Abstract
Regulatory T (Treg) cells are a specialized CD4+ T cell subpopulation that are essential for immune homeostasis, immune tolerance, and protection against autoimmunity. There is evidence that sex-steroid hormones estrogen and progesterone modulate Treg cell abundance and phenotype in women. Since natural oscillations in these hormones are modified by hormonal contraceptives, we examined whether oral contraception (OC) use impacts Treg cells and related T cell populations. T cells were analyzed by multiparameter flow cytometry in peripheral blood collected across the menstrual cycle from healthy women either using OC or without hormonal contraception and from age-matched men. Compared to naturally cycling women, women using OC had fewer Treg cells and an altered Treg cell phenotype. Notably, Treg cells exhibiting a strongly suppressive phenotype, defined by high FOXP3, CD25, Helios, HLADR, CTLA4, and Ki67, comprised a lower proportion of total Treg cells, particularly in the early- and mid-cycle phases. The changes were moderate compared to more substantial differences in Treg cells between women and men, wherein women had fewer Treg cells—especially of the effector memory Treg cell subset—associated with more T helper type 1 (Th1) cells and CD8+ T cells and lower Treg:Th1 cell and Treg:CD8+ T cell ratios than men. These findings imply that OC can modulate the number and phenotype of peripheral blood Treg cells and raise the possibility that Treg cells contribute to the physiological changes and altered disease susceptibility linked with OC use.
Keywords: tolerance, autoimmunity, sex hormones, T cells, regulatory T cells
In reproductive-aged women, the sex-steroid hormones progesterone (P4) and estradiol [1,3,5(10)-estratriene-3,17β-diol; E2] are released by the ovary in a dynamic cyclic pattern to regulate ovulation and prepare uterine receptivity for pregnancy. These hormones exert effects on the development, maturation, and functional competence of immune cells of both the innate and adaptive immune compartments to support their participation in homeostasis and function of reproductive tissues, while maintaining defence against infectious agents and tumour surveillance (1). There are cyclic variations in activity and competence of the immune response associated with oscillating E2 and P4 levels (2, 3), reflected in small but physiologically relevant fluctuations in peripheral blood leukocyte numbers and phenotypes over the course of the menstrual cycle (4, 5). This regulation by E2, P4, and other sex hormones imparts sexual dimorphism in immune function that manifests as greater resistance to microbial infection and cancer—but, conversely, higher rates of autoimmune disease—in women compared to men (6-8).
Immune regulation by sex hormones is an important enabler of viviparous reproduction in mammals. In the adaptive immune compartment, sex hormones affect the balance of effector vs regulatory T (Treg) cells that in turn underpins uterine receptivity to embryo implantation and placental formation (9). Of particular interest are effects of sex hormones on FOXP3+CD4+ Treg cells. Treg cells are critical for maternal fetal tolerance (10) and important modulators of many other physiological processes through their potent anti-inflammatory, immune-regulatory, and vasoregulatory functions (11-13). A reduced abundance and/or impaired function of Treg cells in women is associated with recurrent miscarriage (14) and development of pregnancy complications such as preeclampsia (15, 16) and preterm labor (17). The Treg cell pool has been reported to expand in response to E2 in the proliferative phase of each cycle (18, 19), with an E2-driven increase peaking at ovulation (4) to support uterine receptivity for embryo implantation in the postovulatory, luteal phase. Studies in mice indicate the modulating effects of sex hormones on Treg cells are comparable across species (20, 21) and likely helped propel the evolution of T cell-mediated immune tolerance to mitigate maternal-fetal conflict in placental mammals (22, 23).
Sex hormones E2 and P4 appear to mediate direct and indirect effects on immune cell development, proliferation, homing, and function. E2 receptors are expressed by several leukocyte lineages (24, 25), enabling direct E2 regulation of proinflammatory CD4+ Th1 and Th17 T cells, as well as regulatory CD4+ Th2 and Treg cells (26-29), CD8+ T cells (30), and dendritic cells (31). P4 receptors are detectable in CD4+ and CD8+ T cells, macrophages, and dendritic cells (32-34). P4 acts to skew T cells away from an inflammatory Th1 response by repressing Ifng gene expression to elicit a protolerogenic or regulatory Th2-like response (35-37). Notably, P4 promotes generation of stable Treg cells (38-40) while suppressing development of Th17 immunity (38, 39, 41).
Globally, 8% of women of reproductive age use oral contraception (OC), with rates exceeding 30% in some countries (42). Monophasic and multiphasic combination OC pill use overrides oscillating E2 levels, by suppressing endogenous E2 across the cycle. Consistently high exogenous progestin prevents the mid-cycle surge in luteinizing hormone that stimulates ovulation, thus corpus lutea do not develop, and endogenous P4 production is also suppressed. Given the effects of sex hormones on T cells and that OC interferes with endogenous sex hormone synthesis and bioavailability (43), we reasoned that OC use may cause systemic changes in T cell populations and that Treg cells may be particularly vulnerable. Here, we report flow cytometry data indicating that OC use is associated with altered Treg cells and notably fewer highly suppressive Treg cells in the peripheral blood of women.
Materials and Methods
Study Participants
Study participants were healthy adult women of reproductive age (18-40 years) either not using OC (natural cycling, n = 23), or using a combined progestin and estrogen OC with a 21/7 day cycle regimen (21 days of hormone pill and 7 days of placebo pill; n = 18). OC medications are listed in Supplemental Table 1 (44). Healthy adult men (18-40 years, n = 17) were recruited as a comparison group. Descriptive parameters were provided by participants including height, weight, age, the type of OC pill used, and general health. Mean and range of age and body mass index of the 3 study groups are provided in Table 1. The study was approved by the University of Adelaide Human Research Ethics Committee (H-2013-037), and all participants provided written informed consent.
Table 1.
Clinical characteristics of study participants
| Men | Women | ||
|---|---|---|---|
| Naturally cycling | OC | ||
| Participants, n | 17 | 23 | 17 |
| Age, years | 28.3 ± 1.7 (18.3-40.7) |
28.0 ± 1.4 (18.9-40.4) |
26.0 ± 1.2 (18.9-34.8) |
| BMI,a kg/m2 | 23.4 ± 0.8 (20.1-33.1) |
22.1 ± 0.6 (18.1-29.3) |
21.9 ± 0.6 (17.7-27.3) |
Unless otherwise noted, data are given as mean ± SE of the mean (range).
Abbreviations: BMI, body mass index. OC, oral contraception.
aDifferences in age and BMI were analyzed by 1-way analysis of variance and post hoc Tukey test. There was no significant difference in age or BMI observed between participant groups.
Blood Collection
Venous peripheral blood samples (20 mL) were obtained at 3 time points across the menstrual or OC pill cycle. Time points were identified as (1) early cycle (days 2 and 3 after the start of menstruation for the naturally cycling group and days 2 and 3 after the start of withdrawal bleed for the OC group); (2) mid-cycle [1 day after the luteinizing hormone surge (ovulation) in the naturally cycling group and 14 days after the start of withdrawal bleed in the OC group]; and (3) late cycle [7 days after the luteinizing hormone surge in the natural cycling group (mid-luteal phase) and 21 days after the start of withdrawal bleed in the OC group]. The timing of the luteinizing hormone surge was determined by participant self-testing of urine samples using a First Response™ ovulation test kit (Church & Dwight, Ewing, NJ, USA).
Venipuncture was performed on the median cubital vein between 10 am and 2 pm, with blood collected in EDTA Vacutainer® tubes (BD Biosciences, Franklin Lakes, NJ, USA). Within 4 hours of sample collection, blood was centrifuged at 2000 × g for 10 minutes at room temperature, with the top plasma layer removed in 1 mL aliquots and stored at −80°C. Dulbecco’s modified phosphate-buffered saline (DPBS, Sigma-Aldrich, St. Louis MO, USA) was added to the blood sample in a volume equal to the plasma removed, and then further DPBS was added to the sample to double the volume, before layering over Lympholyte-H cell separation media (Cedarlane Laboratories, Burlington, Canada) and centrifugation at 600 × g for 30 minutes at room temperature without brake. The peripheral blood mononuclear cell layer, containing T cells, was removed by pipette, and then cells were washed in DPBS containing 2% heat-inactivated fetal calf serum (HI-FCS) and counted using a hemocytometer (Hawksley, London, UK) following trypan blue (Sigma-Aldrich) staining. Cells were resuspended in ice cold RPMI-1640 medium + 10% HI-FCS (both Thermo Fisher, Waltham, MA, USA) before adding cold freezing media [20% dimethyl sulfoxide (Sigma-Aldrich) + 80% HI-FCS] drop-wise until the volume was doubled. Cells were aliquoted into 1 mL cryopreservation tubes at a density of 2-6 × 106 cells/mL and then placed into a Mr FrostyTM (Thermo Fisher) controlled-rate freezing container filled with isopropanol (Sigma-Aldrich) and stored at −80°C for 24 hours before transfer into vapor-phase liquid nitrogen storage.
Plasma Sex Steroid Hormones
Plasma P4 and E2 were analyzed at the Adelaide Research Assay Facility. P4 was measured by coated-tube radioimmunoassay (IM1188, Beckman Coulter, Brea, CA, USA) [Supplemental Table 2 (44)], according to the manufacturer’s instructions. This assay detects P4 with an analytical sensitivity of 40 pg/mL. The lowest detection limit of the assay was 110 pg/mL. The intra-assay coefficient of variation of the assay was 3.8%. The assay is highly specific for P4, with low cross-reactivity for 17a-hydroxy P4 (1.15%), 6b-hydroxy P4 (5.1%), 16a-hydroxy P4 (1.82%), 5a-pregnanedione (15.2%), and 5b-pregnanedione (8.12%) but does not detect synthetic progestins.
E2 was measured by enzyme-linked immunosorbent assay (cat no. E-2000, LDN GmbH, Nordhorn, Germany) [Supplemental Table 2 (44)] in duplicate according to the manufacturer’s instructions. This assay detects E2 with an analytical sensitivity of 10.6 pg/mL. The lowest detection limit of the assay was 20 pg/mL. The intra-assay coefficient of variation of the assay was 3.0%. The interassay coefficient of variation of the assay was 5.0%. The assay is highly specific for E2 and has low cross-reactivity with estriol (2.27%), estrone (6.86%) but does not detect synthetic estrogens.
Flow Cytometry
To reduce intersample variation, blood samples were thawed and analyzed in batches of 10 to 15. Frozen vials of peripheral blood mononuclear cells were transferred from liquid nitrogen storage into a 37°C water bath to thaw. Warm RPMI-1640 medium containing 10% FCS was added drop-wise to double the volume. The cell suspension was transferred to a new tube and washed in 10 mL media by centrifugation at 300 × g for 7 minutes at room temperature, and then cells were resuspended in flow cytometry staining (FACS) buffer (1 × phosphate-buffered saline/0.1% bovine serum albumin/0.5% sodium azide) and counted. Staining of 1 × 106 cells per sample was performed in a U-bottom 96-well plate (Corning). First, cells were incubated with 50 μL of a 1:1000 dilution of fixable viability dye FVS575 (BD Biosciences) for 20 minutes in the dark at room temperature. Cells were then washed twice in FACS buffer and resuspended in 50 μL of a 1:100 dilution of human Fc block (BD Biosciences) in FACS buffer at room temperature for 15 minutes. For staining, 10 µL/well of Brilliant Stain Buffer Plus (BD Biosciences) was added to each well, followed by 50 uL antibody cocktail containing CCR7-BUV395, CD3-APCH7, CD4-BUV496, CD8-BUV737, HLADR-V500, CD25-BV786, and CD45RA-BB515 (all BD Biosciences) and CD127-PECy7 and Helios-APC (both BioLegend, San Diego, CA, USA) [see Supplemental Table 2 (44)]. Cells were incubated for 25 minutes in the dark at 4°C, washed twice in ice-cold FACS buffer, and fixed and permeabilized using the Foxp3 Staining Buffer Set (Thermo Fisher) according to the manufacturer’s instructions. Cells were washed in ice cold 1× permeabilization wash and then resuspended in 50 μL of antibody cocktail for intracellular markers, including Tbet-BV421, RORγt-PE, FOXP3-PECF594, and CTLA4-PECy5 (all BD Biosciences) and Ki67-APCR700 (Thermo Fisher) [see Supplemental Table 2 (44)]. Samples were incubated in the dark at 4°C for 30 minutes, washed twice, and resuspended in permeabilization wash. Data were acquired on a BD LSR Fortessa X-20, using FACS Diva Software. In each experiment, single-color controls were run to enable compensation, and fluorescence minus one and unlabeled controls were run to assist with gating positive and negative populations.
