration) were tested to establish a standard curve. All samples were analyzed in triplicate. The total flavonoid content was expressed as mg quercetin equivalents (QE) per 100 g dry extract using the standard curve established previously.
2.6. HPLC analysis of flavonoid compositions
The extracts of different solvents were subjected to a HPLC analysis with a reverse phase column (Lichrospher C18e, 250 mm × 4.6 mm, 5 μm; Merck, Darmstadt, Germany) using a gradient with 2% (v/v) acetic acid in water as solvent A and 0.5% acetic acid in water/acetonitrile (1:1, v/v) as solvent B [16]. The gradient was carried out as follows: 0–10 minutes, 5–10% B; 10–55 minutes, 10–55% B; 55–60 minutes, 55–80% B; 60–65 minutes, 80–100% B; 65–70 minutes, 100% B; and 70–75 minutes, 100–5% B. The flow rate was 1 mL/min. Photodiode array (PDA) detection was performed between 220 nm and 350 nm, with a resolution of 2 nm. Sixteen flavonoids used as standards were available in our laboratory as follows: neoeriocitrin, rutin, diosmin, diosmetin, naringin, hesperidin, neohesperidin, naringenin, hesperetin, sinensetin, luteolin, quercetin, kaempferol, nobiletin, tangeretin, and DGPP. The flavonoids were identified by their retention times and the UV spectra of standards were quantified from the peak area at 280 nm by an external standard method using calibration curves. Their concentrations were expressed as milligrams per 100 g dry weight.
2.7. DPPH radical scavenging activity
The DPPH radical scavenging activity of calamondin extracts was estimated according to a slightly modified method of Yamaguchi et al [17]. After 0.5 mL of calamondin extract was mixed with 0.5 mL of 0.5 mM DPPH in methanol for 30 minutes, the mixture was subjected to a HPLC analysis with a reverse phase column (Hypersil ODS-2, Thermo Scientific, MA, USA, 250 mm × 4.6 mm, 5 μm) under PDA detection at 517 nm. The mobile phase was methanol/water (7/3, v/v) and the flow rate was 1 mL/min. The change in peak area of DPPH was determined after the reaction. Radical scavenging activity was expressed as percent inhibition and was calculated using the following formula:
2.8. Statistical analysis
The data were subjected to analysis of variance (ANOVA) and the significance of the difference between means was determined by Duncan’s multiple range test (p < 0.05), using SAS (SAS Institute, Cary, NC, USA).
3. Results and discussion
3.1. Total phenolic and flavonoid content of calamondin in different extracts
The total phenolic contents of calamondin extracted by different solvents are shown in Table 1. The content of phenolic compounds in peel was 1054–1894 mg/100 g dry extract, which was higher than that in pulp with a range of 189–1053 mg/100 g dry extract. It is well known that dried citrus peel is a traditional Chinese medicine [3]. Citrus peel contains a large quantity of bioactive compounds, such as flavonoids including polymethoxyflavones, phenolic acids, and limonoids [12,18]. The highest total phenolic content in peel was observed in boiling water extract (1894 mg/100 g dry extract). There were no significant differences between extractions of hot water (80°C and 90°C) and ethanol (50, 60, 70, and 80%), which were the second highest concentrations (1641–1743 mg/100 g dry extract). The content in methanol extract was 1586 mg/100 g dry extract. However, the 95% ethanol extract contained significantly lower phenolic content than the extraction of methanol and ethanol with lower concentration. The smallest content of phenolic compounds was obtained in ethyl acetate extract. This result indicates that most of the phenolic compounds in calamondin peel were hydrophilic. In addition, heat treatment might enhance the release of phenolic compounds in calamondin peel.
Table 1.
