Abstract
The complete 184,457-bp sequence of the aromatic catabolic plasmid, pNL1, from Sphingomonas aromaticivorans F199 has been determined. A total of 186 open reading frames (ORFs) are predicted to encode proteins, of which 79 are likely directly associated with catabolism or transport of aromatic compounds. Genes that encode enzymes associated with the degradation of biphenyl, naphthalene, m-xylene, and p-cresol are predicted to be distributed among 15 gene clusters. The unusual coclustering of genes associated with different pathways appears to have evolved in response to similarities in biochemical mechanisms required for the degradation of intermediates in different pathways. A putative efflux pump and several hypothetical membrane-associated proteins were identified and predicted to be involved in the transport of aromatic compounds and/or intermediates in catabolism across the cell wall. Several genes associated with integration and recombination, including two group II intron-associated maturases, were identified in the replication region, suggesting that pNL1 is able to undergo integration and excision events with the chromosome and/or other portions of the plasmid. Conjugative transfer of pNL1 to another Sphingomonas sp. was demonstrated, and genes associated with this function were found in two large clusters. Approximately one-third of the ORFs (59 of them) have no obvious homology to known genes.
Sphingomonas aromaticivorans F199 was isolated from sediments collected 410 m below the land surface near Allendale, S.C., in 1988 (4, 21). It was established that this bacterium possessed the novel ability to degrade a variety of aromatic compounds including toluene, all isomers of xylene, p-cresol, naphthalene, biphenyl, dibenzothiophene, fluorene, salicylate, and benzoate (20, 22). In recent years, there have been many reports of other Sphingomonas strains that are capable of degrading aromatic compounds (12, 17, 30, 36, 40, 44, 45, 62–65, 68–70, 88, 91). Studies of Sphingomonas strains suggest that members of this genus are well adapted for the degradation of high-molecular-weight polycyclic aromatic hydrocarbons and other aromatic contaminants. The inability to detect Sphingomonas biodegradative genes via hybridization with catabolic genes from phylogenetically distinct bacteria suggested that biodegradative genes from Sphingomonas sp. evolved independently from phylogenetically distinct bacteria such as those within the genus Pseudomonas (46, 47). Some Sphingomonas strains are further distinguished in that the genes necessary for degradation of one type of aromatic compound are distributed into multiple operons that also possess genes for the degradation of other aromatic compounds (107). This unusual gene arrangement suggests that a highly complex regulatory network is responsible for the expression of aromatic degradative pathways in some Sphingomonas spp.
S. aromaticivorans F199 was shown to possess two plasmids (20, 22), which are designated pNL1 (∼180 kbp) and pNL2 (∼480 kbp). We reported earlier that catechol meta ring cleavage activity, a central step in the catabolism of aromatic rings, was associated with the smaller plasmid, pNL1 (86), and we described a physical map for this plasmid. To further probe the catabolic functions and accessory genes encoded on pNL1, we undertook the complete sequencing and annotation of this plasmid. This approach has allowed a thorough genetic analysis of pNL1-associated catabolic genes, a comparison of these genes with analogous chromosomally located ones in S. yanoikuyae B1, and the development of hypotheses regarding functions of pNL1-encoded genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
S. aromaticivorans F199, originally isolated by our laboratory, is also maintained in the U.S. Department of Energy’s Subsurface Microbial Culture Collection at Florida State University (3). Sphingomonas sp. strain S-88 (ATCC 31554) mutant m260 was obtained from Thomas Pollock (77). Its resistance to bacitracin and inability to degrade aromatic compounds examined in this study was useful for plasmid transfer experiments. S. paucimobilis ATCC 298377 was purchased from the American Type Culture Collection. Bacteria were maintained on either full-strength (Escherichia coli) or half-strength (Sphingomonas) Luria-Bertani medium (LB).
SB354, a Tn5 derivative of the suicide vector pRK600 (85) was maintained on LB containing kanamycin to select for the transposon and chloramphenicol to select for the pRK600 plasmid containing the transposon. Plasmid pRK2013 (18) was used as a helper plasmid in triparental matings. Antibiotics were supplemented at levels of 25 μg/ml for kanamycin and chloramphenicol; 500 μg/ml for bacitracin, prepared from (Sigma) powder at 73,000 U/g; and 12.5 μg/ml for polymyxin B.
Transposon mutagenesis.
A portion (50 μl) of an overnight culture of the S. aromaticivorans F199 recipient was spotted and allowed to dry on half-strength LB agar. An equivalent amount of overnight cultures of the donor strains, E. coli with either SB354 or pRK2013, were then overlaid, dried, and incubated overnight at 30°C. The resulting bacterial spots were resuspended in 1 ml of saline, and 100-μl aliquots were plated onto 0.5× LB containing kanamycin to select for transposition and polymyxin B to select against the E. coli donor strains. Sphingomonas colonies are readily distinguished from E. coli colonies because of their characteristic yellow pigmentation. The resulting Sphingomonas insertion mutants were screened for loss of dioxygenase activity by placing a small crystal of indole in the lid of the petri dish; the colonies were then examined for the absence of blue coloring (i.e., lack of indigo formation). Conditions for testing the ability to grow on aromatic compounds or to produce colored intermediates from them by Sphingomonas strains have been described elsewhere (20).
Conjugal transfer of pNL1.
Recipient Sphingomonas sp. S-88 m260 and donor S. aromaticivorans F199 strains were mated as described above. Exconjugants were isolated on 0.5× LB plates containing bacitracin to select against donor cells and kanamycin to select for transfer of transposon-mutagenized pNL1. After incubation, a few crystals of indole were added to the lid of the plate and further incubated to allow the development of blue color. The presence of pNL1 in exconjugants was confirmed by plasmid isolation (39) and analysis by pulse field gel electrophoresis (1% agarose, 0.5× TBE, 6 V/cm, included angle of 120, 0.475-s initial switch time, 21.79-s final switch time, linear ramping factor, 20.5-h run time) with the Bio-Rad Chef system (Bio-Rad, Hercules, Calif.).
Construction and isolation of small and large insert libraries.
The procedure for isolation of pNL1 has been described elsewhere (86). For construction of the large insert library, a partial Sau3A digestion of pNL1 was used to produce fragments of approximately 40 kb, which were then dephosphorylated and ligated into the BamHI site of the sCos-1 cosmid vector (16). Ligation mixtures were packaged into lambda particles by using the Gigapack II packaging extract (Stratagene, La Jolla, Calif.), infected into E. coli DH5α, and plated onto LB agar supplemented with 100 μg of kanamycin per ml. Cosmids were isolated from 6-ml overnight cultures of the resulting kanamycin-resistant colonies by using a modified boiling lysis procedure (55). Briefly, bacterial pellets were dissolved in 300 μl of solution A (8% sucrose; 5% Triton X-100; 50 mM Tris, pH 8.0; 50 mM EDTA) and 10 μl of solution B (10 mg RNase per ml; 1 mg of lysozyme per ml; 50 mM Tris, pH 8) and boiled for 1 min. After removal of the cellular debris by centrifugation, 10 μl of a 10-mg/ml concentration of pronase was added to the supernatant and incubated for 30 min at 65°C to remove any remaining bacterial proteins. The cosmid DNA was precipitated by adding 20 μl of 5 M ammonium acetate and 900 μl of isopropanol–1 mM phenylmethylsulfonyl fluoride and was then resuspended in 75 μl of TE (pH 8.0). Restriction enzyme digestion of approximately 300 cosmid clones with NotI and SspI demonstrated uniform coverage of pNL1.
Five small insert libraries were constructed to ensure complete representation of pNL1. The construction of a small insert library from a small amount of pNL1 DNA by using a variation on the random PCR amplification method described by Grothues and Tummler (28) has been previously described (101). pNL1 DNA for the second library was obtained from nine cosmid clones whose inserts covered most of pNL1. Approximately 100 μg of cosmid DNA was sheared by sonication (6) to fragments of 0.5 to 1 kb, whose ends were then repaired with exonuclease I and then cloned into pCR-Blunt (Invitrogen, Carlsbad, Calif.). The remaining three libraries were constructed by subcloning pNL1 DNA digested with BamHI, EcoRI, or PstI into the cognate sites of pBluescript II SK(+) vector (Stratagene).
Sequencing.
Plasmid DNA was purified with the Qiagen QIAwell 96 Ultrawell plasmid kit (Qiagen, Inc., Valencia, Calif.). Cosmid DNA was purified by standard alkaline lysis (55), denatured at room temperature for 10 min in 0.5 M NaOH, neutralized with 1.2 M (final concentration) sodium acetate, and ethanol precipitated. For sequencing reactions, 500 ng of plasmid DNA or 1 μg of cosmid DNA were used for both the cosmid and the plasmid libraries, and primers complementary to the T3 and T7 vector promoter sequences were used to generate the sequence from the insert ends by using dideoxy chain-termination sequencing reactions (83) with Perkin-Elmer/Applied Biosystems Dye Terminators and Ampli-Taq DNA polymerase. For gap closure, primers were designed from the ends of assembled sequences by using Sequencher software (Gene Codes Corp., Ann Arbor, Mich.), MacVector sequence analysis software (Eastman Kodak, Rochester, N.Y.) and Gap4 from the Staden software package. Primers were synthesized by using standard phosphoramidite chemistry on the Applied Biosystems model 392 DNA synthesizer. Sequencing reactions were analyzed with an ABI 377 DNA sequencer.
Sequence assembly.
Sequences were assembled by using tools from the Staden Sequence Analysis software package (10). Sequences were initially processed with PREGAP to automate the generation of compressed files for assembly and to mark vector and other non-pNL1 sequences that appear as a result of library construction. These files were assembled and manually edited by using GAP4. Upon completion, a FASTA file was generated for automated sequence analysis.
Automated sequence analysis.
Automated sequence analyses were completed by using MAGPIE (multipurpose automated genome project investigation environment) (27). Initial assignments of putative open reading frames (ORFs) were made by using the criteria that (i) the start codon was ATG, GTG, or TTG; (ii) the stop codon was TAA, TAG, or TGA; and (iii) the ORF size was between 150 and 30,000 bp in length. Default MAGPIE settings were used to characterize the 1,346 potential ORFs identified. Data collected was manually surveyed to identify the ORFs most likely to encode a gene. The position of each start codon was estimated manually by identification of the Shine-Dalgarno sites within 15 bp of a potential start codon, the position of upstream genes, and a comparison to the start position of the gene homologues. Protein sequences were aligned with CLUSTALW. ORFs predicted to encode genes were adjusted in size to reflect the manually predicted start position and realigned with GenBank sequences by using BLASTP2 and TBLASTN.
Nucleotide sequence accession number.
The sequence of the S. aromaticivorans F199 plasmid pNL1 has been deposited with GenBank under accession number AF079317.
Oxygenase activity measurements.
Two cosmids were selected from the E. coli XL1-Blue large insert library which contained either bphC (cosmid 6) or xylE (cosmid 18) for oxygenase activity measurements. The sCOS-1 T3 primer target site in these clones is positioned 5,887 and 288 bp upstream of the start codons of bphC and xylE, respectively. Overnight cultures were harvested and washed in 20 mM sodium phosphate buffer (pH 7.5). After the addition of a final 0.1-mg/ml concentration of DNase, the cells were lysed by the application of two passes through a French pressure cell at 16K lb/in2. Cellular debris was removed by centrifugation (JA-20; 10,000 rpm for 25 min). The protein content was estimated with a Bio-Rad protein assay kit. Enzymatic production of colored intermediates was measured spectrophotometrically by monitoring the increase in the absorbance at the corresponding wavelength of each meta cleavage product formed from the following substrates: catechol, 375 nm; 3-methyl catechol, 388 nm; 4-methyl catechol, 382 nm, and 2,3-dihydroxybiphenyl, 434 nm. The reaction mixture contained 0.1 M sodium phosphate (pH 8) and between 0.5 and 1 mg of cellular lysate. The reaction was initiated by the addition of the appropriate substrate at a final concentration of 0.4 mM for catechol-type substrates or 0.5 mM for 2,3-dihydroxybiphenyl.
Oxidation of 1,2-dihydroxynaphthalene was measured with lysates prepared in 20 mM KH2PO4-NaOH (pH 6.2), 1 mM 2-mercaptoethanol, and 10% ethanol. The lysate was treated with oxygen-free ferrous sulfate at a concentration of 0.5 mM for 1 h on ice in an anaerobic glovebox prior to assay. The reaction mixtures contained between 0.5 and 1 mg of cellular lysate in 0.1 M acetic acid-NaOH buffer (pH 5.5) at a total volume of 1.8 ml. The reaction was initiated by the addition of 0.5 μmol of 1,2-dihydroxynaphthalene in 10 μl of tetrahydrofuran. A decrease in oxygen over time as a percentage of the total dissolved oxygen was measured polarographically with a YSI model 5300 dissolved oxygen monitor (Yellow Springs Instrument Co., Yellow Springs, Ohio).
RESULTS
Association of conjugative and biodegradative functions with pNL1.
Attempts to transfer native pNL1 to nonaromatic degrading S. paucimobilis and Sphingomonas sp. strain S88m260 in conjugation experiments were unsuccessful. In order to provide a more robust selectable marker on the plasmid, S. aromaticivorans F199 was subjected to transposon mutagenesis. A single mutant, designated F199 tn349, with an altered phenotype that was linked to plasmid function, was identified. S. aromaticivorans F199 tn349 was unable to convert indole to indigo, a property commonly affiliated with oxygenases, including styrene monooxygenase (71), xylene monooxygenase (11), toluene dioxygenase (37), and naphthalene dioxygenase (13). This mutant was also unable to grow on naphthalene or biphenyl and could not produce orange metabolites from dibenzothiophene or yellow metabolites from fluorene or biphenyl. It retained its ability to grow on p-cresol, m-xylene, salicylate, and benzoate and to produce yellow metabolites from catechol.
F199 tn349 was mated with Sphingomonas sp. strain S-88 m260 and kanamycin-resistant exconjugants identified. Plasmids were extracted from seven exconjugants and analyzed by gel electrophoresis. Each exconjugant possessed a plasmid the size of pNL1 in addition to the plasmids native to Sphingomonas sp. S-88 m260. The degradative properties of the exconjugant were identical to S. aromaticivorans F199 tn349, except that they were unable to grow on p-cresol. These results suggest that pNL1 encodes the entire m-xylene and benzoate catabolic pathways, as well as pathways for growth on salicylate. The inability of Sphingomonas S-88 m260 exconjugants to grow on p-cresol suggests that either none or only portions of the p-cresol degradative pathway were encoded by pNL1.
Nucleotide composition and restriction sites.
