Abstract
Baicalein and pentamethyl-hydroxychromane (PMC) have been investigated for use as antioxidants. However, antioxidants may stimulate free radical formation under certain conditions. The aim of our study was to determine whether PMC and baicalein exhibit both pro-oxidant and antioxidant activities in human promyelocytic leukemia (HL-60) cells. In this study, electron spin resonance spectrometry was used to investigate the effects of baicalein and PMC on free radical formation. In HL-60 cells, baicalein and PMC produced hydroxyl and phenoxyl radicals, respectively, but each inhibited radical formation by the other. The PMC pro-oxidant activity required H2O2, whereas baicalein produced hydroxyl radicals during the cell resting state only. The antioxidant effect of baicalein on PMC-induced oxidative stress in HL-60 cells may involve myeloperoxidase inhibition, which produces the myeloperoxidase-protein radical. Our investigation of the antioxidant effects of baicalein on arachidonic acid (AA)-induced oxidative stress in HL-60 cells showed that the baicalein-phenoxyl radical was the primary product, and that either carbon-centered or acyl radicals were the secondary products. However, the antioxidant effects of PMC on AA-induced oxidative stress produced only nonradical products. In conclusion, we showed that baicalein displayed both pro-oxidant and antioxidant activities in HL-60 cells. PMC exhibited no pro-oxidant activity during the cells’ resting state but produced the PMC-phenoxyl radical in the presence of H2O2. The reaction of baicalein with AA in HL-60 cells produced baicalein-derived phenoxyl radicals that may initiate various pro-oxidative reactions. However, PMC does not produce radicals when it acts as an antioxidant. Thus, PMC is more beneficial as an antioxidant than baicalein.
Keywords: Antioxidant, Baicalein, Free radical, MPO, PMC, Pro-oxidant
1. Introduction
Reactive oxygen species (ROS) act as second messengers in cell signaling pathways and are involved in numerous disease states, including inflammation, immune injury, and myocardial infarction [1]. Several age-related diseases and the aging process itself are thought to involve the ROS-mediated degeneration of cell components [2], and ROS may also be involved in the initiation, progression, and metastasis of cancer [3,4]. Paradoxically, ROS production is the mechanism of cell death in chemotherapy, radiotherapy, and photodynamic therapy for cancer [5,6]. Consequently, both pro-oxidant and antioxidant therapies have been proposed for cancer treatment [7,8].
Baicalein (5,6,7-trihydroxyflavone; Fig. 1) is a flavonoid extracted from the traditional Chinese herb Scutellaria baicalensis Georgi (Huangqin). It is commonly used in China and Japan to treat chronic hepatitis. In recent years, studies have shown that baicalein may inhibit the growth of cancer cells in vitro and may act as a potent inducer of apoptotic cell death [9–15]. However, the precise mechanism of action remains unclear. Several studies have shown that baicalein has anti-oxidant properties and protects cells from oxidative stress [16–19]. By contrast, the pro-oxidant properties of baicalein have been shown to be cytotoxic [15,20–22]. Our previous study showed that baicalein induced hydroxyl-radical formation through 12-lipoxygenase (12-LOX) in human platelets [23]. In B16F10 cells, baicalein produced hydroxyl radicals and superoxide anion radicals through 12-LOX, which resulted in a reduction in cell viability [24].
Fig. 1.
Structural features of baicalein (5,6,7-trihydroxyflavone), a naturally occurring alkaloid, and 2,2,5,7,8-pentamethyl-6-hydroxychromane, a novel α-tocopherol derivative.
A derivative of α-tocopherol, 2,2,5,7,8-pentamethyl-6-hydroxychromane (PMC; Fig. 1), is more hydrophilic than other α-tocopherol derivatives, and has potent antiplatelet and free radical-scavenging properties [25]. In a previous study, we showed that PMC treatment may produce both preventative and therapeutic effects in ischemia–reperfusion brain injury [26]. We recently demonstrated that PMC inhibits vascular smooth muscle cell proliferation in vitro and balloon injury-induced neointimal formation in vivo, and we found that the inhibitory mechanism of PMC may involve the inhibition of hydroxyl radical-mediated PLCγ1–PKCδ and JAK2–STAT3 activation, which causes cell cycle arrest at the G2/M phase [27]. By contrast, Tyurin et al [28] used myeloperoxidase-containing human promyelocytic leukemia (HL-60) cells to generate phenoxyl radicals from PMC.
Antioxidants function as redox agents, protecting against free radicals under some conditions, and stimulating free radical formation in others [29]. The aim of this study was to determine whether PMC and baicalein exhibit both pro-oxidant and antioxidant activities in HL-60 cells.
2. Materials and methods
2.1. Materials
Arachidonic acid (AA); baicalein; dimethyl sulfoxide (DMSO); 5,5-dimethyl-1-pyrroline N-oxide (DMPO); reduced nicotinamide-adenine dinucleotide (NADH); myeloperoxidase (MPO); N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulfonamide (NS-398); PMC; and 4-aminobenzoic acid (ABAH) were purchased from Sigma (St. Louis, MO, USA). Cyclooxygenase-2 (COX-2), AA (sodium salt), hematin, phenol, and 0.1 M Tris buffer (pH 8.3) were obtained from Oxford Biochemical Research (Rochester Hills, MI, USA). Amphotericin B (Fungizone), fetal calf serum (FCS), and Gibco RPMI 1640 medium were purchased from Invitrogen (Carlsbad, CA, USA).
2.2. Cell cultivation
The HL-60 cells were provided by Dr. R.T. Wu at the National Yang-Ming University, Taipei, Taiwan, and were cultured at 37°C at a cell density ranging from 1 × 106 cells/mL to 5 × 106 cells/mL in an RPMI (Roswell Park Memorial Institute) 1640 medium containing 10% FCS in a humidified atmosphere of 5% CO2. To ensure optimal growth conditions, the cell culture medium was replaced when necessary by centrifuging the cells and resuspending them in fresh RPMI 1640 supplemented with 10% FCS.
