Abstract
In this study we have evaluated the changes in arterial blood pressure in spontaneously hypertensive rats (SHR) caused by the short-term intake of Bacillus subtilis B060-fermented beans with significant γ-aminobutyric acid (GABA) and nattokinase activity. After being weaned, 7-week-old male SHR and 7-week-old male Wistar–Kyoto (WKY) rats were randomized into seven groups. Until the 8th week of life, the rats in each group were given one of the following: Group 1, high dose of GABA and nattokinase in the SHR (SHD); Group 2, medium dose of GABA and nattokinase in the SHR (SMD); Group 3, low dose of GABA and nattokinase in the SHR (SLD); Group 4, negative control in the SHR (SD); Group 5, positive control in the SHR (SM); Group 6, high dose of GABA and nattokinase in the WKY (WHD); and Group 7, negative control in the WKY (WD). Distilled water served as the negative control, and captopril (50 mg/kg), a known ACE inhibitor, served as the positive control. Systolic blood pressure and diastolic blood pressure values were measured weekly from the 8th week to the 16th week of life using the tail-cuff method. A definite decrease in systolic and diastolic blood pressure values could be observed in the rats treated with captopril and in the rats that received GABA and nattokinase. The greatest antihypertensive effect was observed when the pharmacological treatment was administered. The effect of the daily intake of fermented beans containing GABA and nattokinase may be helpful in controlling blood pressure levels in hypertensive model animals. The fermentation of beans with B. subtilis B060 may therefore constitute a successful strategy for producing a functional food with antihypertensive activity.
Keywords: Bacillus subtilis, Blood pressure, GABA, Nattokinase
1. Introduction
Recently, the relationship between food and health has drawn considerable attention; of specific interest are the physiological actions of food components against certain ailments. Hypertension is a worldwide problem of epidemic propotions and presents in 15–20% of all adults. It is the most common serious chronic health problem because it carries a high risk of cardiovascular complication [1]. As an insidious and ubiquitous disease, hypertension and its complications may contribute to the deaths of 1.5 million and the disability of another 1.5 million Americans each year [1]. Hypertension is associated with increased vascular oxidative stress and signal transduction; however, there is still a debate whether oxidative stress and signal transduction are a cause or a result of hypertension. Animal studies have generally supported the hypothesis that increased blood pressure (BP) is associated with increased oxidative stress and signal transduction; however, human studies have been inconsistent. Oxidative stress and signal transduction promote vascular smooth muscle cell proliferation and hypertrophy and collagen deposition, leading to thickening of the vascular media and narrowing of the vascular lumen. In addition, increased oxidative stress and signal transduction may damage the endothelium and impair endothelium-dependent vascular relaxation and increases vascular contractile activity. All these effects on the vasculature may explain how increased oxidative stress and signal transduction can cause hypertension. Treatment with antioxidants has been suggested to lower oxidative stress, signal transduction, and therefore, BP. However, to date, clinical studies investigating antioxidant supplements have failed to show any consistent benefit. It is noteworthy that lowering BP with antihypertensive medications is associated with reduced oxidative stress and signal transduction [2]. Moreover, it has been suggested that hypertension is closely related to the components of food, specifically sodium chloride and protein, and that antihypertensive peptides may contain an antihypertensive peptide motif.
Some bioactive peptides are angiotensin-converting enzyme inhibitors (ACEIs), and they may be used for preventing hypertension and for other therapeutic purposes [3]. Although most ACEI peptides have been detected in functional dairy products, some have recently been identified and characterized from cereal proteins. In addition to ACEI peptides, γ-aminobutyric acid (GABA) also shows potential for lowering BP in spontaneously hypertensive rats (SHR) and hypertensive humans [4]. GABA, a nonprotein amino acid, possesses well-known physiological functions, such as neurotransmission, induction of hypotension, and diuretic and tranquilizer effects [5]. GABA is synthesized by the irreversible α-decarboxylation of L-glutamate by glutamic acid decarboxylase. Several GABA-enriched cereal foods have been characterized, including rice germ soaked in water, germinated brown rice treated by high-pressure, germinated wheat, and red mold rice [5].
