Abstract
Background
Venous leg ulcers are a chronic health problem that cause considerable economic impact and affect quality of life for those who have them. Primary wound contact dressings are usually applied to ulcers beneath compression therapy to aid healing, promote comfort and control exudate. There are numerous dressing products available for venous leg ulcers and hydrogel is often prescribed for this condition; however, the evidence base to guide dressing choice is sparse.
Objectives
To assess the effects of hydrogel wound dressings on the healing of venous leg ulcers in any care setting.
Search methods
In May 2021, we searched the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies, reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs), either published or unpublished, that compared the effects of hydrogel dressing with other dressings on the healing of venous leg ulcers. We excluded trials evaluating hydrogel dressings impregnated with antimicrobial, antiseptic or analgesic agents as these interventions are evaluated in other Cochrane Reviews.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included four RCTs (10 articles) in a qualitative analysis. Overall, 272 participants were randomised, in sample sizes ranging from 20 to 156 participants. The mean age of the included population in the trials ranged from 55 to 68 years, 37% were women based on studies that reported the sex of participants. The studies compared hydrogel dressings with the following: gauze and saline, alginate dressing, manuka honey and hydrocolloid. Two studies were multicentre and the others were single‐centre trials. Length of treatment using hydrogel dressing was four weeks in three studies and two weeks in one study. The follow‐up period was the same as the duration of treatment in three studies and in one study the follow‐up for wound healing was at 12 weeks after four weeks of treatment. Overall risk of bias was high for all trials because at least one of the three key criteria (selection bias, detection bias and attrition bias) was at high risk.
Hydrogel compared with gauze and saline
It is uncertain whether there is a difference in complete wound healing (risk ratio (RR) 5.33, 95% confidence interval (CI) 1.73 to 16.42; 1 trial, 60 participants) or change in ulcer size (mean difference (MD) –1.50, 95% CI –1.86 to –1.14; 1 trial, 60 participants) between interventions because the certainty of the evidence is very low. Data reported from one trial were incomplete for time‐to‐ulcer healing.
Hydrogel compared with alginate dressing
It is uncertain whether there is a difference in change in ulcer size between hydrogel and alginate gel because the certainty of the evidence is very low (MD –41.80, 95% CI –63.95 to –19.65; 1 trial, 20 participants).
Hydrogel compared with manuka honey
It is uncertain whether there is a difference in complete wound healing (RR 0.75, 95% CI 0.46 to 1.21; 1 trial, 108 participants) or incidence of wound infection (RR 2.00, 95% CI 0.81 to 4.94; 1 trial, 108 participants) between interventions because the certainty of the evidence is very low.
Hydrogel compared with hydrocolloid
One study (84 participants) reported on change in ulcer size between hydrogel and hydrocolloid; however, further analysis was not possible because authors did not report standard errors or any other measurement of variance of a set of data from the means. Therefore, it is also uncertain whether there is a difference in change in ulcer size between hydrogel and hydrocolloid because the certainty of the evidence is very low.
No studies provided evidence for the outcomes: recurrence of ulcer, health‐related quality of life, pain and costs.
Overall, independent of the comparison, the certainty of evidence is very low and downgraded twice due to risk of bias and once or twice due to imprecision for all comparisons and outcomes.
Authors' conclusions
There is inconclusive evidence to determine the effectiveness of hydrogel dressings compared with gauze and saline, alginate dressing, manuka honey or hydrocolloid on venous leg ulcer healing. Practitioners may, therefore, consider other characteristics such as costs and symptom management when choosing between dressings. Any future studies assessing the effects of hydrogel on venous wound healing should consider using all the steps from CONSORT, and consider key points such as appropriate sample size with the power to detect expected differences, appropriate outcomes (such as time‐to‐event analysis) and adverse effects. If time‐to‐event analysis is not used, at least a longer follow‐up (e.g. 12 weeks and above) should be adopted. Future studies should also address important outcomes that the studies we included did not investigate, such as health‐related quality of life, pain and wound recurrence.
Keywords: Aged, Female, Humans, Male, Middle Aged, Alginates, Alginates/therapeutic use, Bandages, Hydrogels, Hydrogels/therapeutic use, Pain, Pain/drug therapy, Randomized Controlled Trials as Topic, Ulcer, Ulcer/drug therapy, Varicose Ulcer, Varicose Ulcer/therapy
Plain language summary
Hydrogel dressings for venous leg ulcers
Key messages
We cannot be certain whether hydrogel dressings are any more effective for healing of venous leg ulcers than other types of dressing such as gauze and saline, alginate, manuka honey or hydrocolloid. There was not enough information to be sure how hydrogel dressings compare with other dressings in terms of potential side effects.
What are venous leg ulcers?
Venous leg ulcers are wounds or sores on the leg caused by alterations in the circulation of blood in the veins. They are hard‐to‐heal wounds. Venous leg ulcers may cause pain, itching and swelling. There may be changes to the skin around the ulcer, and it may also produce fluids. The standard treatment for this type of wound is compression therapy (bandages or stockings) to improve blood flow in the legs. Dressings are applied underneath compression bandages to protect the wound and aid healing. Different types of dressings vary in their ability to: maintain a moist environment; absorb excess fluid from the wound; soften dead tissue; cushion the wound; keep the wound clean and free of germs and keep newly healed skin intact. Hydrogel dressings are filled with a watery gel and can be used to keep the wound moist; they are intended to help remove dead tissue and help healthy skin to grow.
What did we want to find out?
We wanted to find out if hydrogel dressings compared to other dressings:
– heal venous leg ulcers;
– have any unwanted effects;
– have any effect on changes in ulcer size, time‐to‐ulcer healing or recurrence of ulcers;
– improve people's quality of life;
– reduce pain;
– impact the costs of treatment.
What did we do?
We searched the medical literature and collected and analysed all relevant randomised controlled trials (clinical studies where the treatment people receive is chosen at random) to answer this question. This type of trial provides the most reliable health evidence. There were no restrictions on publication language, settings where treatments were used, or sex or age of the participants, as long as they had venous leg ulcers. We excluded trials evaluating hydrogel dressings impregnated with antimicrobial (which reduce the presence of bacteria), antiseptic (which stop or slow down the growth of germs) or analgesic (painkiller) agents as these interventions are evaluated in other Cochrane Reviews.
What did we find?
We found four studies dating from 1994 to 2008, involving 272 participants with an average age ranging from 55 to 68 years. Two studies provided no information on participants' sex and the other two included 29 women and 51 men. The studies investigated the use of hydrogel dressings for either two or four weeks. Hydrogel dressings were compared with gauze and saline (salt water), alginate, manuka honey or hydrocolloid.
– It is uncertain whether there is a difference in complete wound healing when hydrogel is compared with gauze and saline or manuka honey.
– It is uncertain if the incidence of wound infection is different between hydrogel dressings and manuka honey or whether there is difference between hydrogel and gauze and saline, alginate or hydrocolloid dressings in terms of change in ulcer size.
– None of the studies reported useable results for time‐to‐ulcer healing, recurrence of ulcer, health‐related quality of life, pain and costs, so we cannot establish the impact of hydrogel on these outcomes.
What limited our confidence in the evidence?
Most studies were small (only one with more than 100 participants) and all used methods likely to introduce errors in their results. The duration of follow‐up was short (ranging from two to 12 weeks) and studies were not designed to assess time to complete healing.
How up to date is the review?
We searched for studies published up to 10 May 2021.
Summary of findings
Summary of findings 1. Hydrogel dressings compared with gauze and saline for venous leg ulcers healing.
| Hydrogel dressings compared to gauze and saline for venous leg ulcers healing | ||||||
| Patient or population: people with venous leg ulcers Setting: not reported Intervention: hydrogel dressings Comparison: gauze and saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gauze and saline | Risk with hydrogel dressings | |||||
| Complete wound healing | Study population | RR 5.33 (1.73 to 16.42) | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in complete ulcer healing between hydrogel and gauze and saline. | |
| 100 per 1000 | 533 per 1000 (173 to 1000) | |||||
| Incidence of wound infection | Not reported. | |||||
| Changes in ulcer size (cm²) | The mean difference form follow‐up to baseline was –0.8 cm² | The mean difference form follow‐up to baseline was –2.3 cm² | MD 1.50 lower (1.86 lower to 1.14 lower) | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in change in ulcer size between hydrogel and gauze and saline. |
| Time‐to‐ulcer healing | Not reported. | |||||
| Health‐related quality of life | Not reported. | |||||
| Costs | Not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice due to high risk of bias for attrition, incomplete outcome data and other bias, and unclear risk for the remaining bias assessments. bDowngraded once for imprecision due to small sample size.
Summary of findings 2. Hydrogel dressings compared with alginate gel for venous leg ulcers healing.
| Hydrogel dressings compared to alginate gel for venous leg ulcers healing | ||||||
| Patient or population: people with venous leg ulcers Setting: hospital Intervention: hydrogel dressings Comparison: alginate gel | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with alginate gel | Risk with hydrogel dressings | |||||
| Complete wound healing | Not reported. | |||||
| Incidence of wound infection | Not reported. | |||||
| Change in ulcer size: percent reduction in ulcer area at 4 weeks | The mean percentage reduction in ulcer area at 4 weeks was 61.2% | The mean percentage reduction in ulcer area at 4 weeks was 19.4% | MD 41.80% lower (63.95 lower to 19.65 lower) | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in percentage of ulcer reduction comparing hydrogel and alginate gel. |
| Time‐to‐ulcer healing | Not reported. | |||||
| Health‐related quality of life | Not reported. | |||||
| Costs | Not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for risk of bias due to high risk for blinding of participants and personnel and blinding of outcome assessment, and unclear risk for randomisation and allocation. bDowngraded once for imprecision due to small sample size.
Summary of findings 3. Hydrogel dressings compared with manuka honey for venous leg ulcers healing.
| Hydrogel dressings compared with manuka honey for venous leg ulcer healing | ||||||
| Patient or population: people with venous leg ulcers Setting: hospital and community leg ulcer clinics Intervention: hydrogel dressings Comparison: manuka honey | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with manuka honey | Risk with hydrogel dressings compared with manuka honey | |||||
| Complete wound healing | Study population | RR 0.75 (0.46 to 1.21) | 108 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in complete ulcer healing between hydrogel and manuka honey. | |
| 444 per 1000 | 333 per 1000 (204 to 538) | |||||
| Moderate | ||||||
| 333 per 1000 | 250 per 1000 (153 to 403) | |||||
| Incidence of wound infection | Study population | RR 2.00 (0.81 to 4.94) | 108 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in wound infection between hydrogel and manuka honey. | |
| 111 per 1000 | 222 per 1000 (90 to 549) | |||||
| Change in ulcer size | Not reported. | |||||
| Time‐to‐ulcer healing | Not reported. | |||||
| Health‐related quality of life | Not reported. | |||||
| Costs | Not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice due to high risk of bias for blinding of participants and personnel, blinding of outcome assessment and unclear risk for other bias. bDowngraded twice for imprecision as the optimal information size was not met and confidence intervals were wide.
Summary of findings 4. Hydrogel dressing compared with hydrocolloid for venous ulcer healing.
| Hydrogel dressing compared to hydrocolloid for venous ulcer healing | ||||||
| Patient or population: people with venous leg ulcers Setting: not reported Intervention: hydrogel dressing Comparison: hydrocolloid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hydrocolloid | Risk with hydrogel | |||||
| Complete ulcer healing | Not reported. | |||||
| Incidence of wound infection | Not reported. | |||||
| Change in ulcer size | The authors reported percent change in ulcer size after 4 weeks; however, further analysis was not possible because authors did not report standard error or standard deviation for the means. | — | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | It is uncertain if there is any difference in change in ulcer size comparing hydrogel and hydrocolloid. | |
| Time‐to‐ulcer healing | Not reported. | |||||
| Health‐related quality of life | Not reported. | |||||
| Costs | Not reported. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded twice for risk of bias due to high risk of attrition bias and other bias. bDowngraded twice due to small numbers of participants and methodological issues reporting confidence intervals.
Background
A glossary of medical terms is available in Appendix 1.
Description of the condition
Venous leg ulcers present as open wounds or sores on the lower limb. The ulcers are generally irregular and shallow. They are associated with sustained venous hypertension and microcirculatory alterations resulting from chronic venous insufficiency (Grey 2006; Wollina 2006). Venous leg ulceration is a chronic health problem that can take years to heal completely, and venous leg ulcers have a high rate of recurrence and often require life‐long treatment (Margolis 2002; van Hecke 2011). Wound size and wound duration (longer than 18 months) are major contributors to a risk of not healing (Margolis 2004). Additionally, the presence of lipodermatosclerosis, evidence of deep vein thrombosis, superficial thrombophlebitis or poor ankle mobility are individually associated with delayed healing (Lantis 2013). This condition has psychological and financial impact and also impairs the physical functioning of affected people (Platsidacki 2017). The major problems reported by patients are: pain, immobility (difficult moving about), sleep disturbance, lack of energy, limitations in work and leisure activities, odour, drainage, worry, frustration and lack of self‐esteem (Herber 2007; Persoon 2004; Valencia 2001). Thus, venous leg ulcers will ultimately impact quality of life (Platsidacki 2017).
The estimated prevalence of venous leg ulcers ranges between 0.6% and 1.9% in the adult population of the UK, USA and Europe (Briggs 2003), and is most common with increasing age (De Araujo 2003; Wipke‐Tevis 2000), especially in people who are 65 years old and older (Margolis 2002). Venous leg ulcers account for 70% to 80% of all ulcers of the lower limbs in the USA (De Araujo 2003). One retrospective time‐series study in Barcelona, Spain found a trend towards an increase in the incidence of venous leg ulcers from 0.5 new cases per 1000 people/year in 2010 to 1 new case for every 1000 people/year in 2014 (Pérez 2019). As venous leg ulcers are hard‐to‐heal wounds, treating them requires significant healthcare resources. Data from Germany revealed that the mean total cost per year for a person with chronic venous leg ulceration was EUR 9569, of which 92% was estimated to be direct costs (non‐drug treatment, inpatients costs and outpatient care) and 8% indirect costs (inability to work) (Purwins 2010).
The diagnosis of venous ulceration is usually based on clinical examination. Additional tests such as colour duplex ultrasonography (measurement of blood flow in the veins and arteries of the leg), plethysmography (measures variations in the size or volume of a limb), venography (x‐ray test that provides an image of the leg veins) and ankle brachial pressure index (ABPI, provides the ratio of systolic blood pressure at the ankle to that in the arm) (Medical Dictionary; Merriam‐Webster Medical Dictionary) may be helpful if the diagnosis is unclear (Collins 2010; Robson 2006). An ABPI equal or lower than 0.9 is the diagnosis criterion for peripheral arterial disease (Rooke 2011); however, ABPI measurement greater than 0.8 is generally used to exclude peripheral arterial disease as the cause of a leg ulceration, leaving the most likely diagnosis venous ulceration (RCN 2006).