Flow Cytometry Data Analysis
All flow cytometry data were analyzed using FlowJo software (Treestar, Ashland, OR, USA). Gates were established to identify viable single lymphocytes and to exclude debris, dead cells, and doublets. Plots were used to identify CD3+CD4+ and CD3+CD8+ T cells. Tbet and RORγt were used to identify Th1 and Th17 T cells, respectively. Treg cells defined as CD3+CD4+CD25+FOXP3+CD127− were further assessed for phenotype, using Helios, CTLA4, HLADR, and CD45RA, surrogate markers for Treg cell functional capacity, and using Ki67 as a marker of proliferation. Within all T cell compartments, CCR7 and CD45RA expression was analyzed to determine the memory/naïve status of cells. Data are presented as the percentage of cells expressing specific markers or the geometric mean fluorescence intensity (MFI) of an individual marker within a specified population.
Flow cytometry data were also analyzed using the nonbiased, nonlinear dimensionality reducing t-distributed stochastic neighbor embedding (tSNE) algorithm as previously described (45). Briefly, for the CD3+ T cell tSNE analysis, compensated .fcs files were exported from FACSDiva software, and gates were established to exclude dead cells, debris, and doublets using physical parameters (FSC-H, -W, -A and SSC-H, -W, -A) and gated to include events that were viable T cells, defined as FVS575-negative and CD3-positive, and any off-scale (0.5% high and low fluorescent) events were gated out. From each data file 11 437 random events were concatenated within FlowJo. A subsample of 200 000 events from the concatenated file was assessed by the clustering algorithm X-Shift (version 1.3) (46), using the markers CD4, CD8, FOXP3, CD25, Helios, CD127, HLADR, CD45RA, CCR7, CTLA4, Tbet, RORγt, and Ki67. X-Shift defined 14 unique clusters. The concatenated file was transformed using the tSNE algorithm with Barns-Hut approximation, 1000 iterations, and a perplexity of 30 in FlowJo. The clusters defined by X-Shift were applied to the tSNE plot. The MFI of each cell marker in each cluster was acquired from FlowJo and used to generate a heat map in GraphPad Prism version 8.0.0 for Windows (GraphPad Software, www.graphpad.com). The proportion of cells within each of the 14 clusters was assessed for each individual sample.
Similarly, for the Treg cell tSNE analysis, gates were established to exclude dead cells, debris, doublets, and off-scale events, while capturing CD3+CD4+FOXP3+CD25+CD127− Treg cells. From each data file 619 random Treg cell events were concatenated and transformed using the tSNE algorithm with Barns-Hut approximation, 1000 iterations, and a perplexity of 30 in FlowJo by assessing expression of FOXP3, CD25, Helios, HLADR, CD45RA, CCR7, CTLA4, and Ki67. X-Shift defined 12 unique clusters. MFI data were used to generate a heat map, and the proportion of cells within each of the 12 clusters was assessed as described in the previous discussion.
Statistical Analysis
IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA) was used for all data analysis. Data sets were tested for normality of distribution by Shapiro-Wilk and Kolmogorov-Smirnov tests. A small number of data points (<3 per parameter), defined as definitive outliers by both ROUT test (Q = 0.1%) and Grubb’s test (P < 0.01), were excluded from the analysis. A linear mixed model analysis was used to evaluate the relative effects of sex, cycle phase, OC, and OC × cycle interaction (Table 2). Two models were tested. In Model 1 (male and female participants), effects of sex (male, female) and group (men, naturally cycling women, and women taking OC) were evaluated. In Model 2 (female participants), effects of OC, cycle phase, and OC × cycle interaction were evaluated. When OC, cycle, and/or OC × cycle interaction were identified as relevant factors in Model 2 (P < 0.1), specific effects of OC on T cell parameters at different cycle phases were identified by pairwise comparisons with OC and cycle phase as factors, and adjustment for multiple comparisons [Figs. 1-5; Supplemental Figures 1 and 2 (44)]. For all statistical tests, differences between groups were considered significant when P < 0.05.
Table 2.
Effect of sex, oral contraception (OC), and cycle phase on T cell parameters (reanalysis, OL removed)
| Model 1a | Model 2b | ||||
|---|---|---|---|---|---|
| Parameter | Female sex | OC use | OC use | Cycle phase |
OC × Cycle interaction |
| T cells | NS | NS | NS | NS | NS |
| CD4+ (%CD3+) | NS | NS | NS | NS | NS |
| CD8+ (%CD3+) | NS | NS | NS | NS | NS |
| CD8−CD4- (%CD3+) | NS | NS | NS | NS | NS |
| CD8+CD4+ (%CD3+) | ↑P = 0.009 | ↑P = 0.006 | ↑P = 0.012 | NS | NS |
| Treg cells | |||||
| CD4+CD25+FOXP3+CD127-(%CD4+) (Treg cells) | ↓P = 0.019 | NS | NS | NS | P = 0.065 |
| Ki67+ (%Treg) | NS | NS | NS | NS | NS |
| FOXP3 MFI | NS | NS | NS | NS | NS |
| Helios+ (%Treg) | NS | ↓P = 0.024 | ↓P = 0.055 | NS | NS |
| Helios MFI | NS | NS | NS | NS | NS |
| CTLA4+ (%Treg) | NS | NS | NS | NS | NS |
| CTLA4 MFI | NS | NS | NS | NS | NS |
| HLADRhiCD45RA− (%Treg) | NS | ↓P = 0.021 | ↓P = 0.035 | NS | NS |
| HLADRloCD45RA− (%Treg) | NS | ↓P = 0.029 | ↓P = 0.052 | NS | NS |
| HLADR- CD45RA− (%Treg) | NS | NS | NS | NS | NS |
| HLADR-CD45RA+ (%Treg) | NS | NS | NS | NS | NS |
| CD45RA+CCR7+ (%Treg) (naïve) | NS | NS | NS | NS | NS |
| CD45RA-CCR7+ (%Treg) (CM) | NS | NS | NS | NS | NS |
| CD45RA−CCR7− (%Treg) (EM) | ↓P = 0.035 | NS | NS | NS | NS |
| CD45RA+CCR7− (%Treg) (EMRA) | NS | ↑P = 0.015 | ↑P = 0.033 | NS | NS |
| CD4+.T cells | |||||
| Ratio Treg:CD4+ | ↓P = 0.074 | NS | NS | P = 0.055 | P = 0.031 |
| Ki67+ (%CD4+) | ↓P = 0.097 | ↓P = 0.021 | ↓P = 0.032 | NS | NS |
| Tbet+ (%CD4+) (Th1 cells) | ↑P = 0.011 | NS | NS | NS | NS |
| ratio Treg:Tbet+CD4+ | ↓P = 0.026 | NS | NS | P = 0.062 | NS |
| RORγt+ (%CD4+) (Th17 cells) | NS | NS | NS | NS | NS |
| Ratio Treg:RORγt+CD4+ | NS | NS | NS | P = 0.017 | NS |
| CD45RA+CCR7+(%CD4+) (naïve) | NS | NS | NS | P = 0.017 | P = 0.083 |
| CD45RA−CCR7+ (%CD4+) (CM) | NS | NS | NS | NS | P = 0.000 |
| CD45RA−CCR7-(%CD4+) (EM) | NS | NS | NS | P = 0.063 | NS |
| CD45RA+CCR7− (%CD4+) (EMRA) | NS | NS | NS | NS | NS |
| CD8+ T cells | |||||
| Ratio Treg:CD8+ | ↓P = 0.096 | NS | NS | NS | P = 0.080 |
| Ki67+ (%CD8+) | NS | NS | NS | NS | NS |
| Tbet+ (%CD8+) | ↑↑P = 0.005 | NS | NS | NS | NS |
| Ratio Treg:Tbet+ CD8+ | ↓↓P = 0.000 | NS | NS | NS | NS |
| RORγt+ (%CD8+) | ↑ (P = 0.090) | NS | NS | NS | NS |
| Ratio Treg:RORγt+CD8+ | ↓↓P = 0.000 | NS | NS | NS | NS |
| CD45RA+CCR7+ (%CD8+) (naïve) | ↑P = 0.033 | NS | NS | NS | NS |
| CD45RA−CCR7+ (%CD8+) (CM) | NS | NS | NS | NS | NS |
| CD45RA−CCR7− (%CD8+) (EM) | ↓P = 0.005 | NS | NS | NS | NS |
| CD45RA+ CCR7− (%CD8+) (EMRA) | NS | NS | NS | NS | NS |
Data were analyzed by linear mixed model analysis for all participants (Model 1, to determine effect of sex) or for women (Model 2, to determine effect of OC, cycle phase, and the interaction between OC and cycle phase).
Abbreviations: CM, central memory T cells; EM, effector memory T cells; EMRA, CD45RA+ effector memory T cells; naïve, naïve T cells; Treg cells, regulatory T cells.
aModel 1: male and female participants, 3 groups, effects of sex and group.
bModel 2: female participants, 6 groups, effects of OC and cycle phase.
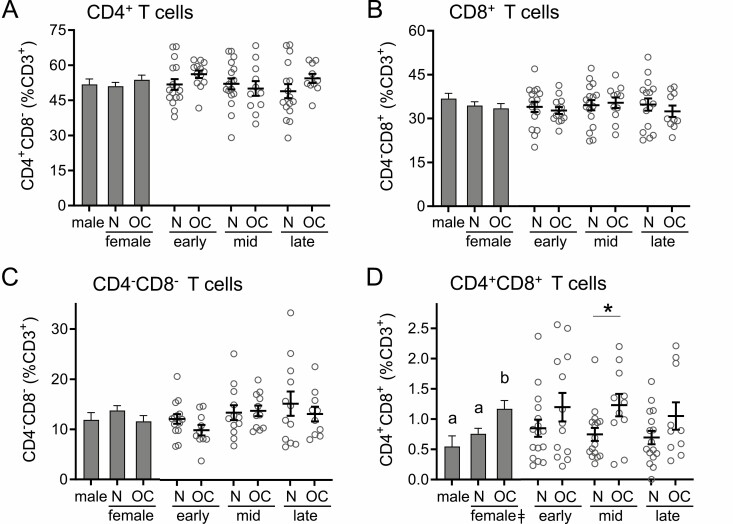
Figure 1.
Oral contraception increases CD4+CD8+ T cells but does not alter CD4+, CD8+, or CD4−CD8− T cells in peripheral blood. The proportions of CD3+ T cells classified as CD4+CD8− (A), CD8+CD4− (B), CD4−CD8− (C), and CD4+CD8+ (D) were assessed by flow cytometry in peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men. Data are shown as estimated marginal means (left side, bar graphs) calculated by linear mixed model analysis (Model 1, all participants) with cycle phase as a covariate for women. Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side), with mean ± SE of the mean indicated. The effects of participant group, female sex, OC use, and cycle phase were assessed by linear mixed model analysis and pairwise comparisons (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). *Significant effect of OC at specific cycle phases (Model 2). All Ps < 0.05.
Figure 5.
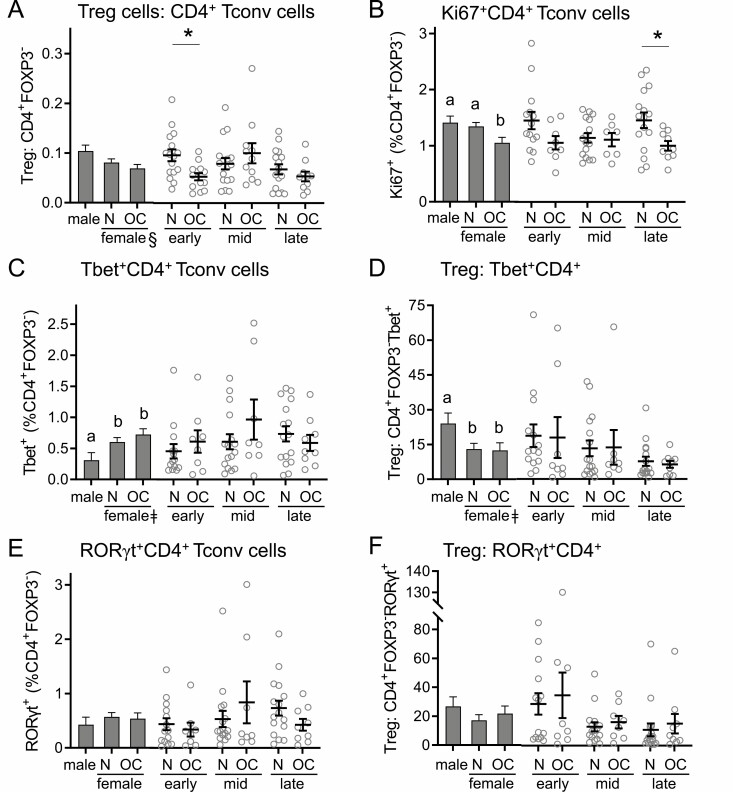
Oral contraception alters Ki67 expression but not other activation phenotype markers in CD4+ Tconv cells. Peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to analyze CD3+CD4+FOXP3− Tconv cells. Panels show the number of Tconv cells (A), the number of Ki67+Tconv cells (B), the number of Tbet+ Tconv cells (C), the ratio of Treg cells to Tbet+ Tconv cells (D), the number of RORγt+ Tconv cells (E), and the ratio of Treg cells to RORγt+ Tconv cells (F). Data are shown by group as estimated marginal means (left side, bar graphs) calculated by linear mixed model analysis (Model 1, all participants). Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side), with mean ± SE of the mean indicated. The effects of participant group, female sex, OC use, and cycle phase were assessed by linear mixed model analysis and pairwise comparisons (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). ϕSignificant effect of cycle phase (Model 2). *Significant effect of OC at specific cycle phases (Model 2). All Ps < 0.05.