Natto is a traditional soybean-fermented food from Japan and contains nattokinase. Kim et al [6] tested the effect of nattokinase on BP for a large number of participants (n = 86) with prehypertension or stage 1 hypertension and observed a reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP) from nattokinase supplementation. The result of this trial has suggested that nattokinase supplementation may be beneficial for hypertension. However, the mechanism by which nattokinase prevents hypertension, particularly the necessity of protease activity, has not yet been revealed. Proteins are, in general, decomposed into peptides or amino acids under the action of digestive enzymes, such as trypsin, chymotrypsin, and carboxypeptidase, in the intestines. By contrast, a previous study using rats has shown that nattokinase escapes the action of digestive enzymes, is absorbed by the small intestine, and cleaves fibrinogen directly in plasma [7].
In this study, both GABA and nattokinase were obtained from fermented beans with a starter culture containing Bacillus subtilis B060. The effects of the administration of GABA and nattokinase on lowering BP in SHR were investigated. To facilitate the interpretation of our results, we also measured the gain in weight and the consumption of solid, freely accessible feed by the animals.
2. Methods
2.1. Bacteria and fermentations
B. subtilis B060 was used in the present study to produce fermented beans with GABA and nattokinase. This microorganism was maintained as a stock culture in agar slants of Tryptic soy agar (Merck, Darmstadt, Germany).
Precultures of B. subtilis B060 were prepared from 10% yeast extract, 4% fructose, 0.3% CaCl2, 0.5% NaCl, and 0.25% K2HPO4 for the production of nattokinase and from 0.3% yeast extract, 0.1% fructose, 1% monosodium glutamate, 0.5% NaCl, and 0.25% K2HPO4 for the production of GABA. The desired mixture was heated to 121°C for 15 min, cooled to 42°C, and inoculated with a loop of the stock culture to yield an initial bacterial concentration of 105–107 colony-forming units/mL. Incubation was performed at 37°C for 18 hours in a shaking incubator. This preculture was used to inoculate the bean base to obtain the stock of fermented beans.
Black beans, soybeans, and wheat bran were purchased from a local market and used as the substrate for GABA and nattokinase production under solid-state cultivation; the optimum mixing ratio was 1:1:1 (w/w/w). Except for the parameters under investigation, the substrates were prepared as follows. Beans were soaked in distilled water for 8 hours, and then the water was removed. The soaked beans were autoclaved for 15 minutes at 121°C. After cooling, the substrates were inoculated with a 2% (v/w) bacterial suspension of B. subtilis B060 and incubated at 37°C overnight. The maximum yields of GABA and nattokinase were 22.07 mg/g and 146.70 units/g, respectively. The fermented beans were blended with distilled water, centrifuged, and finally lyophilized. The final product was the GABA and nattokinase powder.
2.2. Experimental procedure in rats
Thirty male SHR (7 weeks old) and 12 male Wistar–Kyoto (WKY) rats (7 weeks old) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). The rats were housed in individual cages in a room under controlled 12 hours light–dark cycles at 25 ± 1°C and a relative humidity of 55 ± 5%. During the experimental period, the rats were fed a solid standard diet for rats (Labdiet 5001; St. Louis, MO, USA). The rats were divided into seven groups: Group 1, high dose of GABA and nattokinase in the SHR (2 g/kg; SHD); Group 2, medium dose of GABA and nattokinase in the SHR (1.2 g/kg; SMD); Group 3, low dose of GABA and nattokinase in the SHR (0.4 g/kg; SLD); Group 4, negative control in the SHR (SD); Group 5, positive control (SM); Group 6, high dose of GABA and nattokinase in the WKY (2 g/kg) (WHD); and Group 7, negative control in the WKY (WD). Distilled water served as the negative control, and captopril (50 mg/kg), a known ACEI, served as the positive control. All the rats were orally administered by gastric intubation. During the experimental period, food intake and body weight were recorded once per week.
2.3. Data collection
2.3.1. Blood sampling
After 8 weeks, the rats were sacrificed, and blood samples were collected from the abdominal aorta into heparinized tubes to measure total cholesterol, triacylglycerol, high density lipoprotein, low density lipoprotein, uric acid, Na, K, and Cl. The blood was immediately centrifuged (600g for 5 minutes), and then plasma samples were stored at 4°C until analysis.