Total phenolic content of pulp and peel in calamondin extracted by different solvents.
| Solvents | Total phenolic content (GAE mg/100 g dry extract) | |
|---|---|---|
|
| ||
| Pulp | Peel | |
| Hot water | ||
| 80°C | 939 ± 12b | 1762 ± 94b |
| 90°C | 948 ± 32b | 1690 ± 58b,c |
| 100°C | 1017 ± 26a | 1894 ± 66a |
| Ethanol | ||
| 50% | 1034 ± 3a | 1641 ± 31b,c |
| 60% | 1040 ± 27a | 1686 ± 44b,c |
| 70% | 1053 ± 3a | 1706 ± 59b,c |
| 80% | 967 ± 17b | 1743 ± 84b |
| 95% | 926 ± 19b | 1285 ± 10d |
| Methanol | 1013 ± 43a | 1586 ± 28c |
| Ethyl acetate | 189 ± 7c | 1054 ± 88e |
Values (mean ± SD, n = 3) in the same column with different superscripted letters are significantly different (p < 0.05).
GAE = gallic acid equivalent; SD = standard deviation.
In the pulp of calamondin, the highest phenolic content was obtained in boiling water extracts, which was similar to the peel of calamondin. No significant difference could be observed among the extracts of boiling water, ethanol (50%, 60%, and 70%), and methanol. In the extracts of ethyl acetate, the lowest total phenolic content (189 mg/100 g dry extract) was found. This implies that the phenolic compounds with more hydrophobic characteristics might occur in lower amounts than those with hydrophilic characteristics in the pulp of the calamondin.
The total flavonoid contents of peel and pulp in calamondin extracted by different solvents are shown in Table 2. The highest content was obtained by hot water extract in the range of 318–659 mg/100 g dry extract. The extracts of ethyl acetate in peel with some pigments showed very high flavonoid contents, which might be due to interference in the detection absorbance for flavonoids [9]. Therefore, the data for ethyl acetate in peel and pulp were not discussed. In the pulp of calamondin, the highest flavonoid content was observed in the extract of boiling water at a level of 659 mg/100 g dry extract. For the hot water extract, the total content of flavonoids in pulp was higher than that in peel. However, the total content of flavonoids in peel was higher than in pulp when extracted with ethanol and methanol.
Table 2.
Total flavonoid content of pulp and peel in calamondin extracted by different solvents.
| Solvents | Total flavonoid content (QE mg/100 g dry extract) | |
|---|---|---|
|
| ||
| Pulp | Peel | |
| Hot water | ||
| 80°C | 437 ± 28c | 356 ± 25a,b,c |
| 90°C | 539 ± 25b | 318 ± 15a,b,c,d |
| 100°C | 659 ± 23a | 356 ± 14a,b,c |
| Ethanol | ||
| 50% | 101 ± 9d | 226 ± 9e |
| 60% | 81 ± 9d | 243 ± 3d,e |
| 70% | 90 ± 4d | 269 ± 14c,d,e |
| 80% | 94 ± 6d | 301 ± 18b,c,d,e |
| 95% | 100 ± 6d | 397 ± 16a |
| Methanol | 112 ± 5d | 375 ± 1a,b |
| Ethyl acetate | 361 ± 8 | 5821 ± 161 |
Values (mean ± SD, n = 3) in the same column with different superscripted letters are significantly different (p < 0.05).
QE = quercetin equivalents; SD = standard deviation.
3.2. Determination of flavonoid compositions in different extracts of calamondin
The flavonoid profiles of calamondin peel extracted by different solvents are shown in Table 3. Seven flavonoids, including naringin, diosmin, hesperidin, neohesperidin, 3′,5′-di-C-β-glucopyranosyl-phloretin (DGPP), nobiletin, and tangeretin, were identified in various solvent extracts. The major flavonoid was DGPP in various extracts, except ethyl acetate extracts where nobiletin and tangeretin were the only two flavonoids detected. Naringin was the second major flavonoid (94–414 mg/100 g dry extract) except in the extract of 95% ethanol and methanol, where the second major flavonoid was hesperidin. In the hot water extracts, the content of DGPP was in the range 1405–1581 mg/100 g dry extract, which was higher than other extracts by different solvents and the highest value was observed in the 90°C hot water extract. However, nobiletin and tangeretin were not found in the hot water extract. DGPP, the dihydrochalcone derivative in immature calamondin peel, has been isolated and identified from hot water extracts in remarkably large amounts [4,8]. It has also been reported that DGPP contains good tyrosinase inhibitory activity [8]. The content of naringin, diosmin, nobiletin, and tangeretin in 50–80% ethanol extracts were more than that in hot water extracts. Interestingly, only two major compounds, nobiletin and tangeretin, were observed in ethyl acetate extracts. The flavanones naringin and hesperidin that exist commonly in citrus are more polar [19–21]. Nobiletin and tangeretin are polymethoxyflavones with six and five methoxy groups, respectively. Because of the hydrophobic nature of methoxy groups, polymethoxyflavones are less polar than hydroxylated flavonoids, such as DGPP, naringin, and hesperidin [9,12]. The content of nobiletin was higher than tangeretin in all of the extracts. For the total flavonoid compositions, the highest level was obtained in the 90°C hot water extract.
