Abstract
Limnophila aromatica is commonly used as a spice and a medicinal herb in Southeast Asia. In this study, water and various concentrations (50%, 75%, and 100%) of methanol, ethanol, and acetone in water were used as solvent in the extraction of L. aromatica. The antioxidant activity, total phenolic content, and total flavonoid content of the freeze-dried L. aromatica extracts were investigated using various in vitro assays. The extract obtained by 100% ethanol showed the highest total antioxidant activity, reducing power and DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity. The same extract also exhibited the highest phenolic content (40.5 mg gallic acid equivalent/g of defatted L. aromatica) and the highest flavonoid content (31.11 mg quercetin equivalent/g of defatted L. aromatica). The highest extraction yield was obtained by using 50% aqueous acetone. These results indicate that L. aromatica can be used in dietary applications with a potential to reduce oxidative stress.
Keywords: Antioxidants activity, Effect of solvent, Food analysis, Limnophila aromatica, Total phenol content
1. Introduction
Many plants, particularly medicinal plants, have been extensively studied for their antioxidant activity in recent years. It is believed that an increased intake of food rich in natural antioxidants is associated with lower risks of degenerative diseases, particularly cardiovascular diseases and cancer [1]. Antioxidants from aromatic, spicy, medicinal, and other plants were studied to develop natural antioxidant formulations for food, cosmetic, and other applications [2]. There are three major classes of plant chemicals: terpenoids, phenolic metabolites, and alkaloids [3]. Among these three groups, phenolic compounds are the most important for dietary applications and the most extensively researched [4]. Phenolic compounds include phenolic acids (hydroxybenzoic and hydroxycinnamic acids), polyphenols (hydrolyzable and condensed tannins), and flavonoids. These compounds protect plants, fruits, and vegetables from oxidative damage and have been used as antioxidants by humans. Finding new and safe antioxidants from natural sources is of great interest for applications in natural antioxidants, functional foods, and neutraceuticals. Phytochemical screening is one of the methods that have been used to explore antioxidant compounds in plants.
There are many techniques to recover antioxidants from plants, such as Soxhlet extraction, maceration, supercritical fluid extraction, subcritical water extraction, and ultrasound-assisted extraction. However, extraction yield and antioxidant activity not only depend on the extraction method but also on the solvent used for extraction. The presence of various antioxidant compounds with different chemical characteristics and polarities may or may not be soluble in a particular solvent [5]. Polar solvents are frequently used for recovering polyphenols from plant matrices. The most suitable solvents are aqueous mixtures containing ethanol, methanol, acetone, and ethyl acetate. Ethanol has been known as a good solvent for polyphenol extraction and is safe for human consumption. Methanol has been generally found to be more efficient in extraction of lower molecular weight polyphenols, whereas aqueous acetone is good for extraction of higher molecular weight flavanols [6]. The maximum total phenolic content was obtained from barley flour by extraction using a mixture of ethanol and acetone [7]. For extracting flavonoids from tea, aqueous ethanol performed better than aqueous methanol and aqueous acetone [8]. Extracts with the greatest antioxidant activity were obtained in mate tea and black tea by using 50% aqueous ethanol and 50% aqueous acetone, respectively [5].
Limnophila aromatica (Lamk.) Merr. (syn. Limnophila grastissima Blume) belongs to the Scrophulariaceae (figwort, snapdragon) family. Several species of Limnophila are found in Southeast Asia. It has been used as a spice and a medicinal herb in Southeast Asia. In Vietnam, L. aromatica is easily cultivated in flooded rice fields and soggy land. It is used in Vietnamese cuisine to add flavour in soup broths, sauces, and other foods. L. aromatica has been shown to have negligible toxicity and possesses diuretic, muscle relaxant, and antispasmodic activities. It has been used to treat kidney stones, painful cramps, wound, care and ulcer [9]. A colorless antibacterial essential oil (0.1 wt.%) that contains limonene and perillaldehyde as the principal constituents was obtained from dry leaves of L. aromatica (cited as L. gratissima) [10]. Chlorogenic, caffeic acids, and uncommon 8-oxygenated flavonoids were also observed in this species [11,12]. However, there are no reports on the phenolic content and contribution of phenolic compound to the overall antioxidant activities of L. aromatica. The objective of this work was to investigate the effects of solvents on the extraction of polyphenol from defatted L. aromatica (DFLA) and investigate the antioxidant activity of the extracts by in vitro methods.