2.3. Measurement of free radicals by electron spin resonance spectrometry
Electron spin resonance (ESR) spectrometry was performed using a Bruker EMX ESR spectrometer (Bruker BioSpin, Billerica, MA, USA) as described previously [24], with modifications. The culture medium was replaced with phosphate buffered saline (PBS) prior to each experiment. The reagents or enzyme inhibitors were added to a suspension of 1.5 × 106 HL-60 cells in 150 μL of 100 mM DMPO in PBS, and incubated for 3 minutes prior to the addition of baicalein or PMC. Baicalein and PMC were dissolved in DMSO, and the final concentration of DMSO in the suspension was 0.1% (v/v). The ESR spectra were recorded at room temperature using a flat quartz cell designed for aqueous solutions. The sample preparation and ESR analysis were performed within a period of exactly 3 minutes. The ESR experimental conditions were as follows: 20 mW power at 9.78 GHz, a scan range of 100 G; a receiver gain of 5.02 × 104; modulation amplitude, 1 G; time constant, 164 milliseconds; and a scan duration of 42 seconds, with six scans recorded for each sample.
2.4. Statistical analysis
The experimental results are expressed as the mean ± the standard error of the mean, with the number of observations shown as n. The data were analyzed using analysis of variance. If a significant difference among the group means was noted, the difference between two groups was assessed using the Newman–Keuls method. A p value <0.05 was considered statistically significant.
3. Results
3.1. ESR investigation of free radicals induced by PMC with myeloperoxidase in the presence of H2O2
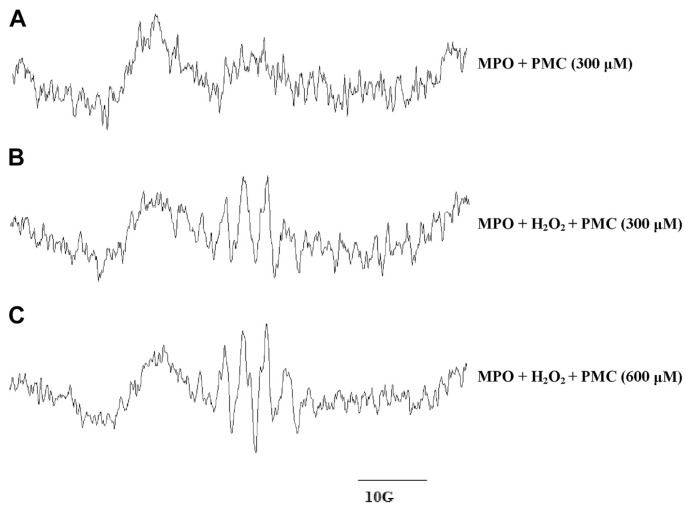
Experiments using MPO/H2O2 were performed to test the effect of PMC on the formation of phenoxyl radicals. The reaction was conducted in 0.1 M of a sodium phosphate buffer containing 600 μM H2O2 and 20 μM MPO, at a pH of 7.4. A typical partially resolved ESR signal of PMC phenoxyl radicals [30] was detected (Fig. 2B and C). No EPR signals were observed for MPO incubated with PMC in the absence of H2O2 (Fig. 2A) or for PMC incubated with H2O2 but without MPO (data not shown).
Fig. 2.
Electron spin resonance spectra of free radicals produced during myeloperoxidase (MPO)-induced oxidation of pentamethyl-hydroxychromane (PMC) in the presence or absence of H2O2. (A) MPO (20 μM) was incubated with 300 μM PMC in the absence of H2O2. (B) Oxidation was initiated for the reaction conditions in (A) by the addition of 600 μM H2O2. (C) MPO (20 μM) was incubated with 600 μM PMC and 600 μM H2O2. All experiments were conducted in a 0.1 M phosphate buffer at pH 7.4, and spectra were recorded after a 3-minute incubation period after the addition of the final reactant (A, PMC; B and C, H2O2).
3.2. ESR investigation of free radicals induced by baicalein and myeloperoxidase
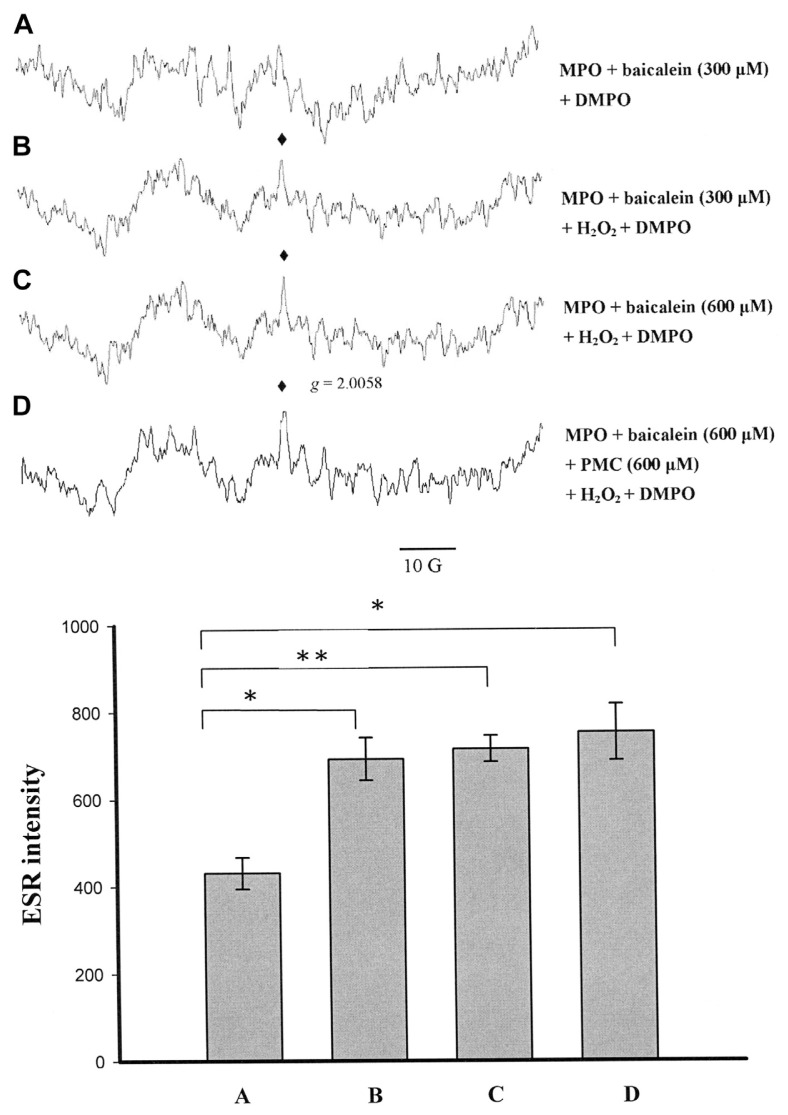
Baicalein was converted by MPO to produce a g = 2.0058 radical (Fig. 3B and C), whereas the formation of this radical was decreased in the controls without H2O2 (Fig. 3A). Baicalein (600 μM) inhibited PMC-phenoxyl radical formation completely (Fig. 3D). The intensity of the ESR signal was not significantly altered for 600 μM baicalein with or without PMC, relative to that of 300 μM baicalein (Figs. 3B–D, 4A and B). Previous studies have shown that aminoglutethimide produced protein radicals with MPO in HL-60 cells [31]. Thus, we concluded that the g = 2.0058 radical might be a protein radical formed by MPO interacting with baicalein.