Table 3.
Flavonoid compositions of peel in calamondin extracted by different solvents.
| Solvents | Flavonoids (mg/100 g dry extract) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Naringin | Diosmin | Hesperidin | Neohesperidin | DGPP | Nobiletin | Tangeretin | Total content | |
| Hot water | ||||||||
| 80°C | 286 ± 39b | N.D. | 145 ± 22c | N.D. | 1405 ± 51b,c | N.D. | N.D. | 1836 ± 68 |
| 90°C | 233 ± 8c | N.D. | 47 ± 3d | N.D. | 1581 ± 21a | N.D. | N.D. | 1861 ± 23 |
| 100°C | 94 ± 6d | N.D. | 44 ± 7d | N.D. | 1480 ± 37b | N.D. | N.D. | 1618 ± 38 |
| Ethanol | ||||||||
| 50% | 369 ± 22a | 74 ± 7a | N.D. | N.D. | 1291 ± 69d,e | 86 ± 9c | 7 ± 1c | 1826 ± 73 |
| 60% | 414 ± 27a | 62 ± 7b | 480 ± 74b | N.D. | 1269 ± 24d,e | 107 ± 7b,c | 36 ± 12b | 1888 ± 68 |
| 70% | 238 ± 23c | 44 ± 3c | N.D. | N.D. | 1335 ± 20c,d | 113 ± 6b | 29 ± 1b | 1759 ± 31 |
| 80% | 385 ± 19a | 70 ± 9a,b | N.D. | N.D. | 1227 ± 56e | 111 ± 12b,c | 32 ± 8b | 1825 ± 62 |
| 95% | N.D. | N.D. | 664 ± 29a | 349 ± 67 | 1031 ± 11f | 117 ± 16b | 20 ± 13b,c | 1168 ± 77 |
| Methanol | 72 ± 17d | N.D. | 452 ± 31b | N.D. | 1238 ± 52e | 131 ± 19b | 28 ± 4b | 1921 ± 66 |
| Ethyl acetate | N.D. | N.D. | N.D. | N.D. | N.D. | 270 ± 16a | 195 ± 9a | 465 ± 18 |
Values (mean ± SD, n = 3) in the same column with different superscripted letters are significantly different (p < 0.05).
DGPP = 3′,5′-di-C-β-glucopyranosylphloretin; N.D. = not detected; SD = standard deviation.
Total flavonoid compositions in pulp were lower than that in peel (Table 4). The three major flavonoids in pulp were DGPP, naringin, and hesperidin with levels at 653–788 mg/100 g dry extract, 18–264 mg/100 g dry extract, and 6–226 mg/100 g dry extract, respectively. Naringin and hesperidin are more soluble in ethanol. Therefore, the content of naringin and hesperidin in hot water extracts was much lower than that in ethanol extracts. The content of DGPP in pulp was lower than in peel, while both nobiletin and tangeretin were not detected. The polymethoxyflavones, nobiletin and tangeretin, existed mainly in the peel. It has been reported that the key role of polymethoxyflavonoids in citrus is for the defense against microbes [22]. Ramful et al [7] showed that the extract of calamondin pulp contained flavonoids, such as poncirin, dydimin, neohesperidin, hesperidin, narirutin, diosmin, and isorhoifolin. They failed to identify the main compound, DGPP, which might be due to the different growth environments.
Table 4.