Description of the intervention
Standard treatment for venous leg ulcers should include therapeutic compression (which may be applied by bandages or stockings) in addition to a dressing, except when otherwise indicated (O'Meara 2009; Robson 2006). There is a broad choice of dressings available to treat wounds such as venous leg ulcers. For ease of comparison this review has classed dressings into groups according to the broad categories of the Nurse Prescribers' Formulary 2011, that is, basic, advanced, antimicrobial and specialist wound dressings (BNF 2018; see Appendix 2). Dressing names, manufacturers and distributors may vary between countries. Dressings are applied underneath bandages or stockings with the aim of protecting the wound and providing a moist environment to aid healing. Nowadays, several types of dressing seek to achieve a moist environment, the aim of which is to promote re‐epithelialisation of the wound, provide comfort, control exudate, and help to prevent bandages and stockings adhering to the wound bed. The ideal conditions required for wound healing in terms of dressing application have been explained as follows: maintenance of a moist wound environment without risk of maceration; avoidance of toxic chemicals, particles or fibres in the dressing fabric; minimisation of number of dressing changes and therefore reduced episodes of pain during changes and also reduced costs; and maintenance of an optimum pH level for healing (BNF 2018).
The primary intervention of interest in this review is hydrogel dressings used in the treatment of venous leg ulcers. The dressings consist of a starch polymer and up to 96% water. They are supplied in two forms; flat sheets (e.g. ActiFormCool (Activa Healthcare)), or amorphous hydrogel (e.g. Aquaflo (Covidien)). The interval between dressing changes varies according to the type of hydrogel dressing: amorphous hydrogel may require daily changes, while hydrogel sheet dressings may last for up to seven days (Mandelbaum 2003).
How the intervention might work
The most appropriate dressing for wound management depends not only upon the type of wound but also on the stage of the healing process. Dressings for moist wound healing need to ensure that the wound remains moist, and free of clinical infection and excessive slough (dead tissue), but that peri‐wound maceration is avoided (BNF 2018).
Hydrogel dressings, classified as an advanced wound dressing by the Nurse Prescribers' Formulary, are designed to control the environment for wound healing by donating fluids to dry sloughy wounds, and by facilitating autolytic debridement of necrotic tissue. Some hydrogel dressings may also have the ability to absorb limited amounts of exudate or rehydrate a wound, depending on the wound's moisture levels (BNF 2018). There are types of hydrogel that are associated with alginates (e.g. Nu‐Gel, Purilon Gel) (BNF 2018), which have a higher capacity for absorption and chemical debridement (Mandelbaum 2003).
The advantages of hydrogel dressings are that they can be used during several phases of healing, and may promote relief and comfort, however, they do require a secondary covering. Hydrogel dressings also may reduce pain in painful wounds (Bradbury 2008).
Why it is important to do this review
Chronic venous ulcer healing is a complex clinical situation that causes considerable economic impact, and adversely affects the quality of life for those who have these ulcers.
Hydrogel dressings can be used to deslough wounds by promoting autolytic debridement through moisture to rehydrate, soften and liquefy non‐viable tissue present on the wound surface. This effect may impact ulcer healing. There is no current up‐to‐date evidence to inform clinicians of the effects of hydrogel dressings in treating venous leg ulcers, or to support policymakers in decisions regarding whether to use these dressings (Norman 2018; Palfreyman 2007). The effect of hydrogel dressings compared with other dressings for venous ulcers needs to be established.
Objectives
To assess the effects of hydrogel wound dressings on the healing of venous leg ulcers in any care setting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), either published or unpublished, that evaluated the effects of any type of hydrogel wound dressing in the treatment of venous leg ulcers, irrespective of language of publication. Trials reported in abstract form were only eligible for inclusion if adequate information was either presented in the abstract or available from the trial author. We excluded studies using quasi‐randomisation.
Types of participants
We included RCTs of people of any age, managed in any care setting with a diagnosed venous leg ulcer determined either by clinical evaluation, or complementary laboratory tests (e.g. duplex ultrasonography, plethysmography and venography), or both (Collins 2010), using the definition of a positive diagnosis given by the authors. We included trials that had people with wounds of other aetiology (e.g. pressure ulcers), or trials of mixed populations (venous ulcers along with arterial or diabetic ulcers) that presented the results for the subgroup of people with venous leg ulcers separately, or if the majority of participants (75% or greater in each group) had leg ulcers of venous aetiology. We attempted to contact trial authors to obtain the relevant data, if data from subgroups of people with venous leg ulcers were not reported separately. We excluded studies of people with infected wounds, because hydrogel dressings are not indicated (prescribed) for this type of wound. Trials evaluating skin grafting are covered elsewhere and were excluded from this review (Jones 2007).
Types of interventions
The primary intervention of interest was hydrogel dressings used as a treatment for venous leg ulcers. We included any RCT in which the presence or absence of a hydrogel dressing was the only systematic difference between treatment groups; and in which a hydrogel dressing was compared with other wound dressings, non‐dressing treatments (e.g. topical applications) or another hydrogel dressing. We included RCTs of hydrogel dressings, irrespective of whether compression therapy was reported as a concurrent therapy. For ease of comparison, we categorised dressings according to the Nurse Prescribers' Formulary (BNF 2018). We reported generic names for all products where possible, also providing trade names and manufacturers, where available. However, it is important to note that manufacturers and distributors of dressings may vary from country to country, and dressing names may also differ. We excluded trials evaluating hydrogel dressings impregnated with antimicrobial, antiseptic or analgesic agents as these interventions are evaluated in other Cochrane Reviews (Briggs 2012; O'Meara 2010). We excluded trials that use larval therapy.
Types of outcome measures
Primary outcomes
Complete wound healing measured by the number of ulcers completely healed within the duration of the trial.
Incidence of wound infection, using diagnosis of infection as described in individual trials.
Secondary outcomes
Changes in ulcer size measured by reduction in original wound area within the duration of the trial expressed as absolute (e.g. surface area changes in centimetre squared (cm²) since baseline) or relative (e.g. percentage change in area relative to baseline) changes.
Time‐to‐ulcer healing.
Recurrence of ulcer.
Health‐related quality of life (measured using a standardised generic questionnaire such as EQ‐5D, 36‐item Short Form (SF‐36), 12‐item Short Form (SF‐12) or 6‐item Short Form (SF‐6) or disease‐specific questionnaire). We did not include ad‐hoc measures of quality of life as these were likely to be unvalidated and would not have been common to multiple trials.
Pain (e.g. at dressing change, between dressing changes or over the course of treatment) was included only if measured by reliable and validated instruments such as surveys, questionnaires, data capture process or visual analogue scale).
Costs (including measurements of resource use, such as number of dressing changes, nurse time or health professional time costs, or both, if reported by the authors).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials on 10 May 2021:
the Cochrane Wounds Specialised Register;
the Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 4) in the Cochrane Library;
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (from 1946);
Ovid Embase (from 1974);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; from 1937).
The search strategies can be found in Appendix 3. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2022). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2022). We combined the CINAHL Plus searches with the trial filters developed by Glanville 2019. There were no restrictions with respect to language, date of publication or study setting.
We searched the following clinical trial registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 10 May 2021);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; trialsearch.who.int/; searched 10 May 2021);
ISRCTN registry (www.isrctn.com/; searched 14 Sep 2021).
Search strategies for clinical trial registries can be found in Appendix 3.
Searching other resources
Searching reference lists of included trials and relevant reviews
We aimed to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, as well as relevant systematic reviews, meta‐analyses and health technology assessment reports.
Searching by contacting individuals or organisations
When necessary, we contacted authors of key papers and abstracts to request further information about their trials. We contacted manufacturers to request information about ongoing or unpublished RCTs (for a list of manufacturers, see Appendix 4).
Adverse effects
We did not perform a separate search for adverse effects of interventions used; we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Ribeiro 2013), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Selection of studies
Two review authors (CR and FD) independently assessed the titles and abstracts of the studies identified from the search strategy against the inclusion criteria. We obtained full versions of articles that appeared to fulfil the inclusion criteria for further assessment. Two review authors then independently checked the full papers for eligibility, with all disagreements resolved by discussion with another review author (GF). We recorded all reasons for exclusion.
We presented our study selection process as a PRISMA flow diagram (Liberati 2009).
Data extraction and management
We extracted and summarised details of the eligible RCTs using a standardised data extraction form. Two review authors (CR and FD) performed independent data extraction of all included RCTs after which both data extractions were compared for agreement. We resolved any disagreements by discussion. If data were missing from reports, we contacted study authors to obtain the missing information. Data from studies that were published in duplicate were only included once. According to methods described in the Cochrane Handbook for Systematic Reviews of Interventions, we extracted the following information (Lefebvre 2022; Li 2022):
country of origin;
study authors and year of publication;
care setting;
type of ulcer;
unit of investigation (per participant) – single ulcer or foot or participant, or multiple ulcers on the same participant;
number of participants randomised to each treatment group;
eligibility criteria and key baseline participant data (gender, age, ethnicity, baseline ulcer area, ulcer duration, prevalence of comorbidities such as diabetes);
details of the dressing/treatment regimen received by each group;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions);
outcome data for primary and secondary outcomes (by group);
overall sample size and methods used to estimate statistical power (relates to the target number of participants to be recruited, the clinical difference to be detected and the ability of the trial to detect this difference);
duration of treatment;
duration of follow‐up;
number of withdrawals (by group with reasons);
statistical methods used for data analysis;
risk of bias criteria (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting);
source of funding.
Data analysis was performed according to Cochrane guidelines. One review author entered quantitative data into Review Manager Web (Review Manager Web 2020), another checked it, and the data were then analysed.
Assessment of risk of bias in included studies
Two review authors independently assessed each included RCT using the Cochrane RoB 1 tool. This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding of participants and care providers; blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other issues that may potentially bias the study (Appendix 5). For this review, we considered other risk of bias issues as follows: comparability of treatment groups in relation to baseline ulcer surface area; choice of analysis where multiple ulcers on the same individual(s) are studied and choice of analysis in cluster randomised trials. We completed a risk of bias table for each eligible study and classified each study at overall high, low or unclear risk of bias, according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We discussed any disagreement among all review authors to achieve a consensus. We presented narrative discussion of the risk of bias, in addition to a risk of bias summary figure, which presents all the judgements in a cross‐tabulation of study by risk of bias domain. We classified trials at high risk of bias overall if they were rated high for any of three key criteria, namely, allocation concealment, blinding of outcome assessors and completeness of outcome data. For trials that had at least one of the three key domains rated as 'unclear' but none of these were at high risk of bias, we classified the trial at overall unclear risk of bias. Trials could only be classified at low risk of bias overall if all three key domains were rated as low risk individually.
Measures of treatment effect
We presented a narrative overview of all included RCTs, with results grouped according to the comparator intervention. We planned to combine trials when sufficiently alike in terms of population and comparison interventions, but this was not possible for this review. We reported mean differences (MD) and 95% confidence intervals (CI) for continuous outcomes (such as absolute or relative changes in ulcer area), and risk ratio (RR) and 95% CI for dichotomous variables (e.g. ulcers healed during time period, number of infected ulcers). We planned to pool standardised mean differences (SMD) estimates where studies measured the same outcome using different methods. In future updates, for time‐to‐event data, we plan to plot estimates of hazard ratios with associated 95% CIs where available from trial reports. In a trial that did not present data for change in ulcer healing but presented data for ulcer area at baseline and at the end of treatment, we estimated the change in ulcer area calculating the MD between these time points and therefore comparing treatment groups; however, caution should be used interpreting the results because samples were treated as independent.
Unit of analysis issues
We treated the number of ulcers as the unit of analysis in this review; however, we recorded whether outcomes in relation to an ulcer were measured on a per‐participant or per‐ulcer basis, and, in studies where multiple ulcers on a person were treated as being independent, we recorded that as part of our risk of bias assessment. We planned to include data from cluster‐randomised trials; however, we identified no studies with this characteristic. For future updates, we will adjust results when the unit of analysis in the trial is presented as the total number of individual participants instead of the number of clusters. Results will be adjusted using the mean cluster size and intracluster correlation coefficient (ICC) (Deeks 2022). For meta‐analysis, data would have been combined from individually randomised trials using the generic inverse‐variance method as described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022); however, meta‐analysis was not possible in this review.
Dealing with missing data
In the case of missing data, we contacted the original investigators to request these data whenever possible. No additional data were provided by study authors and therefore no data inclusions are reported. If trials had reported complete healing outcomes for only those participants who completed the trial (i.e. participants withdrawing and lost to follow‐up were excluded from the analysis), we planned to treat the participants who were not included in the analysis as if their wound had not healed. Where results were reported for participants who completed the trial without specifying the numbers initially randomised per group, we presented complete‐case data. The trials included in the review either had no dropouts, or analysed data on an intention‐to‐treat basis, excepting one that had withdrawals, but did not fully report data or withdrawals per group; therefore, we presented complete‐case data for this.
Assessment of heterogeneity
If, for future updates, we are able to include a sufficient number of studies, we will pool data for meta‐analysis using Review Manager Web (Review Manager Web 2020). We will consider clinical heterogeneity (i.e. where trials appear similar in terms of level of participants, intervention type and duration, and outcome type) and statistical heterogeneity. We will assess statistical heterogeneity using the Chi² test (a significance level of P < 0.10 is considered to indicate significant heterogeneity) in conjunction with the I² measure (Deeks 2022). The I² measure examines the percentage of total variation across trials due to heterogeneity rather than variation due to chance (Deeks 2022). Heterogeneity will be categorised as follows: I² values of 40% or less indicate a low level of heterogeneity and 75% or greater to represent substantial heterogeneity (Deeks 2022).
Assessment of reporting biases
If, for future updates, we are able to include a sufficient number of studies (10 RCTs or more), we will investigate publication bias using funnel plots as described in the Cochrane Handbook for Systematic Reviews of Interventions (Page 2022). If there is asymmetry, we will explore possible causes including publication bias, risk of bias and true heterogeneity.