2. Methods
2.1. Materials
The L. aromatica plant used in this study was bought at a Vietnamese store in Taiwan. Folin–Ciocalteu (FC) reagent, sodium carbonate anhydrous, gallic acid, sodium nitrite, sodium hydroxide, aluminum chloride anhydrous, 2,2-diphenyl-1-picrylhydrazyl (DPPH), trichloroacetic acid, ferric chloride anhydrous, ascorbic acid, and catechin were purchased from Sigma-Aldrich GmbH (Sternheim, Germany). Sulfuric acid and potassium-hexacyanoferrate were obtained from Merck (Darmstadt, Germany). Anhydrous monobasic potassium phosphate was purchased from Fisher Chemicals (Fair Lawn, NJ, USA), whereas HPLC-grade methanol, ethanol, and acetone were supplied by Sigma-Aldrich (St. Louis, MO, USA).
2.2. Sample preparation
After the roots were removed, a fresh L. aromatica plant (2 kg) was washed and freeze-dried. The freeze-dried sample was ground into powder using a kitchen milling machine and passed through a 60-mesh sieve. The powder (100 g) was then defatted using n-hexane as the solvent. The solid–liquid mixture was filtered with ADVANTEC qualitative filter paper number 2, and the residue was dried in an oven at 50°C for 6 hours. The dried defatted L. aromatica (DFLA) was stored at 4°C prior to extraction.
2.3. Extraction of DFLA
DFLA (30 mg) was mixed with the solvent (1.6 mL), and the mixture was vigorously stirred for 5 minutes. The mixture was then centrifuged (13,000g, 25°C) for 20 minutes. The supernatant obtained was concentrated under vacuum at 45°C and freeze-dried for 24 hours. The freeze-dried extract was weighed to determine the extraction yield. All freeze-dried extracts were kept at 4°C prior to analyses.
2.4. Determination of polyphenol content
2.4.1. Total phenol content
Total phenol content (TPC) in each extract was determined using the FC method described by McDonald et al [13], with minor modifications. The freeze-dried extract was dissolved in distilled water to a concentration of 50 μg/mL. The calibration curve was established using gallic acid (0–60 μg/mL). The diluted extract or gallic acid (1.6 mL) was added to 0.2 mL FC reagent (5-fold diluted with distilled water) and mixed thoroughly for 3 minutes. Sodium carbonate (0.2 mL, 10% w/v) was added to the mixture and the mixture was allowed to stand for 30 minutes at room temperature. The absorbance of the mixture was measured at 760 nm using a UV–VIS spectrophotometer V-550 model (Jasco, Tokyo, Japan). TPC was expressed as milligram gallic acid equivalent per gram defatted L. aromatica (mg GAE/g DFLA).
2.4.2. Total flavonoid content
The total flavonoid content (TFC) of each extract was investigated using the aluminum chloride colorimetry method described by Chang et al [14] with slight modifications. In brief, the extract sample was diluted with methanol until 100 μg/mL. The calibration curve was prepared by diluting quercetin in methanol (0–100 μg/mL). The diluted extract or quercetin (2.0 mL) was mixed with 0.1 mL of 10% (w/v) aluminum chloride solution and 0.1 mL of 0.1 mM potassium acetate solution. The mixture was kept at room temperature for 30 minutes. Then the maximum absorbance of the mixture was measured at 415 nm using a UV–VIS spectrophotometer. TFC was expressed as milligram quercetin equivalent per gram defatted L. aromatica (mg QCE/g DFLA).
2.5. Antioxidant activity
2.5.1. Total antioxidant activity
The total antioxidant activity (TAA) of the freeze-dried extract was determined by adapting the method used by Govindarajan et al [15] and Subhasree et al [16] with slight modifications. In brief, the freeze-dried extract was diluted with distilled water (60–220 μg/mL). The diluted extract (0.2 mL) was then mixed with 1.8 mL of reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) in a capped plastic tube. The tube was incubated in a water bath at 90°C for 90 minutes, then cooled down to room temperature. The absorbance of this solution was measured at 695 nm using a UV–VIS spectrophotometer against a blank. Ascorbic acid (5–60 μg/mL) was used as the standard. TAA is expressed as equivalent of ascorbic acid.