Results
Oral Contraception Alters CD4+CD8+ T Cells but Not CD4+ or CD8+ T Cells
Peripheral blood from naturally cycling women, women using OC, and men was analyzed by multicolor flow cytometry to assess the lineage and phenotype composition of the T cell population, using an antibody panel designed to comprehensively characterize Treg cells. A linear mixed model analysis was used to evaluate the relative effects of sex, cycle phase, OC, and OC × cycle interaction. Neither the percentage of CD4+ T cells or CD8+ T cells within the viable CD3+ T cell pool (Fig. 1A and 1B) nor the ratio between them (data not shown) were impacted by sex or OC use. CD4−CD8− double-negative T cells were also unaffected by sex or OC use (Table 2, Fig. 1C). In contrast, sex and OC use were both identified as determinants of the rare double positive CD4+CD8+ T cell population (Table 2). CD4+CD8+ T cells were increased in women taking OC compared to naturally cycling women, particularly in the mid-cycle phase when the mean was elevated by 35% (P = 0.023) (Fig. 1D). CD4+CD8+ T cells were also increased in women taking OC compared to men but were similar in naturally cycling women and men.
Oral Contraception Alters Abundance and Suppressive Phenotype of CD4+ Treg Cells
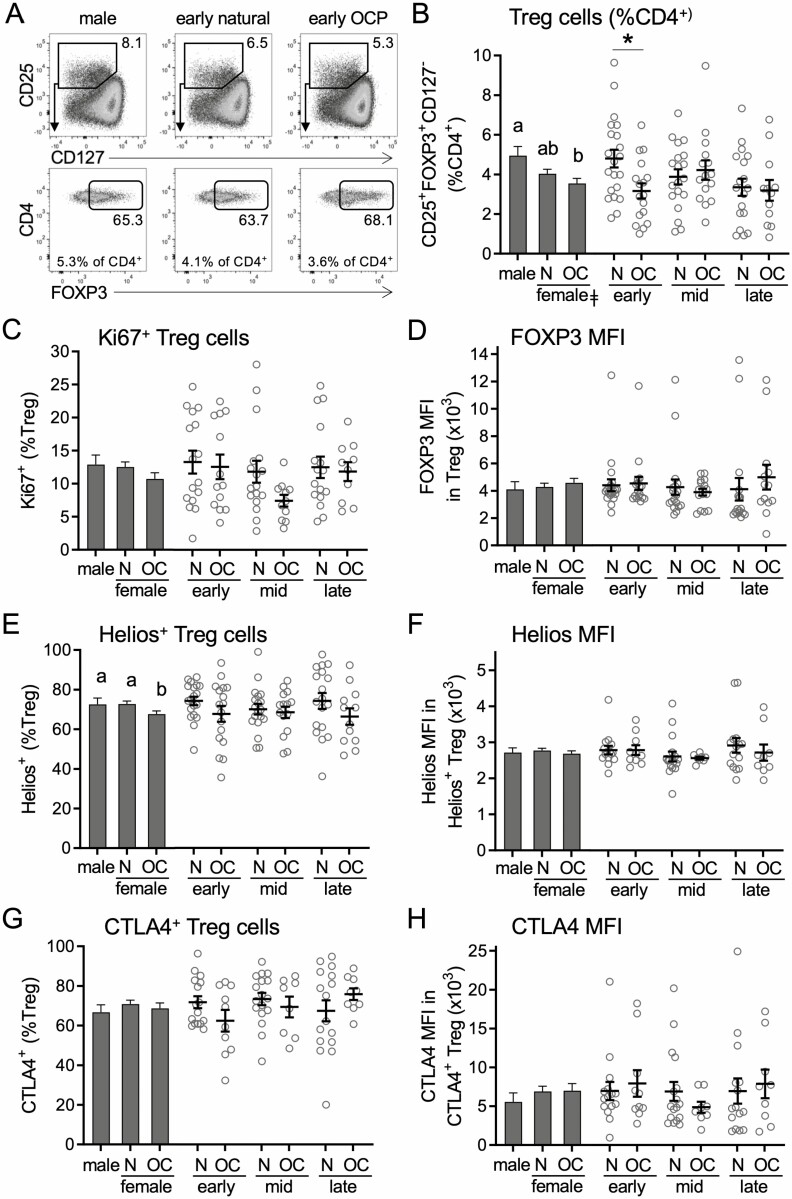
Treg cells, defined as CD25+FOXP3+CD127−/lo cells within the CD3+ CD4+ T cell population, were then assessed. Sex and OC × cycle interaction were identified as determinants of the proportion of Treg cells among CD3+CD4+ T cells (Table 2). Women taking OC had 28% fewer Treg cells (P = 0.009) compared to men (Fig. 2A and 2B), but numbers in naturally cycling women were not different to men. When individual cycle phases were considered, a difference between women using OC and naturally cycling women was evident, such that women using OC had 37% fewer Treg cells (P = 0.011) in the early-cycle phase. Analysis of the proliferative marker Ki67 did not reveal significant effects of OC use or sex (Table 2, Fig. 2C).
Figure 2.
Oral contraception alters the number of and Helios expression in CD4+ Treg cells in peripheral blood. Peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to analyze CD3+CD4+CD25+FOXP3+CD127−/lo Treg cells (A). Panels show the number of Treg cells (B), the number of Ki67+ Treg cells (C), the mean fluorescence intensity of FOXP3 in Treg cells (D), the number of Helios+ Treg cells (E), the mean fluorescence intensity of Helios in Helios+ Treg cells (F), the number of CTLA4+ Treg cells (G), and the mean fluorescence intensity of CTLA4 in CTLA4+ Treg cells (H). Data are shown by group as estimated marginal means (left side, bar graphs) calculated by linear mixed model analysis (Model 1, all participants). Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side), with mean ± SE of the mean indicated. The effects of participant group, female sex, OC use, and cycle phase were assessed by linear mixed model analysis and pairwise comparison (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). ϕSignificant effect of cycle phase (Model 2). *Significant effect of OC at specific cycle phases (Model 2). All Ps < 0.05.
Treg cells were further evaluated for markers of Treg cell suppressive function. The MFI of FOXP3 within Treg cells correlates with Treg cell suppressive potential (47). There was no difference according to sex in FOXP3 MFI and no effect of OC use in women (Table 2, Fig. 2D). Helios is a marker of Treg cell stability and suppressive capacity (48). The percentage of Treg cells expressing Helios was not affected by sex (Table 2). However, a significant effect of OC use was seen (P = 0.024), such that women taking OC had 7% fewer Helios+ Treg cells (Table 2, Fig. 2E). Helios MFI was not affected by sex or OC use (Fig. 2F). CTLA4, a key marker of Treg cell suppressive function, was also assessed. Neither the proportion of Treg cells expressing CTLA4 (Fig. 2G) or the MFI of CTLA4 (Fig. 2H) were affected by sex or by OC use in women.
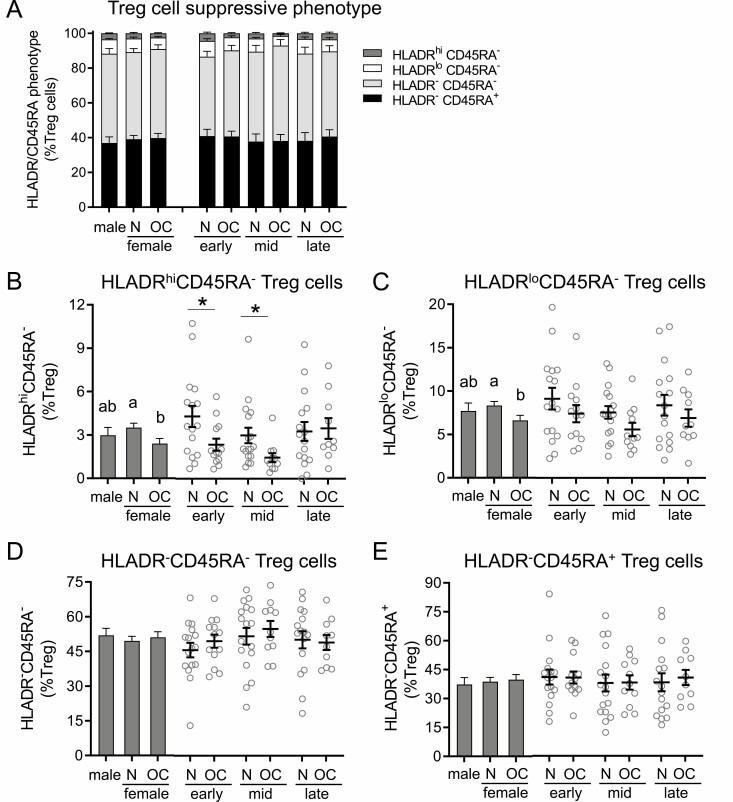
HLADR and CD45RA are additional markers of Treg cell suppressive capacity. HLADRhiCD45RA− Treg cells have the greatest suppressive capacity, followed by HLADRloCD45RA− Treg cells, double-negative HLADR−CD45RA− Treg cells, and finally HLADR-CD45RA+ Treg cells (49, 50). The overall balance of phenotypes of Treg cells expressing HLADR and CD45RA was not affected by sex or OC use (Fig. 3A). However, when considered separately, the HLADRhiCD45RA− subset of highly suppressive Treg cells was decreased in women taking OC compared to naturally cycling women (P = 0.021) (Fig. 3B). HLADRhiCD45RA− Treg cells were 45% fewer at both early- (P = 0.040) and mid-cycle phase (P = 0.042). Similar effects of OC were seen in the more common HLADRloCD45RA− cells where naturally cycling women had significantly higher HLADRloCD45RA− cell numbers compared to the OC group (P = 0.029) (Fig. 3C). There were nonsignificant trends toward fewer HLADRloCD45RA− cells in the OC group in the early and mid-phases of the cycle compared to the naturally cycling women. Similar trends were also seen when total HLADR+CD45RA− Treg cells were considered (data not shown), although corresponding changes in HLADR−CD45RA− Treg cells and HLADR−CD45RA+ Treg cells were not discernible (Fig. 3D and 3E).
Figure 3.
Oral contraception alters HLADR/CD45RA suppressive marker expression in CD4+ Treg cells in peripheral blood. Peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to assess HLADR and CD45RA expression in CD3+CD4+CD25+FOXP3+CD127−/lo Treg cells. Panels show the relative proportion of HLADRhiCD45RA−, HLADRloCD45RA−, HLADR−CD45RA−, and HLADR−CD45RA+ cells among Treg cells (A), the number of HLADRhiCD45RA− cells (B), the number of HLADRloCD45RA− Treg cells (C), the number of HLADR−CD45RA− Treg cells (D), and the number of HLADR−CD45RA+ Treg cells (E). Data are shown by group as estimated marginal means (A and left side, bar graphs B-D) calculated by linear mixed model analysis (Model 1, all participants). Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side, bar graphs B-D), with mean ± SE of the mean indicated. In (A), the effects of group were analyzed by Chi-square analysis. In (B) to (D), the effects of participant group, female sex, OC use, and cycle phase were assessed by linear mixed model analysis and pairwise comparisons (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). ϕSignificant effect of cycle phase (Model 2). *Significant effect of OC at specific cycle phases (Model 2). All Ps < 0.05.