Data synthesis
We combined details of included studies in a narrative review according to type of comparator. There were an insufficient number of included studies to pool data for meta‐analysis. Clinical and methodological heterogeneity would have been considered and pooling undertaken when studies appeared appropriately similar in terms of wound type, intervention type, duration of follow‐up and outcome type. We anticipated using a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow CIs. We would only have used a fixed‐effect approach when clinical and methodological heterogeneity was minimal. Chi² and I² would have been used to quantify heterogeneity but would not have been used to guide choice of model for meta‐analysis. For dichotomous outcomes, we presented the summary estimate as an RR with 95% CI. Where continuous outcomes were measured, we presented an MD with 95% CI. We planned to pool SMD estimates where studies measured the same outcome using different methods, such as health‐related quality of life data; however, this outcome was not reported in the included studies. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the study reports using the generic inverse variance method in Review Manager Web (Review Manager Web 2020). Where time‐to‐healing was analysed as a continuous measure, but it was not clear if all wounds healed, use of the outcome in the study would have been documented, but data would not have been summarised or used in any meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If, for future updates, we are able to include sufficient data and identify substantial heterogeneity, we will conduct subgroup analyses (Page 2022). We planned to conduct subgroup analysis according to presence or absence of compression therapy independent of type (elastic or inelastic) or level (moderate or high) compression.
Sensitivity analysis
For future updates, in case there are a sufficient number of included studies, we plan to undertake sensitivity analyses for each comparison that has a meta‐analysis according to the overall risk of bias. RCTs with overall high or unclear risk of bias will be excluded and the difference between estimates of treatment effect from this analysis and the main analysis considered.
Summary of findings and assessment of the certainty of the evidence
Two review authors (CR, FD) graded the certainty of the evidence using GRADE (GRADEpro GDT), using four levels of certainty: high, moderate, low and very low (Schünemann 2022). We carried out a GRADE assessment on all outcomes summarised in this review, rather than limiting this to complete wound healing and incidence of wound infection (as proposed in the published protocol). We presented the main results of the review in summary of findings tables. The review protocol did not specify outcomes for the summary of findings tables. We included the following outcomes: complete wound healing, incidence of wound infection, change in ulcer size, time‐to‐ulcer healing, health‐related quality of life and costs, because we consider them critical or important for decision‐making in health care; these will be prespecified for any future updates. These tables present key information concerning the certainty of evidence, the magnitude of the effects of the interventions examined and the sum of the available data for the main outcomes (Schünemann 2022). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022). When making decisions for the risk of bias domain, we downgraded one level if studies presented unclear risk of bias for all outcomes, and downgraded once or twice when studies were at high risk of bias for one or more domains (Schünemann 2022). We followed the methods described in Guyatt 2011 when downgrading for imprecision: either considering both the optimal information size (OIS) and the 95% CI of each meta‐analysis if they were estimable; or considering the sample size, the number of events and other eIectiveness indicators if the calculation of OIS and undertaking a meta‐analysis were not applicable. We downgraded twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We identified 470 records in total (371 from electronic bibliographic databases and 99 from registers of ongoing trials). No references were obtained as a result of contact with wound dressing manufacturers (Appendix 4). Three manufacturers out of 16 confirmed that there were no ongoing or recently completed RCTs of hydrogel dressings; we received no replies from the others.
After screening the titles and abstracts, we identified 47 articles as potentially relevant. After obtaining the full text from those articles, we excluded 28. There are three ongoing trials that potentially could be included in future updates (ISRCTN47349949; NCT03275831; RBR‐5d4s4f), and six studies awaiting classification (Altman 1993; Gago 2002; Hofman 1994; Hofman 1996; Semenic 2018; Sparholt 2002). Therefore, four RCTs (10 publications) met the inclusion criteria. See Figure 1 for full details on the results of the study selection process.
1.
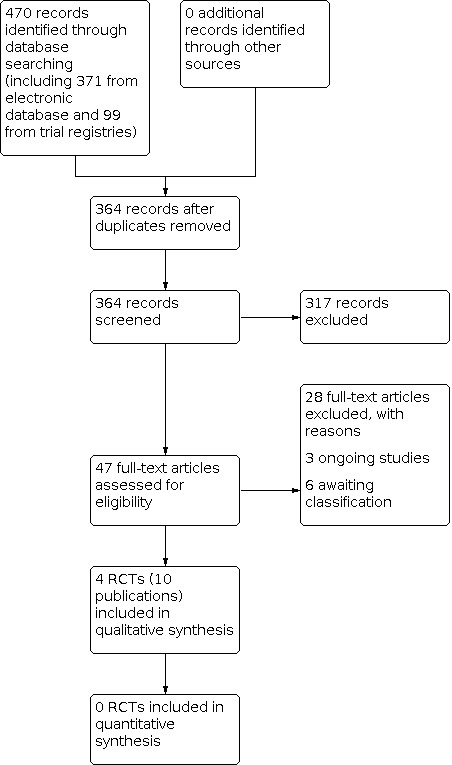
Study flow diagram.
Included studies
We included 10 publications originating from four RCTs with 272 participants, dating from 1994 to 2008 (De la Brassinne 2006; Gethin 2007 (see Appendix 6); Grotewohl 1994; He 2008; see Characteristics of included studies table).
Study design and settings
All studies used a parallel‐group design. Two studies were multicentre (Gethin 2007; Grotewohl 1994), and two were single‐centre trials. Sample sizes ranged from 20 participants to 156 participants. Only one study reported a priori sample size estimation, but failed to recruit the number of participants required (Gethin 2007). One study was conducted in China and was published in Chinese (He 2008). The other three studies were published in English; one was undertaken in Germany (Grotewohl 1994), one in Belgium (De la Brassinne 2006), and one in Ireland (Gethin 2007).
Type of participants
Studies randomised a total of 272 participants with venous leg ulcers. The mean age of the included population in the trials ranged from 55 to 68 years, 37% were females, based on studies that reported sex of participants. Only one study did not report the age of included participants (Grotewohl 1994).
One RCT reported that the method of diagnosis included clinical examination and exclusion of other causative aetiologies including the result of the ABPI (0.8 or greater) (Gethin 2007). The remaining studies stated that participants had venous disease but the method of determination of this diagnosis was not stated. Two RCTs reported that compression therapy was used for treatment (Gethin 2007; Grotewohl 1994).
Wound duration varied among studies. He 2008 reported mean wound duration of 2.9 (standard deviation (SD) 0.7) years in the hydrogel group and 3.3 (SD 0.9) years in the gauze and saline groups. De la Brassinne 2006 reported overall mean duration of ulcer of 2.25 years (range 0.5 to 10 years). In Gethin 2007 wound duration was 39.46 (SD 40.5) months in the manuka honey group and 29.93 (SD 35.2) in the hydrogel group. Grotewohl 1994 did not report wound duration.
Type of interventions
The studies investigated the effects of hydrogel compared with gauze and saline (He 2008), alginate gel (De la Brassinne 2006), manuka honey (Gethin 2007), and hydrocolloid (Grotewohl 1994).
Length of treatment using hydrogel dressing and follow‐up periods were four weeks in two RCTs (De la Brassinne 2006; Grotewohl 1994), and two weeks in one RCT (He 2008). One RCT reported four weeks of treatment with hydrogel and a follow‐up period of 12 weeks (Gethin 2007). Only one RCT described details regarding the secondary dressing (Allevyn) (Gethin 2007).
Type of outcomes
Two studies reported the primary outcome of complete wound healing (Gethin 2007; He 2008). Only one trial reported the primary outcome of incidence of wound infection (Gethin 2007).
All four trials reported the secondary outcome of reduction in ulcer size; however, we were unable to analyse data in one trial because the report was incomplete (Grotewohl 1994). One RCT reported that the ulcers were measured by planimetry after two weeks of treatment and at the end of treatment at four weeks (Grotewohl 1994). One RCT reported that the surfaces of the ulcers were measured using acetate tracing sheets (Opsite Fexigrid – Smith & Nephew Healthcare) and the volumes of the ulcers were measured by Jeltrate (Dentsply Caulk International) (De la Brassinne 2006). The other publications did not describe details of the method used.
None of the included studies reported data for the following secondary outcomes: recurrence of ulcer, quality of life, pain and cost.
Funding sources
Only two studies reported funding of source for the trial (De la Brassinne 2006; Gethin 2007).
Excluded studies
See Characteristics of excluded studies table for further details. We excluded 28 studies from the review.
Seventeen studies did not randomise the participants; six studies did not include the treatment of interest; in three RCTs the hydrogel dressings was not the only systematic difference across treatment groups; one study included leg ulcers with mixed aetiology of which less than 75% were venous leg ulcers; and one study did not report the results for venous leg ulcers separately.
Studies awaiting classification
See Characteristics of studies awaiting classification table.
Six studies were awaiting classification. Five were from conference proceedings or personal communication with limited information to be included or judged for the review (Altman 1993; Gago 2002; Hofman 1994; Hofman 1996; Sparholt 2002). Another study did not provide separate data for venous ulcer healing, we have contacted trial authors and are awaiting a response (Semenic 2018).
Ongoing studies
See Characteristics of ongoing studies table.
We identified three ongoing studies that need to be assessed at review update (ISRCTN47349949; NCT03275831; RBR‐5d4s4f).
Risk of bias in included studies
A summary of the risk of bias assessment is presented in Figure 2 and Figure 3 and summarised in the Characteristics of included studies table.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of the randomisation sequence
All studies were characterised as randomised; however, only one study had information describing the method of randomisation and was at low risk of bias (Gethin 2007). The other RCTs did not describe the method for generation of the randomisation sequence and were at unclear risk of bias (De la Brassinne 2006; Grotewohl 1994; He 2008).
Concealment of the allocation process
One RCT described that allocation concealment was based on serially numbered, sealed and opaque envelopes; therefore, we judged this at low risk of bias (Gethin 2007). The other three studies did not report the allocation concealment and were at unclear risk of bias (De la Brassinne 2006; Grotewohl 1994; He 2008).
Blinding
Two RCTs did not provide statements regarding blinding of participants or study personnel and were at unclear risk of bias (Grotewohl 1994; He 2008). Two RCTs were open‐label studies and were at high risk of bias (De la Brassinne 2006; Gethin 2007). One RCT stated that participants could not be blinded to the treatment because the intervention was an orange/brown colour compared with the other, which was a clear gel (Gethin 2007).
Blinding of outcome assessment
Two RCTs did not provide statements regarding blinding of outcome assessment and were at unclear risk of bias (Grotewohl 1994; He 2008). Two RCTs were open‐label studies and were at high risk of bias (De la Brassinne 2006; Gethin 2007). In one RCT, authors alleged that blinding was not possible because the intervention (manuka honey) was an orange/brown ointment, while the comparator, IntraSite Gel, was a clear gel; however, after four weeks of treatment with the intervention of interest, the participants received follow‐on treatment based on clinical assessment of the wound by the local investigator, which varied, up to week 12, when the outcome of interest of the present review was assessed (Gethin 2007).
Incomplete outcome data
One included RCT described analysis on an intention‐to‐treat basis and was at low risk of bias (Gethin 2007). One RCT did not describe the analysis on an intention‐to‐treat basis but all the participants were included in final analysis and this was at low risk of bias (De la Brassinne 2006). One RCT contained no statement regarding withdrawals, but apparently all participants were included in the final analysis at the 14 days follow‐up; however, there was no description for the number of participants followed up to total ulcer healing (He 2008). Based on the text, authors followed a subgroup of participants. The study was considered at high risk of bias. One RCT was at high risk of bias because the study did not report withdrawals per groups (around 1/4 participants were excluded) and data were not fully reported (Grotewohl 1994). In this trial, authors only presented percentage of healing without presenting SD, standard error, CI or any other statistical analysis, precluding further analysis of data.
Selective reporting
In three included RCTs the study protocol was not available, but all trial outcomes described in the methods section of the report were included in the results section and so they were judged at low risk of bias (De la Brassinne 2006; Gethin 2007; Grotewohl 1994). Only one study described one outcome (time‐to‐complete ulcer healing) in the results section which had not been properly described in the methods section. We were unable to obtain the study protocol and judged the study at high risk of bias (He 2008).
Other potential sources of bias
One RCT described that three participants had bilateral ulcers; furthermore, one participant with bilateral ulcers was treated with both dressings (hydrogel and hydrocolloid), characterising a unit of analysis issue; therefore, the study was at high risk of bias (Grotewohl 1994). Another RCT mentioned the mean baseline ulcer surface area did not differ between groups; however, in our independent analysis using a two‐tailed independent t‐test we found a statistical difference between groups (He 2008). Additionally, there was a small imbalance in groups regarding sex (more men in the hydrogel group); therefore, the study was classified at high risk of bias. One study was at unclear risk because of imbalance in ulcer size, duration and area covered by slough between groups; and because the randomised treatments were for only four weeks and then treatment was given according to clinical assessment; this was not reported, so there was no information on comparability of treatments (Gethin 2007). We did not identify other sources of bias in De la Brassinne 2006 and we classified this study at low risk for this domain.
Overall risk of bias
Overall risk of bias was high for all RCTs because at least one of the three key criteria (selection bias, detection bias and attrition bias) was at high risk (De la Brassinne 2006; Gethin 2007; Grotewohl 1994; He 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See Table 1; Table 2; Table 3; and Table 4 for the main comparisons.
Comparison 1: hydrogel dressing compared with gauze and saline
One RCT with a 14‐day follow‐up compared hydrogel dressing (Quitosana) with gauze and saline in 60 participants (He 2008). See Table 1.
Primary outcomes
1. Complete wound healing
There was complete wound healing in 16/30 participants in the hydrogel group compared with 3/30 in the gauze and saline group. It is uncertain whether there is a difference in complete wound healing between hydrogel dressing and gauze and saline because the certainty of the evidence is very low (RR 5.33, 95% CI 1.73 to 16.42; 1 trial, 60 participants; Analysis 1.1). The certainty of evidence was downgraded twice due to risk of bias and once due to imprecision.
1.1. Analysis.

Comparison 1: Hydrogel dressings compared with gauze and saline, Outcome 1: Complete wound healing during 15 days' follow‐up
2. Incidence of wound infection
No studies provided evidence for incidence of wound infection.
Secondary outcomes
1. Changes in ulcer size
He 2008 reported the mean ulcer size in both groups at baseline and at seven and 14 days of follow‐up. After 14 days of treatment, compared with baseline values, there was a reduction in ulcer area from 3.1 (SD 0.4) cm² to 2.3 (SD 0.7) cm² (30 participants) in the gauze and saline group and a reduction from 3.4 (SD 0.6) cm² to 1.1 (SD 0.2) cm² (30 participants) in the hydrogel group. The authors did not report percent area reduction or the change in ulcer area; however, we were able to estimate the values based on data provided for mean ulcer area. It is important to note that this is the best estimate we could reach using the data provided by authors; however, it was calculated considering that the baseline data and the 14‐day data were independent samples.
The mean reduction in ulcer area from baseline to 14 days was –2.3 (SD 0.61) cm² in the hydrogel group and –0.80 (SD 0.81) cm² in the gauze and saline group. The estimated MD between groups was –1.50 (95% CI –1.86 to –1.14; 1 trial, 60 participants; Analysis 1.2). It is uncertain whether there is a difference in change in ulcer size between hydrogel dressing and gauze and saline because the certainty of the evidence is very low. The certainty of evidence was downgraded twice due to risk of bias and once due to imprecision.
