Abstract
Dolichos biflorus (Muthira) is a branched, suberect, and downing herb, native to most parts of India, and found at altitudes of up to 1000 m, whose seeds can be cooked and eaten. Nutrition plays a key role in building immunity and preventing noncommunicable diseases to a certain extent. The purpose of this study was to evaluate the anti-inflammatory and antioxidant effects of 70% methanolic extract of seeds of D. biflorus (DME) in carrageenan-induced inflammation. DME exhibited maximum percentage of oedema inhibition at a dose of 50 mg/kg at the 3rd hour of carrageenan induction. The effect was higher than that of the standard drug Voveran. The activities of cyclooxygenase, lipoxygenase, nitric oxide synthase, myeloperoxidase, and malondialdehyde showed significant (p < 0.05) reduction whereas the activities of antioxidant enzymes, vitamins C, and reduced glutathione level were increased significantly (p < 0.05) on treatment with DME. Also levels of the acute phase protein, ceruloplasmin, were brought to their normal range in DME-treated rats. Phytochemical analysis showed that the extract contains alkaloids, flavonoids, carbohydrates, proteins, and tannins, which may contribute to its anti-inflammatory and antioxidant activity. Thus the results demonstrate the potential beneficiary effect of DME on carrageenan-induced inflammation in rats.
Keywords: Antioxidant enzymes, Cyclooxygenase, Inflammation, Lipoxygenase
1. Introduction
Inflammation is known to contribute to physiological and pathological processes of arthritis, cancer, stroke, neurodegenerative, and cardiovascular disease by the activation of the immune system and various cells within the damaged tissue [1]. The classical characteristics of inflammation are pain, swelling, edema, redness, and heat [2]. The treatment of inflammatory diseases especially remains an important research area because the available therapeutic drugs are often not adequately efficient or side effects are intolerable, and all the more so in cases of long-time therapy [3].
Prostaglandins, thromboxanes and leukotrienes are inflammatory mediators called eicosanoids, biosynthesized by cyclooxygenases (COX) and lipoxygenases (LOX) in cell types that are associated with inflammatory disorders. COX-2-catalyzed synthesis of PGE2 plays a key role in inflammation and its associated diseases [4]. LOX is a family of iron-containing enzymes involved in the metabolism of prostaglandins and leukotrienes, which utilize arachidonic acid as the substrate. These are of great biological and medical relevance, because of the function of products in signaling or in inducing structural or metabolic changes in the cell.
Certain levels of reactive oxygen species (ROS) are required for normal cell function, they are an important part of the defense mechanism against infection, but if in surplus they cause oxidative stress [5]. They are commonly produced during inflammatory processes and the host defense is also involved in signal transduction and gene activation. ROS cause the peroxidation of membrane polyunsaturated fatty acids, protein, DNA, hyaluronic acid, and cartilage, yielding reactive aldehydes and lipid peroxides that are involved in the pathophysiological changes associated with oxidative stress in cells and tissues [6]. In order to prevent harmful accumulation of damaged DNA, lipids, and proteins, the cell possesses a complex and highly effective system of antioxidant defense that allows an immediate response to oxidative stress [7].
Traditional nonsteroidal anti-inflammatory drugs (NSAIDs) block the COX pathway that contributes to cell-mediated prostaglandin (PGE2) production. Several modern drugs are used to treat inflammatory disorders, but their prolonged use may cause severe adverse side effects. Thus there is a need to develop new anti-inflammatory agents with minimum side effects. The use of natural remedies for treatment of inflammatory and painful conditions has a long history starting with Ayurvedic treatment of traditional medicines. The present study evaluates the anti-inflammatory and free radical scavenging activity of methanolic extract of Dolichos biflorus seeds (DME) in carrageenan-induced inflammation.
In Ayurveda, the seed of D. biflorus is used in the treatment of piles, pain, constipation, wounds, urinary calculi, cough, edema, asthma, etc. Soup prepared from seeds is also beneficial in enlarged liver and spleen. The seeds of D. biflorus have been reported to show antilithiatic [8], antihepatotoxic [9], and hypolipidemic [10] activity, and are involved in lowering the level of blood sugar and total cholesterol [11]. Two Ayurvedic preparations [12,13], having D. biflorus as an ingredient, have shown their antinephrotoxic and free radical scavenging activity. The present study is aimed to evaluate the antioxidant and anti-inflammatory effect of 70% DME.