Oral Contraception Alters the Memory Phenotype of CD4+ Treg Cells
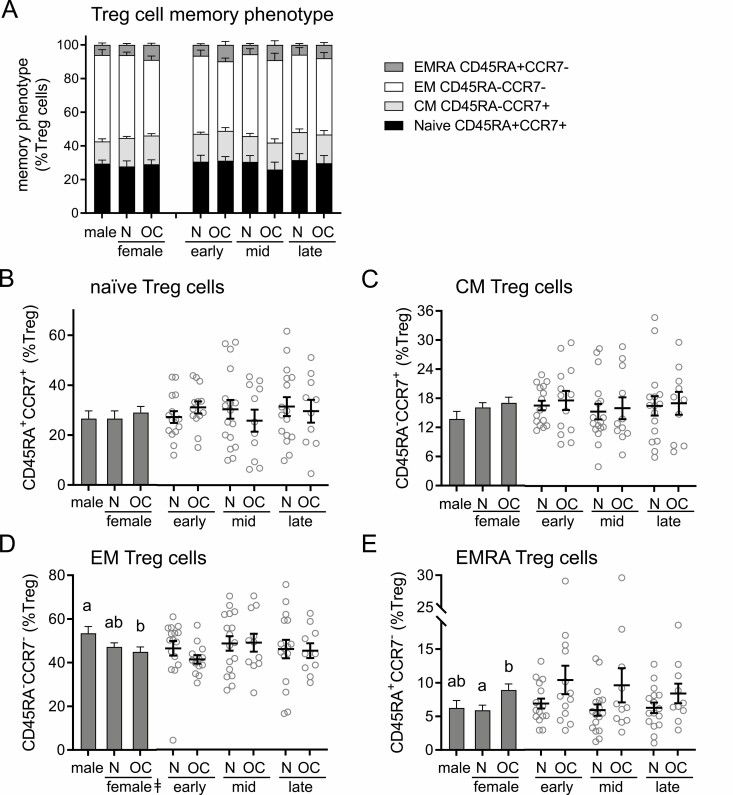
The markers CCR7 and CD45RA are widely used to define the memory status of T cells (51-53), allowing classification as naïve (CD45RA+CCR7+), central memory (CM; CD45RA−CCR7+), effector memory (EM; CD45RA−CCR7−), or terminally differentiated EM (EMRA; CD45RA+CCR7−) cells. Within Treg cells, there was no difference in the overall distribution of subsets according to subject sex or OC use (Fig. 4A). When subsets were considered individually, sex was identified as a determinant of EM cell number, and OC was a determinant of EMRA number (Table 2). While naturally cycling women had similar proportions of EM cells to men, women taking OC had fewer EM Treg cells than men (P = 0.030) (Fig. 4D). Conversely, women taking OC had a significantly higher proportion of EMRA Treg cells compared to naturally cycling women (P = 0.015) (Fig. 4E).
Figure 4.
Oral contraception alters CD45RA/CCR7 memory phenotype marker expression in CD4+ Treg cells. Peripheral blood drawn at early-, mid-, and late-cycle stages from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to assess CD45RA and CCR7 expression in CD3+CD4+CD25+FOXP3+CD127−/lo Treg cells, allowing classification of cells into naïve (CD45RA+CCR7+), central memory (CM; CD45RA−CCR7+), effector memory (EM; CD45RA-CCR7−), or terminally differentiated effector memory (EMRA; CD45RA+CCR7−) subsets. Panels show the relative proportion of naïve, CM, EM, and EMRA cells among Treg cells (A), the number of naïve cells (B), the number of CM Treg cells (C), the number of EM Treg cells (D), and the number of EMRA Treg cells (E). Data are shown by group as estimated marginal means (A, and left side, bar graphs B-D) calculated by linear mixed model analysis (Model 1, all participants). Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side, bar graphs B-D), with mean ± SE of the mean indicated. In (A), the effects of participant group were analyzed by Chi-square analysis. In (B) to (D), the effects of participant group, female sex, OC use, and time of cycle were assessed by linear mixed model analysis and pairwise comparisons (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). ϕSignificant effect of cycle stage (Model 2). *Significant effect of OC at specific cycle stages (Model 2). All Ps < 0.05.
Oral Contraception Alters CD4+ Th1 and Th17 Cells
The abundance and phenotypes of subsets of CD4+ T cells exhibiting phenotypic features of conventional T cells (Tconv; CD4+FOXP3-) were then assessed, and the ratios of Treg cells to Tconv cells, Th1, and Th17 cells calculated as a measure of anti-inflammatory to proinflammatory CD4+ T cell activity. Linear modeling showed the ratio of Treg:CD4+ Tconv cells was impacted by OC × cycle phase interaction (P = 0.031) (Table 2), and this was most evident in the early-cycle phase when a 40% lower ratio was seen in women taking OC (P = 0.007) (Fig. 5A). When cycle phase was considered, the ratio was higher mid-cycle than in late cycle, but this was independent of OC use (P = 0.017) (Fig. 5A).
The percentage of CD4+ T cells expressing Ki67 was not affected by sex, but within women a significant effect of OC use was seen (P = 0.032) (Fig. 5B). Notably, the proportion of CD4+ T cells expressing Ki67 was reduced by 38% in the late-cycle phase in women taking OC (P = 0.011) (Fig. 5B), compared to naturally cycling women, and a trend toward a similar effect was seen in the early-cycle phase (P = 0.057).
Proinflammatory Th1 cells were defined as CD4+FOXP3- Tconv expressing the transcription factor Tbet. There was a significant impact of sex on the proportion of Th1 cells (Table 2), with a 95% and 134% increase in Th1 cells observed in naturally cycling and OC women, respectively (Fig. 5C). To determine the balance between anti-inflammatory and proinflammatory T cells, the Treg:Th1 ratio was calculated (Fig. 5D). Naturally cycling women had a 46% lower Treg:Th1 ratio compared to males (P = 0.036), and women using OC had a 48% lower Treg:Th1 ratio compared to males (P = 0.042) (Fig. 5D). When the 2 groups of women were compared, there was no discernable impact of OC.
Next, the expression of RORγt within the CD4+FOXP3− Tconv cell population was investigated to define Th17 cells. Neither the proportion of RORγt+CD4+ Th17 cells (Fig. 5E) nor the Treg:Th17 ratio (Fig. 5F) was affected by sex or by OC use in women. Although the proportion of Th17 cells did not vary, the Treg:Th17 ratio fluctuated according to cycle phase (P = 0.017) (Table 2). This was most notable in naturally cycling women, where a trend toward a lower Treg:Th17 ratio in late cycle compared to early cycle was seen (P = 0.053).
Tbet+ and RORγt+ CD8+ T Cells Are Elevated in Women but Unaffected by OC Use
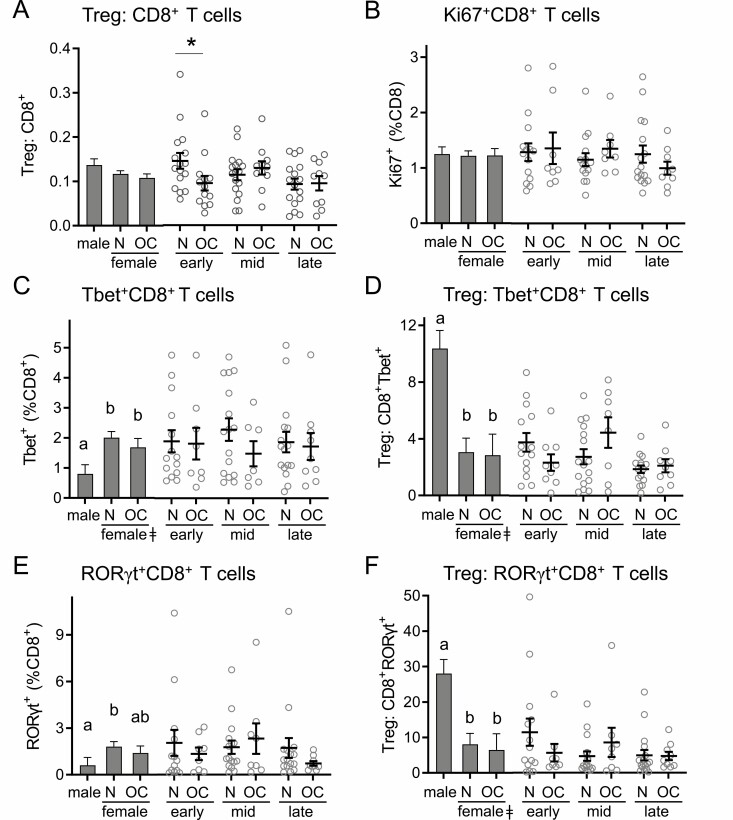
Next, the abundance and phenotypes of subsets of CD8+ T cells were assessed, and the ratios of CD4+ Treg cells to CD8+ T cell subsets were calculated as a measure of anti-inflammatory to proinflammatory CD8+ T cell activity. The ratio of Treg:CD8+ T cells was not impacted by sex (Fig. 6A). In women, OC use caused a significant reduction in Treg:CD8+ T cell ratio in the early phase of the cycle (P = 0.048) (Fig. 6A). The percentage of CD8+ T cells expressing Ki67 was not affected by sex or by OC use in women (Fig. 6B).
Figure 6.
Oral contraception does not alter CD8+ T cell number or phenotype markers. Peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to analyze CD3+CD8+ T cells. Panels show the number of CD8+ T cells (A), the number of Ki67+CD8+ T cells (B), the number of Tbet+CD8+ T cells (C), the ratio of Treg cells to Tbet+CD8+ T cells (D), the number of RORγt+CD8+ T cells (E), and the ratio of Treg cells to RORγt+CD8+ T cells (F). Data are shown by group as estimated marginal means (left side, bar graphs) calculated by linear mixed model analysis (Model 1, all participants). Individual data points are also shown for each woman at each cycle phase in the N and OC groups (right side), with mean ± SE of the mean indicated. The effects of participant group, female sex, OC use, and cycle phase were assessed by linear mixed model analysis and pairwise comparisons (n = 10-17 participants/group). a,b,cDifferent superscripts indicate differences between participant group (Model 1). ǂSignificant effect of sex (Model 1). ϕSignificant effect of cycle phase (Model 2). `Significant effect of OC × cycle interaction (Model 2). *Significant effect of OC at specific cycle phases (Model 2). All Ps < 0.05.
The transcription factors Tbet and RORγt were then assessed in CD8+ T cells. Women had significantly higher numbers of CD8+Tbet+ T cells than men, with a 60% (P = 0.042) and 64% (P = 0.031) increase in naturally cycling and OC women, respectively (Fig. 6C). There was no effect of sex or OC use on the proportion of CD8+Tbet+ cells in women (Fig. 6C). This resulted in a substantially greater Treg:CD8+Tbet+ cell ratio in men than either group of women (both Ps < 0.001) (Fig. 6D).
Men had 70% fewer CD8+RORγt+ T cells than naturally cycling women (P = 0.043) (Fig. 6E), resulting in a 2.4-fold and 3.6-fold greater Treg:CD8+RORγt+ ratio than in naturally cycling or OC women, respectively (Fig. 6F). There was no effect of OC use on the proportion of CD8+RORγt+ T cells or Treg:CD8+RORγt+ ratio in women, nor was there any impact of cycle phase (Fig. 6E and 6F).
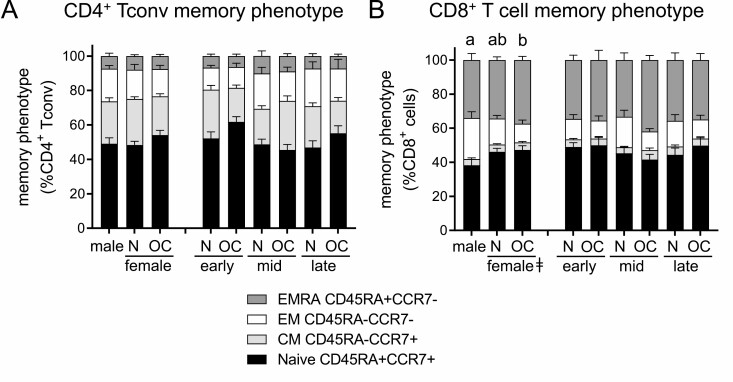
Oral Contraception Alters the Memory Phenotype of CD4+ Tconv but Not CD8+ T Cells
Within CD4+ Tconv cells, there was no difference in the distribution of naive and memory subsets according to subject sex or OC use (Fig. 7A). When subsets were considered separately, interactions between OC and cycle phase were seen to influence naïve and CM CD4+ Tconv cells (Table 2). In women taking OC, CM Tconv cells were lower in the early cycle (P = 0.013) but higher in mid-cycle (P = 0.021), compared to naturally cycling women [Supplemental Figure 2B (44)]. This reflected a decline from early to mid-cycle in naturally cycling women (P = 0.036) that was not present in women taking OC, who conversely showed an increase from early to mid-cycle (P = 0.024) and then a decline from mid- to late cycle (P = 0.015). This was associated with a decline from early to mid-cycle in the number of naïve CD4+ Tconv cells in women taking OC that was not evident in naturally cycling women (Fig. 7A).
Figure 7.
Oral contraception has minor effects on CD45RA/CCR7 memory phenotype marker expression in CD4+ Tconv cells and CD8+ T cells. Peripheral blood drawn at early-, mid-, and late-cycle stages from naturally cycling women (N), women using oral contraception (OC), and men was analyzed by flow cytometry to assess CD45RA and CCR7 expression in CD4+FOXP3− Tconv cells and CD8+ T cells, allowing classification of cells into naïve (CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), effector memory (EM; CD45RA-CCR7−), or terminally differentiated effector memory (EMRA; CD45RA+CCR7−) subsets. Panels show the relative proportion of naïve, CM, EM, and EMRA cells among Tconv cells (A) and CD8+ T cells (B). Data are shown by group as estimated marginal means calculated by linear mixed model analysis (Model 1, all participants). The effects of participant group were analyzed by Chi-square analysis. Additional data showing individual subsets are provided in Supplemental Figures 2 and 3 (44). a,b,cDifferent superscripts indicate differences between participant group (Model 1; P < 0.05).