2.5.2. Reducing power
The method described by Chu et al [17] was applied in this work to determine the reducing power of freeze-dried extract. This reducing power was investigated by observing the transformation of Fe3+ to Fe2+. The extract was diluted with distilled water (60–220 μg/mL). The diluted extract (0.5 mL) was mixed with phosphate buffer (2.5 mL, pH 6.6) and potassium ferricyanide (2.5 mL, 1% w/w) in a test tube, followed by incubating in a water bath at 50°C for 30 minutes. After the tube was removed from the water bath, trichloroacetic acid (2.5 mL, 10% w/v) was added into the tube and centrifuged (13,000g, 10 minutes). The supernatant (2.5 mL) was diluted with distilled water (2.5 mL), and freshly prepared ferric chloride (0.5 mL, 0.1% w/w) was added. The mixture was mixed thoroughly and its absorbance was measured at 700 nm using a UV–VIS spectrophotometer.
2.5.3. DPPH radical scavenging activity
The antioxidant activity of the extract was measured with the DPPH method [18] with slight modifications. A solution of DPPH was freshly prepared by dissolving 6 mg DPPH in 50 mL methanol (about 0.3 mM). The extract (2.5 mL) with varying concentrations (60–220 μg/mL) and DPPH solution (2.5 mL) was mixed together in a test tube. The test tube was then incubated in the dark for 20 minutes at room temperature. The decrease in absorbance was measured at 517 nm using a UV–VIS spectrophotometer. The percentage inhibition of radicals was calculated using the following formula:
where Acontrol is the absorbance of DPPH solution without extract and Asample is the absorbance of sample with DPPH solution. The half-maximal inhibitory concentration (IC50) was reported as the amount of antioxidant required to decrease the initial DPPH concentration by 50%. All tests were performed at least in triplicate, and graphs were plotted using the average of three determinations.
2.6. Statistical analyses
All analyses were done at least in triplicate, and these values were then presented as average values along with their standard derivations. Data were analyzed using the Minitab software. Statistical comparisons were performed with one-way analysis of variance, and p values < 0.05 were regarded as significant. The correlation coefficients (R) between TPC and TFC were calculated to determine their relationship.
3. Results and discussion
3.1. Effects of solvent on extraction yield and polyphenol content
3.1.1. Extraction yield
There are many steps to obtain phytochemicals from plant such as milling, grinding, homogenization, and extraction. Among these steps, extraction is the main step for recovering and isolating phytochemicals from plant materials. Extraction efficiency is affected by the chemical nature of phytochemicals, the extraction method used, sample particle size, the solvent used, as well as the presence of interfering substances [19]. The yield of extraction depends on the solvent with varying polarity, pH, temperature, extraction time, and composition of the sample. Under the same extraction time and temperature, solvent and composition of sample are known as the most important parameters. In this work, L. aromatica extracts were obtained by using water and different concentrations of aqueous methanol, ethanol, and acetone (50%, 75%, and 100%). Extraction yields ranged from 12.33% for acetone extract to 33.67% for 75% aqueous acetone extract (Table 1). The yields of extraction by various solvents decreased in the following order: 50% aqueous acetone > 50% aqueous ethanol > 75% aqueous methanol > 50% aqueous methanol > 75 % aqueous acetone > 75% aqueous ethanol > 100% methanol > RO water > 100% ethanol > 100% acetone. It can be seen that the extraction yield of pure methanol (26.06%) is higher than that of pure ethanol (17.03%) and pure acetone (12.33%). This shows that the extraction yield increases with increasing polarity of the solvent used in extraction. It can also be found that the yield of the water extract (25.58%) is only slightly less than that of the pure methanol extract (26.06%), whereas the yield of aqueous solvent extract (from 26.08 % for 75% aqueous ethanol to 33.67 % for 50% aqueous acetone) is higher than that of the pure solvent extracts (from 12.33% for acetone to 26.06% for methanol). These results indicate that increasing the water concentration in the solvent enhances extraction yield. Compounds other than phenolics may have been extracted and contribute to higher yield. This may be attributable to the higher solubility of proteins and carbohydrates in water and methanol than in ethanol and acetone [20]. The combined use of water and organic solvent may facilitate the extraction of chemicals that are soluble in water and/or organic solvent. This may be the reason why yields of aqueous methanol, ethanol, and acetone extracts are higher than yields of water, methanol, ethanol, and acetone extracts. The results of this study are in agreement with the extraction yields of rice bran [21] and some medicinal plants [22].