Fig. 3.
Electron spin resonance spectra obtained from the reaction of myeloperoxidase (MPO) with baicalein and pentamethyl-hydroxychromane (PMC) in the presence or absence of H2O2. In all groups, DMPO (5,5-dimethyl-1-pyrroline N-oxide; 100 mM) was added. (A) MPO (20 μM) in phosphate-buffered saline (PBS) was incubated with 300 μM baicalein in the absence of H2O2. (B) MPO (20 μM) in PBS was incubated with 300 μM baicalein. (C) MPO (20 μM) in PBS was incubated with 600 μM baicalein. (D) MPO (20 μM) in PBS was incubated with 600 μM baicalein and 600 μM PMC, with PMC and baicalein added simultaneously. The reactions in (B–D) were initiated by the addition of 600 μM H2O2. The spectra are labeled to indicate the components: protein radical (♦). The data are shown as the mean ± SEM of three independent experiments. *p < 0.05 and **p < 0.01 compared with the control (A).
Fig. 4.
Electron spin resonance spectra of free radicals generated by a suspension of human promyelocytic leukemia cells (1.5 × 106 in 150 μL) that were (A) incubated with 300 μM baicalein in the presence of 100 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO), with the reaction initiated by the addition of 600 μM H2O2; (B) incubated with 600 μM baicalein, 100 mM DMPO and 600 μM H2O2; (C) incubated with 600 μM baicalein, 100 mM DMPO in the absence of H2O2; or (D) incubated with 600 μM PMC, 100 mM DMPO, 600 μM PMC, and 600 μM H2O2. The spectra are labeled to indicate the components: protein radical (♦); DMPO-hydroxyl radical adduct (*). The data are shown as the mean ± SEM for the hydroxyl radical signals of four independent experiments. ***p < 0.001.
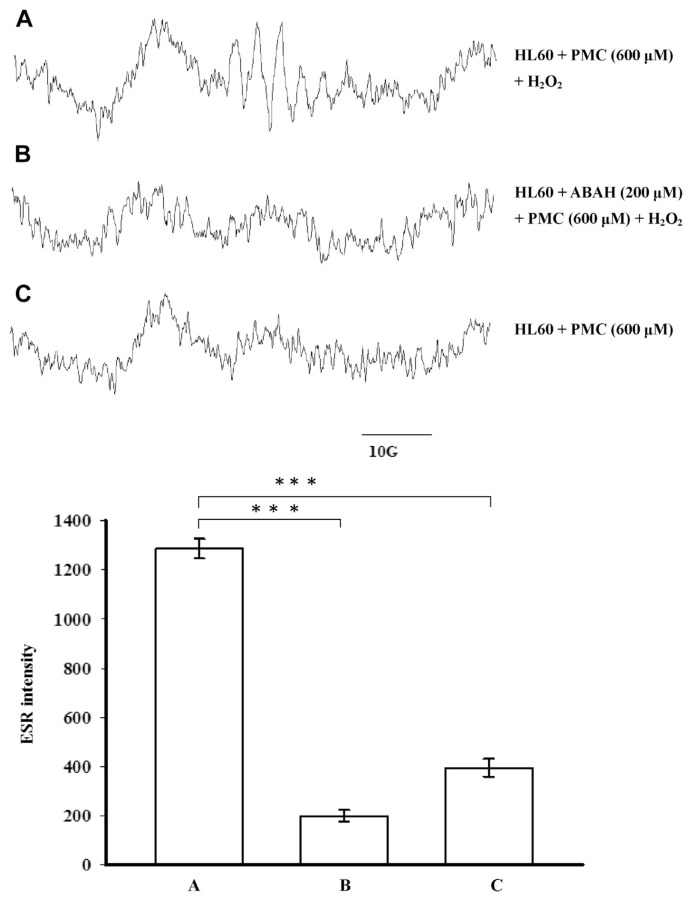
3.3. Effect of PMC on phenoxyl radical formation in HL-60 cells
We investigated whether the endogenous MPO in HL-60 cells catalyzes the single-electron oxidation of PMC. The results showed that HL-60 cells/H2O2 catalyzed PMC to produce a phenoxyl radical (Fig. 5A). Controls without H2O2 or with ABAH (an MPO inhibitor) failed to form this radical adduct (Fig. 5B and C). These results suggest that the phenoxyl radical formation resulted from the MPO-mediated catalysis of H2O2 in the HL-60 cells.
Fig. 5.
Electron spin resonance spectra of free radicals produced in a suspension of HL-60 cells (1.5 × 106 in 150 μL) that were (A) incubated with 600 μM pentamethyl-hydroxychromane (PMC) in the presence of 600 μM H2O2; (B) pre-incubated in 200 μM 4-aminobenzoic acid (ABAH) for 5 minutes prior to the addition of PMC; or (C) incubated with 600 μM PMC in the absence of H2O2. The data are shown as the mean ± SEM of four independent experiments. ***p < 0.001 compared with the control.