Finally, the naïve and memory populations within CD8+ T cells were assessed. Within CD8+ T cells, women had a different phenotype distribution compared to men, particularly in women using OC (Fig. 7B). In both groups of women, a higher proportion of naïve CD8+ T cells was counterbalanced by a lower number of EM CD8+ T cells [Table 2; Supplemental Figure 3A and 3C (44)]. Sex and OC use did not impact the relative numbers of CM or EMRA CD8+ T cell subtypes [Supplemental Figure 3B and 3D (44)].
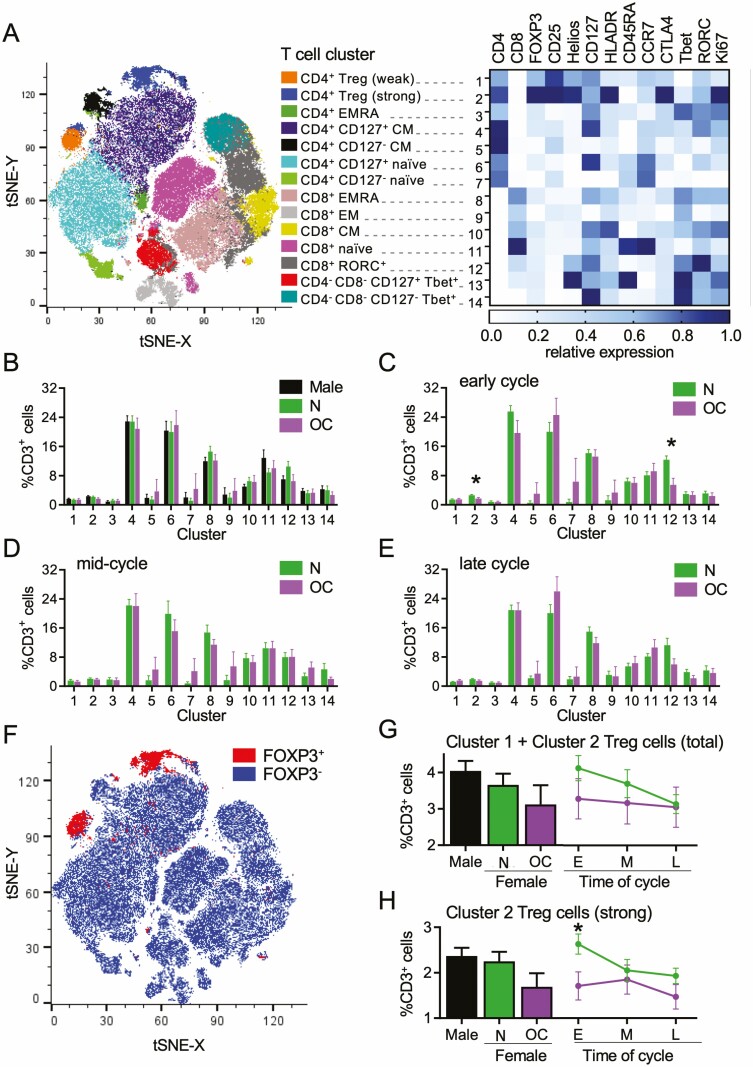
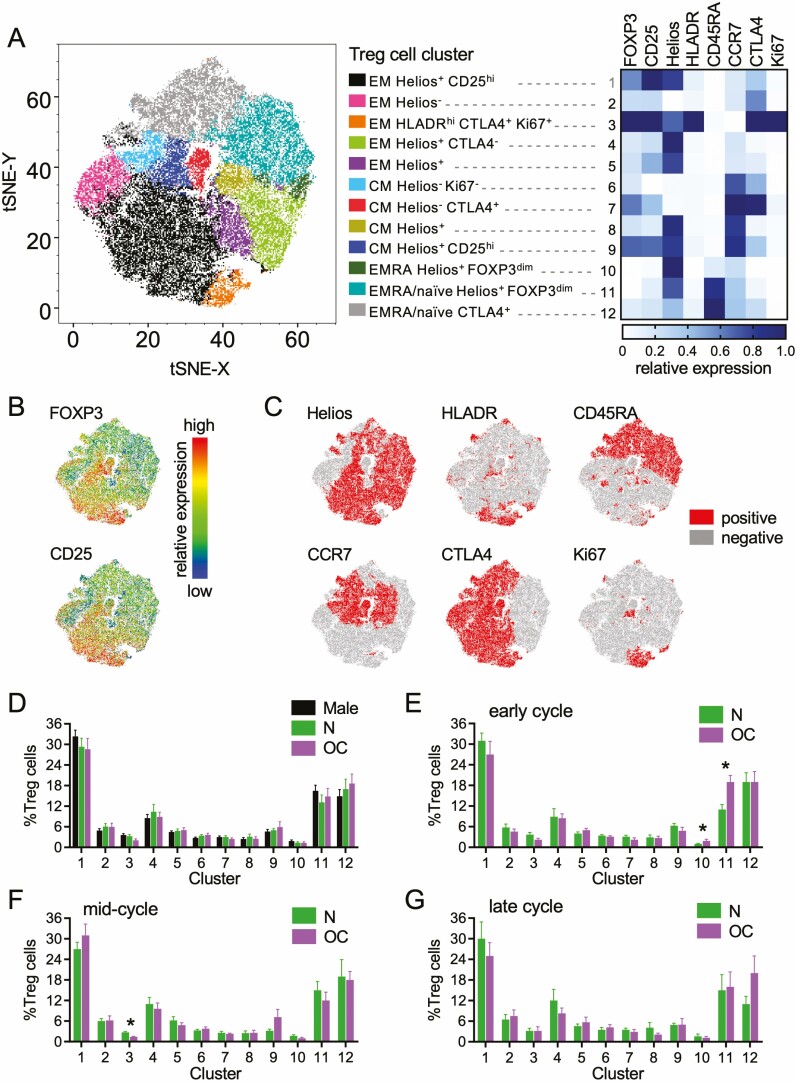
tSNE Analysis of the Impact of Oral Contraception on T Cell Subsets
T cells were also analyzed using the tSNE algorithm, an unbiased assessment of all T cell markers in combination (45), as an alternative approach to investigate effects of sex and OC use on T cell phenotypes. Within the CD3+ T cell population, tSNE analysis identified 14 separate clusters, within which the MFI of each marker was determined (Fig. 8A). CD4+ and CD8+ T cells were clearly distinguished on the tSNE plot and contained identifiable memory and naïve populations, as well as 2 distinct clusters of CD4+ Treg cells (Clusters 1 and 2) (Fig. 8A).
Figure 8.
Dimensionality-reducing t-distributed stochastic neighbor embedding (tSNE)algorithm shows effects of oral contraception on peripheral blood CD3+ T cells. Peripheral blood drawn at early-, mid-, and late-cycle phases from naturally cycling women (N), women using oral contraception (OC), and men. Data for flow cytometry phenotyping of CD3+ T cells were concatenated into a single file and transformed by the tSNE algorithm. X-shift identified 14 unique cell clusters, and the relative expression intensity of each marker within each cluster was calculated using FlowJo software (A). The proportion that each cluster contributes to total CD3+ cells was calculated using linear mixed model analysis, and estimated marginal means were generated for men, N, and OC groups (B). The mean ± SE of the mean cluster proportions for N and OC groups are shown at the early- (C), mid- (D), and late-cycle (E) phases. FOXP3+ Treg cells within the concatenated file were manually gated to view their distribution (F). Two Treg cell populations were identified, Cluster 1 and Cluster 2, with phenotypes typical of “mild” and “strong” suppressive capability, respectively. Total Treg cells (Cluster 1 + Cluster 2) (G) and strong Treg cells (Cluster 2) (H) are shown as estimated marginal means for men and the N and OC groups (bar graphs) and line graphs of mean ± SE of the mean for N and OC groups in early- (E), mid- (M) and late-cycle (L) phases. The effects of participant group, OC use, and cycle phase on Treg cell clusters were assessed by 1-way analysis of variance and post hoc Tukey t-test (n = 8-17 participants/group; *P < 0.05).
No differences in cluster proportions between men, naturally cycling women, and women taking OC were seen when time points were combined (Fig. 8B). However, differences between naturally cycling women and women taking OC were evident when individual cycle phases were considered (Fig. 8C-8E). At the early-cycle phase, 2 clusters were significantly reduced in women taking OC—Cluster 2 (P = 0.025) and Cluster 12 (P = 0.002). Cluster 2 represents Treg cells with strong expression of FOXP3, CD25, Helios, HLADR, CTLA4, and Ki67 and low CD127, a phenotype associated with strong suppressive potential and high proliferative activity. Cluster 12 identifies RORγt+CD8+ T cells. No effects of OC use were seen on these or other clusters in mid- (Fig. 8D) and late-cycle (Fig. 8E) phases.
Treg cell Clusters 1 and 2 could be clearly distinguished on the tSNE plot (Fig. 8F). When “total” Treg cells were examined by combining Cluster 1 and Cluster 2 (Fig. 8G), significant effects of sex and OC were not seen. The effect of OC on Treg cells was evident only in “strong” Treg cells (P = 0.025) (Fig. 8H). This is consistent with the finding of fewer highly suppressive HLADRhiCD45RA− Treg cells in women taking OC seen by conventional flow cytometry analysis (Fig. 3B and 3C).
Treg cells (CD25+FOXP3+CD127−/lo cells within the CD3+CD4+ T cell population) were also analyzed by tSNE, yielding 12 clusters that exhibited features of several distinct Treg cell subsets (Fig. 9A-C). The low numbers of Treg cells in samples limited the degree of subset resolution, so that some subsets, such as naïve and EMRA Treg cells and Helios+ and Helios− HLA-DRhi Treg cells, could not be distinguished. Nevertheless, differences in Treg cell phenotypes associated with OC use were evident and were most prominent in the early- and mid-cycle phases (Fig. 9D-9G). Notably, Treg cells with strong expression of FOXP3, CD25, HLADR, CTLA4, and Ki67 (Cluster 3) comprised a smaller proportion of the total Treg cell population in mid-cycle phase (P = 0.001) (Fig. 9F), and a similar trend was seen in early-cycle phase (P = 0.134) (Fig. 9E). Furthermore, Treg cells with an EMRA and/or naïve phenotype (CD45RA+, CCR7+/−; Clusters 10 and 11) were elevated in the early-cycle phase (P < 0.05) (Fig. 9E). This aligns with the reduction in highly suppressive HLADRhiCD45RA− Treg cells (Fig. 3B and 3C) and the shift toward a greater proportion of Treg cells with an EMRA phenotype (Fig. 4E) seen by conventional analysis in women taking OC.
Figure 9.
Plasma 17β-estradiol (A) and progesterone (B) levels in naturally cycling women and women taking oral contraception. Peripheral blood was drawn at early-, mid-, and late-cycle stages from naturally cycling women (N), women using oral contraception (OC), and men. Data for flow cytometry phenotyping of CD3+CD4+FOXP3+CD25+CD127− Treg cells was concatenated into a single file and transformed by the t-distributed stochastic neighbor embedding (tSNE) algorithm. X-shift identified 12 unique cell clusters and the relative expression intensity of each marker within each cluster was calculated using FlowJo software and is shown on a heat map (A). Heat map tSNE plots show the relative expression of FOXP3 and CD25 within the Treg cells (B) and the Treg cells positive for Helios, HLADR, CD45RA, CCR7, CTLA4, and Ki67 within the tSNE plot are shown (C). The proportion that each cluster contributes to total Treg cells was calculated, and estimated marginal means were generated for men and the N, and OC groups (D). The mean ± SE of the mean proportion of each cluster for N and OC groups at the early- (E), mid- (F), and late-cycle (G) stages were calculated. The effects of participant group, OC use, and cycle phase on Treg cell clusters were assessed by 1-way analysis of variance (ANOVA; n = 8-15 participants/group). The effects of participant group, OC use, and cycle phase on Treg cell clusters were assessed by 1-way ANOVA and post hoc Tukey t-test (n = 8-15 participants/group; *P < 0.05).