Table 1.
Extraction yield, TPC, TFC of freeze-dried L. aromatica extracts.
| Solvent system a | Extraction yield (%)b | TPC (mg GAE/g) | TFC (mg QCE/g) |
|---|---|---|---|
| Water | 25.58 ± 1.04 | 6.25 ± 0.24 | 4.04 ± 0.08 |
| Methanol | |||
| 100% (M) | 26.06 ± 1.64 | 31.50 ± 1.60 | 15.42 ± 0.40 |
| 75% aqueous methanol (M3) | 32.92 ± 1.32 | 35.70 ± 1.95 | 22.51 ± 0.97 |
| 50% aqueous methanol (M1) | 29.69 ± 1.07 | 20.20 ± 0.38 | 11.11 ± 0.56 |
| Ethanol | |||
| 100% (E) | 17.03 ± 2.66 | 40.50 ± 0.88 | 31.11 ± 0.43 |
| 75% aqueous ethanol (E3) | 26.08 ± 1.35 | 30.60 ± 1.36 | 19.47 ± 0.35 |
| 50% aqueous ethanol (E1) | 32.94 ± 1.86 | 30.30 ± 0.54 | 17.19 ± 0.15 |
| Acetone | |||
| 100% (A) | 12.33 ± 1.53 | 40.30 ± 0.20 | 30.86 ± 0.14 |
| 75% aqueous acetone (A3) | 27.14 ± 1.58 | 39.10 ± 0.87 | 29.34 ± 0.64 |
| 50% aqueous acetone (A1) | 33.67 ± 1.61 | 29.60 ± 0.15 | 19.22 ± 0.68 |
GAE = gallic acid equivalent; QCE = quercetin; TFC = total flavonoid content; TPC = total phenol content.
Expressed as fraction of solvents to water.
Expressed as 100 × (g dry extract/g dry leaves).
3.1.2. TPC
Table 1 shows the TPC of the extracts measured using the FC method. TPC values were obtained from the calibration curve y = 50x + 7.75 with R2 = 0.9894, where x is the absorbance and y is the concentration of gallic acid solution (μg/mL) expressed as mg GAE/g DFLA.
The TPC values of the extracts range from 6.25 mg GAE/g DFLA for water extract to 40.5 mg GAE/g DFLA for 100% ethanol extract (Table 1) and they decrease in the following order: 100% ethanol > 100% acetone > 75% aqueous acetone > 75% methanol > 100% methanol > 75% ethanol > 50% ethanol > 50% acetone > 50% methanol > water. The TPC of the 100% ethanol extract is not significantly higher than that of the 100% acetone extract, whereas the TPC of the water extract is significantly less than that of other solvents (p < 0.01). It was also found that the TPC of the extracts decreased with increasing water content in the aqueous solvent except for the methanol system. The TPC of the 75% aqueous methanol extract (35.7 mg GAE/g DFLA) is higher than that of the 100% methanol extract (31.5 mg GAE/g DFLA) and the 50% aqueous extract (20.20 mg GAE/g DFLA). This may be attributable to the content of more nonphenol compounds such as carbohydrate and terpene in water extracts than in other extracts. It may also be caused by the possible complex formation of some phenolic compounds in the extract that are soluble in methanol, acetone, and ethanol. These phenolic compounds may possess more phenol groups or have higher molecular weights than the phenolics in the water extract. Based on the results of TPC, the best extracting solvent was ethanol.