2.3.2. Blood pressure
We measured the SBP, the DBP, and the heart rate (HR) of the rats using the tail-cuff method with a BP-98A (Softron, Tokyo, Japan) once per week. Before the measurements, the rats were maintained at 30°C for 10 minutes to make the pulsations of the tail arteries detectable.
2.4. Statistical analysis
The results are expressed as the means with standard errors for a minimum of six rats. All data are reported as means ± standard deviation. One-way analysis of variance with Duncan’s post hoc test was used to analyze the data, and statistical significance was accepted at p < 0.05 (SAS Software; SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Body weight and feeding efficiency
Table 1 lists the average values of dietary consumption and body weight gain of the rats for 1 week after the beginning of treatment using the continuous oral administration test. Each average value of dietary consumption was significantly different from that of the control group. However, the gain in body weight for each treatment group was also significantly different from that of the control group.
Table 1.
Dietary consumption and body weight gain of rats in the experimental groups.*
| SHR | SMD | SLD | SD | SM | WHD | WD | |
|---|---|---|---|---|---|---|---|
| Diet consumption (g/cage/wk) | 373.50 ± 0.048e | 403.83 ± 0.004b | 394.50 ± 0.006c | 409.84 ± 0.006* | 388.61 ± 0.004d | 346.20 ± 0.004g | 366.39 ± 0.004f |
| Body weight gain (g/wk) | 10.14 ± 0.007g | 10.98 ± 0.005f | 11.32 ± 0.012d | 11.62 ± 0.007c | 11.19 ± 0.006e | 14.33 ± 0.007b | 15.04 ± 0.005* |
Different letters indicate a significant difference (p < 0.05, mean ± SD).
Animals were divided into the following seven groups: Group 1, high dose of γ-aminobutyric acid (GABA) and nattokinase in the SHR (SHD); Group 2, medium dose of GABA and nattokinase in the SHR (SMD); Group 3, low dose of GABA and nattokinase in the SHR (SLD); Group 4, negative control in the SHR (SD); Group 5, positive control (SM); Group 6, high dose of GABA and nattokinase in the WKY(WHD); Group 7, negative control in the WKY (WD). Rats divided into each group were housed three per cage throughout the experiment.
3.2. Effect on the content of serum lipids
In the blood analysis, there was no significant difference observed for both total cholesterol and low density lipoprotein among all seven groups (Table 2). However, a significant difference between the SHR and WKY groups was observed for triacyglycerols. There was also a significant difference observed for high density lipoprotein between the WKY (WHD and WD groups) and SHD groups. Additionally, the SMD and SLD groups showed no significant differences from the SHD, SM, and SD groups, but the SHD group was significantly different from the SM and SD groups, as shown in Table 2. The content of uric acid can be ranked highest to lowest as SD > WD > SLD > WHD > SHD > SM > SMD.
Table 2.
Effect of oral administration of GABA and nattokinase on serum lipids.
| TG (mg/dL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | HDL/LDL (mg/dL) | UA (mg/dL) | |
|---|---|---|---|---|---|---|
| SHD | 64.5 ± 0.778b | 48.5 ± 0.707a | 24.0 ± 0.707b | 4.0 ± 0.414a | 6.0 | 2.1 ± 0.243bc |
| SMD | 59.0 ± 0.414b | 43.5 ± 0.707a | 22.5 ± 0.707bc | 4.5 ± 0.707a | 5.0 | 1.9 ± 0.141c |
| SLD | 61.5 ± 0.192b | 37.0 ± 0.828a | 22.5 ± 0.707bc | 4.0 ± 0.121a | 5.6 | 2.3 ± 0.424bc |
| SD | 62.5 ± 0.778b | 53.0 ± 0.556a | 21.0 ± 0.121c | 3.5 ± 0.707a | 6.0 | 3.6 ± 0.919a |
| SM | 59.0 ± 0.414b | 43.5 ± 0.607a | 21.0 ± 0.414c | 3.5 ± 0.707a | 6.0 | 2.0 ± 0.141bc |
| WHD | 102.5 ± 0.607a | 56.5 ± 0.364a | 33.5 ± 0.707a | 5.5 ± 0.707a | 6.1 | 2.2 ± 0.141bc |
| WD | 101.5 ± 0.707a | 58.0 ± 0.485a | 32.0 ± 0.414a | 5.5 ± 0.707a | 5.8 | 3.0 ± 0.283ab |
Different letters indicate a significant difference (p < 0.05, mean ± SD).