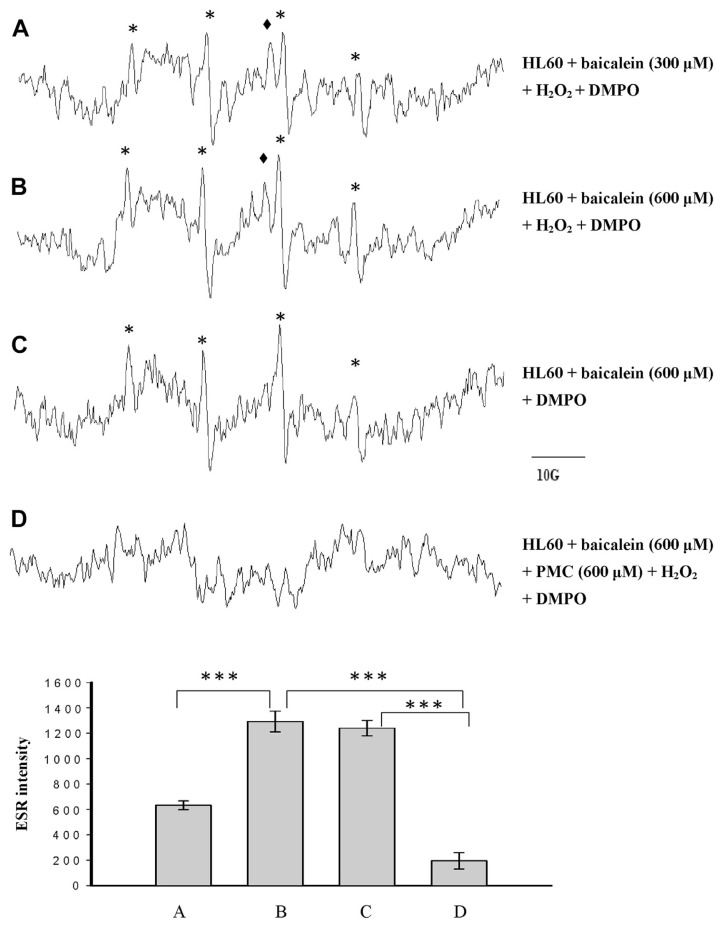
3.4. Free radicals induced by baicalein in the presence of DMPO in HL-60 cells
The addition of baicalein to the HL-60 cells in the presence of 600 μM of H2O2 and the spin trapper DMPO produced a four-line ESR signal, which is typical for the hydroxyl radical (aN = aH = 14.8 G), which was baicalein-dose-dependent (Fig. 4A, control; Fig. 4B). In controls without H2O2, the formation of a hydroxyl radical was not affected (Fig. 4C), suggesting that MPO was not involved in this reaction. Our previous studies showed that baicalein induced hydroxyl radical formation through 12-LOX in human platelets [23] and B16F10 cells [24]. Therefore, baicalein might induce hydroxyl radical formation through 12-LOX in HL-60 cells as well. However, baicalein-induced MPO-protein radical formation was also detectable in HL-60 cells (Fig. 4A and B). The formation of this radical was decreased in controls without H2O2 (Fig. 4C). Furthermore, PMC (600 μM) significantly reduced the baicalein-induced hydroxyl radical signals, suggesting that PMC acted as an antioxidant under this condition (Fig. 4D).
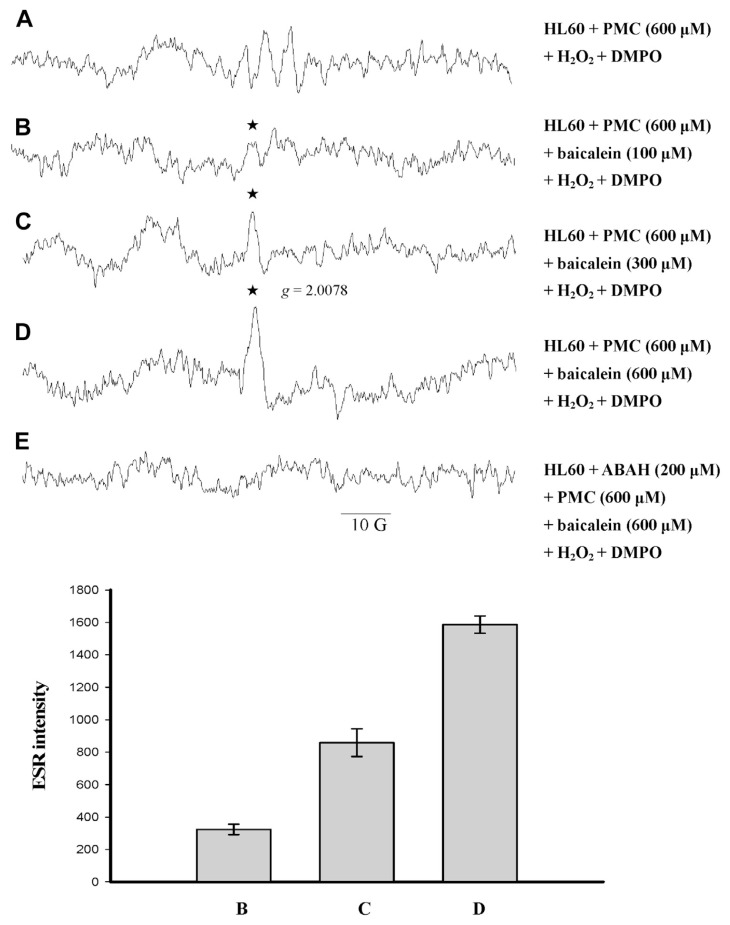
3.5. Effect of baicalein on PMC-phenoxyl radical formation in HL-60 cells
We investigated whether baicalein inhibits the MPO-mediated single-electron oxidation of PMC in HL-60 cells in the presence of 600 μM H2O2. The results showed that baicalein produced a radical with a g value of 2.0078, and completely inhibited PMC-induced phenoxyl radical formation in the presence of H2O2 in HL-60 cells (Fig. 6C and D). The radical produced from baicalein under these conditions differed from the radical (g = 2.0058) for which formation was inhibited by ABAH, indicating that endogenous MPO catalyzed the reaction (Fig. 6E). The intensity of the g = 2.0078 radical signal was baicalein-dose-dependent, suggesting that it was a baicalein-phenoxyl radical.