3.1.3. TFC
The TFC of the extracts are reported in Table 1. The TFCs can be grouped into four levels. The first and the highest level (29.34–31.11 mg QCE/g DFLA) with the highest value belongs to the 100% ethanol extract (31.11 mg QCE/g DFLA), followed by the 100% acetone extract (30.86 mg QCE/g DFLA) and the 75% aqueous acetone extract (29.36 mg QCE/g DFLA). The second level (19.22–22.51 mg QCE/g DFLA) includes the 75% aqueous methanol extract (22.51 mg QCE/g DFLA), the 75% aqueous ethanol extract (19.47 mg QCE/g DFLA), and the 50% aqueous acetone extract (19.22 mg QCE/g DFLA). The third level (11.11–17.19 mg QCE/g DFLA) includes the 50% aqueous ethanol (17.19 mg QCE/g DFLA), the 100% methanol extract (15.42 mg QCE/g DFLA), and the 50% aqueous extract (11.11 mg QCE/g DFLA). The final level with the lowest TFC, is the water extract (4.04 mg QCE/g DFLA). It was observed that the effect of solvents on TFC is similar to that on TPC. The highest TFC was obtained in the 100% ethanol extract, followed by the 100% acetone extract, the 100% methanol extract, and the water extract. A similar trend was observed in the amount of TPC. As the concentration of water in ethanol or acetone increases, the TFC in the extract decreases. By contrast, the TFC in the 75% aqueous methanol extract is higher than that in the 100% methanol extract and the 50% aqueous methanol extract.
A correlation analysis was performed on polyphenolic contents (TPC and TFC) of L. aromatica extracts. The correlation between TPC and TFC assay was found to be 0.923. This indicates that flavonoids are the dominating phenolic group in L. aromatica. The result is similar to the extraction of phenolics from guava and pisang mas, a variety of banana [23]. Some flavonoids that were isolated from L. aromatica have been identified by Khrisnan et al [11] and Bui et al [12].
3.2. Solvent effects on antioxidant activities
3.2.1. TAA
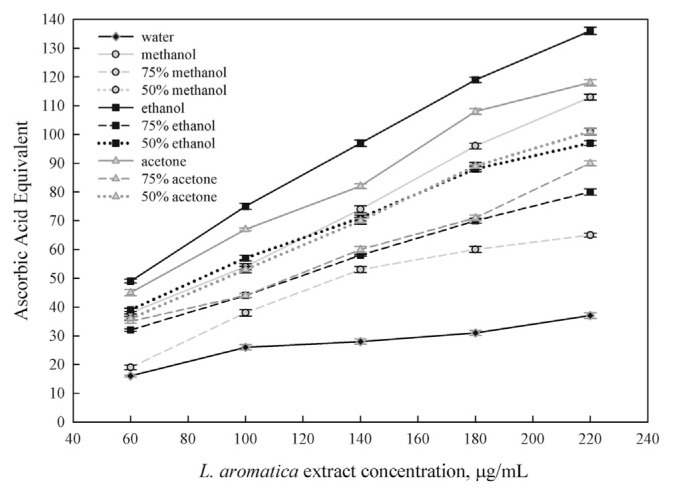
The TAA of the extract was calculated based on the formation of the phosphomolybdenum complex. This activity was measured spectrophotometrically at 695 nm [15] and expressed as equivalents of ascorbic acid. Fig. 1 shows the TAA of extracts in a concentration-dependent manner. The extract of 100% ethanol gave the highest TAA, followed by the extracts of 100% acetone, 100% methanol, 75% aqueous acetone, 75% aqueous methanol, 50% aqueous acetone, 50% aqueous ethanol, and 50% aqueous methanol. The TAA of the water extract is the lowest and is significantly different from that of the others (p < 0.05). The TAA of the 100% ethanol extract is higher than that of the 100% acetone extract. However, both extracts show significantly higher TAA than that of the other extracts (p < 0.05). By contrast, TAAs of the other extracts do not show significant difference from each other.
Fig. 1.
Total antioxidant activity of Limnophila aromatica extracts in different solvents.