GABA = γ-aminobutyric acid; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SD = negative control in the SHR; SHD = high dose of GABA and nattokinase in the SHR; SLD = low dose of GABA and nattokinase in the SHR; SM = medium dose of GABA and nattokinase in the SHR; SMD = medium dose of GABA and nattokinase in the SHR; TC = total cholesterol; TG = triacyglycerols; UA = uric acid; WD = negative control in the WKY; WHD = high dose of GABA and nattokinase in the WKY.
3.3. Effect on the content of minerals
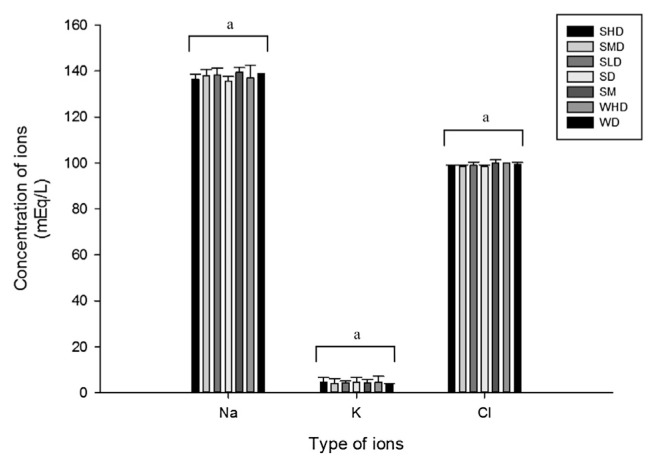
The content of serum sodium, potassium, and chloride remained stable, as shown in Fig. 1. Despite the considerable skepticism that remains among some scientists concerning the relationship between sodium and BP, there is compelling epidemiological evidence to support a relationship between dietary sodium intake and BP and the cardiovascular consequences [8]. Interventional studies have provided further confirmation of this relationship [9] and have led to the recommendation that dietary sodium intake be restricted in hypertensive patients. Independent of the effects of potassium on BP, this ion is known to affect blood vessles [10] and the occurrence of vascular disease [11]. Chloride intake has also emerged as a confounding element in some studies of the effect of sodium on BP, similar to that previously described for potassium [12]. The effect of GABA and nattokinase on the serum content of these mineral elements reflected the plausible mechanism of lowering BP. On the basis of the theory of the renin–angiotensin system, an increase in the level of Ang II would increase the concentration of Na+, whereas the intake of ACEI would decrease the formation of Ang II; thus, the concentration of Na+ decreased. This result suggested that the antihypertensive effect of peptides from food proteins on the BP of SHR was most likely due to the improvement or regulation of the salt balance in addition to the simplicity of the inhibition of ACE from this perspective.
Fig. 1.
Concentration of serum cation of spontaneously hypertensive rats (SHR) after different treatments. Values are the means with standard errors for six animals per group. aNo significant difference was observed in each group (p < 0.05).
3.4. BP and heartbeat
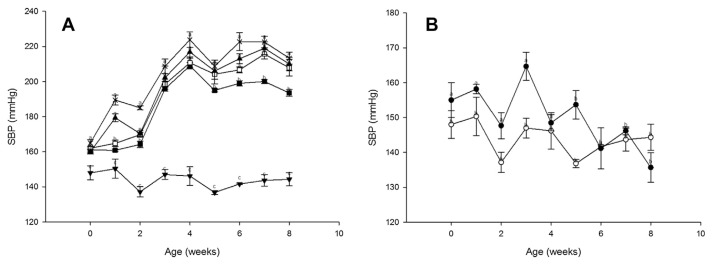
Fig. 2 presents the time courses of the SBP values after the daily intake of GABA and nattokinase. Whereas the SBP levels decreased from negative baseline control values during the intake period in the SHD group, these levels did not similarly change in the SMD and SLD groups.