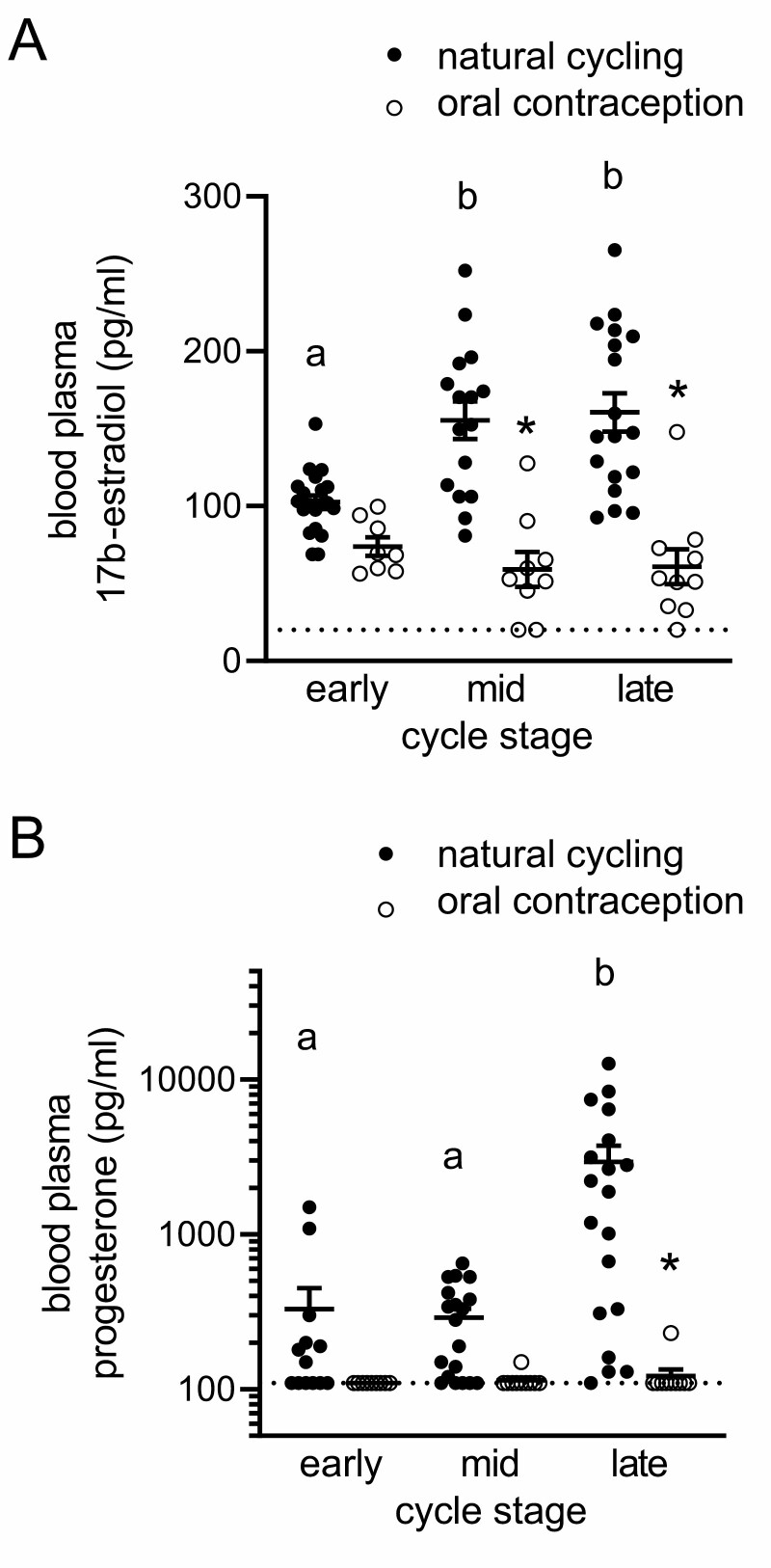
Effects of Oral Contraception on Plasma Sex Steroid Hormones
To examine effects of OC on endogenous sex steroid hormones, we measured E2 and P4 by enzyme-linked immunosorbent assay and radioimmunoassay in plasma of both groups of participants at each cycle phase. As expected, in naturally cycling women, plasma E2 was higher in the mid- and late-cycle phases than in the early cycle, and plasma P4 was more variable between women but consistently higher in the late-cycle phase than in the early or mid-cycle (Fig. 10A and 10B). In women taking OC, the mid- and late-cycle rise in E2 did not occur, and mean E2 levels were 60% and 63% lower, respectively, at these 2 phases (Fig. 10A). For P4, OC caused levels to be undetectable or very low at every phase, and mean levels were 25-fold lower in late-cycle phase in women taking OC compared to the corresponding time in naturally cycling women (Fig. 10B). These findings are consistent with the expected pattern of E2 and P4 fluctuation in healthy cycling women (54) and the suppression of endogenous E2 and P4 production by OC (43, 55, 56).
Figure 10.
Plasma 17β-estradiol (A) and progesterone (B) levels in naturally cycling women and women taking OC. Plasma from venous blood samples taken at early-, mid-, and late-cycle phases from naturally cycling women (closed circles) or women using oral contraception (open circles) was analyzed for 17β-estradiol (A) and progesterone (B) content. Data were analyzed by 1-way analysis of variance with post hoc Sidak’s multiple comparisons test. a,b,cDifferent superscripts indicate difference between cycle phases within a participant group. *Significant effect of OC at specific cycle phase.
Discussion
There is growing evidence that sex-steroid hormones E2 and P4 exert systemic influence on T cell generation, phenotypes, and function, modulating T cell subsets that affect immune defense and control inflammation. In this study, we investigated whether oral contraceptive medication, which suppresses endogenous sex hormone synthesis, alters the size and composition of the anti-inflammatory Treg cell compartment in peripheral blood in women. OC use was found to exert subtle but likely biologically significant effects on specific subsets within the T cell pool that varied in degree depending on cycle phase. Collectively, the analysis showed that Treg cells of the most strongly immune suppressive phenotype, defined by high levels of FOXP3, CD25, Helios, HLADR, CTLA4, and Ki67, were most affected. In women taking OC, these potent anti-inflammatory immune regulators comprised a lower proportion of the total Treg cell population, particularly in the early and mid-cycle phases. A shift in the balance of Treg cell memory phenotypes was also seen, such that women taking OC had a greater proportion of mature EMRA Treg cells, which suggests altered kinetics of Treg cell generation or maturation (57). In addition, minor changes were also seen in the memory phenotypes of CD4+ Tconv cells and CD8+ T cells in the early- and mid-cycle phases, which may reflect a broader impairment of sex hormone modulation of T cell generation or maturation (58, 59).
Another surprising effect of OC use was to increase the abundance of CD4+CD8+ T cells, a subset of cells implicated in pathogenic processes in cancer and chronic autoimmune conditions (60, 61). CD4+CD8+ T cells make up a small proportion of T cells in healthy individuals. Their physiological functions are not fully understood, although both cytotoxic and suppressive capacities have been described (61). The OC-associated increase in double-positive CD4+CD8+ T cells resulted in levels almost 2-fold those seen in men, while no change was observed in double-negative CD4−CD8− T cells. It is possible that OC causes changes to subpopulations within the double-positive and double-negative T cell populations, but further detailed phenotypic analysis would be required to discern this.
Of the changes seen, the effects on Treg cells were of greatest interest considering the major significance of these cells in health and disease susceptibility. Both conventional analysis and the unbiased tSNE analyses support the interpretation that Treg cells with the most suppressive phenotype were predominantly affected by OC. Conventional analysis revealed that the phenotype markers most affected by OC were HLADR and Helios. HLADRhiCD45RA− Treg cells, which comprise only around 3% of the total Treg cell pool, were reduced by 45% in women taking OC compared to naturally cycling women. Helios+ Treg cells were reduced to a smaller degree, while changes in FOXP3, CTLA4, and Ki67 were not detected by conventional analysis. Greater insight was provided by the 2 tSNE analyses, which allows unbiased analysis of all markers simultaneously, both of which supported the interpretation that the subset of Treg cells most affected by OC are those expressing high levels of all the key suppressive markers—that is, the most potent immune regulatory subset.
Both analytical approaches pointed to the Treg cell changes being most evident in the early-cycle phase (2-3 days after commencement of menstruation or withdrawal bleeding), which follows cessation of exogenous hormone intake (in women taking OC) or the late luteal phase decline in endogenous P4 and E2 (in naturally cycling women). The significance of this cycle phase and whether fewer circulating Treg cells in women using OC reflects reduced generation or increased uptake into peripheral tissues are unknown. Treg cells are sequestered into the uterus under the influence of P4 in the luteal phase of naturally cycling women to facilitate receptivity for embryo implantation (62, 63) and potentially to modulate the inflammation and tissue remodeling that accompanies menstruation (64). It is therefore possible that greater Treg cell uptake into the uterus in response to extended duration of progestin exposure contributes to fewer peripheral blood Treg cells in women using OC.
An effect on Treg cell generation and/or maturation due to altered E2 seems the most biologically plausible explanation for the effect of OC on peripheral blood Treg cells. As reported previously and confirmed here, OC overrides the E2-dominant proliferative (or follicular) phase of the menstrual cycle (43). We speculate that the consistently lower E2 levels across the cycle and/or the absence of an E2-dominated phase may be critical. Unopposed E2 is reported to promote Treg cell proliferation (18, 19) and probably accounts for expansion of the Treg pool in the proliferative phase (4). The nuclear E2 receptor estrogen receptor α is expressed in human Treg cells and enables E2 binding to the promoter and the conserved noncoding sequence 2 and 3 enhancer regions of the FOXP3 locus to promote FOXP3 expression and Treg cell function (65). Estrogen receptor β likely also has a role since mice with genetic deficiency in the nuclear receptor estrogen receptor β have impaired transforming growth factor β–dependent differentiation of Treg cells and Foxp3 induction (66). In the absence of E2 signaling, Treg cells fail to downregulate expression of GILZ, a glucocorticoid-responsive transcription factor that is normally suppressed in mature Treg cells (66). Impaired production of thymic Treg cells in the absence of unopposed E2 would fit with the observed shift in phenotype markers and the early cycle timing of the deficit.
The reduction in endogenous P4 that occurs with OC use may also contribute to effects on Treg cells. P4 acts to skew T cell differentiation away from proinflammatory Th1 and Th17 and toward protolerogenic Th2 and Treg cell phenotypes (39, 67, 68), promoting anti-inflammatory interleukin (IL) 10 expression over proinflammatory cytokines tumor necrosis factor, interferon-gamma (IFNG) and IL12 (36). Elevated P4 in the luteal phase of natural cycles is thought to embed Treg stability and promote uterine Treg cell accumulation in readiness for potential pregnancy (16, 69) through systemic effects reflected in peripheral blood (70, 71). There is good evidence of nuclear P4 receptor expression in Treg cells (72), suggesting direct effects of P4 on Treg cell differentiation or function. P4 may also regulate Treg cells via membrane P4 receptor α (73) and glucocorticoid receptors (74). The extent to which synthetic progestins override or recapitulate the signaling effects of endogenous P4 is unclear.
Thus, in natural menstrual cycles, the sequence of unopposed E2 followed by elevated P4 may exert a sequence of regulatory actions, promoting Treg cell proliferation followed by Treg cell maturation. We speculate that OC use interferes with both the generation and maturation of Treg cells by subverting this dynamic cycle.
Our findings are consistent with a previous study indicating an effect of OC on Treg cell numbers in US women (75). This cross-sectional study showed that previous use of OC is a negative predictor of Treg cell levels, with a 22% reduction in mean Treg cell values for women who reported previous or current OC use (75). However, the study did not distinguish previous from current OC use, indicate the type of OC, or evaluate effects on Treg cell phenotype. In contrast with another earlier study (4), we did not observe menstrual cycle–associated changes in the proportion of Treg cells in peripheral blood, perhaps due to the smaller sample size and different timing of sampling, as well as inclusion of CD127 as a lineage-defining Treg cell marker, in the current study.
We included a comparison group of men to evaluate how the effects of OC on immune phenotype in women compare to the sexually dimorphic differences between men and women. Treg cells and ratios of Treg cells to effector T cells were found to be substantially higher in men than in women. This is consistent with a previous report that men have 15% more CD4+CD25hi Treg cells than women (76). Other studies indicate the effect of sex on Treg cells is diminished after menopause (75, 77), implying a significant role for sex hormones as opposed to genetic differences. In comparison to sex, the effects of OC were relatively minor, although for some parameters OC use amplified a sex-associated difference. For example, OC use in women caused their total Treg cells, Helios+ Treg cells, and EM Treg cells to be more distinctly different from those of men.
Treg cells are key regulators of immune balance, critical in the prevention of autoimmunity, limiting and resolving inflammation, and establishing immune tolerance, especially tolerance to the fetus during pregnancy (9, 78, 79). Reduced abundance or dysfunction of Treg cells is associated with many autoimmune diseases including type 1 diabetes (80), systemic lupus erythematosus (81), multiple sclerosis (82), and rheumatoid arthritis (83). A lower Treg cell prevalence and reduced ratio of Treg to effector T cells in women is thought to render them more susceptible to inflammatory activation and to explain the clear skewing toward higher incidence of autoimmune diseases in women compared to men (84, 85).
The physiological significance of the changes in Treg cell phenotype due to OC use for women’s health is unclear. We did not have sufficient cells to measure immune suppressive function, but the shift to fewer HLADRhiCD45RA− Treg cell phenotype we report is expected to result in lower suppressive capability (49, 50). Consistent with this, we saw a decrease in the ratio of Treg cells to both CD4+ Tconv cells and CD8+ T cells in the early-cycle phase, suggesting lower Treg cell suppressive activity may result in higher effector T cell activity for at least a number of days each month.