3.2.2. Reducing power
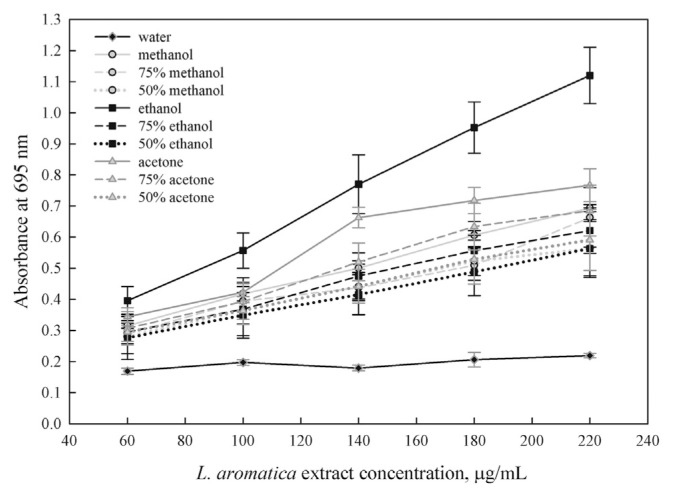
The reducing power of the extract, which may serve as a reflection of its antioxidant activity, was determined using a modified Fe3+ to Fe2+ reduction assay, whereby the yellow color of the test solution changes to various shades of green and blue, depending on the reducing power of the sample. The presence of antioxidants in the sample causes the reduction of Fe3+/ferricyanide complex to the Fe2+ form, which is monitored by measuring the formation of Perl’s Prussian blue at 700 nm [24]. In Fig. 2, all extracts show some degrees of electron-donating capacity in a concentration-dependent manner. The 100% ethanol extract once again gave the highest reducing power and is significantly higher (p < 0.05) than that of the other extracts at all concentrations studied, followed by that of the 100% acetone extract, the 100% methanol extract, and the extracts obtained by different concentrations of aqueous acetone, aqueous ethanol, and aqueous methanol. The reducing power of the 100% acetone extract at concentrations ranging from 60 μg/mL to 100 μg/mL is insignificantly higher than that of the 100% methanol extract and the extracts of aqueous ethanol, aqueous methanol, and aqueous acetone. However, at concentrations higher than 100 μg/mL, the reducing power of the 100% acetone extract is significantly higher (p < 0.05) than that of the extracts of 100% methanol, aqueous ethanol, aqueous acetone, and aqueous methanol. The lowest reducing power was found in the water extract. Its value is also significantly lower than that of the other extracts at all concentrations studied.
Fig. 2.
Reducing power of Limnophila aromatica extracts in different solvents.
3.2.3. DPPH radical scavenging activity
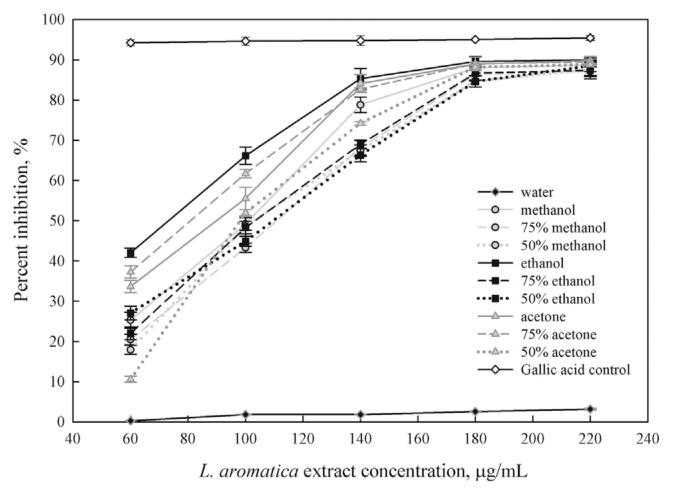
DPPH radical is a stable organic free radical with an absorption band at 517 nm. It loses this absorption when accepting an electron or a free radical species, which results in a visually noticeable discoloration from purple to yellow. It can accommodate many samples in a short period and is sensitive enough to detect active ingredients at low concentrations [25]. Fig. 3 shows the DPPH scavenging activities of the extracts in a concentration-dependent manner. The extract obtained by 100% ethanol yielded the highest DPPH radical scavenging activity at concentrations ranging from 60 μg/mL to 180 μg/mL. However, at concentrations ranging from 180 μg/mL to 220 μg/mL, its DPPH radical scavenging activity is not significantly different from those of the other extracts. All extracts obtained by using a pure and aqueous organic solvent gave stronger radical scavenging capacity than that of the water extract. A similar trend was observed in the study of DPPH radical scavenging activity of pineapple crude extract [23] and defatted wheat germ [26].
Fig. 3.
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity of Limnophila aromatica extracts in different solvents.