The circular plasmid pNL1 from S. aromaticivorans F199 was 184,457 bp in length. The overall G+C content was 62% and the A+G content was 49%; this value is consistent with an earlier report of a G+C content of 62.9 to 65.4 mol% for S. aromaticivorans F199 total DNA (5), which suggests that this plasmid was not laterally transferred from another genus.
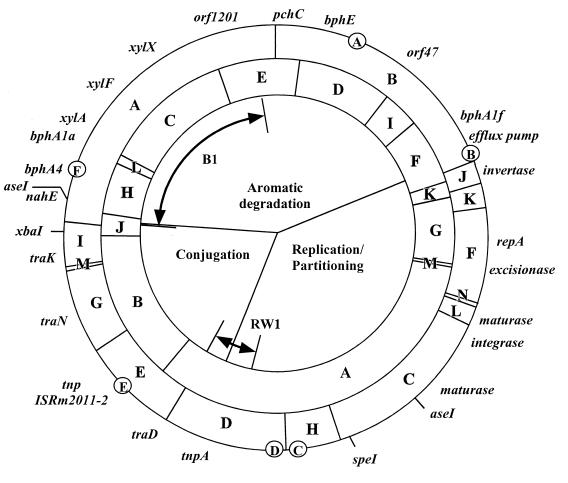
Examination of the sequence for restriction enzyme sites revealed that there were no PmeI sites, single XbaI and SpeI sites, and two AseI sites in this plasmid; this finding is also consistent with earlier results (86). The reported SspI digestion pattern included 12 fragments (designated A through L) which were mapped in the order ILFKGABJHCED and sized at 6, 1.8, 12, 2.7, 11, 51, 30, 3.5, 8.5, 25, 14, and 16 kb, respectively. Thirteen SspI sites were found in the plasmid DNA sequence, with an extra 321 bp SspI fragment (designated M) present between fragments G and A. The position of fragment L was moved between fragments H and C, which results in an updated SspI fragment order of IFKGMABJHLCED. The calculated fragment sizes based on DNA sequence analysis are 6,166, 11,510, 2,540, 11,150, 321, 61,361, 26,058, 3,330, 8,596, 1,917, 22,034, 13,655, and 15,717 bp, respectively. The reported NotI restriction analysis revealed 14 fragments (designated A through N), and these were mapped in the order BJKLNFCHDEGMIA and sized at 35, 3.5, 3.2, 2.5, 0.6, 13, 24, 7.4, 17, 14, 12, 1.1, 6, and 45 kb, respectively. Fourteen NotI restriction sites were also found in the DNA sequence, but the order of LNF was reversed to FNL. The calculated NotI fragment sizes for BJKFNLCHDEGMIA are 35,958, 3,266, 2,945, 13,219, 571, 2,649, 24,426, 7,682, 17,289, 13,863, 11,888, 836, 5,734, and 44,259 bp, respectively. Data from the original restriction analyses were reassessed and found to support the changes indicated by the sequence analysis. Agreement between the calculations of restriction fragment sizes and the order based on DNA sequence and agarose gel analyses indicates that the sequence assembly is robust. A revised physical map is depicted in Fig. 1.
FIG. 1.
Physical map of pNL1. Fragments predicted by computational analysis of the pNL1 sequence for NotI and SspI digests are shown in the outer and inner rings, respectively. The positions of selected genes are shown outside the rings, and putative regulators encoded by orf7 (A), orf158 (B), orf569 (C), orf597 (D), orf758 (E), and orf994 (F) are shown in circles on the perimeter of the outer ring. Also depicted are the regions with homology to S. yanoikuyae B1 and Sphingomonas sp. strain RW1 (bidirectional arrows) and the regions encoding genes associated with aromatic degradation, conjugation, and plasmid partitioning and replication.
ORF analysis.
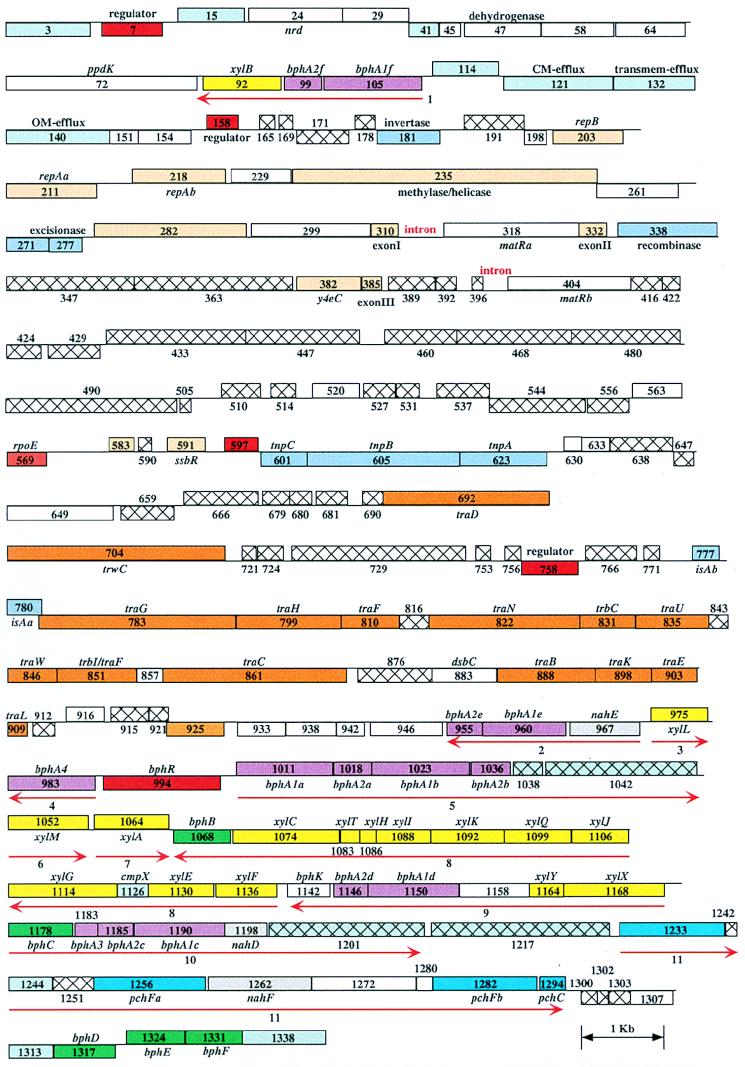
A graphical representation of the ORFs predicted to encode genes is depicted in Fig. 2. Similarities between representative homologs and pNL1 ORFs are detailed in Table 1. Based on gene homolog analysis, functions associated with the plasmid were divided into three general categories: catabolism of aromatic compounds, plasmid replication and partition, and conjugation. The genes associated with each of these general categories are discussed in detail below, with emphasis on those associated with the catabolism of aromatic compounds.
FIG. 2.
Graphical depiction of ORFs. Putative ORFs are depicted as boxes placed either above (frames 1 to 3) or below (frames 4 to 6) the axis. The numbers correspond to MAGPIE number assignments for each of 1,394 potential ORFs found on pNL1. Red arrows indicate putative transcripts (1 to 11) which encode enzymes involved in biphenyl, m-xylene, and naphthalene degradation. Colors are used to highlight putative regulators (red); aromatic oxygenase subunits (purple); enzymes in biphenyl (green), naphthalene (gray), m-xylene (yellow), and p-cresol (turquoise) pathways; possible aromatic transport proteins (light blue); transposases and recombinases (medium blue); plasmid partitioning and replication (light orange); and conjugative genes (dark orange). Hatched boxes depict ORFs with no detected homologs outside pNL1.
TABLE 1.
Homology between pNL1 proteins and representative homologs
| pNL1 | Functional description of closest relative | pNL1a (alignment region) | Homologa (alignment region) | % Identityb | Source | GenBank access no.c |
|---|---|---|---|---|---|---|
| orf003 | Bcr; sulfonamide-bicyclomycin resistance protein | 394 (1–378) | 396 (14–390) | 25 | E. coli K-12 | 584831 |
| orf007 | CatR; putative regulator of catechol degradative operon | 283 (33–283) | 256 (3–256) | 26 | R. opacus 1CP | 2398776 |
| orf015 | DesA; fatty acid desaturase | 309 (27–283) | 287 (22–268) | 29 | C. caldarium RK1 chloroplast | 2465769 |
| nrd | Nrd; aerobic-microaerobic growth with nitrates | 439 (3–438) | 444 (4–442) | 69 | B. japonicum USDA 110 | 2961296 |
| orf029 | YkpB; conserved hypothetical protein | 310 (3–303) | 303 (2–297) | 20 | B. subtilis 168 | 2633815 |
| ORF58; conserved hypothetical protein | 310 (4–294) | 337 (7–322) | 28 | S. aromaticivorans F199 (pNL1) | 3378270 | |
| orf041 | ORF1; conserved hypothetical protein | 136 (1–131) | 139 (1–131) | 51 | B. cepacia LB400 | 586492 |
| orf045 | YtvB; hypothetical protein | 94 (18–52) | 111 (65–99) | 34 | B. subtilis | 2293180 |
| orf047 | ImdH; probable tartrate dehydrogenase | 355 (1–353) | 361 (2–360) | 60 | E. coli K-12 | 3024767 |
| DlpA; isocitrate-isopropylmalate dehydrogenase family | 355 (8–350) | 615 (9–362) | 32 | L. pneumophila | 2497266 | |
| orf058 | YkpB; thiamin biosynthesis-like hypothetical protein | 337 (11–335) | 301 (7–295) | 28 | P. horikoshii OT3 | 3257813 |
| orf064 | DapA; putative dihydropicolinate synthase | 320 (18–317) | 289 (4–285) | 25 | A. fulgidus | 2649690 |
| ppdK | PpdK; pyruvate phosphate dikinase | 888 (21–885) | 874 (16–871) | 57 | C. symbiosum | 1084302 |
| xylB | XylB; aryl alcohol dehydrogenase | 365 (1–365) | 366 (1–365) | 51 | P. putida (pWW53) | 731175 |
| bphA2f | ORFG6; small subunit | 174 (23–173) | 176 (22–175) | 38 | Sphingomonas sp. strain RW1 | 3618275 |
| bphA1f | PahA3; large subunit component naphthalene dioxygenase | 459 (7–436) | 449 (8–439) | 44 | P. aeruginosa PaK1 | 1255669 |
| orf114 | AF010496; conserved hypothetical protein | 309 (17–306) | 289 (9–295) | 32 | R. capsulatus SB1003 | 3128363 |
| orf121 | VceB; putative multidrug resistance pump gene 2 | 509 (21–502) | 511 (22–504) | 43 | V. cholerae 569B | 2815578 |
| orf132 | VceA; putative multidrug resistance pump gene 1 | 381 (19–380) | 395 (16–376) | 46 | V. cholerae 569B | 2815577 |
| orf140 | OprJ; outer membrane component of multidrug resistance efflux pump | 483 (12–473) | 479 (12–468) | 30 | P. aeruginosa PAO | 2497711 |
| orf151 | ORF151; conserved hypothetical protein | 137 (25–135) | 151 (25–145) | 32 | H. halobium | 141254 |
| orf154 | BpoA; conserved hypothetical protein | 247 (2–242) | 261 (21–259) | 43 | M. tuberculosis H37Rv | 2104396 |
| orf158 | YybA; hypothetical regulator, Marr family | 148 (24–148) | 150 (11–135) | 25 | B. subtilis 168 | 2636618 |
| orf181 | Y4cG; probable DNA invertase | 293 (1–292) | 305 (16–303) | 45 | Rhizobium sp. strain NGR234(pNGR234α) | 2497425 |
| orf198 | Y4dW; conserved hypothetical protein | 103 (21–85) | 204 (91–155) | 36 | Rhizobium sp. strain NGR234(pNGR234α) | 2496610 |
| repB | QsopB; trans-acting replication protein | 327 (54–299) | 334 (62–296) | 28 | C. burnetii (QpH1) | 1075479 |
| repAa | RepA; DNA replicase | 420 (22–412) | 405 (2–402) | 32 | A. tumefaciens (pTiB6S3) | 95114 |
| repAb | RepA; DNA replicase | 434 (52–312) | 307 (29–284) | 28 | R. marinus R-21(pRM21) | 527643 |
| orf229 | ORF248; conserved hypothetical protein | 280 (103–174) | 248 (165–239) | 41 | C. burnetii (QpDV) | 1075477 |
| orf235 | R31180_1; conserved hypothetical protein | 1425 (375–1146) | 1384 (197–1015) | 31 | H. sapiens | 3399676 |
| HsdM; StySKI methylase | 1425 (121–298) | 493 (167–373) | 23 | S. enterica | 1841495 | |
| orf261 | AF056496; conserved hypothetical protein | 379 (100–288) | 252 (93–252) | 22 | H. pylori OU145B(pHP0100) | 3046602 |
| orf271 | Rv3749c; hypothetical protein | 196 (99–177) | 169 (60–138) | 35 | M. tuberculosis H37Rv | 2960173 |
| orf277 | Rv3750c; hypothetical protein similar to excisionases | 152 (53–144) | 130 (23–114) | 38 | M. tuberculosis H37Rv | 2960174 |
| orf282 | ORF1; 20.4-kDa plasmid stabilizing protein | 705 (119–288) | 180 (1–168) | 29 | R. meliloti GR4 | 46317 |
| ORF2; 38.5-kDa plasmid stabilizing protein | 705 (310–624) | 356 (21–324) | 26 | R. meliloti GR4 | 46318 | |
| AF053947; conserved hypothetical protein | 705 (4–632) | 669 (21–624) | 27 | Y. pestis (pMT1) | 2996364 | |
| orf299 | AF0450; hypothetical signal transducing His kinase | 555 (30–128) | 329 (229–329) | 31 | A. fulgidus | 2650174 |
| orf310 | ORF382; conserved hypothetical protein | 127 (5–109) | 300 (9–118) | 44 | S. aromaticivorans F199(pNL1) | 3378306 |
| Y4eC; conserved hypothetical protein | 127 (8–108) | 307 (9–118) | 35 | Rhizobium sp. strain NGR234(pNGR234α) | 2496614 | |
| matRa | MatRb; putative maturase-related protein | 633 (45–611) | 571 (3–570) | 52 | S. aromaticivorans F199(pNL1) | 3378311 |
| MatR; maturase-related protein | 633 (26–601) | 599 (26–594) | 38 | L. lactis MG1363 | 1296829 | |
| orf332 | Y4eC; conserved hypothetical protein | 130 (13–115) | 307 (137–235) | 37 | Rhizobium sp. strain NGR234(pNGR234α) | 2496614 |
| ORF382; conserved hypothetical protein | 130 (13–118) | 300 (134–235) | 37 | S. aromaticivorans F199(pNL1) | 3378306 | |
| orf338 | XerD; putative phage-type integrase-recombinase | 463 (202–459) | 316 (143–346) | 23 | M. leprae | 2065218 |
| orf382 | Y4eC; conserved hypothetical protein | 300 (6–292) | 307 (7–295) | 56 | Rhizobium sp. strain NGR234(pNGR234α) | 2496614 |
| orf385 | Y4eC; conserved hypothetical protein | 90 (14–90) | 307 (225–307) | 36 | Rhizobium sp. strain NGR234(pNGR234α) | 2496614 |
| ORF382; conserved hypothetical protein | 90 (22–80) | 300 (235–293) | 32 | S. aromaticivorans F199(pNL1) | 3378306 | |
| matRb | MatRa; putative maturase-related protein | 571 (3–570) | 633 (45–611) | 52 | S. aromaticivorans F199(pNL1) | 338301 |
| MatR; maturase related protein | 571 (6–561) | 599 (48–594) | 36 | L. lactis MG1363 | 1296829 | |
| orf447 | ORF480; hypothetical protein | 533 (18–386) | 507 (14–368) | 29 | S. aromaticivorans F199(pNL1) | 3378320 |
| orf480 | ORF447; hypothetical protein | 507 (14–368) | 533 (18–386) | 29 | S. aromaticivorans F199(pNL1) | 3378317 |
| orf520 | Rv3226c; conserved hypothetical protein | 220 (20–189) | 252 (1–200) | 28 | M. tuberculosis H37Rv | 2072693 |
| orf563 | HglK; protein involved in glycolipid localization | 229 (12–206) | 245 (52–240) | 33 | Synechocystis sp. strain PCC6803 | 1653115 |
| orf569 | RpoE; RNA polymerase sigma-E factor (sigma 24) | 181 (4–170) | 202 (11–192) | 26 | E. coli K-12 | 987648 |
| orf583 | ORFL1; conjugal transfer region | 114 (16–113) | 152 (69–151) | 43 | A. tumefaciens (Ti) | ATU43674 |