Interestingly, the nature and scale of the difference in Treg cells associated with OC use are reminiscent of differences reported for several autoimmune diseases (86, 87), although direct comparisons are difficult as patient sex and hormone status are often not provided, and many older studies use a limited range of lineage and phenotype markers. Treg cells expressing Helios are thought to be particularly susceptible due to impaired thymic output and greater phenotypic instability (86, 88). For example, patients with multiple sclerosis have fewer naïve thymus-derived Treg cells and higher memory Treg cells in peripheral blood (57), associated with reduced suppressive competence in both immature HLADR− and mature HLADR+ subsets (89). Patients with sarcoidosis or systemic lupus erythematosus are also reported to have altered maturation (memory) subsets (90). There is clear evidence from animal studies that Treg cell impairments cause elevated susceptibility to autoimmune disease (86, 87), but whether small changes similar to those reported herein are sufficient to have a substantial pathophysiological role is unclear. It is important to consider that many other factors impact Treg cell parameters in healthy individuals, including age, genetics, and a range of environmental and lifestyle factors (57, 75, 88).
Several studies have investigated whether OC use is causally linked to autoimmune or inflammatory disorders in women. Two large prospective studies provide compelling evidence that Crohn’s disease and ulcerative colitis are more prevalent in women using OC (91, 92). Two studies have reported an increased risk of multiple sclerosis with OC use (93, 94), but this was contradicted by a large cohort study that concluded no change (95). Two separate meta-analyses demonstrate that OC use has no impact on the risk of rheumatoid arthritis (96, 97), a finding confirmed in another recent cohort study (98).
In addition to autoimmune disorders, alterations in Treg cell populations are implicated in unexplained infertility and obstetric disorders. Treg cell deficiency causes pregnancy loss in animals models (10, 99), and in women, aberrant Treg cell populations are linked to pregnancy pathologies including preterm labor (17), preeclampsia (15, 16), and miscarriage (14). It is well-established that prior OC use does not impair capacity to later conceive a healthy pregnancy (100), but a short-term delay of 2 to 6 months in the return of fertility after OC use has been reported (101). Other studies suggest that OC use prior to pregnancy can elevate the risk of pregnancy complications (102, 103), and there is conflicting evidence on links between OC use and miscarriage (104-106). Whether any effects of OC on maternal Treg cells could carry through into pregnancy remains to be investigated.
There are considerable health benefits of OC for contraception and protection from abnormal uterine bleeding, with decreased rates of uterine and ovarian cancer in OC users (107). Counterbalancing these benefits are well-documented side effects including breast tenderness, nausea, weight gain, headaches, and decreased libido (108, 109). A serious, although rare, side effect is the increased risk of cardiovascular pathologies including venous thromboembolism, thrombotic stroke, and myocardial infarction (110-112). Whether any of these effects involve Treg cells is not clear but is biologically plausible given the central roles for Treg cells in tissue homeostasis through their potent anti-inflammatory, immune-regulatory and vasoregulatory functions (11-13). OC use has also been linked with depression and use of antidepressant medication (113-115). It will be important to investigate whether this is associated with altered Treg cell parameters, given the emerging association between mental health and immune and inflammatory status (116, 117).
A limitation of this study is that women in the OC group used several different OC formulations, with varying content of synthetic E2 ethinylestradiol and progestins [Supplemental Table 1 (44)]. Whether formulation affects the degree of impact is not possible to discern in this small data set. Our findings also raise the question of how T cell populations may be altered in women with constant hormone delivery, such as those utilizing OC without a monthly break for withdrawal bleed or women using a progestin-releasing intrauterine device or contraceptive implant (eg, Implanon).
In summary, this study reports a previously undescribed link between OC use and perturbations in Treg cells in peripheral blood of women. By overriding fluctuations in E2 and P4 that in natural cycles facilitate immune tolerance for reproductive success, OC use may alter the dynamics of Treg cell generation and maturation. However, given the critical importance of OC in women’s reproductive planning, it is essential to emphasize that further studies, ideally using a placebo case-control design, are necessary to confirm the immune changes we report and to elucidate the underlying mechanisms. Determining whether effects of OC mediated via Treg cells or other immune cells may be involved in altered autoimmune, reproductive, or cardiovascular disease risks and whether and, if so, how this might interact with other predisposing or causal factors will require detailed investigation. Evaluation of the relationship between prior OC use and Treg cells in reproductive tissues is particularly warranted, given the clear links between Treg cell insufficiency and infertility and adverse pregnancy outcomes in women.
Contributor Information
Lachlan M Moldenhauer, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
Min Jin, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia; Center for Reproductive Medicine, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Jasmine J Wilson, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
Ella S Green, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
David J Sharkey, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
Mark D Salkeld, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
Thomas C Bristow, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
M Louise Hull, Robinson Research Institute and Adelaide Medical School, University of Adelaide, Adelaide, Australia.
Gustaaf A Dekker, Robinson Research Institute and Adelaide Medical School, University of Adelaide, Adelaide, Australia; Division of Women’s Health, Lyell McEwin Hospital, Elizabeth Vale, Australia.
Sarah A Robertson, Robinson Research Institute and School of Biomedicine, University of Adelaide, Adelaide, Australia.
Funding
This work was supported by the National Health and Medical Research Council of Australia (APP1041332 and APP1139509, awarded to S.A.R and G.D.).
Author Contributions
S.A.R., G.D., M.J., and M.L.H. designed the study; M.J., D.J.S., and T.C.B. collected clinical materials; L.M.M., M.J., J.J.W., E.S.G., D.J.S., M.D.S., and T.C.B. performed research; L.M.M., M.J., and J.J.W. analyzed data; G.D. and M.L.H. provided clinical expertise; L.M.M. and S.A.R. wrote the paper, and all authors provided critical comments on drafts.
Disclosures
The authors have no conflict of interest to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the references. Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request
References
- 1. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217-230. Doi: 10.1038/nri3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626-638. Doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 3. Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10:509-516. Doi: 10.1038/gene.2009.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572-2578. Doi: 10.4049/jimmunol.178.4.2572 [DOI] [PubMed] [Google Scholar]
- 5. Nowak J, Borkowska B, Pawlowski B. Leukocyte changes across menstruation, ovulation, and mid-luteal phase and association with sex hormone variation. Am J Hum Biol. 2016;28:721-728. Doi: 10.1002/ajhb.22856 [DOI] [PubMed] [Google Scholar]
- 6. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777-780. Doi: 10.1038/ni0901-777 [DOI] [PubMed] [Google Scholar]
- 7. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347-369. Doi: 10.1016/j.yfrne.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 8. Pollard KM. Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun. 2012;38: J177-J186. Doi: 10.1016/j.jaut.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018;128:4224-4235. Doi: 10.1172/JCI122182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13:23-33. Doi: 10.1038/nri3361 [DOI] [PubMed] [Google Scholar]
- 11. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. Doi: 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 12. Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241 :260-268. Doi : 10.1111/j.1600-065X.2011.01018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamashita T, Sasaki N, Kasahara K, Hirata K. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol. 2015;66:1-8. Doi: 10.1016/j.jjcc.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 14. Inada K, Shima T, Ito M, Ushijima A, Saito S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J Reprod Immunol. 2015;107:10-19. Doi: 10.1016/j.jri.2014.09.053 [DOI] [PubMed] [Google Scholar]
- 15. Santner-Nanan B, Peek MJ, Khanam R, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183(11):7023-7030. Doi: 10.4049/jimmunol.0901154 [DOI] [PubMed] [Google Scholar]
- 16. Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM. The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol. 2011;91:76-82. Doi: 10.1016/j.jri.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 17. Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935-944. Doi: 10.1038/icb.2012.33 [DOI] [PubMed] [Google Scholar]
- 18. Polanczyk MJ, Carson BD, Subramanian S, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227-2230. Doi: 10.4049/jimmunol.173.4.2227 [DOI] [PubMed] [Google Scholar]
- 19. Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58-65. Doi: 10.1111/j.1365-2567.2006.02339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS One. 2007;2:e382. Doi: 10.1371/journal.pone.0000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036-1045. Doi: 10.1095/biolreprod.108.074658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29-38. Doi: 10.1016/j.cell.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102-106. Doi: 10.1038/nature11462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63-69. Doi: 10.1016/j.cellimm.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107-113. Doi: 10.1016/j.imlet.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 26. Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88:4711-4720. Doi: 10.1128/JVI.02081-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Priyanka HP, Krishnan HC, Singh RV, Hima L, Thyagarajan S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes. Mol Immunol. 2013;56:328-339. Doi: 10.1016/j.molimm.2013.05.226 [DOI] [PubMed] [Google Scholar]
- 28. Lelu K, Laffont S, Delpy L, et al. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386-2393. Doi: 10.4049/jimmunol.1101578 [DOI] [PubMed] [Google Scholar]
- 29. Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023-6029. Doi: 10.4049/jimmunol.174.10.6023 [DOI] [PubMed] [Google Scholar]
- 30. Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ansar Ahmed S. Effects of long-term estrogen treatment on IFN-gamma, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208-217. Doi: 10.1006/cyto.2001.0876 [DOI] [PubMed] [Google Scholar]
- 31. Liu HY, Buenafe AC, Matejuk A, et al. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70 :238-248. Doi : 10.1002/jnr.10409 [DOI] [PubMed] [Google Scholar]
- 32. Butts CL, Shukair SA, Duncan KM, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007;19:287-296. Doi: 10.1093/intimm/dxl145 [DOI] [PubMed] [Google Scholar]
- 33. Szekeres-Bartho J, Reznikoff-Etievant MF, Varga P, Pichon MF, Varga Z, Chaouat G. Lymphocytic progesterone receptors in normal and pathological human pregnancy. J Reprod Immunol. 1989;16 :239-247. Doi : 10.1016/0165-0378(89)90053-3 [DOI] [PubMed] [Google Scholar]
- 34. Solano ME, Kowal MK, O’Rourke GE, et al. Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J Clin Invest. 2015;125:1726-1738. Doi: 10.1172/JCI68140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. J Leukoc Biol. 2013;93:369-375. Doi: 10.1189/jlb.1012491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128-133. [PubMed] [Google Scholar]
- 37. Piccinni M-P, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109:30-33. Doi: 10.1016/s0165-5728(00)00299-x [DOI] [PubMed] [Google Scholar]
- 38. Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42:2683-2696. Doi: 10.1002/eji.201142317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011;187 :1778-1787. Doi : 10.4049/jimmunol.1003919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mao G, Wang J, Kang Y, et al. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477-5488. Doi: 10.1210/en.2010-0426 [DOI] [PubMed] [Google Scholar]
- 41. Xu L, Dong B, Wang H, et al. Progesterone suppresses Th17 cell responses, and enhances the development of regulatory T cells, through thymic stromal lymphopoietin-dependent mechanisms in experimental gonococcal genital tract infection. Microbes Infect. 2013;15:796-805. Doi: 10.1016/j.micinf.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 42. United Nations, Department of Economic and Social Affairs. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). United Nations; 2019. [Google Scholar]
- 43. Kjeld JM, Puah CM, Joplin GF. Changed levels of endogenous sex steroids in women on oral contraceptives. Br Med J. 1976;2:1354-1356. Doi: 10.1136/bmj.2.6048.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moldenhauer LM, Jin M, Wilson JJ, et al. Supplemental data for: Regulatory T cell proportion and phenotype are altered in women using oral contraception. J Cont Figshare. 2022. Doi: 10.25909/20324019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579-2605. [Google Scholar]