Flavonoid compositions of pulp in calamondin extracted by different solvents.
| Solvents | Flavonoids (mg/100 g dry extract) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Naringin | Diosmin | Hesperidin | Neohesperidin | DGPP | Nobiletin | Tangeretin | Total content | |
| Hot water | ||||||||
| 80°C | 68 ± 8e | N.D. | 29 ± 11b | N.D. | 747 ± 18a,b | N.D. | N.D. | 844 ± 23 |
| 90°C | 28 ± 1f | N.D. | 14 ± 1b | N.D. | 688 ± 7b,c,d | N.D. | N.D. | 730 ± 7 |
| 100°C | 18 ± 3f | N.D. | 6 ± 1b | N.D. | 653 ± 20d | N.D. | N.D. | 677 ± 20 |
| Ethanol | ||||||||
| 50% | 166 ± 8d | N.D. | N.D. | N.D. | 731 ± 42a,b | Trace | N.D. | 897 ± 43 |
| 60% | 264 ± 29a | N.D. | 205 ± 8a | N.D. | 721 ± 34b,c | Trace | N.D. | 1190 ± 45 |
| 70% | 195 ± 12c | N.D. | 225 ± 19a | N.D. | 788 ± 16a | Trace | N.D. | 1208 ± 28 |
| 80% | 172 ± 9d | N.D. | N.D. | N.D. | 732 ± 29a,b | Trace | N.D. | 904 ± 30 |
| 95% | N.D. | N.D. | 226 ± 27a | 186 ± 9 | 663 ± 61c,d | Trace | N.D. | 888 ± 67 |
| Methanol | 224 ± 3b | N.D. | 208 ± 1a | N.D. | 665 ± 25c,d | 3 ± 1 | N.D. | 1100 ± 25 |
| Ethyl acetate | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
Values (mean ± SD, n = 3) in the same column with different superscripted letters are significantly different (p < 0.05).
DGPP = 3′,5′-di-C-β-glucopyranosylphloretin; N.D. = not detected; SD = standard deviation.
3.3. Antioxidant activity of different extracts from calamondin
Phenolic compounds serve as antioxidants due to their free radical scavenging activity or metal chelating ability. Therefore, the high content of phenolic compounds in extracts from calamondin might result in the high antioxidant activity. In order to investigate the antioxidant activity of different extracts, the various extracts obtained were subjected to a DPPH free radical scavenging assay. The results indicated that the DPPH scavenging potency of extracts from calamondin peel was significantly higher than calamondin pulp (p < 0.05) (Fig. 1). This suggested that the three major flavonoids, including DGPP, naringin, and hesperidin, might play an important role for DPPH scavenging potency of extracts because the contents of these three compounds in peel were higher than in pulp. The antioxidant activities of naringin and hesperidin have previously been reported [10,11]. Naringin and hesperidin contained 4′-OH and 3′-OH, respectively, which can probably increase the antioxidant power of flavonoids [23]. In our previous study, naringin and hesperidin showed good oxygen radical absorbance capacity in hot water extracts from immature calamondin peel [9].
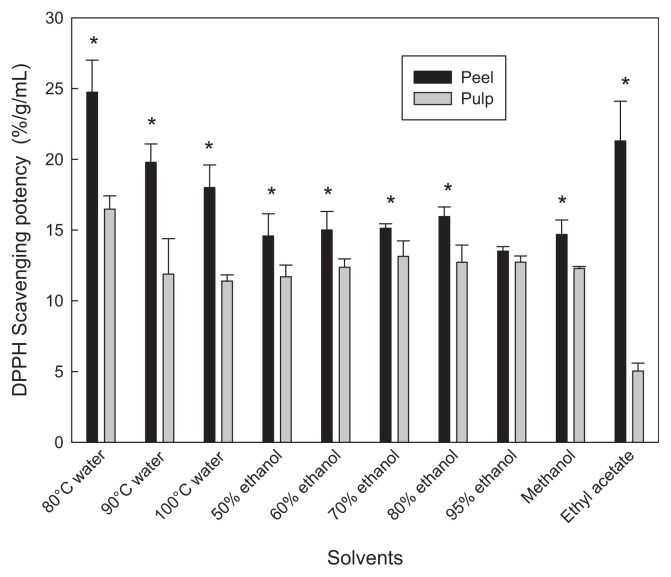
Fig. 1.