| ssbR | Ssb; homolog of single-strand binding protein | 111 (3–111) | 113 (4–104) | 30 | E. aerogenes (R751) | 1572546 |
| orf597 | Ros; repressor of plant conjugal transfer genes | 159 (2–128) | 142 (9–137) | 53 | A. tumefaciens (Ti) | 1710641 |
| tnpC | TnpC; type Tn554 transposase homolog | 218 (18–109) | 125 (17–106) | 23 | S. aureus | 136146 |
| tnpB | TnpB; Tn554 transposase | 710 (373–622) | 630 (336–578) | 30 | S. aureus | 135956 |
| tnpA | TnpA; type Tn554 transposase homolog | 404 (24–374) | 361 (16–347) | 31 | S. aureus | 135955 |
| orf630 | PHS027; conserved hypothetical protein | 81 (7–68) | 74 (6–66) | 35 | P. horikoshii OT3 | 3257321 |
| orf633 | Rv2759c; conserved hypothetical protein | 129 (4–108) | 131 (1–115) | 27 | M. tuberculosis H37Rv | 2624281 |
| orf649 | ORF2; putative 27.6-kDa protein | 495 (261–440) | 236 (31–212) | 27 | H. pylori (pHPM180) | 530827 |
| Tca; DNA polymerase | 495 (31–262) | 834 (276–528) | 28 | T. aquaticus caldophilus GK24 | 2506365 | |
| traD | TraD; membrane protein involved in DNA export | 776 (237–726) | 738 (183–677) | 30 | E. coli (R100) | 136176 |
| trwC | TrwC; DNA helicase | 1013 (2–955) | 966 (4–929) | 31 | E. coli (R388) | 1084124 |
| orf758 | ORF4; hypothetical regulatory protein | 266 (11–201) | 251 (7–209) | 24 | Acinetobacter sp. strain ADP1 | 2271498 |
| isAb | ORFA; transposase part A ISRm2011-2 | 128 (1–126) | 135 (1–126) | 40 | R. meliloti 2011 | 1583631 |
| isAa | ORFB; transposase part B ISRm2011-2 | 157 (1–155) | 201 (45–199) | 50 | R. meliloti 2011 | 1583632 |
| traG | TraG; pilus biosynthesis-mating aggregate stabilization | 912 (1–830) | 938 (1–802) | 21 | E. coli K-12 (F) | 464922 |
| traH | TraH; F pilus assembly | 484 (21–469) | 458 (10–443) | 32 | E. coli K-12 (F) | 464925 |
| traF | TraF; F pilus assembly | 270 (48–263) | 247 (23–241) | 27 | E. coli (R100-1) | 2226078 |
| traN | TraN; stabilization of mating pairs | 704 (396–695) | 602 (293–593) | 29 | E. coli K-12 (F) | 730985 |
| trbC | TrbC; F pilus assembly | 254 (99–221) | 212 (74–206) | 27 | E. coli (R100-1) | 2226075 |
| traU | TraU; conjugal DNA transfer-F pilus assembly | 340 (19–340) | 330 (13–330) | 44 | E. coli K-12 (F) | 136155 |
| traW | TraW; F pilus assembly | 231 (12–210) | 210 (5–204) | 36 | E. coli (R100-1) | 2226072 |
| orf851 | TraF; F pilus assembly | 368 (209–340) | 177 (9–141) | 32 | E. coli (RK2) | 549119 |
| TrbI; influences F pilus outgrowth and retraction | 368 (85–150) | 128 (56–125) | 31 | Escherichia coli (R100-1) | 2226071 | |
| traC | TraC; F pilus assembly | 856 (5–833) | 875 (24–851) | 25 | E. coli K-12 (F) | 136145 |
| dsbC | DsbC; protein disulfide-isomerase | 298 (182–290) | 235 (110–223) | 32 | E. coli | 96548 |
| traB | TraB; F pilus assembly | 458 (36–453) | 475 (4–406) | 26 | E. coli (ColB2) | 730990 |
| traK | TraK; F pilus assembly | 264 (32–263) | 242 (8–241) | 20 | E. coli (ColB2) | 1293089 |
| traE | TraE; F pilus assembly | 207 (19–183) | 187 (12–186) | 22 | S. typhi (pED208) | 136180 |
| traL | TraL; F pilus assembly | 94 (1–79) | 91 (8–86) | 31 | E. coli (ColB2) | 136149 |
| orf916 | Phbn040; hypothetical regulator | 177 (80–156) | 158 (26–93) | 29 | P. horikoshii OT3 | 3130383 |
| orf925 | YjbJ; conserved hypothetical protein similar to lytic transglycolases | 265 (103–217) | 181 (63–177) | 47 | B. subtilis | 2633511 |
| orf933 | MTH888; hypothetical protein | 222 (7–203) | 220 (22–214) | 32 | M. thermoautotrophicum | 2621982 |
| ORF938; conserved hypothetical protein | 222 (2–216) | 232 (9–226) | 34 | S. aromaticivorans F199(pNL1) | 3378388 | |
| DlpA; isocitrate-isopropylmalate dehydrogenase family | 222 (6–219) | 615 (395–611) | 36 | L. pneumophila | 2497266 | |
| orf938 | MTH888; hypothetical protein | 232 (25–217) | 220 (33–217) | 34 | M. thermoautotrophicum | 2621982 |
| DlpA; isocitrate-isopropylmalate dehydrogenase family | 232 (1–214) | 615 (374–595) | 29 | L. pneumophila | 2497266 | |
| orf942 | PH0854; hypothetical protein | 130 (16–125) | 137 (27–135) | 32 | P. horikoshii OT3 | 3257265 |
| orf946 | Slr0619; hypothetical protein | 336 (53–331) | 348 (66–334) | 23 | Synechocystis sp. strain PCC6803 | 1208451 |
| bphA2e | BphA2e; small subunit component of a dioxygenase | 165 (1–165) | 165 (1–165) | 72 | S. yanoikuyae B1 | |
| bphA1e | BphA1e; large subunit component of a dioxygenase | 391 (1–391) | 391 (1–391) | 90 | S. yanoikuyae B1 | |
| nahE | NahE; 2-hydroxy-benzalpyruvate aldolase | 328 (1–327) | 333 (1–327) | 83 | S. yanoikuyae B1 | |
| xylL | XylL; cis-(methyl)benzoate dihydrodiol dehydrogenase | 262 (7–262) | 263 (8–263) | 76 | S. yanoikuyae B1 | |
| bphA4 | BphA4; reductase component of a dioxygenase | 408 (1–408) | 407 (1–407) | 72 | S. yanoikuyae B1 | |
| bphR | BphR; regulatory protein | 546 (1–542) | 543 (1–541) | 61 | S. yanoikuyae B1 | |
| bphR | BphR; regulatory protein | 546 (1–542) | 543 (1–541) | 61 | S. yanoikuyae B1 | |
| bphA1a | BphA1a; large subunit component of a dioxygenase | 450 (5–450) | 448 (3–448) | 82 | S. yanoikuyae B1 | |
| bpha2a | BphA2a; small subunit component of a dioxygenase | 175 (1–175) | 175 (1–175) | 65 | S. yanoikuyae B1 | |
| bphA1b | BphA1b; large subunit component of a dioxygenase | 455 (1–455) | 455 (1–455) | 89 | S. yanoikuyae B1 | |
| bphA2b | BphA2b; small subunit component of a dioxygenase | 183 (1–183) | 183 (1–183) | 80 | S. yanoikuyae B1 | |
| orf1038 | ORF1217; conserved hypothetical protein | 135 (1–135) | 832 (1–135) | 79 | S. aromaticivorans F199(pNL1) | 3378429 |
| orf1042 | ORF1217; conserved hypothetical protein | 705 (1–705) | 832 (152–832) | 72 | S. aromaticivorans F199(pNL1) | 3378429 |
| xylM | XylM; reductase component of xylene monooxygenase | 367 (1–363) | 375 (1–371) | 67 | S. yanoikuyae B1 | |
| xylA | XylA; oxygenase component of xylene monooxygenase | 346 (1–343) | 346 (1–343) | 63 | S. yanoikuyae B1 | |
| bphB | BphB; cis-biphenyl dihydrodiol dehydrogenase | 266 (1–265) | 266 (1–265) | 80 | S. yanoikuyae B1 | |
| xylC | XylC; benzylaldehyde dehydrogenase | 501 (1–501) | 499 (1–499) | 89 | S. yanoikuyae B1 | |
| xylT | XylT; ferredoxin, plant-type | 93 (3–92) | 92 (2–91) | 68 | S. yanoikuyae B1 | |
| xylH | XylH; 4-oxalocrotonoate tautomerase | 80 (1–69) | 70 (1–69) | 79 | S. yanoikuyae B1 | |
| xylI | XylI; 4-oxalocrotonate decarboxylase | 256 (1–256) | 256 (1–256) | 85 | S. yanoikuyae B1 | |
| xylK | XylK; 4-hydroxy-2-oxovalerate aldolase | 343 (1–342) | 343 (1–342) | 90 | S. yanoikuyae B1 | |
| xylQ | XylQ; acetaldehyde dehydrogenase (acylating) | 312 (1–312) | 312 (1–312) | 82 | S. yanoikuyae B1 | |
| xylJ | XylJ; 2-hydroxypent-2,4-dienoate hydratase | 264 (1–264) | 264 (1–264) | 91 | Pseudomonas sp. strain DJ77 | 2315998 |
| xylG | XylG; 2-hydroxymuconic semialdehyde dehydrogenase | 505 (12–505) | 494 (1–494) | 96 | Sphingomonas sp. strain HV3(pSKY4) | 2293079 |
| cmpX | CmpX; conserved hypothetical protein | 143 (1–143) | 143 (1–143) | 97 | Sphingomonas sp. strain HV3(pSKY4) | 2293078 |
| xylE | XylE; catechol 2,3-dioxygenase | 307 (1–307) | 307 (1–307) | 95 | Sphingomonas sp. strain HV3(pSKY4) | 2293077 |
| xylF | XylF; 2-hydroxymuconic semialdehyde hydrolase | 283 (1–282) | 292 (1–282) | 93 | Sphingomonas sp. strain HV3(pSKY4) | 2293079 |
| bphK | BphK; glutathione S-transferase | 201 (1–201) | 201 (1–201) | 97 | S. yanoikuyae B1 | |
| bphA2d | BphA2d; small subunit component of a dioxygenase | 162 (7–162) | 159 (4–159) | 76 | S. yanoikuyae B1 | |
| bphA1d | BphA1d; large subunit component of a dioxygenase | 426 (1–426) | 424 (1–424) | 80 | S. yanoikuyae B1 | |
| Orf1158 | ORF2; conserved hypothetical protein | 330 (9–327) | 330 (7–325) | 87 | S. yanoikuyae B1 | |
| xylY | XylY; small subunit component of benzene dioxygenase | 164 (1–164) | 164 (1–164) | 69 | S. yanoikuyae B1 | |
| xylX | XylX; large subunit component of benzene dioxygenase | 471 (1–462) | 470 (1–462) | 73 | S. yanoikuyae B1 | |
| bphC | BphC; 2,3-dihydroxybiphenyl 1,2-dioxygenase | 299 (1–298) | 299 (1–298) | 87 | S. yanoikuyae B1 | 115106 |
| bphA3 | PhnR; ferredoxin component of phenanthrene dioxygenase | 108 (1–108) | 108 (1–108) | 97 | Pseudomonas sp. strain DJ77 | 2316014 |
| bphA2c | BphA2c; small subunit component of a dioxygenase | 162 (1–162) | 162 (1–162) | 67 | S. yanoikuyae B1 | |
| bphA1c | BphA1c; large subunit component of a dioxygenase | 420 (18–415) | 419 (19–415) | 79 | S. yanoikuyae B1 | |
| nahD | NahD; 2-hydroxychromene-2-carboxylate isomerase | 197 (1–197) | 197 (1–197) | 79 | S. yanoikuyae B1 | |
| orf1201 | ORF1217; conserved hypothetical protein | 726 (10–726) | 832 (19–832) | 26 | S. aromaticivorans F199(pNL1) | 3378429 |
| orf1201 | ORF1217; conserved hypothetical protein | 726 (10–726) | 832 (19–832) | 26 | S. aromaticivorans F199(pNL1) | 3378429 |
| orf1217 | ORF1042; conserved hypothetical protein | 832 (152–832) | 705 (1–705) | 72 | S. aromaticivorans F199(pNL1) | 3378402 |
| gabD | GabD; succinic semialdehyde dehydrogenase | 490 (8–484) | 482 (3–482) | 57 | E. coli K-12 | 120777 |
| orf1244 | YclB; phenylacrylic acid decarboxylase homolog | 204 (3–190) | 204 (14–200) | 50 | B. subtilis 168 trpC2 | 1805434 |
| pchFa | PchF; flavoprotein subunit, p-cresol methyl-hydroxylase | 519 (5–479) | 530 (9–487) | 42 | P. putida NCIMB 9866 | 294369 |
| nahF | Ydh; vanillin: NAD+ oxidoreductase | 479 (1–473) | 482 (1–475) | 41 | P. fluorescens AN103 | 3116018 |
| orf1272 | YclC; conserved hypothetical protein | 486 (17–457) | 473 (7–448) | 47 | B. subtilis 168 trpC2 | 1805435 |
| orf1280 | YclD; conserved hypothetical protein | 74 (6–62) | 229 (4–57) | 32 | B. subtilis 168 trpC2 | 1805436 |
| pchFb | PchFa; flavoprotein subunit, p-cresol methyl-hydroxylase | 488 (2–486) | 519 (9–504) | 37 | S. aromaticivorans F199(pNL1) | 3378433 |
| PchF; flavoprotein subunit, p-cresol methyl-hydroxylase | 519 (39–463) | 530 (57–487) | 32 | P. putida NCIMB 9866 | 294369 | |
| pchC | PchC; cytochrome c subunit, p-cresol methyl-hydroxylase | 121 (10–115) | 101 (10–101) | 29 | P. putida NCIMB 9866 | 294349 |
| orf1300 | FadE19; acyl-CoA dehydrogenase | 76 (12–83) | 394 (53–124) | 36 | M. tuberculosis H37Rv | 2113933 |
| orf1303 | Rv3548c; hypothetical dehydrogenase | 123 (1–49) | 304 (210–256) | 32 | M. tuberculosis H37Rv | 1666112 |
| orf1307 | Rv0148; steroid dehydrogenase | 200 (9–170) | 286 (9–169) | 66 | M. tuberculosis H37Rv | 1877273 |
| Rv3548c; hypothetical dehydrogenase | 200 (5–169) | 304 (4–178) | 42 | M. tuberculosis H37Rv | 1666112 | |
| orf1313 | NKe; hypothetical protein | 201 (42–182) | 159 (5–142) | 30 | P. aeruginosa PAO1 | 77647 |
| bphD | BphDd; 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase | 286 (1–283) | 285 (1–284) | 60 | Rhodococcus sp. strain RHA1 (390-kb plasmid) | 1906778 |
| bphE | BphEd; 2-hydroxypenta-2,4-dienoate hydratase | 271 (11–270) | 267 (7–266) | 66 | Rhodococcus sp. strain RHA1 (390-kb plasmid) | 1906779 |
| bphF | BphFd; 4-hydroxy-2-oxovalerate aldolase | 262 (1–243) | 258 (1–243) | 55 | Rhodococcus sp. strain RHA1 (390-kb plasmid) | 1906780 |
| orf1338 | BaiF; conserved hypothetical protein | 388 (18–281) | 381 (5–273) | 38 | E. coli | 1799782 |
NK, no gene name designated.