1.2. Analysis.

Comparison 1: Hydrogel dressings compared with gauze and saline, Outcome 2: Changes in ulcer size from baseline to 14 days (cm²)
2. Time‐to‐ulcer healing
The nature of the data was considered inappropriate for the assessment of time‐to‐ulcer healing. Authors reported time‐to‐healing, however, we were unable to confirm that all participants were followed up to complete healing (apparently only the participants classified as having "failure" in treatment, described by authors as participants who presented reduction lower than 25% in ulcer size or no healing during the treatment period). Furthermore, it was unclear if only hydrogel was used after two weeks for continuation of treatment. We attempted to contact authors to obtain further information but were unsuccessful.
3. Recurrence of ulcer
No studies provided evidence for recurrence of ulcers.
4. Health‐related quality of life
No studies provided evidence for health‐related quality of life.
5. Pain
No studies provided evidence for pain.
6. Costs
No studies provided evidence for costs.
Comparison 2: hydrogel dressing compared with alginate dressing
One RCT compared hydrogel dressing (IntraSite) with alginate dressing (Flaminal) use for four weeks in 20 participants (De la Brassinne 2006). See Table 2.
Primary outcomes
1. Complete wound healing
No studies provided evidence for complete wound healing.
2. Incidence of wound infection
No studies provided evidence for incidence of wound infection.
Secondary outcomes
1. Change in ulcer size
The authors reported percent reduction in wound area after four weeks of treatment. It is uncertain whether there is a difference in reduction in ulcer area between hydrogel and alginate dressings because the certainty of evidence is very low (MD –41.80%, 95% CI –63.95 to –19.65; 1 trial, 20 participants; Analysis 2.1). The certainty of evidence was downgraded twice due to risk of bias and once due to imprecision.
2.1. Analysis.

Comparison 2: Hydrogel dressing compared with alginate gel, Outcome 1: Percentage reduction in ulcer area at 4 weeks
2. Time‐to‐ulcer healing
No studies provided evidence for time‐to‐ulcer healing.
3. Recurrence of ulcer
No studies provided evidence for recurrence of ulcers.
4. Health‐related quality of life
No studies provided evidence for health‐related quality of life.
5. Pain
No studies provided evidence for pain.
6. Costs
No studies provided evidence for costs.
Comparison 3: hydrogel dressing compared with manuka honey
One RCT compared hydrogel dressings (IntraSite Gel) with manuka honey in 108 participants (Gethin 2007). The intervention was applied for four weeks with the major aim of assessing desloughing efficacy. Wound healing was assessed as a secondary outcome after 12 weeks. See Table 3.
Primary outcomes
1. Complete wound healing
Gethin 2007 reported complete wound healing after 12 weeks in 18/54 participants in the hydrogel group compared with 24/54 participants in the manuka honey group. It is uncertain whether there was a difference in complete wound healing between hydrogel and manuka honey because the certainty of evidence is very low (RR 0.75, 95% CI 0.46 to 1.21; 1 trial, 108 participants; Analysis 3.1). The certainty of evidence was downgraded twice due to risk of bias and twice due to imprecision.
3.1. Analysis.

Comparison 3: Hydrogel dressing compared with manuka honey, Outcome 1: Complete wound healing
2. Incidence of wound infection
It is uncertain whether there is a difference in incidence of wound infection between hydrogel and manuka honey because the certainty of evidence is very low (RR 2.00, 95% CI 0.81 to 4.94; 1 trial, 108 participants; Analysis 3.2). The certainty of evidence was downgraded twice due to risk of bias and twice due to imprecision.
3.2. Analysis.

Comparison 3: Hydrogel dressing compared with manuka honey, Outcome 2: Incidence of wound infection
Secondary outcomes
1. Changes in ulcer size
No studies provided evidence for changes in ulcer size.
2. Time‐to‐ulcer healing
No studies provided evidence for time‐to‐ulcer healing.
3. Recurrence of ulcer
No studies provided evidence for recurrence of ulcers.
4. Health‐related quality of life
No studies provided evidence for health‐related quality of life.
5. Pain
No studies provided evidence for pain.
6. Costs
No studies provided evidence for costs.
Comparison 4: hydrogel dressing compared with hydrocolloid dressing
One RCT randomised 84 participants to compare the effects of hydrogel dressing (Opragel) versus hydrocolloid dressing in venous ulcer healing (Grotewohl 1994). The authors stated that they were able to observe 62 participants (65 ulcers) over the 28 days of the study and did not provide any explanation for dropouts or withdrawals. There was a unit of analysis issue, because there were more ulcers treated than randomised participants (including one participant with a double‐sided ulcer treated with both products at the same time). See Table 4.
Primary outcomes
1. Complete wound healing
No studies provided evidence for complete wound healing.
2. Incidence of wound infection
No studies provided evidence incidence of wound infection.
Secondary outcomes
1. Changes in ulcer size
Grotewohl 1994 reported changes in ulcer size in both groups. The hydrocolloid group presented 33.3% reduction in ulcer size compared with 44.6% reduction in the hydrogel (Opragel) group after four weeks of treatment (no further information). Further analysis was not possible because authors did not report standard error or any other measurement of dispersion from the means. There was no response to our request that authors provide further data. Therefore, it is uncertain whether there is a difference in change in ulcer size between hydrogel and hydrocolloid because the certainty of evidence is very low. The certainty of evidence was downgraded twice due to risk of bias and twice due to imprecision.
2. Time‐to‐ulcer healing
No studies provided evidence for time‐to‐ulcer healing.
3. Recurrence of ulcer
No studies provided evidence for recurrence of ulcers.
4. Health‐related quality of life
No studies provided evidence for health‐related quality of life.
5. Pain
No studies provided evidence for pain.
6. Costs
No studies provided evidence for costs.
Discussion
Summary of main results
In this review, we reported evidence from four RCTs (10 reports), that involved 272 participants with venous leg ulcers, on the effects of hydrogel dressing compared with gauze and saline (He 2008), alginate gel (De la Brassinne 2006), manuka honey (Gethin 2007; see Appendix 6), and hydrocolloid dressing (Grotewohl 1994).
Using GRADE, we assessed the certainty of the evidence as very low for all outcomes in all comparisons. The reasons for these judgements are outlined in Table 1; Table 2; Table 3; and Table 4.
Primary outcomes
When hydrogel is compared with gauze and saline, it is uncertain whether there is a difference in complete wound healing between interventions because the certainty of the evidence is very low (1 study, 60 participants).
When hydrogel is compared with manuka honey, it is uncertain whether there is a difference in complete wound healing or incidence of infection between interventions because the certainty of the evidence is very low (one study, 108 participants).
Secondary outcomes
When hydrogel is compared with gauze and saline, it is uncertain whether there is a difference in change in ulcer size between interventions because the certainty of the evidence is very low (one study, 60 participants).
There is uncertainty whether there is a difference in change in ulcer size between hydrogel and alginate gel (one study, 20 participants) or hydrogel and hydrocolloid (one study, 84 participants) because the certainty of the evidence is very low in both cases.
In summary, there is uncertain evidence on the relative effectiveness of hydrogel compared with gauze and saline, alginate gel, manuka honey and hydrocolloid dressing for healing of venous leg ulcers. Overall, the certainty of evidence is very low and was downgraded twice due to risk of bias and once or twice due to imprecision for all comparisons and outcomes.
Overall completeness and applicability of evidence
As detailed in Search methods for identification of studies, we ran a comprehensive set of literature searches to maximise the relevant studies included in this review. The objective of this review was to assess the effectiveness of hydrogel in the healing of venous leg ulcers. We identified four RCTs comparing hydrogel with different dressings. Two studies reported the primary outcome of the review of complete wound healing (Gethin 2007; He 2008), and one also reported the other primary outcome of incidence of wound infection (Gethin 2007). Three RCTs reported some measurement of change in ulcer size (De la Brassinne 2006; Grotewohl 1994; He 2008).
None of the studies reported the following secondary outcomes: recurrence of ulcer, health‐related quality of life, pain and cost. Therefore, we could not assess the effects of hydrogel dressings on these outcomes. One study reported incomplete data for time‐to‐ulcer healing (He 2008).
Another potential limitation in the included studies is the short follow‐up for venous ulcer healing in three of four trials (ranging from 14 days to 28 days). It is well‐known these are hard‐to‐heal wounds and a longer follow‐up or time‐to‐event analysis would improve the certainty of evidence. Therefore, our evidence is limited due to the short follow‐up for venous leg ulcer healing assessment. Future trials should consider in their design time‐to‐event analysis and, if this is not possible, they should at least consider longer follow‐up (e.g. 12 weeks and above).
All studies were small, only one recruited more than 100 participants (Gethin 2007). The geographical scope was limited to Europe (three trials in Belgium, Ireland and Germany) and China (one trial). Only two trials described settings and in both cases these were hospitals (De la Brassinne 2006; Gethin 2007), and also community leg ulcer clinics in Gethin 2007.
Due to the certainty of evidence it is uncertain whether there is a difference in the healing of venous leg ulcers when hydrogel is compared with all other interventions assessed in this review. The results of this systematic review clearly show a lack of RCTs of high methodological quality addressing the effect of hydrogel on venous leg ulcer healing.
Quality of the evidence
This systematic review was limited by the quality of existing data. Some points must be taken into consideration when analysing the results of this review: the small number of included studies, small sample size, baseline ulcer size and some methodological aspects that increased the risk of bias.
Using the GRADE approach, we found very low‐certainty of evidence for all comparisons on all outcomes. Downgrading the certainty of evidence was due to the high risk of bias and imprecision.
Overall risk of bias was high for all studies. Studies had unclear or high risk of bias for most of biases assessed, especially for performance and detection bias. Only one study provided a description of the method of randomisation and allocation (Gethin 2007). Therefore, we downgraded studies due to risk of bias. We did not downgrade for indirectness because participants, interventions and outcomes in the included studies were within the scope of the review. We were unable to combine studies and further explore possible unexplained heterogeneity, so we did not downgrade studies for inconsistency. We downgraded the certainty of evidence once for imprecision due to small sample size and twice for imprecision when there were very few events and CIs around effects included both appreciable benefit and appreciable harm.
Potential biases in the review process
We attempted to apply robust methods in the process of analysing the search, collecting data, performing meta‐analysis and assessing risk of bias. Nevertheless, some points must be taken into consideration.
When authors did not report change in ulcer size, we estimated the magnitude of change using data from the longest follow‐up and baseline in one case (He 2008); however, we had to consider the means from those time points as independent groups. We recognised that those calculated data might be inaccurate. We were unable to combine data for meta‐analysis, because the interventions of comparison were different across studies. Therefore, the subgroup and the sensitivity analyses proposed in the review protocol were not performed due to insufficient data.
Agreements and disagreements with other studies or reviews
There is no good‐quality evidence to determine whether hydrogel dressings are better or worse than other dressing treatments for the healing of venous leg ulcers. This observation agrees with one network meta‐analysis (NMA) (Norman 2018), the systematic review performed by Palfreyman 2007, and with the evidence summary discussing advanced wound dressing for chronic wounds reported by the National Institute for Health and Care Excellence (NICE 2016).
Authors' conclusions
Implications for practice.
There is inconclusive evidence to determine the effectiveness of hydrogel dressings compared with gauze and saline, alginate gel, manuka honey and hydrocolloid dressing on healing of venous leg ulcers. Practitioners may, therefore, consider other characteristics such as costs and symptom management when choosing between dressings.
Implications for research.
One network meta‐analysis confirmed that more research is needed to determine whether particular dressings or topical agents improve the probability of healing of venous leg ulcers, as we cannot be certain which are the most effective treatments for these ulcers (Norman 2018). The NMA also found that it was unclear which treatments it would be best to compare in future trials; this decision should be driven by high‐priority questions from patients and other decision‐makers. All the randomised controlled trials included in our review have methodological and reporting problems. Any future studies assessing the effects of hydrogel on venous wound healing should consider using all the steps from CONSORT (Schulz 2010), and consider key points such as appropriate sample size with the power to detect expected differences, appropriate outcomes (such as time‐to‐event analysis) and adverse effects. If time‐to‐event analysis is not used, at least longer follow‐up (as 12 weeks and above) should be adopted. They should also address important outcomes that all the studies included did not investigate, such as health‐related quality of life, pain and wound recurrence.
History
Protocol first published: Issue 9, 2013
Acknowledgements
The review authors would like to acknowledge Elizabeth Royle for copy editing the protocol and Joan Webster, Marian Brady, Marialena Trivella, Mark Corbett, Una Adderley, Vicki Pennick and Amy Zelmer for commenting on the protocol.
They would also like to thank Tanya Walsh, Zhenmi Liu, Victoria Pennick, Michael Gallagher and Janet Wale for their peer review comments on the review and Anne Lawson for copy editing the review.
Appendices
Appendix 1. Glossary of terms
Alginate = a salt of alginic acid.
Chronic venous insufficiency = refers to a long and permanent disease of the veins. Insufficient veins means that the blood does not flow at the normal speed and normal pathways in the veins of the lower limbs (in the case of venous leg ulcer). This causes reflow, stagnation of the blood in the legs that leads to signs and symptoms such as painful legs, swelling and heaviness, itchiness, varicose veins, changes in the skin colour and eventually wounds.
Compression therapy = application of external pressure to a limb, to help venous blood or lymph circulation. Compression can be applied using bandages, elastic stockings or inflatable sleeves.
Debridement = medical removal of dead, damaged or infected tissue to improve the healing potential of the remaining healthy tissue. It is thought to be an important part of the healing process for wounds.
Exudate = fluid that leaks out of a wound.
Lipodermatosclerosis = area of pigmentation and hardened skin caused by leakage of red blood cells into the skin. Occurs in people with chronic venous insufficiency.
Maceration = when skin in contact with moisture for too long becomes soft or soggy to the touch, wrinkly and lighter in colour. It can eventually lead to ulceration or extend a previous wound.
Macrovascular = portion of the vasculature of the body comprising the larger vessels.
Medial gaiter area = area of the lower leg, between the ankle and calf muscle.
Microcirculatory = flow of blood or lymph through the smallest vessels of the body, especially venules, capillaries and arterioles.
Physiopathology = part of the science of disease concerned with disordered function.
Polymer = a large molecule (macromolecule) composed of repeating structural units.
Re‐epithelialisation = growth of the epithelium across a wound from the wound edges.
Skin perfusion = process of nutritive delivery of arterial blood to a capillary bed in the biological tissue.
Thrombophlebitis = inflammation of a vein with blood clot formation inside the vein at the site of the inflammation.
Thrombosis = formation of a thrombus (blood clot) within the heart or blood vessels.