2. Materials and methods
2.1. Chemicals and solvents
Linoleic acid, histopaque, arachidonic acid, and carrageenan were purchased from Sigma–Aldrich Chemicals (St Louis, MO, USA) and Spectrochem Pvt. Ltd. (Mumbai, Maharashtra, India). All the other chemicals and biochemicals used were of the highest grade available.
2.2. Preparation of DME
The seeds of D. biflorus were purchased from the local market. The seeds were washed thoroughly and dried in shade. The finely powdered seeds (100 g) were soaked in 70% methanol and stirred using a magnetic stirrer for 15 hours. The mixture was then centrifuged at 2850g and the supernatant decanted. The process was repeated by adding the solvent with the precipitated pellet. The supernatants were collected and concentrated in a rotary evaporator in low pressure yielding the dried extract. This extract (12 g/100g powdered D. biflorus) was used for the study.
2.3. Animals
Female albino rats (Sprague–Dawley strain) of body weight 100–150 g, which were bred and reared in the department animal house, were used for this study. They were provided with laboratory chow (Hindustan Lever Lab diet) and water ad libitum throughout the experimental period. The rats were housed in polypropylene cages in a room with the temperature maintained at 26 ± 1°C and a 12-hour light–dark cycle. All experiments were conducted as per the guidelines of the Animal Ethics Committee CPCSEA (Registration No. 218/CPCSEA) according to Government of India accepted principles for laboratory animal use.
2.4. Carrageenan-induced acute inflammation
Anti-inflammatory effects of various doses of the DME were evaluated in acute inflammatory model induced by carrageenan in albino rats (150–200 g body weight). Rats were divided into four groups of six rats as follows. Group I: control rats; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + DME (50 mg/kg body weight); and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran (Novartis India Ltd, Thane, Maharashtra, India; 20 mg/kg body weight). The methanolic extract was given as a dose of intraperitonial injection (50 mg/kg body weight) to the rats in Group III. Rats of Group IV were given an intraperitonial injection (20 mg/kg body weight) of Voveran. After 30 minutes of pretreatment with methanolic extract, the inflammatory agent used for the study was Type IV Lambda carrageenan (Spectrochem). A 0.1 mL sample of carrageenan (1% in normal saline) was injected (Groups II–IV) into the plantar aponeurosis of the right hind paw, producing acute inflammatory edema leading to marked increase in the volume of the limb. The control group received only carrageenan (1% in normal saline). The volume of each paw was measured by means of a screw gauge before and 3 hours after carrageenan injection.
The percentage edema was calculated by Winter et al’s method [14].
| [1] |
where Vc and Vt are the average edema volume of control and test. At the end of the experimental period animals were sacrificed after an overnight fast. The blood was collected in test tubes containing anticoagulant (heparin). The paw tissue of the inflamed paw was also taken for the experiment.
2.5. Isolation of peripheral blood mononuclear cells
Isolation of monocytes was carried out as described by Radhika et al [15]. A 3 mL volume of Histopaque 1083 solution was placed in a 15 mL tube and 3 mL blood was layered on top of this density gradient. After the centrifugation (400g for 30 minutes at room temperature) the blood cells were separated into two fractions: an upper white layer consisting of mononuclear cells plus the majority of platelets at the interface region, and a lower layer containing erythrocytes and granulocytes. The plasma layer on top was clear and contained no cells. First the plasma layer was removed and discarded. From the buffy coat, the monocytes were carefully taken off by aspiration and washed with phosphate buffered saline. This was repeated twice. After that, the pellet was resuspended in phosphate buffered saline–Tween and subjected to freeze-–thaw cycle three times. The resulting lysate was used as the enzyme source.
2.6. Assay of COX
COX was assayed by the thiobarbituric acid method of Shimazu et al [16]. The assay mixture contained Tris-HCl buffer, glutathione, hemoglobin, and enzyme. The reaction was started by the addition of arachidonic acid. Then terminated after 1 minute’s incubation at 25°C by addition of 0.2 mL of 10% trichloracetic acid in 1N HCl, mixed and 0.2 mL of thiobarbituric acid was added. The contents heated in a boiling water bath for 20 minutes, cooled and centrifuged at 120g for 3 minutes. The supernatant was measured at 532 nm for COX activity.