Fig. 6.
Electron spin resonance spectra of free radicals generated by a suspension of human promyelocytic leukemia (HL-60) cells (1.5 × 106 in 150 μL) that were (A) incubated with 600 μM pentamethyl-hydroxychromane (PMC), 100 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO), and 600 μM H2O2. After 1 minute, (B) 100 μM, (C) 300 μM, and (D) 600 μM baicalein were added. (E) The HL-60 cells were treated identically to sample (D), except that they were pre-incubated for 5 minutes in 200 μM 4-aminobenzoic acid (ABAH) before PMC was added. The spectra are labeled to indicate the components: phenoxyl radical (★). The data are shown as the mean ± SEM of protein radical signals for four independent experiments.
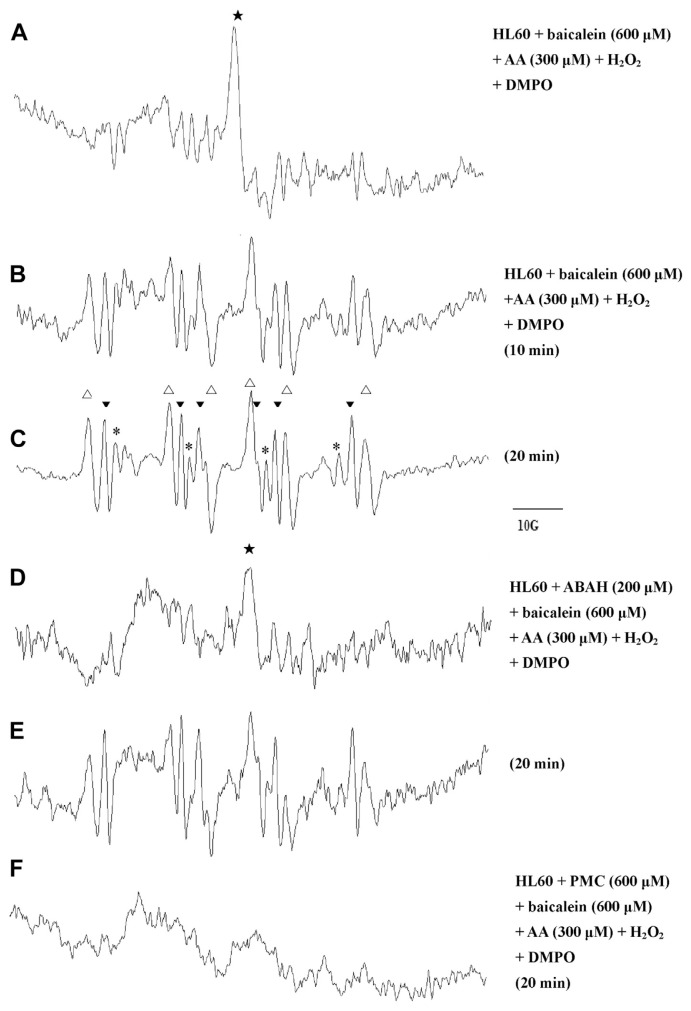
3.6. Effect of baicalein on AA-induced free radical formation in HL-60 cells
Previous studies have shown that AA-induced free radical generation might occur through an oxidative metabolic process involving cyclooxygenase (COX), lipoxygenase (LOX) [32], and NADPH oxidase [33,34]. We investigated whether baicalein or PMC inhibits AA-induced free radical formation in HL-60 cells. Our ESR analysis showed that the incubation of HL-60 cells in 300 μM of AA, 600 μM of H2O2, and 600 μM baicalein produced a narrow signal with a g value of 2.0078 that corresponded to that of the baicalein-phenoxyl radical. After 20 minutes, the following three signals were detected (Fig. 7C): a hydroxyl radical (aN = aH = 14.8 G), a carbon-centered radical (aN = 16.0 G, aH = 22.8 G), and an acyl radical (aN = 14.9 G, aH = 18.3 G). These radicals persisted in the presence of ABAH, suggesting that endogenous MPO in the HL-60 cells was not involved in this reaction (Fig. 7E). By contrast, the formation of these radicals was almost completely inhibited by pre-incubation with 600 μM PMC (Fig. 7F), indicating that PMC acted as an antioxidant in that reaction.
Fig. 7.
Electron spin resonance spectra of free radicals generated by a suspension of human promyelocytic leukemia cells (1.5 × 106 in 150 μL). To each group we added 100 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 600 μM H2O2. The cells were incubated with acid (AA) to trigger free radical formation for (A) 3 minutes, (B) 10 minutes, and (C) 20 minutes. Using these reaction conditions, a (D) 10-minute reaction and (E) a 20-minute reaction were performed in which the cells were pre-incubated in 200 μM 4-aminobenzoic acid (ABAH) for 5 minutes prior to the addition of baicalein, and (F) a 20-minute reaction was performed in which the cells were pre-incubated in 600 μM PMC for 5 minutes prior to the addition of baicalein. The spectra are labeled to indicate the components: DMPO-hydroxyl radical adduct (*), DMPO-carbon-centered adduct (△), and DMPO-acyl radical adduct (▼).