DPPH scavenging potency of calamondin peel and pulp extracted with different solvents. *Significant difference (p < 0.05) between peel and pulp of same extraction. DPPH = α,α-diphenyl-β-picrylhydrazyl.
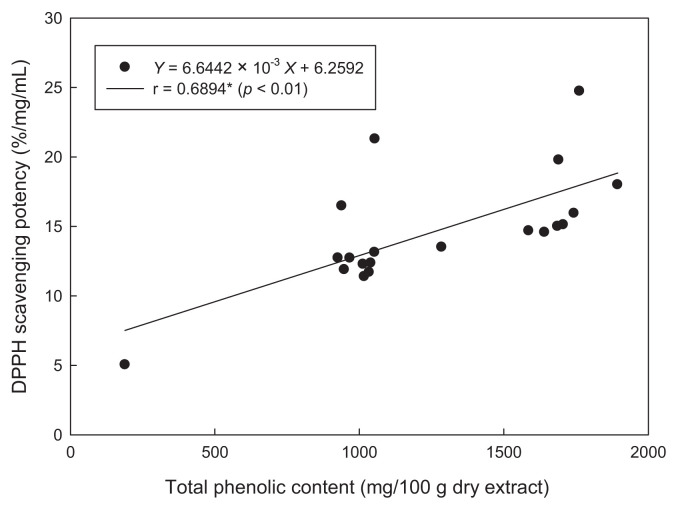
The highest DPPH scavenging potency was observed in the 80°C water extract. The DPPH scavenging potency of hot water extracts from peel was higher than that extracted with the ethanol and methanol (Fig. 1). This might be due to the higher DGPP content in the water extracts. The major component, DGPP, might also contribute some antioxidant activity because of its high quantity, although it showed a lower oxygen radical absorbance capacity (ORAC) value than naringin and hesperidin [9]. For the ethyl acetate extract, a higher DPPH scavenging potency was found in the calamondin peel, while a relative low scavenging potency was observed in the calamondin pulp. The high DPPH scavenging potency of ethyl acetate extract from peel may be due to the effect of co-extracted pigments as mentioned. The content of nobiletin and tangeretin in peel may lead to the high DPPH scavenging potency. However, nobiletin and tangeretin are polymethoxyflavones, which show lower antioxidant activity due to the O-methylation of all phenolic groups [23]. Therefore, some other active compounds, which have not been identified, may also contribute to the antioxidant activity. Polymethoxyflavones exhibit a broad spectrum of biological activity, including anti-inflammatory, anticarcinogenic, antiviral, antithrombogenic, and antiatherogenic properties [12]. The correlation coefficient (r) between total phenolic contents and DPPH scavenging potency in extracts of calamondin peel and pulp was 0.6894, which was considered significant (p < 0.01) (Fig. 2). Total flavonoid compositions also showed significant correlation (p < 0.05) to DPPH scavenging potency with r = 0.4910.
Fig. 2.
Correlation between DPPH scavenging potency and total phenolic content of calamondin. *Significant relationship (p < 0.01). DPPH = α,α-diphenyl-β-picrylhydrazyl.