2.7. Assay of LOX
LOX was measured using Axelrod et al’s method [17]. A 70 mg sample of linoleic acid and equal weight of Tween 20 were dissolved in 4 mL oxygen free water and mixed back and forth with a pipette, avoiding air bubbles. Sufficient amount of 0.5 N NaOH was added to yield a clear solution (0.55 mL) and then made up to 25 mL using oxygen-free water. This was divided into 0.5 mL portions and flushed with nitrogen gas before closing and kept frozen until needed. The assay mixture for 5-LOX contained 2.75 mL of borate buffer pH 9.0, 0.2 mL of sodium linoleate and 50 μL of enzyme. The increase in OD was measured at 234 nm. The assay mixture for 15-LOX contained 2.75 mL of phosphate buffer pH 6.5, 0.2 mL of sodium linoleate, and 50 μL of enzyme. The increase in OD was measured at 280 nm.
2.8. Biochemical estimations
Determination of myeloperoxidase activity was determined by a previously described method [18]. The sample was mixed with 50 mM phosphate buffer (pH 6) containing 1.67 mg/mL O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide; 5N HCL was added to stop the reaction. The change in absorbance at 400 nm was measured. Nitric oxide synthase (NOS) was determined by the method described previously [19]. Malondialdehyde (MDA), Superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), reduced glutathione (GSH), and vitamin C were estimated as reported earlier [20]. Glutathione reductase (GRd) was assayed by the following method of David and Richard [21]. Concentration of ceruloplasmin was measured by Ravin’s method [22]. The protein concentration of the samples was determined by Lowry et al’s method [23].
2.9. Phytochemical analysis
Phytochemical analysis was carried out to ascertain the qualitative composition of secondary metabolites such as sugars, phenolic compounds, tannins, anthroquinones, flavonoids, saponins, steroids, glycosides, and alkaloids by employing coloration and precipitation test [24,25].
2.10. Statistical analysis
The results were analyzed using SPSS/PC+, version 11.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was employed for comparison test of significant differences among groups. Pair fed comparisons between the groups was made by Duncan’s multiple range test p < 0.05 was considered significant.
3. Results
3.1. Anti-inflammatory effect of DME on carrageenan-induced paw edema
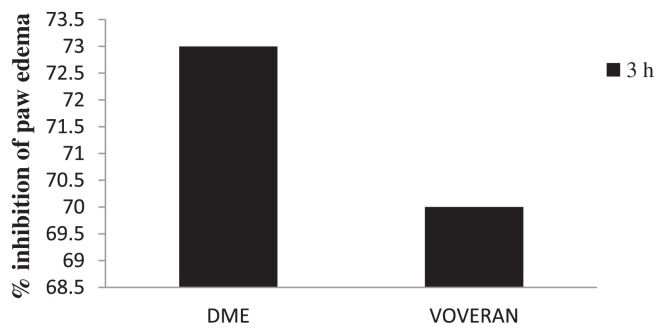
A dose of 50 mg/kg DME showed 73% inhibition of paw edema at the 3rd hour. The result was comparable to the effect produced by the standard anti-inflammatory drug Voveran (20 mg/kg; Fig. 1).
Fig. 1.
Inhibitory effect of methanolic extract of Dolichos biflorus seeds and Voveran on carrageenan-induced paw edema. Values expressed as average of six rats ± standard error of the mean in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran.
3.2. Effect of DME on inflammatory mediators
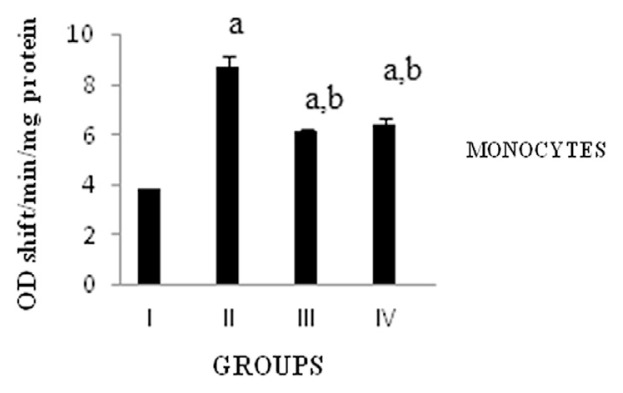
Isolated monocytes from the blood after 3 hours of experiment period and COX activity was assayed. COX is responsible for the synthesis of PGs involved in the inflammatory response. The activity of COX in monocytes was significantly (p < 0.05) increased in carrageenan-induced rats when compared with the normal group. Treatment with DME showed significant (p < 0.05) reduction in COX activity as compared to carrageenan-induced rats (Fig. 2).