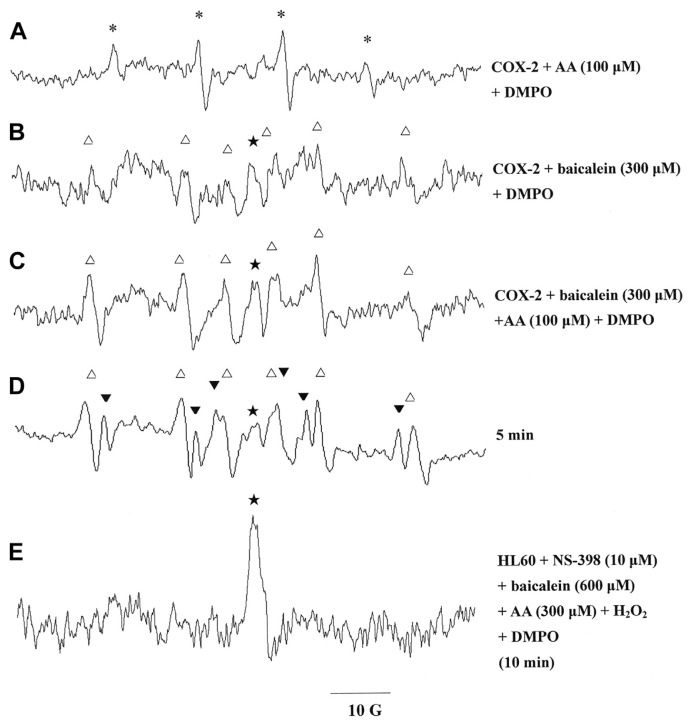
3.7. Free radicals induced by AA and baicalein with COX-2 in the presence of DMPO
We hypothesized that baicalein and AA would produce a hydroxyl radical, a carbon-centered radical, and an acyl radical in HL-60 cells, mainly through COX. We tested this hypothesis using a COX-2 system. ESR analysis showed that treatment with 100 μM AA produced a hydroxyl radical signal (aN = aH = 14.8 G) in cells suspended in the 0.1 M Tris–HCl buffer, pH 8.3, containing 500 μM phenol, 100 μM NADH, 1 μM hematin, and 10 U of COX-2 (Fig. 8A). By contrast, baicalein produced a carbon-centered radical signal (aN = 16.0 G, aH = 22.8 G) and another radical signal with a g value of 2.0078 (Fig. 8B) under the same conditions (COX-2 system). The simultaneous addition of 300 μM baicalein and 100 μM AA to HL-60 cells in the COX-2 system produced a distinct carbon-centered radical signal and another radical signal with a g value of 2.0078 (Fig. 8C). An additional acyl radical signal (aN = 14.9 G, aH = 18.3 G) appeared, and the intensity of the original radical signal (g = 2.0078) decreased, following an additional 5 minutes of incubation (Fig. 8D). We tested the effect of a selective COX-2 inhibitor, NS-398, on the production of radicals in the presence of baicalein/AA in HL-60 cells. Our ESR analysis showed that the incubation of HL-60 cells in 10 μM of NS-398, 300 μM of AA, 600 μM of H2O2, and 600 μM baicalein only produced a narrow signal with a g value of 2.0078 that corresponded to that of the baicalein-phenoxyl radical (Fig. 8E).
Fig. 8.
Electron spin resonance spectra detected from the reaction of cyclooxygenase-2 (COX-2) with (A) 100 μM arachidonic acid (AA), (B) 300 μM baicalein, (C) 100 μM AA and 300 μM baicalein, and (D) 1 mM AA and 1 mM baicalein. (E) Electron spin resonance spectra of free radicals generated by a suspension of human promyelocytic leukemia cells (1.5 × 106 in 150 μL) that were incubated with 10 μM NS-398, 600 μM H2O2, 600 μM baicalein, and 300 μM AA to trigger free radical formation for 10 minutes. The 5,5-dimethyl-1-pyrroline N-oxide (DMPO, 100 mM) was added to all the reactions. The spectra are labeled to indicate the components: phenoxyl radical (★), DMPO-carbon-centered adduct (△), and DMPO-acyl radical adduct (▼).
4. Discussion
We analyzed the free radical formation induced by PMC and baicalein in HL-60 cells to investigate the pro-oxidant and antioxidant properties of these substances. The results showed that baicalein produced five radical species in HL-60 cells, whereas PMC produced a phenoxyl radical only, and both baicalein and PMC mutually inhibited radical formation induced by the other. A radical signal with a g value of 2.0058 was recorded for the reaction of H2O2, MPO, and baicalein (Figs. 3 and 4), but its intensity was independent of the concentration of baicalein. We thus interpreted it to represent the formation of an MPO-protein radical. By contrast, a radical signal with a g value of 2.0078 that was also recorded was found to be baicalein dose-dependent, and did not require the presence of MPO and H2O2 (Fig. 7). We thus assigned it as a baicalein-phenoxyl radical.
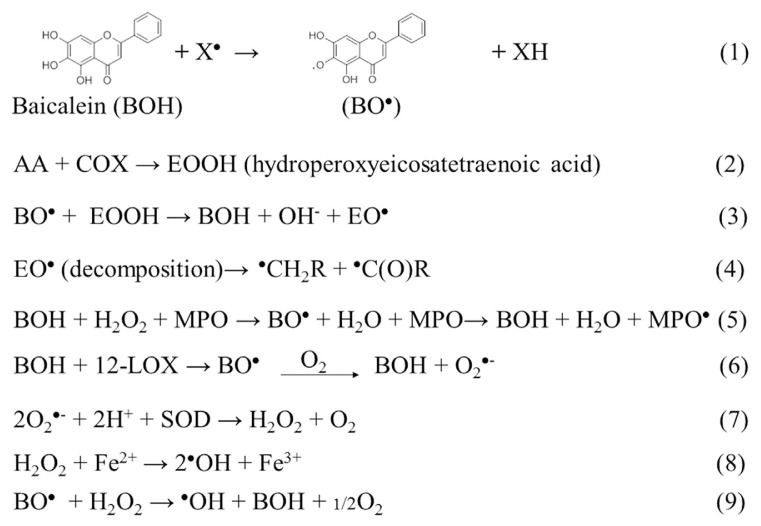
Shen et al [35] showed that baicalein inhibited MPO activity in human leukocytes. When baicalein acts as a free radical scavenger, it produces baicalein-derived radicals, such as phenoxyl, carbon-centered, and acyl radicals. However, our ESR results showed that baicalein completely blocked the production of PMC-derived phenoxyl radicals by MPO in vitro and in HL-60 cells. Under both sets of conditions, an MPO-protein radical was produced (Fig. 9, Equation 5). This result provided further evidence that the antioxidative effects of baicalein on PMC-induced lipid peroxidation may be the result of MPO inhibition.
Fig. 9.
Equations describing the possible reaction pathways for the formation of the five radical species that were induced by baicalein.