Values in parentheses describe the portion of protein used in the alignment.
Percentage of amino acids in aligned region that are identical when the sequences are aligned with BLASTP2.
Accession number 1163200 describing ORFL has been removed from GenBank. The accession number listed refers to the DNA sequence from which the corresponding ORF can be derived. Sequences for S. yanoikuyae B1 genes were derived from the DNA sequences provided by Gerben Zylstra where not available in GenBank.
The gene designation refers to those assigned by Masai et al. (56). In GenBank the same genes are designated etbDEF.
Ring-hydroxylating dioxygenases.
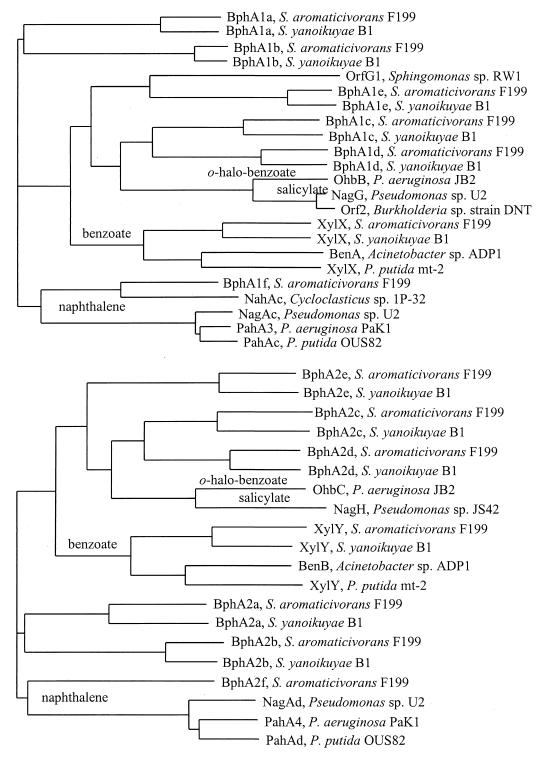
Seven homologs to both large and small substrate binding components of ring hydroxylating dioxygenases were identified (bphA1[a–f]-bphA2[a–f]). Only a single homolog for each the ferredoxin (bphA3) and the ferredoxin reductase (bphA4) components were found. A homolog to XylZ, possessing both ferredoxin and ferredoxin reductase domains, was not found. The Cys-X1-His-X17-Cys-X2-His Rieske-type (2Fe-2S) cluster binding site motif is conserved in each of the seven pNL1-encoded large oxygenase components. All except BphA1e also possess a potential mononuclear non-heme iron coordination site consensus sequence, E-X3/4-D-X2-H-X4/5-H, defined by Jiang et al. (38). In BphA1e (S. aromaticivorans F199 and S. yanoikuyae B1) and OrfG1 from Sphingomonas sp. RW1 (1), two aspartate residues are separated by only one amino acid (D-X1-D-X2-H-X4-H). It is unclear whether these latter proteins can accommodate the Fe(II) ligand.
A dendrogram of representative protein homologs for small- and large-ring hydroxylating oxygenase components is depicted in Fig. 3. On pNL1, the gene homologs for the large and small oxygenase subunits are all located in pairs. Members of each pair cluster with large and small subunits of similar oxygenases from the same bacterium rather than from dissimilar organisms, suggesting that members of a pair function as components of the same enzyme. For example, XylX and XylY from pNL1 cluster with the XylX and XylY benzoate dioxygenase large and small components, respectively, from S. yanoikuyae B1 rather than with components from distinct enzymes or organisms. The distribution of these gene pairs on pNL1 suggests that they are part of at least six different operons. The absence of terminator-like sequences between bphA2a and bphA1b suggests that the bphA1a-bphA2b and bphA1b-bphA2b pairs are cotranscribed. These findings suggest that most or all paired binding components are part of the same oxygenase enzyme complex.
FIG. 3.
CLUSTALW dendrogram of representative large (above) and small (below) oxygenase component amino acid sequences. The GenBank accession numbers associated with these sequences are as follows: Acinetobacter calcoaceticus ADP1 (2996621-2996622), P. putida mt-2 plasmid pWWO (139861-139862), Pseudomonas sp. strain U2 plasmid pWWU2 (2828018-2828019, 2828015-2828016), Sphingomonas sp. strain RW1 (3618270), Burkholderia sp. strain DNT (1477921), Cycloclasticus sp. strain 1P032 (3170521), P. aeruginosa PaK1 (12555669-1255670), P. putida OUS82 (1073044-1073045), and P. aeruginosa JB2 (3643998-3643999). Sequences from S. yanoikuyae B1 were derived from the dissertation thesis of E. Kim (45).
Biphenyl, naphthalene, and m-xylene catabolic gene homologs.
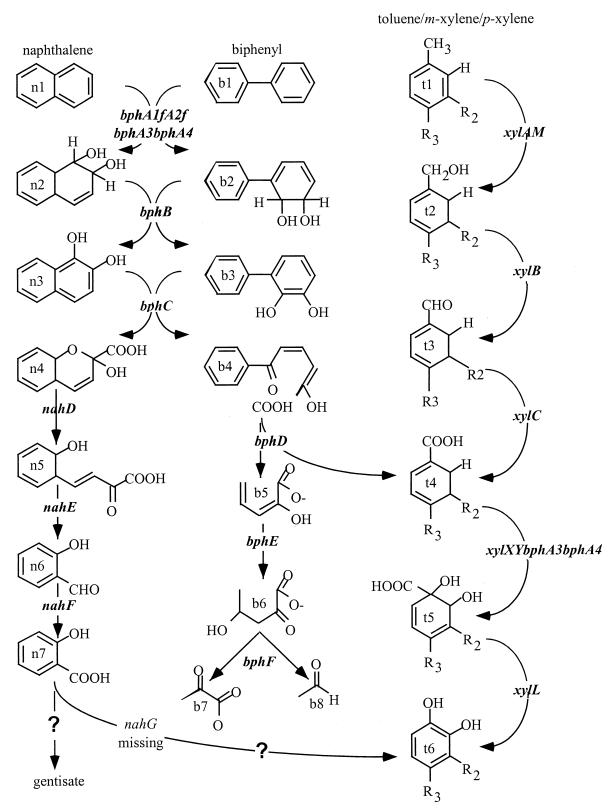
Homologs to genes associated with m-xylene, biphenyl, and naphthalene model degradative pathways were identified on pNL1 (Fig. 4). Based on gene spacing and the occurrence of terminator-like structures, we predict that genes encoding enzymes associated with these pathways are distributed among at least 11 transcriptional units (Fig. 2).
FIG. 4.
Hypothetical naphthalene, biphenyl, and toluene degradative pathways encoded on pNL1. Pathway intermediates include naphthalene (n1), cis-1,2-dihydroxy-1,2-dihydronaphthalene (n2), 1,2-dihydroxynaphthalene (n3), 2-hydroxy-2-(2-oxo-3,5-cyclohexadienyl)-buta-2,4-dienoate (n4), 2-hydroxychromene-2-carboxylate (n5), salicylaldehyde (n6), salicylate (n7), biphenyl (b1), cis-2,3-dihydrodiol (b2), 2,3-dihydroxybiphenyl (b3), 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid (b4), cis-2-hydroxy-penta-2,4-dienoate (b5), 4-hydroxy-2-oxovalerate (b6), pyruvate (b7), acetaldehyde (b8), toluene (t1), m-methylbenzyl alcohol (t2), m-methyl benzaldehyde (t3), benzoate (t4), 1,2-dihydroxycyclohexa-3,5-dienecarboxylate (t5), and catechol (t6). The enzymes predicted to produce these intermediates are dioxygenase (bphA1fA2fbphA3bphA4), cis-dihydrodiol dehydrogenase (bphB), catechol dioxygenase (bphC), 2-hydroxychromene-2-carboxylate dehydrogenase (nahD), 1,2-dihydroxybenzylpyruvate aldolase (nahE), salicylaldehyde dehydrogenase (nahF), salicylate hydroxylase (nahG), salicylate-5-monooxygenase (nagG), 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase (bphD), 2-oxopent-4-enoate hydratase (bphE), 4-hydroxy-2-oxo-valerate aldolase (bphF), xylene monooxygenase (xylAM), benzyl alcohol dehydrogenase (xylB), benzaldehyde dehydrogenase (xylC), toluate oxygenase (xylXYbphA3bphA4), and dihydroxy cyclohexadiene carboxylate dehydrogenase (xylL). R1 = R2 = R3 = H for toluene degradation, R1 = R3 = H and R2 = CH3 for m-xylene degradation, and R1 = R2 = H and R3 = CH3 for p-xylene degradation.
Critical analysis of the pNL1 sequences suggests that some genes from both the m-xylene and the naphthalene degradative pathways are required for complete degradation of biphenyl. Only a single homolog to dihydrodiol dehydrogenase (orf1068; bphB) and ring cleavage oxygenase (orf1178; bphC) are found on pNL1. These enzymes catalyze the second and third steps, respectively, in naphthalene and biphenyl degradation and typically exhibit broad substrate specificity (Fig. 3) (31, 80). Results from analysis of pNL1 subclones encoding bphC or xylE confirmed that bphC is capable of oxidizing both 1,2-dihydroxynaphthalene and 2,3-dihydroxybiphenyl (Table 2). This result further supports the hypothesis that the first three steps in the degradation of both biphenyl and naphthalene are catalyzed by a single set of enzymes whose components are encoded by genes on four different putative transcriptional units: 1 (bphA1e and bphA2e), 4 (bphA4), 8 (bphB), and 10 (bphC and bphA3).
TABLE 2.
Oxygenase activity of cosmid clones encoding bphC (cosmid 6) and xylE (cosmid 18) with various aromatic substrates
| Cosmid | Oxygenase activity (μmol of substrate/min/mg consumed) with:
|
||||
|---|---|---|---|---|---|
| Catechol | 3-Methyl catechol | 4-Methyl catechol | 2,3-Dihydroxy-biphenyl | 1,2-Dihydroxy-naphthalenea | |
| 6 | 1.03 | 4.7 | 1.24 | 2.73 | 6.33 |
| 18 | 10.73 | 14.42 | 5.77 | 0.75 | 0.74 |
Measured as microliters of O2 per minute per milligram consumed.
The BphC degradative product is converted by the BphD hydrolase to cis-2-hydroxy-penta-2,4-dienoate (2HPDA) and benzoate. The 2HPDA intermediate can then be converted by the products of bphE and bphF to pyruvate and acetaldehyde. The benzoate intermediate can presumably be further metabolized via the m-xylene degradative pathway enzymes. Degradation of benzoate requires genes encoded by putative transcripts 3 (xylL) and 9 (xylXY), as well as continued expression of genes on putative transcripts 1 (bphA1e, bphA2e, and xylB), 4 (bphA4), 8 (xylFEGJQKIHTC), and 10 (bphA3). This brings the total number of required putative transcripts to eight for the complete metabolism of biphenyl. Two putative transcripts are required for biphenyl degradation alone (bphD-orf1313-orf130-7orf1303 and bphE-bphF-orf1338), three are required for both biphenyl and m-xylene degradation (transcripts 3, 8, and 9), and three are required for m-xylene, biphenyl, and naphthalene degradation (transcripts 1, 4, and 10).