Valvular incompetence = inability of the valves to keep blood flowing in the direction it should be.
Definitions taken from Medical Dictionary and Merriam‐Webster Medical Dictionary.
Appendix 2. Nurse Prescribers Formulary 2011 categories of dressings
Basic wound contact dressings
Low‐adherence dressings and wound contact materials
These dressings are usually cotton pads that are placed directly in contact with the wound. They can be either non‐medicated (e.g. paraffin gauze dressing) or medicated (e.g. containing povidone iodine or chlorhexidine). Examples include paraffin gauze dressing, BP 1993 and Xeroform (Covidien) dressing – a non‐adherent petrolatum blend with 3% bismuth tribromophenate on fine mesh gauze.
Absorbent dressings
These dressings are applied directly to the wound and may be used as secondary absorbent layers in the management of heavily exuding wounds. Examples include Primapore (Smith & Nephew Healthcare), Megapore (Mölnlycke) and absorbent cotton gauze (BP 1988).
Advanced wound dressings
Hydrogel sheet and amorphous dressings
These dressings consist of a starch polymer and up to 96% water. They can absorb wound exudate or rehydrate a wound, depending on the wound moisture levels. They are supplied in either flat sheets or amorphous hydrogel. Examples of hydrogel sheet dressings include: Actiformcool (Activa Healthcare) and Aquaflo (Covidien). Examples of amorphous hydrogel dressings include: Purilon Gel (Coloplast) and NuGel (Systagenix).
Films‐permeable film and membrane dressings
These dressings are permeable to water vapour and oxygen, but not to liquid water or micro‐organisms. Examples include Tegaderm (3M); Opsite (Smith & Nephew Healthcare).
Soft polymer dressings
These dressings are composed of a soft silicone polymer held in a non‐adherent layer. They are moderately absorbent. Examples include: Mepitel (Mölnlycke) and Urgotul (Urgo).
Hydrocolloid dressings
These dressings are usually composed of an absorbent hydrocolloid matrix on a vapour‐permeable film or foam backing. Examples include: Granuflex (Convatec). NU DERM (Systagenix). Fibrous alternatives have been developed that resemble alginates and are not occlusive: Aquacel (Convatec).
Foam dressings
These dressings contain hydrophilic polyurethane foam and are designed to absorb wound exudate and maintain a moist wound surface. There are various versions; some include additional absorbent materials, such as viscose and acrylate fibres, or particles of superabsorbent polyacrylate, while some are silicone‐coated for non‐traumatic removal. Examples include: Allevyn (Smith & Nephew Healthcare), Biatain (Coloplast) and Tegaderm (3M).
Alginate dressings
These dressings are highly absorbent and consist of calcium alginate or calcium sodium alginate, which can be combined with collagen. The alginate forms a gel when in contact with wound surface. This gel can be lifted off at dressing removal, or rinsed away with sterile saline. Bonding to a secondary viscose pad increases absorbency. Examples include: Curasorb (Covidien), SeaSorb (Coloplast) and Sorbsan (Unomedical).
Capillary‐action dressings
These dressings consist of an absorbent core of hydrophilic fibres held between two low‐adherent contact layers. Examples include: Advadraw (Advancis) and Vacutx (Protex).
Odour‐absorbent dressings
These dressings contain charcoal and are used to absorb wound odour. Often this type of dressing is used in conjunction with a secondary dressing to improve absorbency. Examples include: CarboFLEX (Convatec).
Antimicrobial dressings
Honey‐impregnated dressings
These dressings contain medical‐grade honey, which is supposed to have antimicrobial and anti‐inflammatory properties, and can be used for acute or chronic wounds. Examples include: Medihoney (Medihoney) and Activon Tulle (Advancis).
Iodine‐impregnated dressings
These dressings release free iodine, which is thought to act as a wound antiseptic, when exposed to wound exudate. An example is Iodozyme (Insense).
Silver‐impregnated dressings
These dressings are used to treat infected wounds, as silver ions are thought to have antimicrobial properties. Silver versions of most dressing types are available (e.g. silver foam, silver hydrocolloid, etc.). Examples include: Acticoat (Smith & Nephew Healthcare) and Urgosorb Silver (Urgo).
Other antimicrobial dressings
These dressings are composed of a gauze or low‐adherent dressing impregnated with an ointment thought to have antimicrobial properties. Examples include: chlorhexidine gauze dressing (Smith & Nephew Healthcare) and Cutimed Sorbact (BSN Medical).
Specialist dressings
Protease‐modulating matrix dressings
These dressings alter the activity of proteolytic (protein‐digesting) enzymes in chronic wounds. Examples include: Promogran (Systagenix) and Sorbion (H & R).
Silicone keloid dressing
These dressings reduce or prevent hypertrophic or keloid scarring. Examples include: Cica‐Care (Smith & Nephew Healthcare) and Ciltech (Su‐med).
Appendix 3. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND INREGISTER
2 (varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or (ulcer* next cruris) AND INREGISTER
3 #1 OR #2
4 MESH DESCRIPTOR Hydrogels EXPLODE ALL AND INREGISTER
5 (hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or "suprasorb gel" or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or "Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curate) AND INREGISTER
6 #4 OR #5
7 #3 AND #6
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Leg Ulcer] explode all trees
#2 (varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or (ulcer* next cruris):ti,ab,kw
#3 #1 or #2
#4 MeSH descriptor: [Hydrogels] explode all trees
#5 (hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or "suprasorb gel" or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or "Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curate):ti,ab,kw
#6 #4 or #5
#7 #3 and #6 in Trials
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) searched via the Cochrane Register of Studies
1 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND CENTRAL:TARGET
2 (varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or (ulcer* next cruris) AND CENTRAL:TARGET
3 #1 OR #2 AND CENTRAL:TARGET
4 MESH DESCRIPTOR Hydrogels EXPLODE ALL AND CENTRAL:TARGET
5 (hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or "suprasorb gel" or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or "Askina Transorbent" or "Cutimed Sorbact" or "Intrasite Comformable" or "Xtrasorb HCS" or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or "Actiform cool" or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or "Gel FX" or Geliperm or Novogel or SanoSkin or Vacunet or curate) AND CENTRAL:TARGET
6 #4 OR #5 AND CENTRAL:TARGET
7 #3 AND #6 AND CENTRAL:TARGET
8 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
9 http*:SO AND CENTRAL:TARGET
10 #8 or #9 AND CENTRAL:TARGET
11 #7 AND #10
Ovid MEDLINE
1 exp Leg Ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw.
3 or/1‐2
4 exp Hydrogels/
5 (hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or suprasorb gel or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or Askina Transorbent or Cutimed Sorbact or Intrasite Comformable or Xtrasorb HCS or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or Actiform cool or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or Gel FX or Geliperm or Novogel or SanoSkin or Vacunet or curate).tw.
6 or/4‐5
7 3 and 6
8 randomized controlled trial.pt.
9 controlled clinical trial.pt.
10 randomi?ed.ab.
11 placebo.ab.
12 clinical trials as topic.sh.
13 randomly.ab.
14 trial.ti.
15 or/8‐14
16 exp animals/ not humans.sh.
17 15 not 16
18 7 and 17
Ovid Embase
1 exp leg ulcer/
2 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw.
3 or/1‐2
4 exp HYDROGEL/
5 exp HYDROGEL DRESSING/
6 (hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or suprasorb gel or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or Askina Transorbent or Cutimed Sorbact or Intrasite Comformable or Xtrasorb HCS or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or Actiform cool or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or Gel FX or Geliperm or Novogel or SanoSkin or Vacunet or curate).tw.
7 or/4‐6
8 3 and 7
9 Randomized controlled trial/
10 Controlled clinical study/
11 Random$.ti,ab.
12 randomization/
13 intermethod comparison/
14 placebo.ti,ab.
15 (compare or compared or comparison).ti.
16 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
17 (open adj label).ti,ab.
18 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
19 double blind procedure/
20 parallel group$1.ti,ab.
21 (crossover or cross over).ti,ab.
22 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
23 (assigned or allocated).ti,ab.
24 (controlled adj7 (study or design or trial)).ti,ab.
25 (volunteer or volunteers).ti,ab.
26 human experiment/
27 trial.ti.
28 or/9‐27
29 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
30 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
31 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
32 (Systematic review not (trial or study)).ti.
33 (nonrandom$ not random$).ti,ab.
34 Random field$.ti,ab.
35 (random cluster adj3 sampl$).ti,ab.
36 (review.ab. and review.pt.) not trial.ti.
37 we searched.ab. and (review.ti. or review.pt.)
38 update review.ab.
39 (databases adj4 searched).ab.
40 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
41 Animal experiment/ not (human experiment/ or human/)
42 or/29‐41
43 28 not 42
44 8 and 43
EBSCO CINAHL Plus
S31 S7 AND S30
S30 S29 NOT S28
S29 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S28 S26 NOT S27
S27 MH (human)
S26 S23 OR S24 OR S25
S25 TI (animal model*)
S24 MH (animal studies)
S23 MH animals+
S22 AB (CLUSTER W3 RCT)
S21 MH (crossover design) OR MH (comparative studies)
S20 AB (control W5 group)
S19 PT (randomized controlled trial)
S18 MH (placebos)
S17 MH (sample size) AND AB (assigned OR allocated OR control)
S16 TI (trial)
S15 AB (random*)
S14 TI (randomised OR randomized)
S13 MH cluster sample
S12 MH pretest‐posttest design
S11 MH random assignment
S10 MH single‐blind studies
S9 MH double‐blind studies
S8 MH randomized controlled trials
S7 S3 and S6
S6 S4 or S5
S5 TI ( hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or suprasorb gel or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or Askina Transorbent or Cutimed Sorbact or Intrasite Comformable or Xtrasorb HCS or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or Actiform cool or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or Gel FX or Geliperm or Novogel or SanoSkin or Vacunet or curate ) or AB ( hydrogel* or intrasite or curafil or dermagran or duoderm or hydrosorb or hypergel or normlgel or nu‐gel or nugel or purilon or suprasorb gel or hypligel or elasto‐gel or elastogel or tegagel or aquaform or granugel or curasol or curatec or opragel or Askina Transorbent or Cutimed Sorbact or Intrasite Comformable or Xtrasorb HCS or ActivHeal or Askina or Cutimed or Prontosan or Octenillin or Actiform cool or ActiformCool or Iodozyme or Kerralite or Novogel or Oxyzyme or Flexigran or Aquaflo or Coolie or Gel FX or Geliperm or Novogel or SanoSkin or Vacunet or curate ) 2,257
S4 (MH "Hydrogel Dressings")
S3 S1 or S2
S2 TI (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcer cruris or ulcus cruris) or AB (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcer cruris or ulcus cruris)
S1 (MH "Leg Ulcer+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel | Venous Leg Ulcer
"Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran | Venous Leg Ulcer
Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate | Venous Leg Ulcer
hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel| Ulcer Venous
"Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran | Ulcer Venous
Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate | Ulcer Venous
hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel | Varicose Ulcer
"Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran | Varicose Ulcer
Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate | Varicose Ulcer
hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel | Venous Stasis Ulcer
"Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran | Venous Stasis Ulcer
Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate | Venous Stasis Ulcer
World Health Organization International Clinical Trials Registry Platform
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "venous ulcer"
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "venous ulceration"
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "leg ulcer"
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "crural ulcer"
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "stasis ulcer"
(hydrogel OR intrasite OR curafil OR dermagran OR duoderm OR hydrosorb OR hypergel OR normlgel OR nu‐gel OR nugel OR purilon OR "suprasorb gel" OR hypligel OR elasto‐gel OR elastogel OR tegagel OR aquaform OR granugel OR curasol OR curatec OR opragel OR "Askina Transorbent" OR "Cutimed Sorbact" OR "Intrasite Comformable" OR "Xtrasorb HCS" OR ActivHeal OR Askina OR Cutimed OR Prontosan OR Octenillin OR "Actiform cool" OR ActiformCool OR Iodozyme OR Kerralite OR Novogel OR Oxyzyme OR Flexigran OR Aquaflo OR Coolie OR "Gel FX" OR Geliperm OR Novogel OR SanoSkin OR Vacunet OR curate) AND "varicose ulcer"
ISRCTN registry
"leg ulcer" OR "venous ulcer" OR "venous wound"
Appendix 4. Dressing manufacturers contacted regarding ongoing or recently completed trials
3M
A1 Pharmaceuticals
Activa Healthcare
Aspen Medical
Braun
BSN Medical
Coloplast
Convatec
Covidien
Hartmann
MedLogic
Mölnlycke
Protex
Sinergy Healthcare
Smith & Nephew Healthcare
Systagenix
Appendix 5. Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators described a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators described a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding – was knowledge of the allocated interventions adequately prevented during the study?
1. Blinding of participants and care providers.
2. Blinding of outcome assessors.
Low risk of bias
Any one of the following.
No blinding, but the review authors judged that the outcome and the outcome measurement were unlikely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data had been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol was available and all the study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way.
The study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all the study's prespecified primary outcomes were reported.
One or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis.
The study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
For example:
comparability of treatment groups in relation to baseline ulcer surface area;
choice of analysis where multiple ulcers on the same individuals(s) were studied;
choice of analysis in cluster randomised trials;
Low risk of bias
The study appeared free of other sources of bias.
High risk of bias
There was at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there was either:
insufficient information to assess whether an important risk of bias existed; or
insufficient rationale or evidence that an identified problem would introduce bias.
Appendix 6. Notes
During the production of this review the review authors became aware that the publication Gethin G, Cowman S. Manuka honey vs. hydrogel – a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Journal of Clinical Nursing 2009;18(3):466‐74 (onlinelibrary.wiley.com/doi/10.1111/jocn.12652/abstract) had been retracted by agreement between the journal Editor‐in‐Chief, the authors and John Wiley & Sons, Ltd (Anonymous 2015). The retraction was agreed due to errors in the data analysis that affected the article's findings. The review authors would like to confirm that the data in this review was taken from the source: Gethin G. Manuka honey versus hydrogel – a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Unpublished PhD thesis 2007.