4. Conclusion
The total phenolic contents and DPPH scavenging capacity of various extracts from calamondin peel were higher than that from pulp. A significantly positive relationship (p < 0.01) between total phenolic content and DPPH scavenging potency was observed. DGPP was the major compound in hot water extracts of calamondin peel, however, nobiletin and tangeretin were the main compounds in ethyl acetate extracts. In the ethanol (50–95%) and methanol extracts, DGPP, nobiletin, and tangeretin existed at the same time. The contents of flavonoid compositions in extracts from calamondin peel were in the following order: hot water extract > ethanol, methanol extract > ethyl acetate extract. The hot water extract of calamondin peel might have great potential for beneficial health food and treatment of hyperpigmentation in cosmetic applications.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Myrna ONC, Baldwin EA, Moshonas MG, et al. Determination of volatile flavor components, sugars, and ascorbic, dehydroascorbic, and other organic acids in calamondin (Citrus mitis Blanco) J Agric Food Chem. 1992;40:2464–6. [Google Scholar]
- 2. Moshonas MG, Shaw PE. Volatile components of calamondin peel oil. J Agric Food Chem. 1996;44:1105–7. [Google Scholar]
- 3.Zang KT. Small encyclopedia of Chinese herbal medicine. Taipei: Neptune Culture Publishing Ltd., Heliopolis Culture Group; 2005. [Google Scholar]
- 4. Ogawa K, Kawasaki A, Omura M, et al. 3′, 5′-Di-C-β-glucopyranosylphloretin, a flavonoid characteristic of the genus Fortunella. Phytochemistry. 2001;57:737–42. doi: 10.1016/s0031-9422(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 5. Sadek ES, Makris DP, Kefalas P. Polyphenolic composition and antioxidant characteristics of kumquat (Fortunella margarita) peel fractions. Plant Foods Hum Nutr. 2009;64:297–302. doi: 10.1007/s11130-009-0140-1. [DOI] [PubMed] [Google Scholar]
- 6. Tatum JH, Berry RE. Flavonoids of citrus cultivar calamondin and synthetic 2′-β-dihydroxychalcones. Phytochemistry. 1978;17:447–9. [Google Scholar]
- 7. Ramful D, Tarnus E, Aruoma OI, et al. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res Int. 2011;44:2088–99. [Google Scholar]
- 8. Lou SN, Yu MW, Ho CT. Tyrosinase inhibitory components of immature calamondin peel. Food Chem. 2012;135:1091–6. doi: 10.1016/j.foodchem.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 9. Yu MW, Lou SN, Ho CT. Antioxidant activity and effective compounds of immature calamondin peel. Food Chem. 2013;136:1130–5. doi: 10.1016/j.foodchem.2012.09.088. [DOI] [PubMed] [Google Scholar]
- 10. Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–61. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 11. Yu J, Wang L, Walzem RL, et al. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 2005;53:2009–14. doi: 10.1021/jf0484632. [DOI] [PubMed] [Google Scholar]
- 12. Li S, Lo CY, Ho CT. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J Agric Food Chem. 2006;54:4176–85. doi: 10.1021/jf060234n. [DOI] [PubMed] [Google Scholar]
- 13. Ho CT, Pan MH, Lai CS, et al. Polymethoxyflavones as food factors for the management of inflammatory diseases. J Food Drug Anal. 2012;20:337–41. [Google Scholar]
- 14. Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural antioxidants. J Am Oil Chem Soc. 1984;61:928–31. [Google Scholar]
- 15. Christel QD, Gressier B, Vasseur J, et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrumesculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 16. Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apples and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910:265–73. doi: 10.1016/s0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 17. Yamaguchi T, Takamura H, Matoba T, et al. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–4. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- 18. Choi MY, Chai C, Park JH, et al. Effects of storage period and heat treatment on phenolic compound composition in dried citrus peels (Chenpi) and discrimination of chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J Pharmaceut Biomed. 2011;54:638–45. doi: 10.1016/j.jpba.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 19. Kawail S, Tomono Y, Katase E, et al. Quantitation of flavonoid constituents in Citrus fruits. J Agric Food Chem. 1999;47:3565–71. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- 20. Kawail S, Tomono Y, Katase E, et al. Quantitative study of flavonoids in leaves of Citrus plants. J Agric Food Chem. 2000;48:3865–71. doi: 10.1021/jf000100o. [DOI] [PubMed] [Google Scholar]
- 21. Yoo KM, Lee KW, Park JB, et al. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J Agric Food Chem. 2004;52:5907–13. doi: 10.1021/jf0498158. [DOI] [PubMed] [Google Scholar]
- 22. Del Rio JA, Gomez P, Baidez AG, et al. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (cv. Valencia Late) fruits against Phytophthora citrophthora. J Agric Food Chem. 2004;52:1913–7. doi: 10.1021/jf035038k. [DOI] [PubMed] [Google Scholar]
- 23. Di Majo D, Giammanco M, La Guardia M, et al. Flavanones in Citrus fruit: structure–antioxidant activity relationships. Food Res Int. 2005;38:1161–6. [Google Scholar]