Fig. 2.
Effect of a single oral administration of γ-aminobutyric acid (GABA) and nattokinase on systolic blood pressure (SBP) values in (A) spontaneously hypertensive rats and (B) Wistar–Kyoto rats. Symbols: ■, SHD; □, SMD; ▲, SLD; ×, SD; ▼, SM; ○, WHD; ●, WD. Values are means with standard errors for six animals per group. Mean values were significantly different from the control value at the same time point: p < 0.05. SD = negative control in the SHR; SHD = high dose of GABA and nattokinase in the SHR; SLD = low dose of GABA and nattokinase in the SHR; SM = medium dose of GABA and nattokinase in the SHR; SMD = medium dose of GABA and nattokinase in the SHR; WD = negative control in the WKY; WHD = high dose of GABA and nattokinase in the WKY.
The decreases observed in the SBP levels at 5 weeks, 6 weeks, 7 weeks, and 8 weeks after GABA and nattokinase intake were 13.67 mmHg, 23.67 mmHg, 22.5 mmHg, and 19.83 mmHg, respectively. These changes differed significantly from those of the SD group.
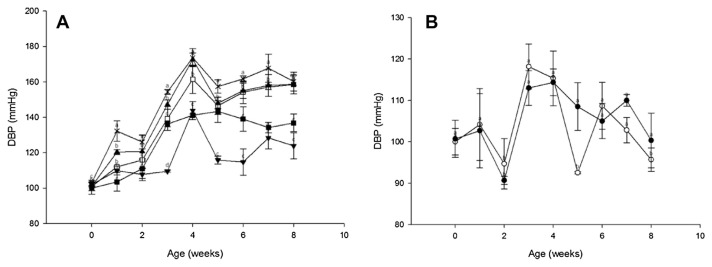
The DBP levels decreased significantly different from the SD group by 32.5 mmHg (p < 0.05) after 4 weeks of consuming GABA and nattokinase (Fig. 3A). In the SMD and SLD groups, the observed decrease was 12.17 mmHg and 1.00 mmHg, respectively. The reduction in the mean blood pressure (MBP) values of the SHD group differed significantly from the SD group after 5 weeks, 6 weeks, 7 weeks, and 8 weeks by 11.66 mmHg (p < 0.05), 19.67 mmHg (p < 0.05), 24.00 mmHg (p < 0.05), and 29.5 mmHg (p < 0.05), respectively. By contrast, this parameter did not change significantly in the SMD and SLD groups throughout the intake period.
Fig. 3.
Effect of a single oral administration of GABA and nattokinase on diastolic blood pressure (DBP) values in (A) spontaneously hypertensive rats and (B) Wistar–Kyoto rats. Symbols: ■, SHD; □, SMD; ▲, SLD; ×, SD; ▼, SM; ○, WHD; ●, WD. Values are means with standard errors for six animals per group. Mean values were significantly different from the control value at the same time point: p <0.05. GABA = γ-aminobutyric acid; SD = negative control in the SHR; SHD = high dose of GABA and nattokinase in the SHR; SLD = low dose of GABA and nattokinase in the SHR; SM = medium dose of GABA and nattokinase in the SHR; SMD = medium dose of GABA and nattokinase in the SHR; WD = negative control in the WKY; WHD = high dose of GABA and nattokinase in the WKY.
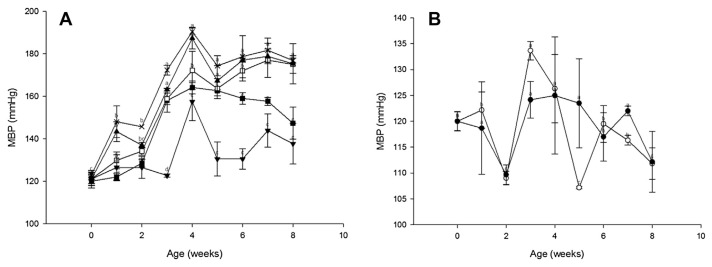
After 4 weeks of intake by the SHD group, the MBP values decreased by at least 26.33 mmHg, as shown in Fig. 4A, and the values increased with time during the intake period. By comparison, GABA and nattokinase did not cause a change in the values of SBP (Fig. 2B), DBP (Fig. 3B), and MBP (Fig. 4B) in the WHD group. In addition, HR did not change significantly in either group at any point.