In addition to benzoate pathway-encoding transcripts 3 and 9, degradation of m-xylene requires the expression of genes encoded on transcripts 6 (xylM) and 7 (xylA), as well as transcript 1 (xylB), bringing the total to five transcripts necessary for m-xylene degradation. Metabolism of naphthalene to salicylate requires expression of genes from six transcripts, including those also required by biphenyl (1, 4, 8, and 10) and transcripts 2 (nahE) and 11 (nahF). Further metabolism of salicylate to catechol is typically catalyzed by salicylate hydroxylase (NahG). However, no nahG homolog was found on pNL1, suggesting that either salicylate degradation does not proceed through a catechol intermediate or that a novel pNL1-encoded enzyme is responsible for converting salicylate to catechol.
p-Cresol catabolic gene homologs.
The prototypical toluene catabolic pathway from P. mendocina KR1 proceeds through a p-cresol intermediate and involves the action of three enzymes to generate protocatechuate from p-cresol (99). The conversion of p-cresol to p-hydroxybenzaldehyde is a two-step process that is catalyzed by a two-component p-cresol methylhydroxylase. Two gene homologs, designated pchFa and pchFb, to the p-cresol methylhydroxylase flavoprotein component and one cytochrome subunit homolog, pchFc, are found on a single cluster in pNL1 (Fig. 2). Subsequent conversion of p-hydroxybenzaldehyde to p-hydroxybenzoate in P. mendocina KR1 is catalyzed by p-hydroxybenzaldehyde dehydrogenase. NahF clusters most closely with p-hydroxybenzaldehyde dehydrogenase (unpublished results; GenBank no. 995954) and presumably is capable of catalyzing the aldehyde dehydrogenase reactions in both the naphthalene and the p-cresol pathways. Homologs to known proteins responsible for catabolism of p-hydroxybenzoate were not found on pNL1.
Membrane proteins.
Several pNL1 ORFs, distributed among the catabolic genes, have extensive homology to bacterial efflux pump genes. Typical efflux pump transport systems are composed of a cytoplasmic and outer membrane protein and a membrane fusion protein (74). Sequence similarity analysis suggests that ORF140, ORF132, and ORF121 encode the outer membrane, membrane fusion, and cytoplasmic membrane components of a novel efflux pump. Psort analysis of ORF140 identified a possible signal cleavage site and supports its localization in the outer membrane. Tmpred analysis of the putative cytoplasmic component of the pNL1 encoded efflux pump, ORF121, predicts the presence of 14 transmembrane helices, a feature common to Family 1 transport exporters such as EmrB (73). A signature for the lysR family of regulatory proteins is present at the N terminus of ORF121 and, like ORF140, it has a membrane lipoprotein lipid A signature.
Two additional putative inner membrane proteins whose function may be related to efflux pumps were also found. The deduced protein sequence of orf114, which is immediately downstream of the pNL1-encoded efflux pump cluster, is predicted to have 10 transmembrane domains. It has a lipocalin signature (PS00213) that is characteristic of proteins which transport small hydrophobic molecules such as steroids, bilins, retinoids, and lipids. ORF3 is predicted to have 11 transmembrane domains and is similar to proteins belonging to the drug resistance translocase subfamily BCR/CMLA.
Nine ORFs that cluster among the pNL1-encoded aromatic catabolic genes are not homologous to functionally characterized genes and are predicted to reside in the membrane. ORF1038 and ORF1042 have extensive homology to the N terminus and the C terminus, respectively, of ORF1217 and to a lesser extent to those of ORF1201. Read-through of the ORF1038 stop codon would result in a single ORF encompassing both ORF1038 and ORF1042. Reanalysis of the raw sequence confirmed that the stop codon separating ORF1038 and ORF1042 was not due to sequence error. The results of Psort analyses predict that ORF1038 (or a fusion of ORF1038 and ORF1042) and ORF1201 are outer membrane proteins and that ORF1217 is localized to the inner (cytoplasmic) membrane.
Homologs to three of the remaining proteins cluster with genes associated with aromatic catabolism and are predicted to reside in the inner membrane. Like the pNL1-encoded cmpX, its three homologs from Pseudomonas sp. strain DJ77 (unpublished results; GenBank no. 2316006), Sphingomonas agrestis HV3 (105), and S. yanoikuyae B1 (45) are all found downstream of a catechol 2,3-dioxygenase. Homologs to ORF41 are clustered with components of ring-hydroxylating dioxygenases in C. oligotrophicus RB1 (95), Pseudomonas sp. strain KKS102 (43), Ralstonia eutropha A5 (58), P. fluorescens (unpublished results; GenBank no. 1256705), Pseudomonas sp. strain JR1 (75), Comamonas testosteroni B-356 (90), P. pseudoalcaligenes KF707 (92), and B. cepacia LB400 (14). ORF1338 is homologous to a partial ORF that is expressed bidirectionally from the Acinetobacter calcoaceticus cis,cis-muconate catechol ortho-cleavage pathway transport protein (100) and to a partial ORF found in a Bordetella pertussis gene cluster along with a salicylate hydroxylase homolog (102).
The remaining two membrane proteins, ORF15 and ORF1313, are also predicted to reside in the inner membrane, but they do not align with proteins encoded in other aromatic catabolic gene clusters. ORF15 is predicted to possess five transmembrane domains and is similar to fatty acid desaturases and beta-carotene ketolases. Aligned proteins share three histidine-rich domains with the signatures H-D-X2-H, H-X2-H-H and H-X2-H-H-[LR]-[CWH]-[PV]-X2-P. ORF1313 has homology to a small hypothetical protein that clusters with regulatory genes that control synthesis of the exopolysaccharide alginate in P. aeruginosa (41).
Other ORFs found in the aromatic catabolic region of pNL1.
Homologs to two additional ORFs, bphK and orf1158, also cluster with aromatic catabolic genes, but their function in aromatic catabolism has not been established. BphK is a glutathione S-transferase, and its homologs are found in polycyclic aromatic catabolic gene clusters of Pseudomonas sp. strain DJ77 (unpublished data), S. paucimobilis epa505 (54), Pseudomonas pseudoalcaligenes KF707 (49), Pseudomonas sp. strain LB400 (34), S. yanoikuyae B1 (45), and Cycloclasticus oligotrophus RB1 (95). Homologs to ORF1158 are found in the B. cepacia Pc701 plasmid-encoded 4-methylphthalate catabolic cluster (82) and in S. yanoikuyae B1 (ORF2) (107). The latter ORFs are also similar to the pyridoxal phosphate biosynthetic protein (PdxA).
The eight genes between orf15 and xylB do not align to genes associated with aromatic catabolic gene clusters. ORF24 is similar to the Nrd protein of Bradyrhizobium japonicum, whose function is involved in aerobic and microaerobic growth with nitrate as the only nitrogen source. A functional role of ORF24 in microaerobic growth on aromatics is correlates well with earlier findings which demonstrated that S. aromaticivorans F199 grew better on aromatic compounds under microaerobic conditions (22). ORF24 is also similar to one member of two component monooxygenases, including nitrilotriacetate monooxygenase (GenBank no. 1119211), dibenzothiophene monooxygenase (GenBank no. 595291), and pristinamycin oxygenase (GenBank no. 940348). A homolog to the second monooxygenase component is not evident on pNL1. Only two of the remaining ORFs in this region, orf72 (pyruvate, orthophosphate dikinase) and orf47 (isopropylmalate-tartrate dehydrogenase), show significant homology to functionally characterized genes.
Six new ORFs are linked with aromatic catabolic genes in pNL1 cluster 11, which includes the p-cresol catabolic genes and nahF. Among these is GabD which, when added to NahF, XylC, XylQ, and XylG, brings the total of pNL1-encoded semialdehyde dehydrogenases to five. ORFS 1244, 1272, and 1280 are similar to first three genes in the B. subtilis 168 yclBCDE gene cluster (52). ORF1280 contains only enough sequence to align with the first third of YclD and therefore probably does not represent a functional homolog. The remaining two ORFs in the pNL1 cluster 11, ORF1242 and ORF1251, are novel genes.
ORF1307 is encoded in the cluster containing bphD and is similar to the N-terminal region of beta-oxoacyl(acyl-carrier protein) reductases, which catalyze the first reduction step in fatty acid biosynthesis, of multifunctional enzymes from eukaryotic organisms that are involved in the beta-oxidation fatty acids and of hypothetical dehydrogenase proteins in M. tuberculosis. Within the region of overlap with these three classes of proteins, it has an imperfect (two mismatches) short-chain dehydrogenase-reductase family signature (PS00061). The region of homology with these proteins is extended into the C-terminal region by including the sequence from ORF1303, which can be achieved by a single frame shift. Two additional small ORFs, ORF1302 and ORF1300, are predicted in the remaining gap between orf1307 and pchC. ORF1300 has homology to the N terminus of acyl coenzyme A (acyl-CoA) dehydrogenases, which are also involved in fatty acid oxidation pathways. A frameshift extends the region of homology and adds the acyl-CoA dehydrogenase signature 1 (PS00072). Although additional frameshifts extend the homology to acyl-CoA dehydrogenases even further, the entirety of this region only extends approximately halfway through the homolog sequences.
Plasmid partition-replication.
Two RepA-like proteins are encoded by the bidirectionally transcribed genes orf211 (repAa) and orf218 (repAb). Their deduced protein sequences are dissimilar and consequently cluster with different families of RepA proteins. The intergenic space between repAa and repAb has six 17-bp direct repeats with the consensus sequence 5′-rsCGATGAwyTCvGwkC. Five repeats are located on the strand that encodes repAb, and one is on the repAa-encoding strand. An additional repeat with this consensus is found at the end of repAb. In the intergenic space between repAa and repB are three inverted repeats with the consensus sequence 5′-AGTTGCCACGTGGCAAC.
Downstream of the repAb gene are several additional genes which may be associated with pNL1 replication and partitioning. ORF229 is similar to the C-terminal region of hypothetical proteins associated with Coxiella burnetii plasmids and is likely cotranscribed with ORF235, the largest protein encoded on pNL1 (1,425 amino acids). The N-terminal 300-amino-acid portion of ORF235 is similar to methylases and includes an N-6 adenine-specific DNA methylase signature sequence (PS00092) typical of enzymes that methylate the amino group of the C-6 position of adenines. The central region of the protein is similar to several eukaryotic proteins, but the presence of a DEAH-box-family ATP-dependent helicase signature distinguishes it from its eukaryotic homologs. It is likely, therefore, that this novel prokaryotic protein is involved in the DNA methylation and unwinding of pNL1 and possibly other functions related to those catalyzed by its eukaryotic homologs.
ORF282 is homologous to what would be a fusion of the Rhizobium meliloti pRmeGr4α plasmid-encoded orf1 and orf2 genes, whose products function in plasmid stabilization (57). ORF583 is homologous to members of the thermonuclease family, including the E. coli ParB protein that is involved in plasmid partitioning. Like other members of this family, ORF583 has three nuclease active-site residues and is likely localized outside of the cytoplasmic membrane.
Two group II-associated maturases, matRa and matRb, were found in the pNL1 replication region. No detectable sequence homology was found in the vicinity of matRb, but a number of ORFs with homology to portions of TraC proteins encoded by plasmid RP4 and R751 (59) flank matRa. The TraC replication primase is required for autonomous replication of the F plasmid in E. coli, catalyzing the synthesis of short oligoribonucleotide primers on single-stranded DNA templates. Alternate start sites in these proteins result in expression of N-terminal truncated proteins. The TraC region lost as a result of translation from the internal start sites is homologous to the Rhizobium sp. pNGR234a plasmid Y4eC protein (23) and pNL1 ORF382. A second ORF382-like protein can be constructed by splicing together the sequences of ORF383 (exon I), ORF332 (exon II), and ORF385 (exon III). Splicing activities associated with matRa could presumably splice together exons I and II. However, a means for the addition of exon III, which is separated from exon II by four genes, is less apparent. Except for matrRb, genes found between orf385 and orf563 do not possess extensive homology to functionally characterized genes.
Integration-recombination-associated genes.
pNL1 ORFs predicted to function in DNA integration or recombination include a resolvase family site-specific recombinase (orf277), an excisionase (orf338), a phage-type integrase-recombinase (orf338), and two transposons (tnpABC and isAab). Since orf277 and orf271 are homologous to adjacent genes in M. tuberculosis (76), it is probable that both ORFs are functionally related. Sequences from the region encoding isAab resemble the ISRm2011-2 transposase of R. meliloti (84). Like ISRm2011-2, there is a potential frameshifting window (5′-AAAAAAAG) near the end of IsAa that would allow its fusion with IsAb to form the mature transposase IsA. IsA is flanked by inverted repeat sequences: 5′-CGCGCTAGAGCGGTTTTCGA ending 79 bp upstream of isAa and 5′-TCGAAAATCGCTCTAGCGCG starting immediately after the TGA stop codon in isAb. These repeats are flanked by an upstream CG and a downstream GC putative target of the duplication sequence.
Conjugative genes.
Three gene clusters encode homologs to E. coli F plasmid genes required for conjugative sex pilus formation and mating-pair stabilization (24). The first cluster includes homologs to the F plasmid genes traL, traE, traK, and traB; to dsbC (61); and to S. typhimurium plasmid R64 trbB (26). DsbC catalyzes disulfide bond formation in some periplasmic proteins. Like DsbC, ORF883 possesses an active site typical of disulfide isomerases (FsdfrCgyC) and is predicted to reside in the periplasm. The second cluster includes homologs to E. coli F plasmid genes traC, trbI, traW, traU, traN, traH, and traG. Homology to the entire TrbI is localized within the N-terminal portion of ORF851. The C-terminal portion is homologous to TraF from E. coli RK2 (97) and to various type-I leader peptidases. The third cluster encodes an F plasmid-like TraD and a protein that is similar to TrwC from E. coli R388 (53) and to the N-terminal portion of F plasmid TraN. A total of six additional ORFs are also present in these clusters but have no identifiable homolog.
The nucleotide sequence spanning positions 89,726 to 90,301 of pNL1, just upstream from traD, is 85% identical to sequences spanning positions 3,990 to 4,576 of a Sphingomonas sp. strain RW1 DNA fragment (2). Five ORFs were reported in the 4,576-bp Sphingomonas sp. strain RW1 fragment, including the ferredoxin component of dioxin dioxygenase. pNL1 ORF683 corresponds to RW1 ORF4 but has 76 rather than 105 amino acids. However, G+C compensation calculations do not support ORF683 encoding a gene. The location of this region on pNL1 suggests that sequences downstream of the Sphingomonas sp. strain RW1 ferredoxin are not related to biodegradation. A preliminary analysis of this region on pNL1 predicts the formation of extensive secondary structure. Among the possible ORFs in the 10-kb gap between the two tra clusters, only ORF758 had detectable homology to genes in the public databases.