3.2.4. IC50
The IC50 of a compound is inversely related to its antioxidant capacity, as it expresses the amount of antioxidant required to decrease the DPPH concentration by 50%, which is obtained by interpolation from a linear regression analysis [27]. A lower IC50 indicates a higher antioxidant activity of a compound. Table 2 shows the IC50 values in the DPPH radical scavenging activity assay of the extracts. It was found that the 100% ethanol extract possesses the strongest DPPH radical activity (IC50 = 70.06 μg/mL).
Table 2.
IC50 in DPPH radical scavenging activity.
| Extracts | IC50 (μg/mL) |
|---|---|
| Water | 679.67 ± 1.4i |
| Methanol | |
| 100% (M) | 95.68 ± 0.8e |
| 75% aqueous methanol (M3) | 80.98 ± 2.5c |
| 50% aqueous methanol (M1) | 137.85 ± 0.4h |
| Ethanol | |
| 100% (E) | 70.06 ± 1.0a |
| 75% aqueous ethanol (E3) | 106.18 ± 1.2f |
| 50% aqueous ethanol (E1) | 109.97 ± 1.0fg |
| Acetone | |
| 100% (A) | 88.06 ± 1.3d |
| 75% aqueous acetone (A3) | 79.98 ± 1.6b |
| 50% aqueous acetone (A1) | 110.36 ± 0.24g |
| Gallic acid1† | 2.6 |
| Ascorbic acid† | 8.4 |
Different superscripted letters (a–i) indicate significant difference from one another (p > 0.05).
DPPH = 2,2-diphenyl-1-picrylhydrazyl; IC50 = half-maximal inhibitory concentration.
The IC50 values of gallic acid and ascorbic acid as controls were taken from “Antioxidant activity and total phenolic content of some Brazilian species”, by Brighente IMC, Dias M, Verdi LG, et al. 2007, Pharm Biol 45, p. 156–61. Copyright 2007, Informa Healthcare. Reprinted with permission.
Phenolics were the main antioxidant components, and their total contents were directly proportional to their antioxidant activity [27]. In this study, there is a correlation between total phenolic content and antioxidant activity of the freeze-dried L. aromatica extracts. With increasing water content in the solvent, yield increased whereas total phenolic content and antioxidant activity decreased. This result is different from the results of previous studies on black tea and mate tea [5] and bunga kantan inflorescence [28]. Almost all of their results showed higher TPC as well as TAA when the organic solvent system contains water. In this study, it was also observed that the TPC, TFC, and antioxidant activity of pure acetone and pure ethanol extracts are higher than those of pure methanol, water, and the other solvents. The differences between the results of this study and those of other studies may be attributed to several factors: (1) the difference in plant matrix; (2) different solvents used in extraction resulted in differences in compositions and antioxidant activities of the extracts [29]; (3) an extract possessing a phenolic compound that contains a higher number of hydroxyl groups has a higher antioxidant activity [30]; (4) the method and conditions of extraction (temperature and time) also affected antioxidant activities [31].
4. Conclusion
The antioxidant activity of L. aromatica was evaluated using the TAA assay, DPPH radical scavenging assay, and reducing power assay. In general, extraction yield increased with increasing water content in ethanol, acetone, and methanol system. This may be caused by the combination of organic solvent and water that facilitates the extraction of all compounds that were soluble in both water and organic solvents. In contrast to extraction yield, the TPC, TFC, and antioxidant activity decreased with increasing water content in organic solvents. The water extract may either contain more non-phenolic compounds or possess phenolic compounds that contain a smaller number of active groups than the other solvents. The TPC of extracts were consistent with the TFC and antioxidant activity of extracts. By considering both yield and antioxidant activity, extraction by using 75% acetone provided significantly better results (extraction yield 27.14 ± 1.58%, TPC 39.10 ± 0.87 mg GAE/g DFLA, TFC 29.34 ± 0.64 mg QCE/g DFLA, IC50 79.98 ± 1.8 μg/mL) than those of the other solvent systems. But despite its extraction yield, extract of L. aromatica in 100% ethanol fraction showed the highest amounts of TPC (40.50 ± 0.88 mg GAE/g DFLA) and TFC (31.11 ± 0.43 mg QCE/g DFLA) and the lowest value of IC50 (70.06 ± 1.0 μg/mL). It is clear that 100% ethanol extract gave the highest antioxidant capacity in all in vitro assays studied. The results of this work indicated that L. aromatica, when a proper extraction solvent is established, could serve as a medicine against free-radical-associated oxidative damage.