Fig. 4.
Effect of a single oral administration of GABA and nattokinase on mean blood pressure (MBP) values in (A) spontaneously hypertensive rats and (B) Wistar–Kyoto rats. Symbols: ■, SHD; □, SMD; ▲, SLD; ×, SD; ▼, SM; ○, WHD; ●, WD. Values are means with standard errors for six animals per group. Mean values were significantly different from the control value at the same time point: p <0.05. GABA = γ-aminobutyric acid; SD = negative control in the SHR; SHD = high dose of GABA and nattokinase in the SHR; SLD = low dose of GABA and nattokinase in the SHR; SM = medium dose of GABA and nattokinase in the SHR; SMD = medium dose of GABA and nattokinase in the SHR; WD = negative control in the WKY; WHD = high dose of GABA and nattokinase in the WKY.
4. Discussion
The SHR and WKY rats were fed the same diet but showed significant differences in food intake, and the body weights of the SHR were significantly lower. Feeding efficiency in hypertensive rats may be caused by excitation of the peripheral sympathetic nervous system [13,14].
In the present study, GABA and nattokinase had no blood lipids or electrolytes. Although there is a report showing that captopril may elevate blood Na level, the IC50 of the drug was much lower than the GABA and nattokinase used in the present study.
The SHR of the negative control groups showed a gradual increase in SBP and DBP values from weaning that reached maxima at 8 weeks of life. From this age, the arterial BPs of the rats remained consistently high, and their DBP values were similar to those of Week 4. A clear decrease in the SBP and DBP values could be observed in the rats of the positive control group treated with captopril from the 5th week to the 8th week of life. In these animals, both the SBP and DBP values remained constant during this period. The antihypertensive effect of these products was less pronounced than that of the pharmacological treatment but could be clearly observed from the 5th week to the 8th week of life.
It was not surprising to find a clear decrease in SBP and DBP values upon administering 50 mg/kg of captopril to SHR because this drug is a potent ACEI with an IC50 value of 0.02 μM [15]. In this context, Fujita and Yoshikawa [15] suggested that, compared with captopril, the ACEIs derived from food protein possess higher in vivo activities than those expected from their in vitro activities, and that this result could be explained by their higher affinity for the tissues and by their slower elimination.
During fermentation, GABA and nattokinase are made from the protein of the beans by B. subtilis B060, which has high productivity of GABA and nattokinase. With respect to GABA production, acidic fermented beans are beneficial because the glutamate decarboxylase activity of B. subtilis B060 is optimal at a pH of approximately 7 (Suwanmanon and Hsieh, unpublished results). Its GABA transaminase (optimal pH 7–10) [16], which catalyzes the transformation of GABA to succinic semialdehyde, is poorly active under acidic conditions. Therefore, GABA accumulates during fermentation.
Although the mechanism underlying the hypotensive action of systemically administered GABA has not yet been fully elucidated, several hypotheses have been postulated. One of them is as follows. Because GABA hardly passes the blood–-brain barrier [17,18], this molecule acts not in the central nervous system but in the peripheral nervous system.
Recently, a few studies have reported that various dietary materials or products containing GABA decreased BP values in hypertensive patients in Japan. Gabaron tea, extracts of Beni-Koji, and a particular germ of rice [19] were included among these supplements. However, these dietary materials or products contain many ingredients in addition to GABA, and although their effects were not ruled out, it was presumed that the hypotensive effect was due to the presence of GABA.
Nattokinase has been investigated as a strong fibrinolytic enzyme in vitro and in vivo [7,20–22]. Recent studies have shown that the oral administration of supplements containing nattokinase decreases both SBP and DBP in hypertensive patients [5]. However, the mechanism by which nattokinase prevents hypertension has yet to be elucidated.