Data and analyses
Comparison 1. Hydrogel dressings compared with gauze and saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complete wound healing during 15 days' follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Changes in ulcer size from baseline to 14 days (cm²) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Hydrogel dressing compared with alginate gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Percentage reduction in ulcer area at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Hydrogel dressing compared with manuka honey.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Complete wound healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Incidence of wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De la Brassinne 2006.
| Study characteristics | ||
| Methods | Design: open; RCT Country: Belgium Setting: hospital Sample size calculation: not reported Ethical approval: was granted after the medical ethics committee approved the project (Ethics Committee of the Medicine Faculty of Liege University, Belgium). Informed consent: was obtained after fully advising the participants about the purpose and consequences of the study. |
|
| Participants | Total sample: 20 hospitalised participants with chronic leg ulcers of venous origin Inclusion criteria: men and women, mobile, aged 40–70 years and chronic leg ulcers of venous origin with exudate Exclusion criteria: none specified Numbers randomised
Gender: 13 women and 7 men Mean age: 61 years (range 45–70 years) ABPI: not reported Unit of randomisation: participant Unit of analysis: participant Ulcer infection: not reported Ulcer duration (current): 2.25 years (range 0.5–10 years) in both groups Baseline ulcer surface
Baseline volume measurement
Data collected: ulcer dimension by planimetry and volume of the ulcer at day 7, 14 and 28 |
|
| Interventions | Group 1: hydrogel dressing (IntraSite Gel) Group 2: alginate dressing (Flaminal gel) *Both groups had the wound debrided and cleaning with saline before the dressing was applied. Description of compression therapy: not reported Length of treatment: 4 weeks Follow‐up: 4 weeks Comments: * The surface measurement of the ulcer was made using acetate tracing (Opsite Flexigrid from Smith & Nephew Healthcare). * The volume measurement of the ulcer was made using high algin impression material (Jeltrate mould from Dentsply Caulk International) |
|
| Outcomes | Complete wound healing: not reported Incidence of wound infection: not reported Change in ulcer size: reported, planimetry used to measure the wound at baseline, 7, 14 and 28 days of treatment and volume measured on same days Mean percentage surface reduction at day 14 (P < 0.01)
Mean percentage surface reduction at day 28 (P ≤ 0.01)
Time to ulcer healing: not reported Recurrence of ulcer: not reported Health‐related quality of life: not reported Pain: not reported Costs: not reported |
|
| Notes | Number of participants withdrawing and reasons: no withdrawals Funding: UCB‐Belgium |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was performed in two‐armed parallel groups, open and randomised". Comment: no further description of method of randomisation provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: open study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: open study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the study started with 20 participants and the authors did not report dropouts. All the participants were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: all trial outcomes described in the methods section of the report were included in the results section. Unable to obtain RCT protocol (no response to email sent to trial author). |
| Other bias | Low risk | Comment: there was no report regarding the balance between men and women allocated on each group; however, the baseline ulcer surface area and volume did not differ between groups. |
Gethin 2007.
| Study characteristics | ||
| Methods | Design: 2‐arm, open‐label, multicentre RCT Country: Ireland Setting: hospital and community leg ulcer clinics Sample size calculation: a sample size of 156 participants randomly allocated to 2 equal groups of 78 estimated as having 80% power to detect a minimum difference of 20% at the 5% 2‐sided significance level. Ethical approval: obtained from each centre involved in the trial. Informed consent: obtained |
|
| Participants | Total sample: 108 participants recruited from February 2003 to January 2006 Inclusion criteria: having an uninfected venous ulcer, aged > 18 years, able to provide written informed consent, having ≥ 50% of wound bed covered in slough, wound area < 100 cm², ABPI ≥ 0.8 Exclusion criteria: aged < 18 years, unable to provide written informed consent, having an ulcer > 100 cm², ulcer diagnosed as being malignant, having a cavity wound, clinical diagnosis of wound infection, currently taking antibiotics for any reason, currently taking oral immunosuppressants, having poorly controlled diabetes, having previously enrolled into the study, pregnant women or lactating mothers Mean age: overall mean not reported
ABPI: results not reported Baseline data collected: gender, age, wound size and duration, ulcer location and history of recurrence Medical information collected: history of deep venous thrombosis, hypertension, trauma or surgery to the affected limb, diabetes, immunosuppression and current medications Ulcer duration
Baseline ulcer surface
Margolis index 0, 1, 2
Wounds covered in slough
Numbers randomised
Unit of analysis: ulcer Ulcer infection: was reported Comments: the trial authors reported that each participant could only contribute with 1 ulcer (the uppermost ulcer or the largest ulcer was selected if > 1 was present) |
|
| Interventions | Group 1: hydrogel dressing (IntraSite Gel, Smith & Nephew Healthcare) at 3 g/20 cm² Group 2: monofloral (manuka) honey dressing (Woundcare 18+) at 5 g/20 cm² Description of compression therapy: all participants were subjected to compression therapy applied weekly Secondary dressing: Allevyn hydrocellular foam (Smith & Nephew Healthcare) Length of treatment: 4 weeks, dressing changes and compression therapy. Quote: "After four weeks received follow up on treatment based on clinical assessment of the wound by the local investigator, which varied from patient to patient." Follow‐up: week 12 |
|
| Outcomes | Complete wound healing: at 12 weeks
The authors' regression analysis was adjusted for Margolis score: they reported an odds ratio of 3.1 (95% CI 1.15 to 8.35) and a risk ratio of 1.38 (95% CI 1.02 to 1.88) Incidence of wound infection
Change in ulcer size: not reported Time to ulcer healing: not reported Recurrence of ulcer: not reported Health‐related quality of life: not reported Pain: not reported Costs: not reported |
|
| Notes | Healing was a secondary outcome. The primary outcome was change in area of slough at 4 weeks. Funding: Research and Education Foundation in Sligo General Hospital, European Wound Management Association and the Health Research Board of Ireland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "78 pieces of both green and yellow card were counted, checked, and shuffled by a person independent of the trial. The card was then inserted into opaque brown envelopes, counted, sealed, and shuffled by another person independent of the trial. The envelopes were then, sequentially numbered. This process represented generation of an unpredictable allocation sequence. Yellow indicated allocation to the Manuka honey treatment and green allocation to the IntraSite treatment." |
| Allocation concealment (selection bias) | Low risk | Quote: "Envelopes were given to a person independent of participant enrolment at a remote location. This process ensured allocation concealment. When a person agreed to participate and having met the inclusion criteria and signed the consent form, the recruiting nurse phoned the remote number, gave details of the patient including, name, gender, trial centre, ABPI, percentage of wound covered in slough and ulcer size. The external person then, using the pre‐generated allocation sequence, allocated the trial number for the patient and the treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The consequence of this for the writers RCT is that patients cannot be blinded to the treatment as the intervention is an orange/brown ointment, while the comparator IntraSite Gel™ is a clear gel, thus the difference between treatments is obvious." Comment: blinding of participants and healthcare providers not done. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinded outcome assessment is not possible for two reasons. As stated, honey leaves an orange staining on the periwound skin (photo 3.1) which would identify which treatment the patient is receiving and secondly, within the main study centre clinic and in community leg ulcer clinics, a person trained in wound management would not be available to assess wounds. Photographs would not be used for blinded outcome assessment, as the ability to achieve high quality photos from each centre for each patient was not possible. Therefore, the trial would be classified as open label." Comment: primary outcome assessors were not blinded. Quote: "However, to add some element of blinding, the laboratory would not be made aware of which treatment the patient was receiving and the statistician would not be aware of the identity of each group." Comment: secondary outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: figure 5.2 shows that no participants were lost to follow‐up, but there were 9/54 withdrawals in the manuka honey group (6 due to infection in the study wound) and 17/54 withdrawals in the hydrogel group (12 due to infection in the study wound). This was relatively small compared with the event rate, so acceptable. Quote: "Data were analysed on an intention to treat (ITT) basis." Comment: ITT analysis had been done, as all the randomised participants were included in the final results. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not available, but all trial outcomes described in the methods section of the report were included in the results section. |
| Other bias | Unclear risk | Quote: "Analysis using independent t‐test determined there was no statistically significant difference between treatment groups for any of the baseline continuous variables such as wound duration, size, patient age, and slough. Chi‐square analysis did not report any statistically significant differences between groups for baseline categorical." Comment: ulcers in the honey group were larger (median ulcer areas: 4.2 cm² with hydrogel dressing vs 5.4 cm² with manuka honey dressing; mean ulcer areas: 9.87 cm² with hydrogel dressing vs 10.52 cm² with manuka honey dressing), present for longer (median durations: 14 weeks with hydrogel dressing vs 18 weeks with manuka honey dressing; mean durations: 29.9 weeks with hydrogel dressing vs 39.4 weeks with manuka honey dressing) and had a greater area covered by slough (78.2% with hydrogel dressing vs 85.5% with manuka honey dressing); however, these factors would affect the likelihood of healing in the honey group. The Margolis index had a higher proportion of score 2 patients in the honey group (22% with hydrogel dressing vs 37% with manuka honey dressing). The study seemed free from other forms of bias. In addition, the randomised treatments were for only 4 weeks and then treatment was given according to clinical assessment; this was not reported, so no information on comparability of treatments. |
Grotewohl 1994.
| Study characteristics | ||
| Methods | Design: multicentre RCT Country: Germany Setting: not reported Sample size calculation: not reported Ethical approval: not reported Informed consent: was obtained |
|
| Participants | Total sample: 84 participants with ulcera crurum Inclusion criteria: none specified Exclusion criteria: none specified Numbers randomised
ABPI: not reported Unit of analysis: ulcer Baseline ulcer size cm² (mean) (before treatment)
Ulcer infection: not reported Data collected: ulcer dimension by planimetry Comments: the study started with 84 participants, but only 62 participants were followed for 28 days of the study. There was no justification for the dropouts. The trial author reported that 3 participants from the hydrogel group had double‐sided ulcers; 1 of these participants was treated with hydrogel and hydrocolloid at once |
|
| Interventions | Group 1: hydrogel dressing (Opragel, Lohmann) Group 2: hydrocolloid dressing (proprietary name not reported, manufacturer not reported) *All participants were subjected to compression therapy Description of compression therapy: not reported Length of treatment: 4 weeks Follow‐up: 4 weeks |
|
| Outcomes | Complete wound healing: reported that some ulcers were completely healed after 28 days and several participants after 14 days (no numbers reported) Incidence of wound infection: not reported Change in ulcer size: reported, planimetry used to measure the wound at day 14 and 28 of treatment
Time to ulcer healing: not reported Recurrence of ulcer: not reported Health‐related quality of life: not reported Pain: not reported Costs: not reported |
|
| Notes | No details of the number of ulcers healed at end of trial were provided. Exclusions post‐randomisation: 22 exclusions, no reason provided for withdrawal and no details provided regarding number of withdrawals per group. The trial author reported no observed cases of irritation or allergic reactions with the use of dressings No details explaining the imbalance in allocation between study groups Group 1: "4 ulcers did not demonstrate signs of healing". Group 2: "3 ulcers did not demonstrate signs of healing". Funding: not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The assignment was carried out to the principle of random selection". Comment: method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment in study report#. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: authors did not report withdrawals per groups (22/84 participants were excluded) and data were not fully reported. Trial authors only presented percentage of healing without present SD, standard error, CI or any other statistical analysis, precluding review authors to perform further analysis of data. Authors described that "In some cases the ulcers were completely healed after 28 days …" but did not provide number of healed ulcers per group. |
| Selective reporting (reporting bias) | Low risk | Comment: all trial outcomes described in the methods section of the report were included in the results section. Unable to obtain RCT protocol (no response from email sent to trial author). |
| Other bias | High risk | Comment: study showed inconsistency of data based on the number of participants with multiple ulcers and also imbalance with baseline ulcer surface area. |
He 2008.
| Study characteristics | ||
| Methods | Design: RCT Country: China Setting: not reported Sample size calculation: not reported Ethical approval: not reported Informed consent: not reported |
|
| Participants | Total sample: 60 participants with chronic venous ulcer of lower extremities Inclusion criteria: aged ≥ 18 years; required to have a venous insufficiency ulcer for > 1 month of duration, with area ≤ 4 cm²; woman not pregnant; not on immunosuppression; no history of allergy to polysaccharides (glycans) Exclusion criteria: none specified Numbers randomised
Sex:
Side of the ulcers: 32 ulcers from the left side and 28 ulcers from the right side Baseline ulcer size (mean) (before treatment): range 2.5–4.0 cm²
Mean age:
Ulcer duration:
ABPI: not reported Unit of randomisation: participant Unit of analysis: participant Ulcer infection: not reported Data collected: diagnosis, sex, age, ulcer dimension and volume |
|
| Interventions | Group 1: hydrogel dressing (Chitosan, Xangai Qisheng) Group 2: gauze and saline Description of compression therapy: not reported Length of treatment: 14 days Follow‐up: not reported. Authors reported that participants who resulted were classified as "failure" continued the treatment until cured (results of treatment were classified as: "cured", "excellent", "regular", "failure"). |
|
| Outcomes | Complete wound healing
Incidence of wound infection: not reported Change in ulcer size: reported at 7 and 14 days.
Time to ulcer healing: reported (reported P < 0.01). Unable to confirm the number of participants followed up to complete ulcer healing. Time for healing reported by authors
Recurrence of ulcer: not reported Health‐related quality of life: not reported Pain: not reported Costs: not reported |
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the authors mentioned that the participants were randomly divided into 2 groups, but they did not describe the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no mention of blinding in study report. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the study report contained no statement regarding withdrawals but all the participants were included in the final analysis at the 14‐day follow‐up; however, there is no description for the number of participants followed up to total ulcer healing. |
| Selective reporting (reporting bias) | High risk | Comment: the study report described 1 outcome (time to complete ulcer healing) in the results section that was not properly described in the methods section. Unable to obtain RCT protocol (no response from email sent to trial author). |
| Other bias | High risk | Comment: unit of randomisation and unit the analysis were the participant. There was a small imbalance in groups regarding sex (more men in hydrogel group). Even though the study mentioned the mean baseline ulcer surface area did not differ between groups, our independent analysis using a 2‐tailed independent t‐test resulted in difference between groups. |
ABPI: ankle brachial pressure index; CI: confidence interval; ITT: intention to treat; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1994 | Not an RCT. |
| Anonymous 1997 | Not an RCT. |
| Beldon 2009 | Not an RCT. |
| Bolton 2008 | Not an RCT. |
| Farina 1997 | Hydrogel was not the only systematic difference between groups. |
| Flanagan 1995 | Not an RCT. |
| Fonder 2008 | Not an RCT. |
| Gibson 1995 | Leg ulcers with mixed aetiology presenting < 75% of venous leg ulcer in each group of the study. |
| Hampton 2004 | Not an RCT. |
| Hofman 2006 | Not an RCT. |
| Hutchinson 1997 | Not a treatment of interest. |
| JNMA 2007 | Not an RCT. |
| Jull 2013 | Not an RCT. |
| Karlsmark 2002 | Not a treatment of interest. |
| Kerstein 2001 | Not an RCT. |
| Kikta 1988 | Hydrogel dressing was not the only systematic difference across treatment groups. |
| Lafferty 2011 | Not an RCT. |
| Larsen 1997 | Not a treatment of interest. |
| Lawall 2012 | Not an RCT. |
| Milne 2014 | Not an RCT. |
| Perez 2000 | Not a treatment of interest. |
| Pirie 2009 | Not an RCT. |
| Robinson 1988 | Not a treatment of interest. |
| Robinson 1996 | No separate report of data for participants with venous leg ulcers. |
| Sironi 2003 | Hydrogel dressing was not the only systematic difference across treatment groups. |
| Timmons 2008 | Not an RCT. |
| Werner‐Schlenzka 1994 | Not a treatment of interest. |
| Young 2005 | Not an RCT. |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Altman 1993.