Fig. 2.
Activity of cyclooxygenase. Values expressed as average of six rats ± standard error of the mean in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran. a = statistical difference compared with Group I at p < 0.05; b =statistical difference when Group II compared with Groups III and IV at p < 0.05.
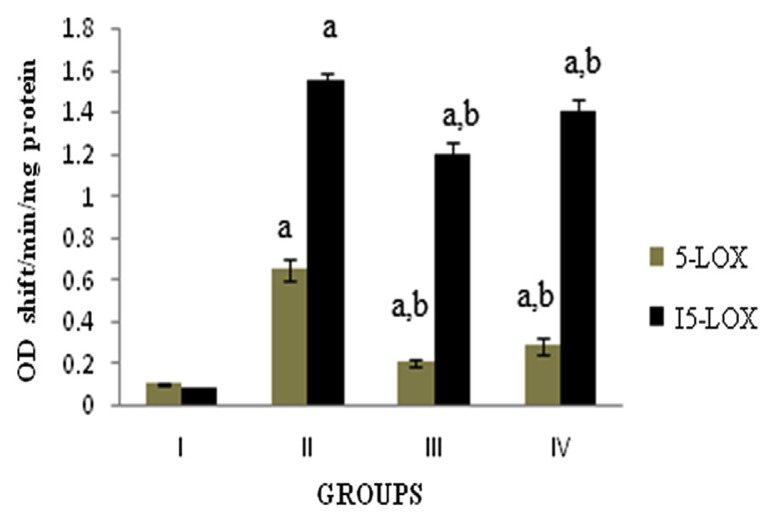
LOX is involved in the synthesis of leukotrienes, which are lipid mediators of inflammation. The activity of 5-LOX and 15-LOX in monocytes was significantly (p < 0.05) increased in the carrageenan-induced rats when compared with normal group. Supplementation of DME showed significant (p < 0.05) decrease in 5-LOX and 15-LOX activity as compared to carrageenan-induced rats (Fig. 3).
Fig. 3.
Activity of 5-lipoxygenase and 15-lipoxygenase. Values expressed as average of six rats ± standard error of the mean in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran. a = statistical difference compared with Group I at p < 0.05; b =statistical difference when Group II compared with Groups III and IV at p < 0.05.
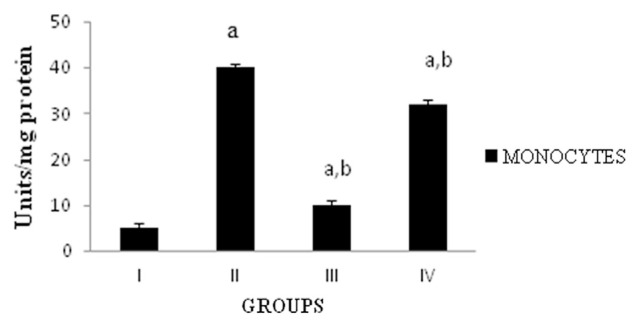
Myeloperoxidase (MPO) is a powerful proinflammatory enzyme considered as a biomarker of neutrophil activity. In paw tissue, the activity of MPO was significantly (p < 0.05) increased in carrageenan-induced rats when compared to the normal group. DME treated group showed significant (p < 0.05) inhibition in MPO activity when compared to the carrageenan-induced rats (Fig. 4).
Fig. 4.
Activity of myeloperoxidase. Values expressed as average of six rats ± standard error of the mean in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran. a = statistical difference compared with Group I at p < 0.05; b =statistical difference when Group II compared with Groups III and IV at p < 0.05. One unit of MPO activity defined as that degrading 1 μm of peroxide per minute at 25°C.