Several reports have noted that B. subtilis produces and secretes enzymes such as subtilisin [23], metalloproteases [24], Epr [25], Mpr [26], Vpr [27], bacillopeptidase F [28,29], and β-glucanase [30]. Nattokinase also digests cross-linked fibrin directly at a concentration of 0.02 mg/mL [21] and prolongs the coagulation time of plasma in parallel with the degradation of fibrinogen in rats with intraduodenal administration (80 mg/ kg body weight) [7]. These reports suggest that the fibrinolytic and antithrombotic effects of nattokinase are similar or superior to those of bacillopeptidase F. Bacillopeptidase F contains the residues Val101–Ala102–Pro103, which corresponds to the ACE-inhibitory tripeptide Val–Ala–Pro, and does not contain the renin-inhibitory dipeptide, Tyr–Tyr [29].
In conclusion, the daily intake of fermented beans containing GABA and nattokinase may be helpful in controlling BP levels in hypertensive model animals. Nattokinase, whether intact or fragmented, is absorbed intestinally and reduces hypertension in SHR. Nevertheless, there are different mechanisms on antihypertensive effects depending on the form of oral administration. Nattokinase retaining its protease activity may lower BP through the decrease of blood viscosity by cleaving plasma fibrinogen. GABA is present in many vegetables and fruits. The low incidence of hypertension among vegetarians, therefore, may be partly attributable to their ingestion of GABA via these foods. Moreover, GABA and nattokinase were contained in food ingested daily and thus meet the requirement for naturalness and safety.
Footnotes
Conflicts of interest
The authors declare that there no conflicts of interest.
REFERENCES
- 1.Manger WM, Rosenthal J, editors. Arterial hypertension: pathogenesis, diagnosis, and therapy. New York: Springer-Verlag; 1982. An overview of current concepts regarding the pathogenesis and pathophysiology of hypertension; pp. 1–40. [Google Scholar]
- 2. Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;30:185–9. doi: 10.2337/dc08-s246. [DOI] [PubMed] [Google Scholar]
- 3. Li GH, Qu MR, Wan JZ, et al. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asis Pacific J Clin Nutr. 2007;16:275–80. [PubMed] [Google Scholar]
- 4. Kajimoto O, Hirata H, Nakagawa S, et al. Hypotensive effect of fermented milk containing γ-aminobutyric acid (GABA) in subjects with high normal blood pressure. J Jpn Soc Food Sci. 2004;51:79–86. [Google Scholar]
- 5. Siragusa S, Angelis DM, Cagno DR, et al. Synthesis of γ-aminobutyric acid (GABA) by lactic acid bacteria isolated from Italian cheese varieties. Appl Environ Microbiol. 2007;73:7283–90. doi: 10.1128/AEM.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim JY, Gum SN, Paik JK, et al. Effects of nattokinase on blood pressure: a randomized, controlled trial. Hypertens Res. 2008;31:1583–8. doi: 10.1291/hypres.31.1583. [DOI] [PubMed] [Google Scholar]
- 7. Fujita M, Hong K, Ito Y, et al. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995;18:1194–6. doi: 10.1248/bpb.18.1194. [DOI] [PubMed] [Google Scholar]
- 8. MacGregor GA. Sodium is more important than calcium in essential hypertension. Hypertension. 1985;7:628–37. doi: 10.1161/01.hyp.7.4.628. [DOI] [PubMed] [Google Scholar]
- 9. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;37:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 10.Tobian L, Laragh JH, Brenner BM, editors. Hypertension: pathophysiology, diagnosis and management. 2nd ed. New York: Raven Press; 1995. The protective effects of high-potassium diets in hypertension, and the mechanisms by which high-NaCl diets produce hypertension. [Google Scholar]
- 11. Khaw KT, Barrett CE. Dietarypotassium and stroke-controlled trail of potassium chloride in the treatment of mild hypertension. Hypertension. 1987;9:445–50. [Google Scholar]
- 12. McCarron DA. Is calcium more important than sodium in the pathogenesis of essential hypertension? J Hypertens. 1985;7:607–27. doi: 10.1161/01.hyp.7.4.607. [DOI] [PubMed] [Google Scholar]