Nine putative genes were found between traL and the bphA2e. ORF925 has homology to lytic transglycosylases. Similar proteins encoded by conjugative plasmids have been proposed to facilitate the passage of plasmid DNA through the peptidoglycan layer during conjugation (7, 51). ORF933 and ORF938 are similar to each other, to hypothetical proteins, and to the C-terminal region of the DlpA protein of L. pneumophila (8). Interestingly, the entire N-terminal region of DlpA not shared with ORF933 or ORF938 is similar to the entire pNL1 isopropylmalate-tartrate dehydrogenase protein sequence.
Regulatory genes.
Six regulatory genes were identified on pNL1. ORF597 is a member of the Ros-MucR family and shares the conserved C2H2 zinc finger-like domain, C-X2-C-X3-(F,M)-X5-H-X4-H associated with this family. ORF569 belongs to the ECF (extracytoplasmic functions) sigma-54 subfamily of regulators (Prosite no. PDOC00814). The ECF sigma factors constitute a diverse group of alternative sigma factors that have been demonstrated to regulate gene expression in response to environmental conditions. ORF758 is a gntR family regulator (Prosite no. PDOC00042) with similarity to regulators that cluster with aromatic catabolic genes in Acinetobacter sp. strain ADP1 (unpublished results; GenBank no. AF009672), P. pseudoalcaligenes KF707 (unpublished results; GenBank no. 1389649), and B. cepacia LB400 (14) and with the regulator for the pNL1 ORF24 homolog, NtaA (94). These similarities suggest that ORF758 may have a role in the regulation of aromatic degradative genes.
ORF154, which is immediately upstream of the cluster of genes encoding the putative pNL1 efflux pump, belongs to the marR family of regulators (Prosite no. PDOC00861). Members of the MarR family respond to environmental signals of the phenolic class such as sodium salicylate (87). Some members of this family, such as EmrR, have been shown to control the expression of efflux pump genes.
BphR is similar to aromatic degradative operon regulators and is a member of the ntcR regulator family (85a). It possesses two of the N-terminal sigma-54 interaction domain signatures (PS00675 and PS00676A) believed to have ATPase activity, as well as a C-terminal HTH lysR regulator family signature (PDOC00043). A region with only a single mismatch to the third sigma-54 interaction domain signature (PS00688) is also present. ORF007 has some similarity to iclR family regulators (Prosite no. PDOC00807) and to Rhodococcus opacus CatR (15), which is the regulator for the catechol intradiol cleavage operon. A seventh regulator may be encoded by ORF916, which has weak similarity to hypothetical regulatory proteins.
DISCUSSION
This is the first report of a complete sequence of a conjugative plasmid that encodes pathways for the complete catabolism of aromatic organic compounds. Nearly one-half of the pNL1 DNA encodes genes likely to be involved in either catabolism or transport of aromatic compounds. The remainder of the plasmid appears to encode functions associated with plasmid replication, maintenance, or transfer. A remarkable conservation in both sequence and gene order is evident between pNL1-encoded aromatic catabolic genes and their homologs in other Sphingomonas strains. DNA sequences from S. agrestis HV3 and Pseudomonas sp. strain DJ77 possess high homology to pNL1. A 4,012-bp DNA fragment encoding cmpFEXC from S. agrestis HV3 plasmid pSKY4 is 90% identical to the pNL1 region encoding xylF-xylE-cmpX-xylG (105). DNA fragments from Pseudomonas sp. strain DJ77 are 88% identical over a 2,363-bp region encoding homologs (phnDEF) to xylF-xylE-cmpX (sequence assembled from GenBank entries PSU83881, PSU88298, and PSU83882) and 91% identical over a 1,520-bp region encoding homologs to xylG-xylJ (48). The high degree of sequence similarity suggests that either strain DJ77 is a member of the genus Sphingomonas or that lateral transfer of genes occurred between Sphingomonas and Pseudomonas species. The genus Sphingomonas is a relatively recent addition to the list of accepted genera (103), and resulted in the renaming of several bacteria (i.e., P. paucimobilis, Flavobacterium capsulatum, and Beijerinkia sp. strain B1).
Thirty-five genes, extending from bphA2e to nahD are found in the same order and transcriptional direction of the chromosome of S. yanoikuyae B1 (107), an organism that degrades a range of aromatic compounds similar to that of S. aromaticivorans F199. The presence of five additional genes (bphA2c, bphA2d, cmpX, xylT, and bphA2e) not listed in the preliminary report of the S. yanoikuyae B1 gene order were confirmed (106). The primary difference between these regions exists at the ends of the 39-kb region. At one end, downstream of the S. yanoikuyae nahD gene, is an insertion sequence element. The bphX gene at the other end of the S. yanoikuyae B1 sequence is a putative membrane protein that is similar to other putative membrane proteins associated with catabolic genes including the P. fluorescens IP01 cumene (29) and the P. putida F1 toluene (96) degradative gene clusters. Absent from the sequenced S. yanoikuyae B1 region were homologs to the putative membrane proteins, ORF1038 and ORF1042, located between xylM and bphA2 in pNL1. Another notable difference between the pNL1 and S. yanoikuyae B1 catabolic sequences was the distance between the 3′ ends of bphB and xylA. The gap between these genes in pNL1 was 60 bp, and in S. yanoikuyae B1 it was 555 bp.
Although DNA sequences from S. yanoikuyae B1 and S. aromaticivorans F199 are similar, significant differences in the catabolic activity of these two strains has been noted. S. yanoikuyae B1 is capable of growth on both monocyclic (toluene, p-ethyltoluene, 1,2,4-trimethyl benzene, and m- and p-xylene) and polycyclic aromatics (biphenyl, naphthalene, phenanthrene, and anthracene) (107). S. aromaticivorans F199 can also grow on toluene, all xylene isomers, naphthalene, and biphenyl (22), but in our studies growth was not as robust as that of S. yanoikuyae B1 on these compounds. Another difference noted was in the ability to express bphC and xylE in E. coli without the need for vector-encoded promoter sequences. E. coli containing cosmid clones of S. yanoikuyae B1 encoding xylE or bphC could not be detected by screening for catechol ring cleavage activity (47). In addition, expression of xylE from pGEM7Zf(−) was dependent on the vector-encoded lac promoter. In contrast, cosmid clones encoding pNL1 xylE or bphC genes were readily detected in E. coli by noting the yellow ring cleavage product that appeared after colonies were sprayed with catechol (86). In sequencing these cosmids, we found that vector-encoded promoters were not required for the expression of ring cleavage activity. Furthermore, the absence of the putative promoter region upstream of xylF in cosmid 18 (the present study) suggests that a secondary promoter recognized by the E. coli transcriptional machinery is located within the C terminus of xylF.
Besides probable differences in gene expression, the presence or absence of specific membrane proteins may also account for the differences in levels of catabolic activity observed in these two Sphingomonas strains. Membrane proteins have been implicated in the transport of aromatic compounds across bacterial cell walls. Functions ascribed to these proteins include the export of toxic aromatic compounds out of the cell (2a, 3, 34, 36a, 39) or the uptake of nontoxic aromatics from the environment (9, 67, 78, 100). Aromatic compounds can be toxic due to the adverse effect of accumulation of the hydrophobic compounds in the cellular membrane. Consequently, bacterial growth on aromatic compounds is typically achieved by allowing the substrate to volatilize from a separate phase and diffuse into the medium rather than by direct addition to the growth medium. Some aromatic compounds are not toxic to the cell but are sparingly soluble in water, making them poorly available to bacteria. Therefore, successful growth on aromatic compounds requires both mechanisms for exclusion of toxic aromatics from the cellular interior and uptake of nontoxic aromatic substrates into the cytoplasm where they can be metabolized. A homolog to the functionally uncharacterized membrane protein, BphX, is absent on pNL1 and may play an important role in modulating growth on aromatic compounds which somehow enables S. yanoikuyae B1 to grow better in laboratory conditions than S. aromaticivorans F199.
The presence of putative efflux pump genes within the aromatic catabolic region of pNL1 suggests that it may function in efflux of aromatic substrates or intermediates associated with pNL1-encoded degradative pathways. Several reports on bacterial efflux pumps associated with solvent (toluene) export and resistance have been published prior to this work (42, 47a, 52a, 79a). The P. putida S12 and putative pNL1 efflux pumps are distinct from each other in that they are members of the major facilitator superfamily and the resistance-nodulation-cell division family, respectively. Both families are predicted to utilize proton motive force to drive substrate export (73). It would be interesting to determine if other Sphingomonas strains contain similar efflux pump genes.
The export and catabolism of aromatic compounds are two biochemical mechanisms for solvent resistance. Physical mechanisms that lead to increases in cell membrane rigidity are also thought to be important in solvent tolerance (79). This alteration is achieved through desaturation of cis fatty acids and subsequent conversion to the trans isomer (33, 35, 98). ORF15 and ORFs that cluster with bphD are similar to fatty acid biosynthetic pathway enzymes and may prove to function in altering the cellular membrane in response to the presence of aromatic compounds.
The occurrence of multiple oxygenase binding subunit homologs also appears to be characteristic of Sphingomonas sp. Although it is tempting to speculate that S. aromaticivorans F199 is able to mix and match these oxygenase binding subunits to theoretically form as many as 49 different four-component enzymes, the fact that (i) every large subunit gene is adjacent to a small subunit gene, (ii) members of each large and small subunit pair align best to subunits of the same enzyme, and (iii) all but two of the oxygenase subunit sets are on different transcripts suggests that this does not normally occur. There have been, however, several reports on the construction of hybrid aromatic oxygenases which were in some cases functionally active (72, 89, 93). This suggests that if S. aromaticivorans F199 simultaneously expresses several sets of oxygenase binding subunits along with BphA3 and BphA4, novel combinations of oxygenase binding subunits and electron transfer components may occur that result in the formation of hybrid enzymes. Experimentation is needed to test this hypothesis and to measure the ability of hybrid enzymes from this bacterium to oxidize aromatic compounds.
Except for the apparent absence of a nahG or xylZ homolog, homologs to all genes found in model degradative pathways for biphenyl, m-xylene, and naphthalene were found on pNL1. We presume that the xylZ ferredoxin and ferredoxin reductase activities are provided by bphA3 and bphA4 for the benzoate dioxygenase encoded by pNL1. The existence of similar four-component benzoate dioxygenases have been described in S. yanoikuyae B1 (107) and P. aeruginosa (81). Since pNL1 conferred the ability to grow with salicylate as the sole carbon and energy source to Sphingomonas sp. strain S-88 m260, the genes for catabolism of salicylate are most likely encoded on pNL1. Salicylate can be degraded to gentisate by some microorganisms via catalysis by salicylate-5-monooxygenase (NagG) (25). The BphA1c-BphA2c and BphA1d-BphA2d oxygenase binding subunits cluster most closely with NagG-NagH, to functionally uncharacterized proteins in dinitrotoluene degradative gene clusters, and to the o-halobenzoate dioxygenase large subunit, OhbB (Fig. 4). Since bphA1cA2c clusters with nahD, it is a prime candidate for an oxygenase catalyzing the degradation of salicylate. Although the gentisate pathway has been described, the sequence for genes in this pathway is limited to two short peptide stretches of 15 and 26 amino acids from gentisate 1,2-dioxygenase (32). No significant homology with these peptides was found among sequences encoded by pNL1. Until more of the gene sequence information is available from the gentisate catabolic pathway, it will not be possible to rule out a gentisate pathway encoded on pNL1.
The putative replication-partitioning region of pNL1 is characteristic of iteron plasmids. The six repeats found between the orf211 and orf218 repA genes are likely iteron sequences to which the RepA binds to regulate plasmid replication. The occurrence of two different repA genes on the same plasmid is unusual and may provide a mechanism for regulating the copy number of pNL1 in response to different environmental stimuli. Bacterial homologs to eukaryotic group II-associated maturases have been previously associated with conjugative elements (60, 66, 104). To our knowledge, this is the first evidence for group II introns on a conjugative plasmid. The unusual distribution of the three putative exons near matRa suggest that both maturase-associated splicing and possibly other novel recombinatorial mechanisms are utilized to generate alternate proteins from pNL1.
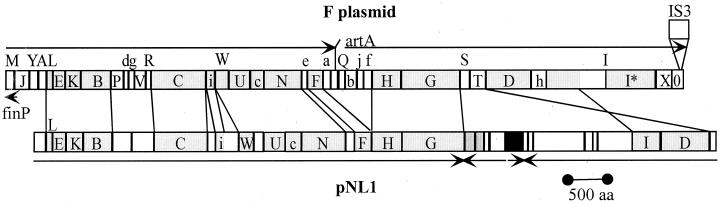
Overall, the conjugative transfer genes of pNL1 are most similar to those from E. coli F plasmid (Fig. 5). The gene order and transcriptional direction of shared genes is the same in pNL1 and F, except that the traD and traI genes are inverted with respect to the rest of the conjugative genes. F plasmid gene homologs not found on pNL1 include the following: (i) the regulatory genes traJ, traY, and finO; (ii) the pilus synthesis and assembly genes traA, traV, traQ, and traX; (iii) the mating-signal gene traM, the origin-nicking gene traY, and the DNA nicking-unwinding gene traI* (the C-terminal transcript within TraI); (iv) the surface exclusion genes traS and traT; and (v) the functionally uncharacterized genes traP, traR, and artA and all trb genes save trbi and trbc. The F plasmid genes are the closest homologs to pNL1 genes except for traE (orf207), which is most similar to S. typhimurium pED208 traE (19); orf851, whose C-terminal region is homologous to the entire traF gene of plasmid RK2 (97); and orf704, which is most similar to E. coli R388 trwC (53).
FIG. 5.
Comparison of F plasmid and pNL1 transfer regions. Capital letters refer to tra genes, and lowercase letters refer to trb genes. The direction of gene transcription is indicated by arrows. Lightly shaded boxes depict shared genes, medium-shaded boxes are pNL1 ORFs encoding the ISRm2011-2-like transposase, and the black box is the putative pNL1 regulator encoded by orf758. I* refers to the alternative transcript within I. The F plasmid portion of this graphic is from Frost et al. (24).
The presence of multiple ORFs with homology to genes that function in DNA integration and recombination suggests that pNL1 sequences can be rearranged and/or integrated into other genetic elements. The ability to promote site-specific recombination between two closely linked sites on pNL1 is probably provided by the ORF181 invertase. Based on other invertase activities, the predicted outcome of ORF181 activity would be an inversion of a segment of pNL1, most likely in the immediate vicinity of ORF181. Site-specific insertion into a different DNA target is the predicted outcome for phage-type integrases encoded by ORF338, ORF277, and TnpABC. In fact, the localization of the S. yanoikuyae B1 aromatic degradative genes on the chromosome may have resulted from such a recombinational event, as early studies with S. yanoikuyae B1 (formerly Beijerinckia sp. strain B1) suggested that the aromatic degradative genes were plasmid encoded (50).