Numerous lipoxygenase (LOX) isoforms exist, including 12-LOX in platelets and leukocytes, 5-LOX in neutrophils, and 1-LOX in soybeans. The LOX inhibitors, phenidone and nordihydroguaiaretic acid, are oxidized to free-radical metabolites during the reduction of the catalytically active ferric LOX to its inactive ferrous form [36]. Baicalein is known to block the activity of 12-LOX that is often overexpressed in tumor tissues, and we showed in a previous study that baicalein produced superoxide anions in B16 F10 cells via a 12-LOX pathway [24]. We suggested that superoxide anions produced by baicalein were promptly converted to hydroxyl radicals through superoxide dismutase and the Fenton reaction in B16 F10 melanoma cells [24]. Similarly, the results of our current study showed that baicalein induced hydroxyl radical formation in HL-60 cells. However, baicalein did not produce hydroxyl radicals through MPO (Fig. 3) or COX-2 (Fig. 8). Therefore, we concluded that baicalein produces hydroxyl radicals through a 12-LOX-mediated pathway in HL-60 cells.
The radical-scavenging activity of flavonoids depends on the availability of phenolic hydrogens and on the stabilization of the resulting phenoxyl radicals [37,38]. Adhikari et al [39] reported that baicalein acted as an effective scavenger of hydroxyl, azidyl, and alkylchloroperoxyl radicals, and produced a baicalein-derived phenoxyl radical. Our study confirmed that baicalein exerted a direct antioxidant effect. Based on theoretical considerations, we expected that baicalein-derived phenoxyl radicals would be the initial intermediates resulting from the interaction between a radical and baicalein (Fig. 9, Equation 1). However, it is difficult to detect phenoxyl radicals in situ at room temperature in aqueous solutions [40]. The transient baicalein-derived phenoxyl radicals can be oxidized by oxygen to produce superoxide radicals (Fig. 9, Equation 6). Hydroxyl radicals may be generated through the reductive homolytic cleavage of H2O2 at the expense of baicalein-derived phenoxyl radicals [41] (Fig. 9, Equation 9) or by reacting with ferrous ions in the Fenton reaction [24] (Fig. 9, Equation 8).
Buettner [42] reported that an interaction between an antioxidant and lipid radicals leads to various secondary radical products, all of which possess pro-oxidant abilities. We observed that the interaction between baicalein and AA in HL-60 cells led to the production of hydroxyl, carbon-centered, and acyl radicals. Substantial amounts of these products were produced within minutes, indicating that they were not primary radicals. Based on the results of the COX-2 experiments, we concluded that phenoxyl radicals were the primary radicals, and that the carbon-centered and acyl radicals were secondary radicals. Nohl et al [41] previously showed that ubisemiquinone reacted with linoleic acid hydroperoxide to produce carbon-centered and acyl radicals. Similarly, COX can convert AA to hydroperoxide [43] (Fig. 9, Eq. 2). The baicalein-derived phenoxyl radical interacts with AA hydroperoxide to produce carbon-centered and acyl radicals (Fig. 9, Equations 3 and 4). The possible pathways, with or without AA, of baicalein-induced radical formation in HL-60 cells are shown in Figure 9.
An antioxidant can act as a pro-oxidant under certain conditions [29]. In this study, we demonstrated that PMC acted as a pro-oxidant in cells in the absence of other free radicals (Fig. 5A), but acted as an antioxidant under conditions of high oxidative stress (Figs. 4D and 7F). Similarly, baicalein produced hydroxyl radicals in HL-60 cells in the resting state (Fig. 4), and inhibited PMC-derived phenoxyl radical and AA-derived hydroxyl radical formation (Fig. 8). The reaction components were the same as those for the reactions described in Figs. 4D and 6A, but the results in each condition differed according to the degree of oxidative stress. We propose that the sequence in which the reagents were added determined the role of the reagent regarding the redox reaction, with the reagents added first acting as pro-oxidants, and those added later acting as antioxidants.
In conclusion, we showed that baicalein displayed both pro-oxidant and antioxidant activities in HL-60 cells. PMC exhibited no pro-oxidant activity during the cells’ resting state but produced the PMC-phenoxyl radical in the presence of H2O2. The reaction of baicalein with AA produced baicalein-derived phenoxyl radicals that may initiate various pro-oxidative reactions. However, PMC does not produce radicals when it acts as an antioxidant. Thus, PMC is more beneficial as an antioxidant than baicalein.
Acknowledgments
This work was supported by a grant from Wan Fang Hospital of Taipei Medical University, Taipei, Taiwan (100TMU-WFH-01-1).