- 46. Samusik N, Good Z, Spitzer MH, Davis KL, Nolan GP. Automated mapping of phenotype space with single-cell data. Nat Methods. 2016;13:493-496. Doi: 10.1038/nmeth.3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo H, Zheng M, Zhang K, et al. Functional defects in CD4(+) CD25(high) FoxP3(+) regulatory cells in ankylosing spondylitis. Sci Rep. 2016;6:37559. Doi: 10.1038/srep37559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chougnet C, Hildeman D. Helios-controller of Treg stability and function. Transl Cancer Res. 2016;5:S338-S341. Doi: 10.21037/tcr.2016.07.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schaier M, Seissler N, Schmitt E, et al. DR(high+)CD45RA(-)-Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLoS One. 2012;7:e34208. Doi: 10.1371/journal.pone.0034208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622-4631. Doi: 10.4049/jimmunol.176.8.4622 [DOI] [PubMed] [Google Scholar]
- 51. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708-712. Doi: 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 52. Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167-171. Doi: 10.1007/978-3-642-57276-0_21 [DOI] [PubMed] [Google Scholar]
- 53. Sallusto F, Kremmer E, Palermo B, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037-2045. Doi: [DOI] [PubMed] [Google Scholar]
- 54. Edelman A, Stouffer R, Zava DT, Jensen JT. A comparison of blood spot vs. plasma analysis of gonadotropin and ovarian steroid hormone levels in reproductive-age women. Fertil Steril. 2007;88:1404-1407. Doi: 10.1016/j.fertnstert.2006.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mishell DR Jr, Thorneycroft IH, Nakamura RM, Nagata Y, Stone SC. Serum estradiol in women ingesting combination oral contraceptive steroids. Am J Obstet Gynecol. 1972; 114(7):923-928. Doi: 10.1016/0002-9378(72)90098-1 [DOI] [PubMed] [Google Scholar]
- 56. Thorneycroft IH, Stone SC. Radioimmunoassay of serum progesterone in women receiving oral contraceptive steroids. Contraception. 1972;5:129-146. Doi: 10.1016/0010-7824(72)90024-8 [DOI] [PubMed] [Google Scholar]
- 57. Haas J, Fritzsching B, Trubswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322-1330. Doi: 10.4049/jimmunol.179.2.1322 [DOI] [PubMed] [Google Scholar]
- 58. Engelmann F, Barron A, Urbanski H, et al. Accelerated immune senescence and reduced response to vaccination in ovariectomized female rhesus macaques. Age (Dordr). 2011;33:275-289. Doi: 10.1007/s11357-010-9178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol. 2014;142:171-175. Doi: 10.1016/j.jsbmb.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev. 2004;3:215-220. Doi: 10.1016/j.autrev.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 61. Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J Leukoc Biol. 2015;97:31-38. Doi: 10.1189/jlb.1RU0814-382 [DOI] [PubMed] [Google Scholar]
- 62. Galgani M, Insabato L, Cali G, et al. Regulatory T cells, inflammation, and endoplasmic reticulum stress in women with defective endometrial receptivity. Fertil Steril. 2015;103:1579-1586 e1571. Doi: 10.1016/j.fertnstert.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 63. Hosseini S, Shokri F, Ansari Pour S, et al. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2016;116:13-22. Doi: 10.1016/j.jri.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 64. Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13:277-288. Doi: 10.1007/s11154-012-9223-7 [DOI] [PubMed] [Google Scholar]
- 65. Adurthi S, Kumar MM, Vinodkumar HS, et al. Oestrogen receptor-alpha binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer. Sci Rep. 2017;7:17289. Doi: 10.1038/s41598-017-17102-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goodman WA, Bedoyan SM, Havran HL, Richardson B, Cameron MJ, Pizarro TT. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc Natl Acad Sci U S A. 2020;117:17166-17176. Doi: 10.1073/pnas.2002266117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087-1094. Doi: 10.4049/jimmunol.168.3.1087 [DOI] [PubMed] [Google Scholar]
- 68. Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502-A514. Doi: 10.1016/j.autrev.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol Reprod. 2010;82:698-705. Doi: 10.1095/biolreprod.109.081208 [DOI] [PubMed] [Google Scholar]
- 70. Steinborn A, Haensch GM, Mahnke K, et al. Distinct subsets of regulatory T cells during pregnancy: is the imbalance of these subsets involved in the pathogenesis of preeclampsia? Clin Immunol. 2008;129:401-412. Doi: 10.1016/j.clim.2008.07.032 [DOI] [PubMed] [Google Scholar]
- 71. Dimova T, Nagaeva O, Stenqvist AC, et al. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25- regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol. 2011;66(Suppl 1):44-56. Doi: 10.1111/j.1600-0897.2011.01046.x [DOI] [PubMed] [Google Scholar]
- 72. Severance AL, Kinder JM, Xin L, et al. Maternal-fetal conflict averted by progesterone- induced FOXP3+ regulatory T cells. iScience. 2022;25:104400. Doi: 10.1016/j.isci.2022.104400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Areia A, Vale-Pereira S, Alves V, Rodrigues-Santos P, Moura P, Mota-Pinto A. Membrane progesterone receptors in human regulatory T cells: a reality in pregnancy. BJOG. 2015;122(11):1544-1550. Doi: 10.1111/1471-0528.13294 [DOI] [PubMed] [Google Scholar]
- 74. Hierweger AM, Engler JB, Friese MA, et al. Progesterone modulates the T-cell response via glucocorticoid receptor-dependent pathways. Am J Reprod Immunol. 2019;81:e13084. Doi: 10.1111/aji.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hampras SS, Nesline M, Wallace PK, et al. Predictors of immunosuppressive regulatory T lymphocytes in healthy women. J Cancer Epidemiol. 2012;2012:191090. Doi: 10.1155/2012/191090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Afshan G, Afzal N, Qureshi S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab. 2012;58:567-571. [PubMed] [Google Scholar]
- 77. Kverneland AH, Streitz M, Geissler E, et al. Age and gender leucocytes variances and references values generated using the standardized ONE-Study protocol. Cytometry A. 2016;89: 543-564. Doi: 10.1002/cyto.a.22855 [DOI] [PubMed] [Google Scholar]
- 78. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541-566. Doi: 10.1146/annurev-immunol-042718-041717 [DOI] [PubMed] [Google Scholar]
- 79. Li B, Zheng SG. How regulatory T cells sense and adapt to inflammation. Cell Mol Immunol. 2015;12:519-520. Doi: 10.1038/cmi.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zoka A, Barna G, Somogyi A, et al. Extension of the CD4(+)Foxp3(+)CD25(-/low) regulatory T-cell subpopulation in type 1 diabetes mellitus. Autoimmunity. 2015;48:289-297. Doi: 10.3109/08916934.2014.992518 [DOI] [PubMed] [Google Scholar]
- 81. Barreto M, Ferreira RC, Lourenco L, et al. Low frequency of CD4+CD25+ Treg in SLE patients: a heritable trait associated with CTLA4 and TGFbeta gene variants. BMC Immunol. 2009;10:5. Doi: 10.1186/1471-2172-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602-7610. Doi: 10.4049/jimmunol.0901881 [DOI] [PubMed] [Google Scholar]
- 83. Morita T, Shima Y, Wing JB, Sakaguchi S, Ogata A, Kumanogoh A. The proportion of regulatory T cells in patients with rheumatoid arthritis: a meta-analysis. PLoS One. 2016;11:e0162306. Doi: 10.1371/journal.pone.0162306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Aldridge J, Pandya JM, Meurs L, et al. Sex-based differences in association between circulating T cell subsets and disease activity in untreated early rheumatoid arthritis patients. Arthritis Res Ther. 2018;20:150. Doi: 10.1186/s13075-018-1648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3(+) Treg cells and gender bias in autoimmune diseases. Front Immunol. 2015;6:493. Doi: 10.3389/fimmu.2015.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744-755. Doi: 10.1016/j.autrev.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 87. Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061-2066. Doi: 10.4049/jimmunol.1003224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19:665-673. Doi: 10.1038/s41590-018-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baecher-Allan CM, Costantino CM, Cvetanovich GL, et al. CD2 costimulation reveals defective activity by human CD4+CD25(hi) regulatory cells in patients with multiple sclerosis. J Immunol. 2011;186:3317-3326. Doi: 10.4049/jimmunol.1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. Doi: 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 91. Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62:1153-1159. Doi: 10.1136/gutjnl-2012-302362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394-2400. Doi: 10.1111/j.1572-0241.2008.02064.x [DOI] [PubMed] [Google Scholar]
- 93. Kotzamani D, Panou T, Mastorodemos V, et al. Rising incidence of multiple sclerosis in females associated with urbanization. Neurology. 2012;78:1728-1735. Doi: 10.1212/WNL.0b013e31825830a9 [DOI] [PubMed] [Google Scholar]
- 94. Hellwig K, Chen LH, Stancyzk FZ, Langer-Gould AM. Oral contraceptives and multiple sclerosis/clinically isolated syndrome susceptibility. PLoS One. 2016;11:e0149094. Doi: 10.1371/journal.pone.0149094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hernan MA, Hohol MJ, Olek MJ, Spiegelman D, Ascherio A. Oral contraceptives and the incidence of multiple sclerosis. Neurology. 2000;55:848-854. Doi: 10.1212/wnl.55.6.848 [DOI] [PubMed] [Google Scholar]
- 96. Romieu I, Hernandez-Avila M, Liang MH. Oral contraceptives and the risk of rheumatoid arthritis: a meta-analysis of a conflicting literature. Br J Rheumatol. 1989;28(Suppl 1):13-17; discussion 18-23. Doi: 10.1093/rheumatology/xxviii.suppl_1.13 [DOI] [PubMed] [Google Scholar]
- 97. Spector TD, Hochberg MC. The protective effect of the oral contraceptive pill on rheumatoid arthritis: an overview of the analytic epidemiological studies using meta-analysis. J Clin Epidemiol. 1990;43:1221-1230. Doi: 10.1016/0895-4356(90)90023-i [DOI] [PubMed] [Google Scholar]
- 98. Adab P, Jiang CQ, Rankin E, et al. Breastfeeding practice, oral contraceptive use and risk of rheumatoid arthritis among Chinese women: the Guangzhou Biobank Cohort Study. Rheumatology (Oxford). 2014;53:860-866. Doi: 10.1093/rheumatology/ket456 [DOI] [PubMed] [Google Scholar]
- 99. Shima T, Sasaki Y, Itoh M, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121-129. Doi: 10.1016/j.jri.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 100. Girum T, Wasie A. Return of fertility after discontinuation of contraception: a systematic review and meta-analysis. Contracept Reprod Med. 2018;3:9. Doi: 10.1186/s40834-018-0064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Sorensen HT. Pre-gravid oral contraceptive use and time to pregnancy: a Danish prospective cohort study. Hum Reprod. 2013;28:1398-1405. Doi: 10.1093/humrep/det023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thadhani R, Stampfer MJ, Chasan-Taber L, Willett WC, Curhan GC. A prospective study of pregravid oral contraceptive use and risk of hypertensive disorders of pregnancy. Contraception. 1999;60:145-150. Doi: 10.1016/s0010-7824(99)00079-7 [DOI] [PubMed] [Google Scholar]
- 103. Tang R, Ye X, Chen S, Ding X, Lin Z, Zhu J. Pregravid oral contraceptive use and the risk of preterm birth, low birth weight, and spontaneous abortion: a systematic review and meta-analysis. J Womens Health (Larchmt). 2020;29:570-576. Doi: 10.1089/jwh.2018.7636 [DOI] [PubMed] [Google Scholar]
- 104. Garcia-Enguidanos A, Martinez D, Calle ME, Luna S, Valero de Bernabe J, Dominguez-Rojas V. Long-term use of oral contraceptives increases the risk of miscarriage. Fertil Steril. 2005;83:1864-1866. Doi: 10.1016/j.fertnstert.2004.11.085 [DOI] [PubMed] [Google Scholar]
- 105. Risch HA, Weiss NS, Clarke EA, Miller AB. Risk factors for spontaneous abortion and its recurrence. Am J Epidemiol. 1988;128:420-430. Doi: 10.1093/oxfordjournals.aje.a114982 [DOI] [PubMed] [Google Scholar]
- 106. Ford JH, MacCormac L. Pregnancy and lifestyle study: the long-term use of the contraceptive pill and the risk of age-related miscarriage. Hum Reprod. 1995;10:1397-1402. Doi: 10.1093/humrep/10.6.1397 [DOI] [PubMed] [Google Scholar]
- 107. Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205:S4-S8. Doi: 10.1016/j.ajog.2011.06.056 [DOI] [PubMed] [Google Scholar]
- 108. Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445. Doi: 10.1016/j.contraception.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 109. Rosenberg MJ, Waugh MS. Oral contraceptive discontinuation: a prospective evaluation of frequency and reasons. Am J Obstet Gynecol. 1998;179:577-582. Doi: 10.1016/s0002-9378(98)70047-x [DOI] [PubMed] [Google Scholar]
- 110. Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266. Doi: 10.1056/NEJMoa1111840 [DOI] [PubMed] [Google Scholar]
- 111. Lidegaard O, Nielsen LH, Skovlund CW, Skjeldestad FE, Lokkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423. Doi: 10.1136/bmj.d6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lidegaard O, Milsom I, Geirsson RT, Skjeldestad FE. Hormonal contraception and venous thromboembolism. Acta Obstet Gynecol Scand. 2012;91:769-778. Doi: 10.1111/j.1600-0412.2012.01444.x [DOI] [PubMed] [Google Scholar]
- 113. Skovlund CW, Morch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154-1162. Doi: 10.1001/jamapsychiatry.2016.2387 [DOI] [PubMed] [Google Scholar]
- 114. Oddens BJ. Women’s satisfaction with birth control: a population survey of physical and psychological effects of oral contraceptives, intrauterine devices, condoms, natural family planning, and sterilization among 1466 women. Contraception. 1999;59:277-286. Doi: 10.1016/s0010-7824(99)00034-7 [DOI] [PubMed] [Google Scholar]
- 115. Kulkarni J, Liew J, Garland KA. Depression associated with combined oral contraceptives--a pilot study. Aust Fam Physician. 2005;34:990. [PubMed] [Google Scholar]
- 116. Ghosh R, Kumar PK, Mitra P, Purohit P, Nebhinani N, Sharma P. Circulating T helper 17 and IFN-gamma positive Th17 cells in Major Depressive Disorder. Behav Brain Res. 2020;394:112811. Doi: 10.1016/j.bbr.2020.112811 [DOI] [PubMed] [Google Scholar]
- 117. Freier E, Weber CS, Nowottne U, et al. Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology. 2010;35:663-673. Doi: 10.1016/j.psyneuen.2009.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the references. Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request