The sequences of proteins associated with aromatic catabolism in Sphingomonas species diverge considerably from those associated with other types of bacteria with similar capabilities. A large proportion of new aromatic catabolic genes sequenced were isolated from Pseudomonas species and, not surprisingly, turn out to be very similar to previously characterized genes. This becomes more apparent when doing a cluster analysis of protein families. Identification and characterization of aromatic catabolic genes from phylogenetically distinct bacteria is invaluable in computational predictions of gene functions associated with large sequences generated from microbial genome projects. For example, the characterization and sequencing of the bphDEF genes from Rhodococcus sp. strain RHA1 (56) allowed us to predict with more reliability that ORFs 1317, 1324, and 1331 encoded bphDEF, rather than xylFJK, whose products perform very similar functions and whose deduced amino acid sequences are very similar. The same rationale is also true of genes associated with plasmid replication and conjugation. Very few homologs to sequences in the corresponding region of pNL1 were found, making it more difficult to predict functions associated with them. This problem was further compounded by the shuffling of domains among different proteins associated with conjugation (i.e., ORF851 has domains associated with plasmid F TrbI and plasmid RK-2 TraF).
In summary, the analysis of pNL1 sequences suggests that this plasmid confers the ability to utilize and/or tolerate a variety of aromatic compounds to its host, S. aromaticivorans. The conferred functions related to aromatic compounds can be divided into three general categories: aromatic catabolism, transport, and cell membrane alteration. The unique codisbursement of genes associated with distinct aromatic catabolic pathways appears to be unique to Sphingomonas species and delineates a novel evolutionary path for biodegradative gene organization. The association of membrane proteins with export and/or import of pathway substrates or intermediates is suggested by the sequence annotation. They may provide a means to prevent accumulation of toxic aromatic compounds in the cytoplasm or to sequester them when present at low concentrations in the environment or when they are otherwise poorly available. A protective effect may also be conferred by pNL1-encoded enzymes that change the membrane fatty acid saturation. It should be emphasized that the many of these hypotheses are derived primarily from sequence comparisons and should be considered hypotheses until evaluated experimentally. Work is currently in progress to test some of these predictions.
ACKNOWLEDGMENTS
This work was supported by a Pacific Northwest National Laboratory Initiative in Microbial Biotechnology under Department of Energy contract DE-AC06-76RL0 1830.
We thank Gerben Zylstra for helpful discussions and sharing unpublished data. We also thank Ray Wildung, Blaine Metting, and Ron Walters for their encouragement.
Footnotes
NRCC publication 42280.
REFERENCES
- 1.Armengaud J, Happe B, Timmis K N. Genetic analysis of dioxin dioxygenase of Sphingomonas sp. strain RW1: catabolic genes dispersed on the genome. J Bacteriol. 1998;180:3954–3966. doi: 10.1128/jb.180.15.3954-3966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armengaud J, Timmis K N. Molecular characterization of Fdx1, a putidaredoxin-type [2Fe-2S] ferredoxin able to transfer electrons to the dioxin dioxygenase of Sphingomonas sp. RW1. Eur J Biochem. 1997;247:833–842. doi: 10.1111/j.1432-1033.1997.00833.x. [DOI] [PubMed] [Google Scholar]
- 2a.Asako H, Nakajima N, Kobayashi K, Kobayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill D L. DOE makes subsurface cultures available. ASM News. 1993;59:504–506. [Google Scholar]
- 4.Balkwill D L. Numbers, diversity, and morphological characteristics of aerobic, chemoheterotrophic bacteria in deep subsurface sediments from a site in South Carolina. Geomicrobiol J. 1988;7:33–52. [Google Scholar]
- 5.Balkwill D L, Drake G R, Reeves R H, Fredrickson J K, White D C, Ringelberg D B, Chandler D P, Romine M F, Kennedy D W, Spadoni C M. Taxonomic study of aromatic-degrading bacteria from deep-terrestrial-subsurface sediments and description of Sphingomonas aromaticivorans sp. nov., Sphingomonas subterranea sp. nov., and Sphingomonas stygia sp. nov. Int J Syst Bacteriol. 1997;47:191–201. doi: 10.1099/00207713-47-1-191. [DOI] [PubMed] [Google Scholar]
- 6.Bankier A T. Generation of random fragments by sonication. Methods Mol Biol. 1993;23:47–50. doi: 10.1385/0-89603-248-5:47. [DOI] [PubMed] [Google Scholar]
- 7.Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A, Koraimann G, Hogenauer G. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 9.Collier L S, Nichols N N, Neidle E L. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dear S, Staden R. A sequence assembly and editing program for efficient management of large projects. DNA Sequence. 1991;19:3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deil H, Saint C M, Williams P A. Gene organization of the first catabolic operon of TOL plasmid pWW53: production of indigo by the xylA gene product. J Bacteriol. 1987;169:764–770. doi: 10.1128/jb.169.2.764-770.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ederer M M, Crawford R L, Herwig R P, Orser C S. PCP degradation is mediated by closely related strains of the genus Sphingomonas. Mol Ecol. 1997;6:39–49. doi: 10.1046/j.1365-294x.1997.00151.x. [DOI] [PubMed] [Google Scholar]
- 13.Ensley B D, Ratzkin B J, Osslund T D, Simon M J, Wackett L P, Gibson D T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983;222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 14.Erickson B D, Mondello F J. Nucleotide sequencing and transcriptional mapping of the genes encoding biphenyl dioxygenase, a multicomponent polychlorinated-biphenyl-degrading enzyme in Pseudomonas strain LB400. J Bacteriol. 1992;174:2903–2912. doi: 10.1128/jb.174.9.2903-2912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eulberg D, Golovleva L A, Schlomann M. Characterization of catechol catabolic genes from Rhodococcus erythropolis 1CP. J Bacteriol. 1997;179:370–381. doi: 10.1128/jb.179.2.370-381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans C A, Lewis K, Rothenberg B E. High efficiency vectors for cosmid microcloning and genomic analysis. Gene. 1989;39:9–20. doi: 10.1016/0378-1119(89)90088-7. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Ou L T, Ogram A. Cloning and sequence analysis of a novel insertion element from plasmids harbored by the carbofuran-degrading bacterium, Sphingomonas sp. CFO6. Plasmid. 1997;37:169–179. doi: 10.1006/plas.1997.1289. [DOI] [PubMed] [Google Scholar]
- 18.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay B B, Frost L S, Paranchych W. Nucleotide sequence of the tra YALE region from IncFV plasmid pED208. J Bacteriol. 1986;168:990–998. doi: 10.1128/jb.168.2.990-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredrickson J K, Balkwill D L, Drake G R, Romine M F, Ringelberg D B, White D C. Aromatic-degrading Sphingomonas isolates from the deep subsurface. Appl Environ Microbiol. 1995;61:1917–1922. doi: 10.1128/aem.61.5.1917-1922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredrickson J K, Balkwill D L, Zachara J M, Li S W, Brockman F J, Simmons M A. Physiological diversity and distributions of heterotrophic bacteria in deep cretaceous sediments of the Atlantic coastal plain. Appl Environ Microbiol. 1991;57:402–411. doi: 10.1128/aem.57.2.402-411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredrickson J K, Brockman F J, Workman D J, Li S W, Stevens T O. Isolation and characterization of a subsurface bacterium capable of growth on toluene, naphthalene, and other aromatic compounds. Appl Environ Microbiol. 1991;57:796–803. doi: 10.1128/aem.57.3.796-803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 24.Frost L S, Ippen-Ihler K, Skurray R A. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuenmayor S L, Wild M, Boyes A L, Williams P A. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J Bacteriol. 1998;180:2522–2530. doi: 10.1128/jb.180.9.2522-2530.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya N, Komano T. Nucleotide sequence and characterization of the trbABC region of the IncI1 plasmid R64: existence of the pnd gene for plasmid maintenance within the transfer region. J Bacteriol. 1996;178:1491–1497. doi: 10.1128/jb.178.6.1491-1497.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaasterland T, Sensen C W. Fully automated genome analysis that reflects user needs and preferences. A detailed introduction to the MAGPIE system architecture. Biochimie. 1996;78:302–310. doi: 10.1016/0300-9084(96)84761-4. [DOI] [PubMed] [Google Scholar]
- 28.Grothues D, Tummler B. New approaches in genome analysis by pulsed-field gel electrophoresis: application to the analysis of Pseudomonas species. Mol Microbiol. 1991;5:2763–2776. doi: 10.1111/j.1365-2958.1991.tb01985.x. [DOI] [PubMed] [Google Scholar]
- 29.Habe H, Kasuga K, Nojiri H, Yamane H, Omori T. Analysis of cumene (isopropylbenzene) degradation genes from Pseudomonas fluorescens IP01. Appl Environ Microbiol. 1996;62:4471–4477. doi: 10.1128/aem.62.12.4471-4477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Happe B, Eltis L D, Poth H, Hedderich R, Timmis K N. Characterization of 2,2′,3-trihydroxybiphenyl dioxygenase, an extradiol dioxygenase from the dibenzofuran- and dibenzo-p-dioxin-degrading bacterium Sphingomonas sp. strain RW1. J Bacteriol. 1993;175:7313–7320. doi: 10.1128/jb.175.22.7313-7320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harayama S, Rekik M, Timmis K N. Genetic analysis of a relaxed substrate specificity aromatic ring dioxygenase, toluate 1,2-dioxygenase, encoded by TOL plasmid pWWO of Pseudomonas putida. Mol Gen Genet. 1986;202:226–234. doi: 10.1007/BF00331641. [DOI] [PubMed] [Google Scholar]
- 32.Harpel M R, Lipscomb J D. Gentisate 1,2-dioxygenase from pseudomonas. Purification, characterization, and comparison of the enzymes from Pseudomonas testosteroni and Pseudomonas acidovorans. J Biol Chem. 1990;265:6301–6311. [PubMed] [Google Scholar]
- 33.Heipieper H J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofer B, Backhaus S, Timmis K N. The biphenyl/polychlorinated biphenyl-degradation locus (bph) of Pseudomonas sp. LB400 encodes four additional metabolic enzymes. Gene. 1994;144:9–16. doi: 10.1016/0378-1119(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 35.Holtwick R, Meinhardt F, Keweloh H. cis-trans Isomerization of unsaturated fatty acids: cloning and sequencing of the cti gene from Pseudomonas putida P8. Appl Environ Microbiol. 1997;63:4292–4297. doi: 10.1128/aem.63.11.4292-4297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai R, Nagata Y, Fukuda M, Takagi M, Yano K. Molecular cloning of a Pseudomonas paucimobilis gene encoding a 17-kilodalton polypeptide that eliminates HCl molecules from gamma-hexachlorocyclohexane. J Bacteriol. 1991;173:6811–6819. doi: 10.1128/jb.173.21.6811-6819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins R O, Dalton H. The use of indole as a spectrophotometric assay substrate for toluene dioxygenase. FEMS Microbiol Lett. 1985;30:227–231. [Google Scholar]
- 38.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kado C I, Liu S-T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamoda S, Saburi Y. Cloning, expression, and sequence analysis of a lignostilbene-alpha,beta-dioxygenase gene from Pseudomonas paucimobilis TMY1009. Biosci Biotechnol Biochem. 1993;57:926–930. doi: 10.1271/bbb.57.926. [DOI] [PubMed] [Google Scholar]
- 41.Kato J, Chu L, Kitano K, DeVault J D, Kimbara K, Chakrabarty A M, Misra T K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989;84:31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 42.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi Y, Nagata Y, Hinata M, Kimbara K, Fukuda M, Yano K, Takagi M. Identification of bphA4 gene encoding ferredoxin reductase involved in biphenyl and polychlorinated biphenyl degradation in Pseudomonas sp. strain KKS102. Appl Environ Microbiol. 1994;176:1689–1694. doi: 10.1128/jb.176.6.1689-1694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kilpi S, Backstrom V, Korhola M. Degradation of 2-methyl-4-chlorophenoxy acetic acid (MCPA), 2,4-dichlorophenoxy acetic acid (2,4-D), benzoic acid and salicylic acid by Pseudomonas sp. HV3. FEMS Microbiol Lett. 1980;8:177–182. [Google Scholar]
- 45.Kim E. Ph.D. thesis. New Brunswick, N.J: Rutgers University; 1996. [Google Scholar]
- 46.Kim E, Aversano P J, Romine M F, Schneider R P, Zylstra G J. Homology between genes for aromatic hydrocarbon degradation in surface and deep-subsurface Sphingomonas strains. Appl Environ Microbiol. 1996;62:1467–1470. doi: 10.1128/aem.62.4.1467-1470.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim E, Zylstra G J. Molecular and biochemical characterization of two meta-cleavage dioxygenases involved in biphenyl and m-xylene degradation by Beijerinckia sp. strain B1. J Bacteriol. 1995;177:3095–3103. doi: 10.1128/jb.177.11.3095-3103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Kim K, Lee S, Lee K, Lim D. Isolation and characterization of toluene-sensitive mutants from the toluene-resistant bacterium Pseudomonas putida GM73. J Bacteriol. 1998;180:3692–3696. doi: 10.1128/jb.180.14.3692-3696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S, Shin H J, Kim Y, Kim S J, Kim Y C. Nucleotide sequence of the Pseudomonas sp. DJ77 phnG gene encoding 2-hydroxymuconic semialdehyde dehydrogenase. Biochem Biophys Res Commun. 1997;240:41–45. doi: 10.1006/bbrc.1997.7595. [DOI] [PubMed] [Google Scholar]
- 49.Kimura N, Nishi A, Goto M, Furukawa K. Functional analyses of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiyohara H, Sugiyama M, Mondello F J, Gibson D T, Yano K. Plasmid involvement in the degradation of polycyclic aromatic hydrocarbons by Beijerinckia species. Biochem Biophys Res Commun. 1983;111:939–945. doi: 10.1016/0006-291x(83)91390-6. [DOI] [PubMed] [Google Scholar]
- 51.Koonin E V, Tatusov R L. Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity—application of an iterative approach to database search. J Mol Biol. 1994;244:125–132. doi: 10.1006/jmbi.1994.1711. [DOI] [PubMed] [Google Scholar]
- 52.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Cummings N J, Daniel R A, Denizot F, Devine K M, Dusterhoft A, Ehrlich S D, Emmerson P T, Entian K D, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim S Y, Glaser P, Goffeau A, Golightly E J, Grandi G, Guiseppi G, Guy B J, Haga K, Haiech J, Harwood C R, Henaut A, Hilbert H, Holsappel S, Hosono S, Hullo M F, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee S M, Levine A, Liu H, Masuda S, Mauel C, Medigue C, Medina N, Mellado R P, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park S H, Parro V, Pohl T M, Portetelle D, Porwollik S, Prescott A M, Presecan E, Pujic P, Purnelle B, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 52a.Li X-Z, Zhang L, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llosa M, Bolland S, de la Cruz F. Genetic organization of the conjugal DNA processing region of the IncW plasmid R388. J Mol Biol. 1994;235:448–464. doi: 10.1006/jmbi.1994.1005. [DOI] [PubMed] [Google Scholar]
- 54.Lloyd-Jones G, Lau P C. Glutathione S-transferase-encoding gene as a potential probe for environmental bacterial isolates capable of degrading polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 1997;63:3286–90. doi: 10.1128/aem.63.8.3286-3290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 56.Masai E, Sugiyama K, Iwashita N, Shimizu S, Hauschild J E, Hatta T, Kimbara K, Yano K, Fukuda M. The bphDEF meta-cleavage pathway genes involved in biphenyl/polychlorinated biphenyl degradation are located on a linear plasmid and separated from the initial bphACB genes in Rhodococcus sp. strain RHA1. Gene. 1997;187:141–149. doi: 10.1016/s0378-1119(96)00748-2. [DOI] [PubMed] [Google Scholar]
- 57.Mercado-Blanco J, Olivares J. A protein involved in stabilization of a large non-symbiotic plasmid of Rhizobium meliloti shows homology to eukaryotic cytoskeletal proteins and DNA-binding proteins. Gene. 1994;139:75–79. doi: 10.1016/0378-1119(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 58.Merlin C, Springael D, Mergeay M, Toussaint A. Organization of the bph gene cluster of transposon Tn4371, encoding enzymes for the degradation of biphenyl and 4-chlorobiphenyl compounds. Mol Gen Genet. 1997;253:499–506. doi: 10.1007/s004380050349. [DOI] [PubMed] [Google Scholar]
- 59.Miele L, Strack B, Kruft V, Lanka E. Gene organization and nucleotide sequence of the primase region of IncP plasmids RP4 and R751. DNA Sequence. 1991;2:145–162. doi: 10.3109/10425179109039685. [DOI] [PubMed] [Google Scholar]
- 60.Mills D J, McKay L L, Dunny G M. Splicing of a group II intron involved in the conjugative transfer of pRS01 in lactococci. J Bacteriol. 1996;178:3531–3538. doi: 10.1128/jb.178.12.3531-3538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Missiakas D, Georgopoulos C, Raina S. The Escherichia coli dsbC (xprA) gene encodes a periplasmic protein involved in disulfide bond formation. EMBO J. 1994;13:2013–2020. doi: 10.1002/j.1460-2075.1994.tb06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore E. Genetic and serological evidence for the recognition of four pentachlorophenol-degrading bacterial strains as species of the genus Sphingomonas. Syst Appl Microbiol. 1995;18:539–548. [Google Scholar]
- 63.Moore, E. R. B., R. M. Wittich, P. Fortnagel, and K. N. Timmis. 16S ribosomal RNA gene sequence characterization and phylogenetic analysis of a dibenzo-p-dioxin degrading isolate within the genus Sphingomonas. Lett. Appl. Microbiol., in press.