Funding Statement
This work was supported by a grant from Wan Fang Hospital of Taipei Medical University, Taipei, Taiwan (100TMU-WFH-01-1).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 2. Kootstra A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol Biol. 1994;26:771–4. doi: 10.1007/BF00013762. [DOI] [PubMed] [Google Scholar]
- 3. Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–96. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 4. Halliwell B. Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med. 2002;32:968–74. doi: 10.1016/s0891-5849(02)00808-0. [DOI] [PubMed] [Google Scholar]
- 5. Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J Biol Chem. 1999;274:19792–8. doi: 10.1074/jbc.274.28.19792. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875–84. doi: 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- 7. Hyoudou K, Nishikawa M, Kobayashi Y, et al. PEGylated catalase prevents metastatic tumor growth aggravated by tumor removal. Free Radic Biol Med. 2006;41:1449–58. doi: 10.1016/j.freeradbiomed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 8. Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–96. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 9. Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–48. doi: 10.1158/1535-7163.MCT-07-0281. [DOI] [PubMed] [Google Scholar]
- 10. Chen CH, Huang LL, Huang CC, et al. Baicalein, a novel apoptotic agent for hepatoma cell lines: a potential medicine for hepatoma. Nutr Cancer. 2000;38:287–95. doi: 10.1207/S15327914NC382_19. [DOI] [PubMed] [Google Scholar]
- 11. Chen FL, Wang XZ, Li JY, et al. 12-Lipoxygenase induces apoptosis of human gastric cancer AGS cells via the ERK1/2 signal pathway. Dig Dis Sci. 2008;53:181–7. doi: 10.1007/s10620-007-9841-1. [DOI] [PubMed] [Google Scholar]
- 12. Ciesielska E, Gwardys A, Metodiewa D. Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. Anticancer Res. 2002;22:2885–91. [PubMed] [Google Scholar]
- 13. Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55:951–5. doi: 10.1016/s0090-4295(00)00467-2. [DOI] [PubMed] [Google Scholar]
- 14. Po LS, Chen ZY, Tsang DS, et al. Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett. 2002;187:33–40. doi: 10.1016/s0304-3835(02)00355-5. [DOI] [PubMed] [Google Scholar]
- 15. Ueda S, Nakamura H, Masutani H, et al. Baicalin induces apoptosis via mitochondrial pathway as prooxidant. Mol Immunol. 2002;38:781–91. doi: 10.1016/s0161-5890(01)00115-8. [DOI] [PubMed] [Google Scholar]
- 16. Gao Z, Huang K, Yang X, et al. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–50. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- 17. Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 18. Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–5. [PubMed] [Google Scholar]
- 19. Furuno K, Akasako T, Sugihara N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull. 2002;25:19–23. doi: 10.1248/bpb.25.19. [DOI] [PubMed] [Google Scholar]
- 20. Miura YH, Tomita I, Watanabe T, et al. Active oxygens generation by flavonoids. Biol Pharm Bull. 1998;21:93–6. doi: 10.1248/bpb.21.93. [DOI] [PubMed] [Google Scholar]
- 21. Yoshino M, Haneda M, Naruse M, et al. Prooxidant activity of flavonoids: copper-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Mol Genet Metab. 1999;68:468–72. doi: 10.1006/mgme.1999.2901. [DOI] [PubMed] [Google Scholar]
- 22. Woo AY, Cheng CH, Waye MM. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc Res. 2005;65:244–53. doi: 10.1016/j.cardiores.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 23. Chou DS, Lee JJ, Hsiao G, et al. Baicalein induction of hydroxyl radical formation via 12-lipoxygenase in human platelets: an ESR study. J Agric Food Chem. 2007;55:649–55. doi: 10.1021/jf062584f. [DOI] [PubMed] [Google Scholar]
- 24. Chou DS, Hsiao G, Lai YA, et al. Baicalein induces proliferation inhibition in B16F10 melanoma cells by generating reactive oxygen species via 12-lipoxygenase. Free Radic Biol Med. 2009;46:1197–203. doi: 10.1016/j.freeradbiomed.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 25. Hsiao G, Yen MH, Lee YM, et al. Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. Br J Haematol. 2002;117:699–704. doi: 10.1046/j.1365-2141.2002.03492.x. [DOI] [PubMed] [Google Scholar]
- 26. Hsiao G, Lee JJ, Chen YC, et al. Neuroprotective effects of PMC, a potent alpha-tocopherol derivative, in brain ischemia–reperfusion: reduced neutrophil activation and anti-oxidant actions. Biochem Pharmacol. 2007;73:682–93. doi: 10.1016/j.bcp.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 27. Hsieh CY, Liu CL, Hsu MJ, et al. Inhibition of vascular smooth muscle cell proliferation by the vitamin E derivative pentamethylhydroxychromane in an in vitro and in vivo study: pivotal role of hydroxyl radical-mediated PLCgamma1 and JAK2 phosphorylation. Free Radic Biol Med. 2010;49:881–93. doi: 10.1016/j.freeradbiomed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 28. Tyurin VA, Carta G, Tyurina YY, et al. Peroxidase-catalyzed oxidation of beta-carotene in HL-60 cells and in model systems: involvement of phenoxyl radicals. Lipids. 1997;32:131–42. doi: 10.1007/s11745-997-0017-0. [DOI] [PubMed] [Google Scholar]
- 29. Herbert V. Prooxidant effects of antioxidant vitamins. Introduction. J Nutr. 1996;126:1197S–200S. [PubMed] [Google Scholar]
- 30. Kagan VE, Kuzmenko AI, Shvedova AA, et al. Myeloperoxidase-catalyzed phenoxyl radicals of vitamin E homologue, 2,2,5,7,8-pentamethyl-6-hydroxychromane, do not induce oxidative stress in live HL-60 cells. Biochem Biophys Res Commun. 2000;270:1086–92. doi: 10.1006/bbrc.2000.2564. [DOI] [PubMed] [Google Scholar]
- 31. Siraki AG, Bonini MG, Jiang J, et al. Aminoglutethimide-induced protein free radical formation on myeloperoxidase: a potential mechanism of agranulocytosis. Chem Res Toxicol. 2007;20:1038–45. doi: 10.1021/tx6003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edderkaoui M, Hong P, Vaquero EC, et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–47. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 33. Kim C, Dinauer MC. Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid. J Leukoc Biol. 2006;79:223–4. doi: 10.1189/jlb.0705371. [DOI] [PubMed] [Google Scholar]
- 34. Shiose A, Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J Biol Chem. 2000;275:13793–801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 35. Shen YC, Chiou WF, Chou YC, et al. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171–81. doi: 10.1016/s0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- 36. Kemal C, Louis-Flamberg P, Krupinski-Olsen R, et al. Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity. Biochemistry. 1987;26:7064–72. doi: 10.1021/bi00396a031. [DOI] [PubMed] [Google Scholar]
- 37. Bors W, Heller W, Michel C, et al. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–55. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- 38. Rice-Evans CA, Miller NJ, Paganga G, et al. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 39. Adhikari S, Tilak JC, Devasagayam TP. Free radical reactions of a naturally occurring flavone baicalein and possible mechanisms towards its membrane protective properties. Indian J Biochem Biophys. 2011;48:275–82. [PubMed] [Google Scholar]
- 40. Goldman R, Claycamp GH, Sweetland MA, et al. Myeloperoxidase-catalyzed redox-cycling of phenol promotes lipid peroxidation and thiol oxidation in HL-60 cells. Free Radic Biol Med. 1999;27:1050–63. doi: 10.1016/s0891-5849(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 41. Nohl H, Gille L, Kozlov AV. Antioxidant-derived prooxidant formation from ubiquinol. Free Radic Biol Med. 1998;25:666–75. doi: 10.1016/s0891-5849(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 42. Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–43. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 43. O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]