Acknowledgments
This work was supported by a grant from the National Taiwan University of Science and Technology (101H451403).
Funding Statement
This work was supported by a grant from the National Taiwan University of Science and Technology (101H451403).
REFERENCES
- 1. Pérez-Jiménez J, Arranz S, Tabernero M, et al. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int. 2008;41:274–85. [Google Scholar]
- 2. Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 3.Harborne JB. Classes and functions of secondary products from plants. In: Walton JN, Brown DE, editors. Chemicals from plants—perspectives on plant secondary products. London, UK: Imperial College Press; 1999. pp. 1–25. [Google Scholar]
- 4. King A, Young G. Characteristics and occurrence of phenolic phytochemicals. J Am Diet Assoc. 1999;99:213–8. doi: 10.1016/S0002-8223(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 5. Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006;99:835–41. [Google Scholar]
- 6. Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–52. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonoli M, Verardo V, Marconi E, et al. Antioxidant phenols in barley (Hordeum vulgare L.) flour: comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J Agric Food Chem. 2004;52:5195–200. doi: 10.1021/jf040075c. [DOI] [PubMed] [Google Scholar]
- 8. Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Res Int. 2001;34:223–7. [Google Scholar]
- 9.Do TL. Vietnamese medicinal plants. Hanoi, Vietnam: Medicine Publishing House; 1999. p. 268. [Google Scholar]
- 10. Venkata Rao J, Shrinivasa Aithal K, Srinivasan KK. Antimicrobial activity of the essential oil of Limnophila gratissima. Fitoterapia. 1989;60:376–7. [Google Scholar]
- 11. Krishnan S, Nair A, Ramachandran G. Uncommon 8-oxygenated flavonoids from Limnophila aromatica (Scrophulariaceae) Indian J Chem. 1999;38B:1009. [Google Scholar]
- 12. Bui ML, Grayer RJ, Veitch NC, et al. Uncommon 8-oxygenated flavonoids from Limnophila aromatica (Scrophulariaceae) Biochem Syst Ecol. 2004;32:943–7. [Google Scholar]
- 13. McDonald S, Prenzler PD, Antolovich M, et al. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. [Google Scholar]
- 14. Chang CC, Yang MH, Wen HM, et al. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 15. Govindarajan R, Rastogi S, Vijayakumar M, et al. Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull. 2003;26:1424–7. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- 16. Subhasree B, Baskar R, Laxmi Keerthana R, et al. Evaluation of antioxidant potential in selected green leafy vegetables. Food Chem. 2009;115:1213–20. [Google Scholar]
- 17. Chu YH, Chang CL, Hsu HF. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric. 2000;80:561–6. [Google Scholar]
- 18. Shimada K, Fujikawa K, Yahara K, et al. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 19. Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30:3268–95. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 20. Zieliński H, Kozłowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–16. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]
- 21. Chatha SAS, Anwar F, Manzoor M, et al. Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. Grasas Aceites. 2006;57:328–35. [Google Scholar]
- 22. Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–8. [Google Scholar]
- 24. Yang QM, Pan X, Kong W, et al. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010;118:84–9. [Google Scholar]
- 25. Hseu YC, Chang WH, Chen CS, et al. Antioxidant activities of toona sinensis leaves extracts using different antioxidant models. Food Chem Toxicol. 2008;46:105–14. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 26. Zhu KX, Lian CX, Guo XN, et al. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011;126:1122–6. [Google Scholar]
- 27. Liu SC, Lin JT, Wang CK, et al. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis sonn.) flowers. Food Chem. 2009;114:577–81. [Google Scholar]
- 28. Wijekoon MMJO, Bhat R, Karim AA. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J Food Compos Anal. 2011;24:615–9. [Google Scholar]
- 29. Pinelo M, Manzocco L, Nuñez MJ, et al. Interaction among phenols in food fortification: negative synergism on antioxidant capacity. J Agric Food Chem. 2004;52:1177–80. doi: 10.1021/jf0350515. [DOI] [PubMed] [Google Scholar]
- 30. Arabshahi-D S, Vishalakshi Devi D, Urooj A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 2007;100:1100–5. [Google Scholar]
- 31. Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J Chromatogr A. 2003;1000:657–91. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]