- 64.Mueller J G, Chapman P J, Blattmann B O, Pritchard P H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. Appl Environ Microbiol. 1990;56:1079–1086. doi: 10.1128/aem.56.4.1079-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mueller J G, Devereux R, Santavy D L, Lantz S E, Willis S G, Pritchard P H. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Leeuwenhoek. 1997;71:329–343. doi: 10.1023/a:1000277008064. [DOI] [PubMed] [Google Scholar]
- 66.Mullant P, Pallen M, Wilks M, Stephen J R, Tabaqchali S. A group II intron in a conjugative transposon from the gram positive bacterium Clostridium difficile. Gene. 1996;174:145–150. doi: 10.1016/0378-1119(96)00511-2. [DOI] [PubMed] [Google Scholar]
- 67.Nichols N N, Harwood C S. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J Bacteriol. 1997;179:5056–5061. doi: 10.1128/jb.179.16.5056-5061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nickel K, Suter M J, Kohler H P. Involvement of two alpha-ketoglutarate-dependent dioxygenases in enantioselective degradation of (R)- and (S)-mecoprop by Sphingomonas herbicidovorans MH. J Bacteriol. 1997;179:6674–6679. doi: 10.1128/jb.179.21.6674-6679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noda Y, Nishikawa S, Shiozuka K-I, Kadokura H, Nakajima H, Yoda K, Katayama Y, Haraguchi N M T, Yamasaki M. Molecular cloning of the protocatechuate 4,5-dioxygenase genes of Pseudomonas paucimobilis. J Bacteriol. 1990;172:2704–2709. doi: 10.1128/jb.172.5.2704-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nohynek L J, Nurmiaho-Lassila E-L, Suhonen E L, Busse H-J, Mohammadi M, Hantula J, Rainey F, Salkinoja-Salonen M S. Description of chlorophenol-degrading Pseudomonas sp. strains KF1T, KF3, and NKF1 as a new species of the genus Sphingomonas, Sphingomonas subarctica sp. nov. Int J Syst Bacteriol. 1996;46:1042–1055. doi: 10.1099/00207713-46-4-1042. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor K E, Dobson A D, Hartman S. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl Environ Microbiol. 1997;63:4287–4291. doi: 10.1128/aem.63.11.4287-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parales R E, Emig M D, Lynch N A, Gibson D T. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J Bacteriol. 1998;180:2337–2344. doi: 10.1128/jb.180.9.2337-2344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paulson I T, Park J H, Choi P S, Saier M H. A family of Gram-negative bacterial outer membrane factors that function in the export of protein, carbohydrates, drugs, and heavy metals from Gram-negative bacteria. FEMS Microbiol Lett. 1997;156:1–8. doi: 10.1111/j.1574-6968.1997.tb12697.x. [DOI] [PubMed] [Google Scholar]
- 75.Pflugmacher U, Averhoff B, Gottschalk G. Cloning, sequencing, and expression of isopropylbenzene degradation genes from Pseudomonas sp. strain JR1: identification of isopropylbenzene dioxygenase that mediates trichloroethene oxidation. Appl Environ Microbiol. 1996;62:3967–3977. doi: 10.1128/aem.62.11.3967-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philipp W J, Poulet S, Eiglmeier K, Pascopella L, Balasubramanian V, Heym B, Bergh S, Bloom B R, Jacobs W R, Jr, Cole S T. An integrated map of the genome of the tubercle bacillus, Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc Natl Acad Sci USA. 1996;7:3132–3137. doi: 10.1073/pnas.93.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pollock T J, Throne L, Yamazaki M, Mikolajczak M J, Armentrout R W. Mechanism of bacitracin resistance in gram-negative bacteria that synthesize exopolysaccharides. J Bacteriol. 1994;176:6229–6237. doi: 10.1128/jb.176.20.6229-6237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prieto M A, Garcia J L. Identification of the 4-hydroxyphenylacetate transport gene of Escherichia coli W: construction of a highly sensitive cellular biosensor. FEBS Lett. 1997;414:293–297. doi: 10.1016/s0014-5793(97)01012-0. [DOI] [PubMed] [Google Scholar]
- 79.Ramos J L, Duque E, Rodríguez-Herva J-J, Godoy P, Haïdour A, Reyes F, Fernández-Barrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1996;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 79a.Ramos J L, Estrella D, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Resnick S M, Lee K, Gibson D T. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol. 1996;17:438–457. [Google Scholar]
- 81.Romanov V, Hausinger R P. Pseudomonas aeruginosa 142 uses a three-component ortho-halobenzoate 1,2-dioxygenase for metabolism of 2,4-dichloro- and 2-chlorobenzoate. J Bacteriol. 1994;176:3368–3374. doi: 10.1128/jb.176.11.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saint C P, Romas P. 4-Methylphthalate catabolism in Burkholderia (Pseudomonas) cepacia Pc701: a gene encoding a phthalate-specific permease forms part of a novel gene cluster. Microbiology. 1996;142:2407–2418. doi: 10.1099/00221287-142-9-2407. [DOI] [PubMed] [Google Scholar]
- 83.Sanger F, Nicklen S, Coulsen A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selbitschka W, Arnold W, Jording D, Kosier B, Toro N, Puhler A. The insertion sequence element ISRm2011-2 belongs to the IS630-Tc1 family of transposable elements and is abundant in Rhizobium meliloti. Gene. 1995;163:59–64. doi: 10.1016/0378-1119(95)00371-c. [DOI] [PubMed] [Google Scholar]
- 85.Sharma S B, Signer E R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in plants revealed by transposon Tn5-gusA. Genes Dev. 1990;4:344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- 85a.Shingler V. Signal sensing by ς54-dependent regulators: derepression as a control mechanism. Mol Microbiol. 1996;19:409–416. doi: 10.1046/j.1365-2958.1996.388920.x. [DOI] [PubMed] [Google Scholar]
- 86.Stillwell L C, Thurston S J, Schnieder R P, Romine M F, Fredrickson J K, Saffer J D. Physical mapping and characterization of a catabolic plasmid from the deep-subsurface bacterium Sphingomonas sp. strain F199. J Bacteriol. 1995;177:4537–4539. doi: 10.1128/jb.177.15.4537-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sulavik M C, Gambino L F, Miller P F. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol Med. 1995;1:436–446. [PMC free article] [PubMed] [Google Scholar]
- 88.Sutton S D, Pfaller S L, Shann J R, Warshawsky D, Kinkle B K, Vestal J R. Aerobic biodegradation of 4-methylquinoline by a soil bacterium. Appl Environ Microbiol. 1996;62:2910–2914. doi: 10.1128/aem.62.8.2910-2914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suyama A, Iwakiri R, Kimura N, Nishi A, Nakamura K, Furukawa K. Engineering hybrid pseudomonads capable of utilizing a wide range of aromatic hydrocarbons and of efficient degradation of trichloroethylene. J Bacteriol. 1996;178:4039–4046. doi: 10.1128/jb.178.14.4039-4046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sylvestre M, Sirois M, Hurtubise Y, Bergeron J, Ahmad D, Shareck F, Barriault D, Guillemette I, Juteau J M. Sequencing of Comamonas testosteroni strain B-356-biphenyl/chlorobiphenyl dioxygenase genes: evolutionary relationships among Gram-negative bacterial biphenyl dioxygenases. Gene. 1996;174:195–202. doi: 10.1016/0378-1119(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 91.Taira K, Hayase N, Arimura N, Yamashita S, Miyazaki T, Furukawa K. Cloning and nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene from the PCB-degrading strain of Pseudomonas paucimobilis Q1. Biochemistry. 1988;27:3990–3996. doi: 10.1021/bi00411a015. [DOI] [PubMed] [Google Scholar]
- 92.Taira K, Hirose J, Hayashida S, Furukawa K. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J Biol Chem. 1992;267:4844–4853. [PubMed] [Google Scholar]
- 93.Tan H M, Cheong C M. Substitution of the ISP alpha subunit of biphenyl dioxygenase from Pseudomonas results in a modification of the enzyme activity. Biochem Biophys Res Commun. 1994;204:912–917. doi: 10.1006/bbrc.1994.2546. [DOI] [PubMed] [Google Scholar]
- 94.Uetz T, Schneider R, Snozzi M, Egli T. Purification and characterization of a two-component monooxygenase that hydroxylates nitrilotriacetate from “Chelatobacter” strain ATCC 29600. J Bacteriol. 1992;174:1179–1188. doi: 10.1128/jb.174.4.1179-1188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Lau P C K, Button D K. A marine oligobacterium harboring genes known to be part of aromatic hydrocarbon degradation pathways of soil pseudomonads. Appl Environ Microbiol. 1996;62:2169–2173. doi: 10.1128/aem.62.6.2169-2173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Rawlings M, Gibson D T, Labbe D, Bergeron H, Brousseau R, Lau P C. Identification of a membrane protein and a truncated LysR-type regulator associated with the toluene degradation pathway in Pseudomonas putida F1. Mol Gen Genet. 1995;246:570–579. doi: 10.1007/BF00298963. [DOI] [PubMed] [Google Scholar]
- 97.Waters V L, Strack B, Pansegrau W, Lanka E, Guiney D G. Mutational analysis of essential IncP alpha plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992;174:6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weber F J, Isken S, de Bont J A. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994;140(Pt. 8):2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- 99.Whited G M, Gibson D T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991;173:3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams P A, Shaw L E. mucK, a gene in Acinetobacter calcoaceticus ADP1 (BD413), encodes the ability to grow on exogenous cis,cis-muconate as the sole carbon source. J Bacteriol. 1997;179:5935–5942. doi: 10.1128/jb.179.18.5935-5942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong K K, Stillwell L C, Dockery C A, Saffer J D. Use of tagged random hexamer amplification (TRHA) to clone and sequence minute quantities of DNA: application to a 180 kb plasmid isolated from Sphingomonas F199. Nucleic Acids Res. 1996;24:3778–3783. doi: 10.1093/nar/24.19.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood M S, Lory C, Lessie T G. Activation of the lac genes of Tn951 by insertion sequences from Pseudomonas cepacia. J Bacteriol. 1990;172:1719–1724. doi: 10.1128/jb.172.4.1719-1724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yabuuchi E, Yano I, Oyaizu H, Hashimoto Y, Ezaki T, Yamamato H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol Immunol. 1990;34:99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 104.Yeo C C, Tham J M, Yap M W, Poh C L. Group II intron from Pseudomonas alcaligenes NCIB9867 (P25X): entrapment in plasmid RP4 and sequence analysis. Microbiology. 1997;143(Pt. 8):2833–2840. doi: 10.1099/00221287-143-8-2833. [DOI] [PubMed] [Google Scholar]
- 105.Yrjala K, Paulin L, Romantschuk M. Novel organization of catechol meta-pathway genes in Sphingomonas sp. HV3 pSKY4 plasmid. FEMS Microbiol Lett. 1997;154:403–408. doi: 10.1111/j.1574-6968.1997.tb12674.x. [DOI] [PubMed] [Google Scholar]
- 106.Zylstra, G. J. Personal communication.
- 107.Zylstra G J, Kim E. Aromatic hydrocarbon degradation by Sphingomonas yanoikuyae B1. J Ind Microbiol Biotechnol. 1997;19:408–414. doi: 10.1038/sj.jim.2900475. [DOI] [PubMed] [Google Scholar]