| Methods | RCT |
| Participants | 60 participants; all races; either sex; aged 18–85 years; with a diagnosis of venous, diabetic or arterial ulcer, or dehisced surgical incision |
| Interventions | NuGel vs gauze dressing with saline |
| Outcomes | Percent reduction of wound size; "wound condition"; pain; granulation tissue |
| Notes | Conference abstract with limited information. The authors did not report outcomes separately for venous leg ulcers. We obtained no response from the authors. |
Gago 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Conference abstract with limited information. The authors did not report the aetiology of leg ulcers. The reported outcome maceration was not within the scope of this review. We are unsure if other outcomes were assessed. We contacted the authors but obtained no further information. |
Hofman 1994.
| Methods | RCT |
| Participants | 62 participants with sloughy leg ulcers |
| Interventions | DuoDerm vs IntraSite Gel under Melonin |
| Outcomes | Reduction in slough and reduction in pain |
| Notes | Conference abstract with limited information. The authors did not report the aetiology of leg ulcers. We contacted the authors but obtained no further information. |
Hofman 1996.
| Methods | Report of clinical trials and case studies |
| Participants | 62 participants with sloughy leg ulcers |
| Interventions | Granugel vs IntraSite Gel |
| Outcomes | Reduction in slough; wound area |
| Notes | Report with limited information. The authors did not report the aetiology of leg ulcers. We contacted the authors but obtained no further information. |
Semenic 2018.
| Methods | Prospectively randomised, double‐blind, placebo controlled trial |
| Participants | Volunteers with venous ulcers (12 participants), arterial ulcers (12 participants), diabetic ulcers (30 participants) and non‐healed ulcers following injury (6 participants) |
| Interventions | Allogenic platelet gel vs hydrogel |
| Outcomes | Percent change in wound area; laboratory blood values and inflammation parameters in the blood; adverse reactions |
| Notes | Venous leg ulcers accounted for 12/60 participants. Data on venous leg ulcer cure were not reported separately. Authors contacted to request data for analysis. |
Sparholt 2002.
| Methods | RCT |
| Participants | 32 participants with venous leg ulcer |
| Interventions | Purilon Gel vs IntraSite Gel |
| Outcomes | Wound area; pain; wear time |
| Notes | Conference abstract with limited information. The authors did not report numerical data for wound area or pain. We contacted the authors but obtained no further information. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN47349949.
| Study name | Comparison of cleaning of leg ulcers using pads or wound dressings |
| Methods | Prospective open‐label randomised controlled exploratory study |
| Participants | Leg ulcer, chronic wound |
| Interventions | Debrisoft (Activa Healthcare) that consists of monofilament polyester fibres, and the reverse side is coated with polyacrylate vs Intrasite Conformable (Smith & Nephew Healthcare), a hydrogel wound dressing. |
| Outcomes | Primary outcomes: changes in the appearance of the wound bed will be based upon quantitative size and colour analysis of photographs taken before debridement and 10 minutes after debridement has occurred with these measures repeated weekly for 2 weeks; wound size within 14 days of treatment. Secondary outcomes: removal of hyperkeratosis; relative costs of each treatment option; narrative reports from clinical staff and participants regarding their experience of the interventions; any pain and discomfort associated with the use of the interventions rated using visual analogue scales. |
| Starting date | |
| Contact information | |
| Notes | Results of the trial are overdue. We will assess inclusion if data are published or provided by authors. |
NCT03275831.
| Study name | PluroGel on wounds of mixed aetiology |
| Methods | Randomised controlled trial |
| Participants | People with a non‐healing venous leg ulcer or mixed aetiology ulcer with a presence of ≥ 25% visible slough within the wound bed |
| Interventions | IntraSite Gel (hydrogel) vs PluroGel Burn |
| Outcomes | Change in ulcer size; change in mean percent reduction of slough in wound bed over 4 weeks of treatment; patient evaluation; staff evaluation |
| Starting date | 8 January 2018 |
| Contact information | Keith G Harding: keith.harding@wwic.wales Kara Cassady: kcassady@medicine.com |
| Notes | clinicaltrials.gov/ct2/show/NCT03275831 |
RBR‐5d4s4f.
| Study name | Effect of therapies combination to healing of venous ulcers, leg pain and self rated health and quality of life of patients in a six‐month follow‐up |
| Methods | Randomised controlled trial, parallel, open, with 2 study arms |
| Participants | People with a leg ulcer with clinical signs of impaired venous circulation with or without the low impairment of arterial circulation |
| Interventions | Intervention followed 3 paths: orientation for all participants on exercising the lower limbs and resting with legs elevated using an educational booklet. Oral supplementary nutrition for all participants using Cubitan 200 mL 3 times a day between meals, for up to 8 weeks, or less in the case of early healing. Application of topical therapy combined with compression therapy, in different ways, for group A (30 participants) and B (30 participants), by random allocation. Topical therapy combined with compression therapy consisted of 3 phases. In the first phase, group A treated with papain 2% combined with Surepress compression therapy, and group B with hydrogel 2% combined with Surepress compression therapy. In the second phase, group A treated with rayon gauze and Unna Boot or Surepress compression therapy and group B with Membracel and Unna Boot or Surepress compression therapy. Third phase covered exercises and elevation with the leg rests and Surepress compression therapy. |
| Outcomes | Primary outcome: complete wound healing Secondary outcomes: "Progress of cictrization" (Pressure Ulcer Scale for Healing); ulcer healing rate; "resolvability" (calculated by dividing the number of participants who had all wounds completely healed by the total number of study participants); "positive self‐evaluation of health"; quality of life; intensity of pain; "quality of pain" (McGill Pain Questionnaire) |
| Starting date | 1 July 2016 |
| Contact information | Maria Márcia Bachion; E‐mail: mbachion@gmail.com; telephone: +55‐062991464878 |
| Notes | If results from first phase are reported separated for people with venous leg wounds without arterial disease (or if most ulcers are not mixed ulcers, as specified in the protocol) we may be able to pool data. |
Differences between protocol and review
Where studies reported outcomes at multiple time points, we preferred results from the longest follow‐up. We carried out a GRADE assessment on all outcomes summarised in this review, rather than limiting this to complete wound healing and incidence of wound infection (as proposed in the published protocol) (Ribeiro 2013). The review protocol did not specify outcomes for the summary of findings tables; therefore, we included the outcomes complete wound healing, incidence of wound infection, change in ulcer size, time‐to‐ulcer healing, health‐related quality of life and costs, because we consider them critical or important for decision‐making in health care.
Contributions of authors
CR: designed the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; wrote to study author/experts/companies; provided data; performed economic analysis; performed translations; approved the final review prior to submission; and is a guarantor of the review.
FD: conceived, designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; provided data; performed economic analysis; performed translations; approved the final review prior to submission; and is a guarantor of the review.
GF: conceived the review; designed the review; co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; contributed to writing or editing the review; advised on the review; secured funding; performed previous work that was the foundation of the current review; provided data; performed economic analysis; performed translations; approved the final review prior to submission; and is a guarantor of the review.
Contributions of the editorial base
Nicky Cullum (Joint Co‐ordinating Editor): edited the protocol; advised on methodology, interpretation and content; and approved the final version of the protocol prior to publication.
Gill Norman (Editor): edited the review; advised on methodology, interpretation and content; and approved the final version of the review prior to publication.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process. Advised on interpretation and content. Edited the protocol and the review.
Sophie Bishop (Information Specialist): designed the search strategy, ran the searches and edited the search methods section.
Tom Patterson (Editorial Assistant): edited the references and the plain language summary.
Sources of support
Internal sources
-
Laboratorio PneumoCardiovascular HUOL / EBSERH – Universidade Federal do Rio Grande do Norte, Brazil
Research Lab dedicated to study and the production of high‐quality health evidence
External sources
-
MCT/CT‐Saúde and Decit/SCTIE/MS (CNPq – process 559416/2009‐1), Brazil
Research grant
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
CR: research was supported by funding from Brazilian Ministry of Science and Technology/Ministry of Health and the Brazilian National Council of Scientific and Technological Development (Grant 559416/2009‐1).
FD: research was supported by funding from Brazilian Ministry of Science and Technology/Ministry of Health and the Brazilian National Council of Scientific and Technological Development (Grant 559416/2009‐1).
GF: research was supported by funding from Brazilian Ministry of Science and Technology/Ministry of Health and the Brazilian National Council of Scientific and Technological Development (Grant 559416/2009‐1).
New
References
References to studies included in this review
De la Brassinne 2006 {published data only}
- De la Brassinne M, Thirion L, Horvat LI. A novel method of comparing the healing properties of two hydrogels in chronic leg ulcers. Journal of the European Academy of Dermatology and Venereology 2006;20(2):131-5. [DOI] [PubMed] [Google Scholar]
Gethin 2007 {published data only}
- Anonymous. Retraction statement: manuka honey vs. hydrogel – a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Journal of Clinical Nursing 2015;24(17-8):2686. [DOI: 10.1111/jocn.12652] [DOI] [PubMed] [Google Scholar]
- Armstrong DG. Manuka honey improved wound healing in patients with sloughy venous leg ulcers. Evidence Based Medicine 2009;14(5):148. [DOI: 10.1136/ebm.14.5.148] [DOI] [PubMed] [Google Scholar]
- Gethin G, Cowman S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: an RCT. Journal of Wound Care 2008;17(6):241-7. [DOI: 10.12968/jowc.2008.17.6.29583] [DOI] [PubMed] [Google Scholar]
- Gethin G, Cowman S. Manuka honey versus hydrogel to deslough venous leg ulcers: a randomised controlled trial. In: 17th Conference of the European Wound Management Association; 2007 May 2-4; Glasgow (UK). 2007:29.
- Gethin G, Cowman S. Manuka honey versus hydrogel to deslough venous leg ulcers: a randomised controlled trial. In: Third Congress of the World Union of Wound Healing Societies Meeting; 2008 Jun 4-8; Toronto (Canada). 2008:4.
- Gethin G, Cowman S. Manuka honey vs. hydrogel – a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous leg ulcers. Journal of Clinical Nursing 2009;18(3):466-74 [withdrawn due to errors in the data analysis that affected the article's finding. Data from this publication not used in review; see Appendix 6]. [DOI: 10.1111/j.1365-2702.2008.02558.x] [DOI] [PubMed] [Google Scholar]
- Gethin G. Manuka Honey Versus Hydrogel – a Prospective, Open Label, Multicentre, Randomised Controlled Trial to Compare Desloughing Efficacy and Healing Outcomes in Venous Ulcers [PhD thesis]. Dublin (Ireland): Royal College of Surgeons in Ireland, 2007. [Google Scholar]
Grotewohl 1994 {published data only}
- Grotewohl JH. The phase-oriented wound dressings of ulcus cruris venosum: experience with the use of new hydrogel Opragel. Zeitschrift fur Allgemeinmedizin 1994;70(9):351-4. [Google Scholar]
He 2008 {published data only}
- He Q, Wu G, Yu B, Zhang T, Wang W, Gu Q. A prospective study on wound-healing hydrogel in treating chronic venous ulcer of lower extremities. Chinese Journal of Reparative and Reconstructive Surgery 2008;22(3):311-3. [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1994 {published data only}
- Anonymous. New therapy concept in leg ulcer [Neues TherapieKonzept bei Ulcus cruris]. TW Dermatologie 1994;24(6):407. [Google Scholar]
Anonymous 1997 {published data only}
- Anonymous. Differential therapy of chronic wounds [Ein neuer weg zur therapie von dermatomykosen]. Hautarzt 1997;48(2 Suppl):1-4. [PMID: ] [PubMed] [Google Scholar]
Beldon 2009 {published data only}
- Beldon P. Topical dressings to manage pain in venous leg ulceration. British Journal of Community Nursing 2009;14(3):S6-10. [DOI: 10.12968/bjcn.2009.14.Sup1.40104] [DOI] [PubMed] [Google Scholar]
Bolton 2008 {published data only}
- Bolton L. Evidence corner: honey on sloughy venous leg ulcers. Wounds 2008;20(12):A13-4. [Google Scholar]
Farina 1997 {published data only}
- Farina M, Bonifati C, Dallavalle E. TEAM system in the treatment of venous leg ulcers. A comparative study. In: European Wound Management Association Conference; 1997 Apr 27-9; Milan (Italy). 1997:50-1.
Flanagan 1995 {published data only}
- Flanagan M. The efficacy of a hydrogel in the treatment of wounds with non-viable tissue. Journal of Wound Care 1995;4(6):264-7. [DOI: 10.12968/jowc.1995.4.6.264] [DOI] [PubMed] [Google Scholar]
Fonder 2008 {published data only}
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. Journal of the American Academy of Dermatology 2008;58(2):185-206. [DOI: 10.1016/j.jaad.2007.08.048] [DOI] [PubMed] [Google Scholar]
Gibson 1995 {published data only}
- Gibson B. A cost effectiveness comparison of two gels in the treatment of sloughy leg ulcers. In: Symposium on Advanced Wound Care and Medical Research Forum on Wound Repair; 1995 April; San Diego (CA). 1995.
Hampton 2004 {published data only}
- Hampton S. A small study in healing rates and symptom control using a new sheet hydrogel dressing. Journal of Wound Care 2004;7:297-300. [DOI: 10.12968/jowc.2004.13.7.26639] [DOI] [PubMed] [Google Scholar]
Hofman 2006 {published data only}
- Hofman D. Practical steps to address pain in wound care. British Journal of Nursing 2006;15(21):10-4. [Google Scholar]
Hutchinson 1997 {published data only}
- Hutchinson J. Prospective study of clinical infections in wounds dressed with hydrocolloid versus conventional dressings. In: Sixth European Conference on Advances in Wound Management; 1996 Oct 1-4; Amsterdam (Netherlands). 1997:263.
JNMA 2007 {published data only}
- JNMA Editorial Office. Dressing choice unimportant for leg ulcers. Journal of the National Medical Association 2007;99(12):1422. [Google Scholar]
Jull 2013 {published data only}
- Jull A. Maggots mixed munching. Nursing Review 2013;13(7):24. [Google Scholar]
Karlsmark 2002 {published data only}
- Karlsmark T, Zillmer R, Agren MS, Gottrup F. Adhesive dressings: effect in healthy and peri-wound skin. In: 12th Conference of the European Wound Management Association; 2002 May 23-25; Granada (Spain). 2002:127.