- 13. Lamont LS. Beta-blockers and their effects on protein metabolism and resting energy expenditure. J Cardiopulm Rehabil. 1995;15:183–5. doi: 10.1097/00008483-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 14. Cabassi A, Vinci S, Cantoni AM, et al. Sympathetic activation in adipose tissue and skeletal muscle of hypertensive rats. Hypertension. 2002;39:656–61. doi: 10.1161/hy0202.103471. [DOI] [PubMed] [Google Scholar]
- 15. Fujita H, Yoshikawa MLKPNM. a prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44:123–7. doi: 10.1016/s0162-3109(99)00118-6. [DOI] [PubMed] [Google Scholar]
- 16. Shelp JB, Bown WB, McLean DM. Metabolism and functions of gamma-aminobutyric. Trends Plant Sci. 1999;4:1360–85. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- 17. Van Gelder MN, Elliott KAC. Disposition of γ-aminobutyric acid administered to mammals. J Neurochem. 1958;3:139–43. doi: 10.1111/j.1471-4159.1958.tb12620.x. [DOI] [PubMed] [Google Scholar]
- 18. Kuriyama K, Sze Y. Blood–brain barrier to H3-γ-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology. 1971;10:103–8. doi: 10.1016/0028-3908(71)90013-x. [DOI] [PubMed] [Google Scholar]
- 19.Onoda A, Saikusa T, Horino T, et al. Accumulative effects of γ-aminobutyric acid (GABA) from defatted rice germ. First International Symposium on Disease Prevention by IP6 and Other Rice Components; 1998; p. 97. [Google Scholar]
- 20. Fujita M, Nomura K, Hong K, et al. Purification and characterization of a strong fibrinolytic enzyme (nattokinase) in the vegetable cheese natto, a popular soybean fermented food in Japan. Biochem Biophys Res Commun. 1993;197:1340–7. doi: 10.1006/bbrc.1993.2624. [DOI] [PubMed] [Google Scholar]
- 21. Fujita M, Ito Y, Hong K, et al. Characterization of nattokinase degraded products from human fibrinogen or crosslinked fibrin. Fibrinolysis. 1995;9:157–64. [Google Scholar]
- 22. Fujita M, Hong K, Ito Y, et al. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. 1995;18:1387–91. doi: 10.1248/bpb.18.1387. [DOI] [PubMed] [Google Scholar]
- 23. Kawamura F, Doi RH. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984;160:442–4. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uehara H, Yamane K, Maruo B. Thermosensitive, extracellular neutral proteases in Bacillus subtilis: isolation, characterization, and genetics. J Bacteriol. 1979;139:583–90. doi: 10.1128/jb.139.2.583-590.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sloma A, Ally A, Ally D, et al. Gene encoding a minor extracellular protease in Bacillus subtilis. J Bacteriol. 1988;170:5557–63. doi: 10.1128/jb.170.12.5557-5563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sloma A, Rudolph CF, Rufo GA, et al. Gene encoding a novel extracellular metalloprotease in Bacillus subtilis. J Bacteriol. 1990;172:1024–9. doi: 10.1128/jb.172.2.1024-1029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sloma A, Rufo GJ, Theriault KA, et al. Cloning and characterization of the gene for an additional extracellular serine protease of Bacillus subtilis. J Bacteriol. 1991;173:6889–95. doi: 10.1128/jb.173.21.6889-6895.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sloma A, Rufo GJ, Rudolph CF, et al. Bacillopeptidase F of Bacillus subtilis: purification of the protein and cloning of the gene. J Bacteriol. 1990;172:1470–7. doi: 10.1128/jb.172.3.1470-1477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Omura K, Hitosugi M, Zhu X, et al. A newly derived protein from Bacillus subtilis natto with both antithrombotic and fibrinolytic effects. J Pharm Sci. 2005;99:247–51. doi: 10.1254/jphs.fp0050408. [DOI] [PubMed] [Google Scholar]
- 30. Murphy N, McConnell DJ, Cantwell BA. The DNA sequence of the gene and genetic control sites for the excreted B. subtilis enzyme beta-glucanase. Nucleic Acids Res. 1984;12:5355–67. doi: 10.1093/nar/12.13.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]