Kerstein 2001 {published data only}
- Kerstein MD, Gemmen E, Rijswijk L, Lyder CH, Philips T, Xakellis G, et al. Cost and cost effectiveness of venous and pressure ulcer protocols of care. Disease Management and Health Outcomes 2001;9(11):651-63. [DOI: 10.2165/00115677-200109110-00005] [DOI] [Google Scholar]
Kikta 1988 {published data only}
- Kikta MJ, Schuler JJ, Meyer JP, Durham JR, Eldrup JJ, Schwarcz TH, et al. A prospective, randomized trial of Unna's boots versus hydroactive dressing in the treatment of venous stasis ulcers. Journal of Vascular Surgery 1988;7(3):478-83. [PubMed] [Google Scholar]
Lafferty 2011 {published data only}
- Lafferty B, Wood L. Improved care and reduced costs with advanced wound dressings. Wounds UK 2011;7(1):14-23. [Google Scholar]
Larsen 1997 {published data only}
- Larsen AM, Andersen KE, Berg BK. A comparative study on the effect and function of Comfeel Purilon versus IntraSite gel on leg ulcers. In: European Wound Management Association Conference; 1997, Apr 27-29; Milan (Italy). 1997.
Lawall 2012 {published data only}
- Lawall H. Treatment of chronic wounds. Vasa 2012;41(6):396-409. [DOI: 10.1024/0301-1526/a000230] [DOI] [PubMed] [Google Scholar]
Milne 2014 {published data only}
- Milne J. A research roundup of recent papers relevant to wound care. Wounds UK 2014;10(2):116-7. [Google Scholar]
Perez 2000 {published data only}
- Perez RC, Aguilar VC, Colome AM, Garcia AG, Torra i Bou J-E. A comparison of the effectiveness and cost of moist environment dressings treatment as compared to traditional dressings treatment [Comparacion de la efectidad y coste de la cura en ambiente humedo frente a la cura tradicional]. Revista de Enfermería 2000;23(1):17-24. [PMID: ] [PubMed] [Google Scholar]
Pirie 2009 {published data only}
- Pirie G, Duguid K, Timmons J. Cutimed Sorbact gel: a new infection management dressing. Wounds UK 2009;5(2):74-8. [Google Scholar]
Robinson 1988 {published data only}
- Robinson BJ. Randomized comparative trial of Duoderm vs Viscopaste PB7 bandage in the management of venous leg ulceration and cost to the community. In: Ryan TJ, editors(s). Beyond Occlusion: Wound Care Proceedings. London (UK): Royal Society of Medicine, 1988:101-4. [Google Scholar]
Robinson 1996 {published data only}
- Robinson B. A cost-effectiveness comparison of two gels in the treatment of sloughy leg ulcers. In: Fifth European Conference on Advances in Wound Management; 1995 Nov 21-24; Harrogate (UK). 1996:149-50.
Sironi 2003 {published data only}
- Sironi G, Losa S, DiLuca G, Pezzoni F. Patients with venous leg ulcers in vascular treatment with Intrasite gel, Opsite Flexigrid, Allevyn and Flexobande Legere/Forte: a randomised comparative clinical evaluation – an interim report. In: 13th Conference of the European Wound Management Association; 2003 May 22-24; Pisa (Italy). 2003.
- Sironi G, Losa S, DiLuca G, Pezzoni F. Treatment of venous leg ulcers with Intrasite gel, OpSite Flexigrid, Allevyn hydrocellular dressings and Flexobande (elastic compression bandage) in vascular surgery. A protocol for clinical evaluations. In: 3rd European Conference on Advances in Wound Management; 1993 Oct 19-22; Harrogate (UK). 1994:164.
Timmons 2008 {published data only}
- Timmons J. Treatment of a bilateral necrotic leg ulcer with Mesitran. Wounds UK 2008;4(4):129. [Google Scholar]
Werner‐Schlenzka 1994 {published data only}
- Werner-Schlenzka H, Lehnert W. Topical treatment of venous leg ulcers with a prostacyclin hydrogel: a double blind trial. Prostaglandins Leukotrienes and Essential Fatty Acids 1994;51(3):203-6. [DOI: 10.1016/0952-3278(94)90135-x] [DOI] [PubMed] [Google Scholar]
Young 2005 {published data only}
- Young SR, Hampton S. Pain management in leg ulcers using ActiFormCool™. Wounds UK 2005;1(3):94-6, 98, 100-1. [Google Scholar]
References to studies awaiting assessment
Altman 1993 {published data only}
- Altman MI, Mulder GD. Multi-centre evaluation of Nu-Gel wound dressing in full-thickness chronic wounds of the lower extremities. In: Third European Conference on Advances in Wound Management; 1993 Oct 19-22; Harrogate (UK). 1993:164-5.
Gago 2002 {published data only}
- Gago FM, Verdu SJ, Garcia-Conzalez RF, Guerrero JJ, Rueda LJ, Gaztelu VV. Maceration of the skin surrounding ulcerous lesions. Comparison of seven groups of moist dressings [Maceración en la piel perilesional de las úlceras en pierna. Comparación de siete grupos de apósitos de cura en ambiente húmedo]. In: 12th Conference of the European Wound Management Association; 2002 May 23-25; Granada (Spain). 2002:132.
Hofman 1994 {published data only}
- Hofman D, Burgess B, Cherry GW, Robinson BJ, Ryan TJ. Management of pressure sores and leg ulcers with Duoderm hydroactive gel. In: Fourth Annual Meeting of the European Tissue Repair Society; 1994 Aug 25-28; Oxford (UK). 1994:198.
Hofman 1996 {published data only}
- Hofman D, Burgess B. Management of leg ulcers and pressure sores with Granugel [personal communication]. Fax from Smith & Nephew Healthcare 1996.
Semenic 2018 {published data only}
- Semenic D, Cirman T, Rozman P, Smrke DM. Regeneration of chronic wounds with allogeneic platelet gel versus hydrogel treatment: a prospective study. Acta Clinica Croatica 2018;57(3):434-42. [DOI: 10.20471/acc.2018.57.03.05] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sparholt 2002 {published data only}
- Sparholt SH, Wilhelmsen F. Outcome evaluation of two hydrogels. Ostomy/Wound Management 2002;48(4):74. [Google Scholar]
References to ongoing studies
ISRCTN47349949 {published and unpublished data}
- ISRCTN47349949. Comparison of cleaning of leg ulcers using pads or wound dressings. isrctn.com/ISRCTN47349949 (first received 14 June 2012). [DOI: 10.1186/ISRCTN47349949] [DOI]
NCT03275831 {unpublished data only}
- NCT03275831. PluroGel on wounds of mixed etiology. clinicaltrials.gov/ct2/show/NCT03275831 (first received 8 September 2017).
RBR‐5d4s4f {published and unpublished data}
- RBR-5d4s4f. Effect of therapies combination to healing of venous ulcers, leg pain and self rated health and quality of life of patients in a six month follow-up. ensaiosclinicos.gov.br/rg/RBR-5d4s4f (first received 7 December 2018).
Additional references
BNF 2018
- BMJ Group, Royal Pharmaceutical Society of Great Britain. British National Formulary (BNF). Wound management products and elasticated garments. bnf.nice.org.uk/wound-management/ (accessed 25 February 2022).
Bradbury 2008
- Bradbury S, Ivins N, Harding K, Turner A. Measuring outcomes with complex patients: an audit of the effect of Actiform Cool on painful wounds. Wounds UK 2008;4(3):22-31. [Google Scholar]
Briggs 2003
- Briggs M, Closs SJ. The prevalence of leg ulceration: a review of the literature. EWMA Journal 2003;3(2):14-20. [Google Scholar]
Briggs 2012
- Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD001177. [DOI: 10.1002/14651858.CD001177.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2010
- Collins L, Seraj S. Diagnosis and treatment of venous ulcers. American Family Physician 2010;81(8):989-96. [PMID: ] [PubMed] [Google Scholar]
De Araujo 2003
- De Araujo T, Valencia I, Federman DG, Kirsner RS. Managing the patient with venous ulcers. Annals of Internal Medicine 2003;138(4):326-34. [DOI: 10.7326/0003-4819-138-4-200302180-00012] [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI: 10.1111/hir.12251] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 2 December 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2021. Available at gradepro.org.
Grey 2006
- Grey JE, Stuart E, Harding KG. Venous and arterial leg ulcers – ABC of wound healing. BMJ 2006;332(7537):347-50. [DOI: 10.1136/bmj.332.7537.347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. rating the quality of evidence – study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64:407-15. [DOI: 10.1016/j.jclinepi.2010.07.017] [DOI] [PubMed] [Google Scholar]
Herber 2007
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;5(44):1-12. [DOI: 10.1186/1477-7525-5-44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Jones 2007
- Jones JE, Nelson EA. Skin grafting for venous leg ulcers. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD001737. [DOI: 10.1002/14651858.CD001737.pub3] [DOI] [PubMed] [Google Scholar]
Lantis 2013
- Lantis JC, Marston WA, Farber A, Kirsner RS, Zhang Y, Lee TD, et al. The influence of patient and wound variables on healing of venous leg ulcers in a randomized controlled trial of growth-arrested allogeneic keratinocytes and fibroblasts. Journal of Vascular Surgery 2013;58(2):433-9. [DOI: 10.1016/j.jvs.2012.12.055] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Li 2022
- Li T, Higgins JP, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandelbaum 2003
- Mandelbaum SH, Di Santis EP, Mandelbaum MH. Cicatrization: current concepts and auxiliary resources – part II [Cicatrização: conceitos atuais e recursos auxiliares – parte II]. Anais Brasileiros de Dermatologia 2003;78(5):525-40. [DOI: 10.1590/S0365-05962003000500002] [DOI] [Google Scholar]
Margolis 2002
- Margolis D, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. Journal of the American Academy of Dermatology 2002;46(3):381-6. [DOI: 10.1067/mjd.2002.121739] [DOI] [PubMed] [Google Scholar]
Margolis 2004
- Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair and Regeneration 2004;12(2):163-8. [DOI: 10.1111/j.1067-1927.2004.012207.x] [DOI] [PubMed] [Google Scholar]
Medical Dictionary
- Medical Dictionary. medical-dictionary.thefreedictionary.com (accessed 4 March 2022).
Merriam‐Webster Medical Dictionary
- Merriam-Webster Inc. Glossary. www.merriam-webster.com/medical (accessed 4 March 2022).
NICE 2016
- National Institute for Health and Care Excellence. Chronic wounds: advanced wound dressings and antimicrobial dressings. Available from www.nice.org.uk/advice/esmpb2/chapter/Key-points-from-the-evidence (accessed 14 May 2018).
Norman 2018
- Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. Cochrane Database of Systematic Reviews 2018, Issue 6. Art. No: CD012583. [DOI: 10.1002/14651858.CD012583.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Meara 2009
- O'Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD000265. [DOI: 10.1002/14651858.CD000265.pub2] [DOI] [PubMed] [Google Scholar]
O'Meara 2010
- O'Meara S, Al-Kurdi D, Ologun Y, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD003557. [DOI: 10.1002/14651858.CD003557.pub3] [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Palfreyman 2007
- Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ 2007;335(7613):244. [DOI: 10.1136/bmj.39248.634977.AE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pérez 2019
- Pérez MB, López-Casanova P, Lavín RS, la Torre HG, Verdú-Soriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre – a time series study (2010–2014). International Wound Journal 2019;16:256-65. [DOI: 10.1111/iwj.13026] [DOI] [PMC free article] [PubMed] [Google Scholar]
Persoon 2004
- Persoon A, Heinen MM, Vleuten CJ, Rooij MJ, de Kerkhof PC, Achterberg T. Leg ulcers: a review of their impact on daily life. Journal of Clinical Nursing 2004;13(3):341-54. [DOI: 10.1046/j.1365-2702.2003.00859.x] [DOI] [PubMed] [Google Scholar]
Platsidacki 2017
- Platsidacki E, Kouris A, Christodoulou C. Psychosocial aspects in patients with chronic leg ulcers. Wounds 2017;29(10):306-10. [DOI: 10.25270/wnds/2017.10.306310] [DOI] [PubMed] [Google Scholar]
Purwins 2010
- Purwins S, Herberger K, Debus ES, Rustenbach SJ, Pelzer P, Rabe E, et al. Cost-of-illness of chronic leg ulcers in Germany. International Wound Journal 2010;7(2):97-102. [DOI: 10.1111/j.1742-481X.2010.00660.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
RCN 2006
- Royal College of Nursing (RCN). Clinical Practice Guidelines: the Nursing Management of Patients with Venous Leg Ulcers. London (UK): Royal College of Nursing, 2006. [Google Scholar]
Review Manager Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Robson 2006
- Robson MC, Cooper DM, Aslam R, Gould LJ, Harding KG, Margolis DJ, et al. Guidelines for the treatment of venous ulcers. Wound Repair and Regeneration 2006;14(6):649-62. [DOI: 10.1111/j.1524-475X.2006.00174.x] [DOI] [PubMed] [Google Scholar]
Rooke 2011
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of Vascular Surgery 2011;54(5):e32-58. [DOI: 10.1016/j.jvs.2011.09.001] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152(11):726-32. [DOI: 10.7326/0003-4819-152-11-201006010-0023] [DOI] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Valencia 2001
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. Journal of the American Academy of Dermatology 2001;44(3):401-20. [DOI: 10.1067/mjd.2001.111633] [DOI] [PubMed] [Google Scholar]
van Hecke 2011
- Hecke A, Verhaeghe S, Grypdonck M, Beele H, Defloor T. Processes underlying adherence to leg ulcer treatment: a qualitative field study. International Journal of Nursing Studies 2011;48(2):145-55. [DOI: 10.1016/j.ijnurstu.2010.07.001] [DOI] [PubMed] [Google Scholar]
Wipke‐Tevis 2000
- Wipke-Tevis DD, Rantz MJ, Mehr DR, Popejoy L, Petroski G, Madsen R, et al. Prevalence, incidence, management, and predictors of venous ulcers in the long-term-care population using the MDS. Advances in Skin and Wound Care 2000;13(5):218-24. [PMID: ] [PubMed] [Google Scholar]
Wollina 2006
- Wollina U, Abdel-Naser MB, Mani R. A review of the microcirculation in skin in patients with chronic venous insufficiency: the problem and the evidence available for therapeutic options. International Journal of Lower Extremity Wounds 2006;5(3):169-80. [DOI: 10.1177/1534734606291870] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ribeiro 2013
- Ribeiro CT, Dias FA, Fregonezi GA. Hydrogel dressings for venous leg ulcers. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD010738. [DOI: 10.1002/14651858.CD010738] [DOI] [PMC free article] [PubMed] [Google Scholar]