Nitric oxide is a ubiquitous biomessenger, used for a number of inter- and intra-cellular signaling functions including blood-vessel dilation, neuronal signal transmission, and cytotoxicity against pathogens. The concentration of NOS in monocytes was significantly (p < 0.05) increased in the carrageenan-induced rats when compared with the normal group. Supplementation of DME showed significant (p < 0.05) reduction in NOS activity as compared to the carrageenan-induced rats (Fig. 5).
Fig. 5.
Activity of NOS. Values expressed as average of 6 rats ± standard error of the mean in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: rats were given 0.1 mL 1% carrageenan + Voveran. a = statistical difference compared with Group I at p < 0.05; b =statistical difference when Group II compared with Groups III and IV at p < 0.05. Units = 1 nmol of NO produced/min at 37°C.
3.3. Effect of DME on oxidative stress
MDA is one of the most frequently used indicators of lipid peroxidation, which is a causative of oxidative stress. The concentration of MDA in paw tissue was significantly (p < 0.05) increased in the carrageenan-induced rats when compared with the normal group. Treatment with DME showed a significant (p < 0.05) decrease in MDA level in paw tissue when compared to the carrageenan-induced rats. The effect was similar in rats treated with both drugs. The concentration of ceruloplasmin in serum was significantly (p < 0.05) increased in the carrageenan-induced rats when compared with normal group. Supplementation of DME showed a significant (p < 0.05) decrease in ceruloplasmin as compared to the carrageenan-induced rats. Glutathione content and vitamin C in serum also decreased significantly in the carrageenan-treated rats when compared with the control rats. Treatment with DME showed a significantly increased concentration of glutathione and vitamin C content as compared to the carrageenan-treated rats. SOD, catalase, GPx, and GRd activities in paw tissue were significantly reduced in the carrageenan-induced rats when compared with the control rats. Administration of DME showed a significant increase in the activities of all these antioxidant enzymes over the carrageenan-induced rats (Table 1).
Table 1.
Effects of methanolic extract of Dolichos biflorus seeds on malondialdehyde and antioxidants.
| Groups | I | II | III | IV |
|---|---|---|---|---|
| Malondialdehyde (mM/g tissue) | 1.60 ± 0.09 | 4.50 ± 0.28a | 2.80 ± 0.14a,b | 2.30 ± 0.10a,b |
| Glutathione (mM/g tissue) | 18 ± 1.52 | 6 ± 0.91a | 12 ± 0.10a,b | 9 ± 0.13a,b |
| Catalase (Units*/mg protein) | 4.80 ± 0.37 | 2.10 ± 0.13a | 2.90 ± 0.16a,b | 2.60 ± 0.11a,b |
| Superoxide dismutase (Units$/mg protein) | 3.80 ± 0.03 | 1.30 ± 0.09a | 2.50 ± 0.14a,b | 2.20 ± 0.10a,b |
| Glutathione peroxidase (Units#/mg protein) | 10.50 ± 0.93 | 6.60 ± 0.79a | 8.02 ± 0.68a,b | 7.50 ± 0.63a,b |
| Glutathione reductase (Units#/mg protein) | 480.08 ± 7.7 | 100.14 ± 2.8a | 360.25 ± 9.1a,b | 320.93 ± 5.6a,b |
| Vitamin C (mg/100 g tissue) | 100.33 ± 1.22 | 38.56 ± 2.53a | 50.12 ± 0.962a,b | 46.72 ± 0.31a,b |
| Ceruloplasmin (mg/dL) | 0.08 ± 0.001 | 0.66 ± 0.005a | 0.21 ± 0.002a,b | 0.30 ± 0.001a,b |
Values expressed as average of six rats ± standard deviation in each group. Group I: normal; Group II: rats were given 0.1 mL 1% carrageenan; Group III: rats were given 0.1 mL 1% carrageenan + methanolic extract of Dolichos biflorus seeds; and Group IV: Rats were given 0.1 mL 1% carrageenan + Voveran.
= statistical difference compared with Group I at p < 0.05;
= statistical difference when Group II compared with Groups III and IV at p < 0.05.
Velocity constant/second;
enzyme concentration to inhibit chromogen production (OD 560 nm) by 50% in 1 minute;
1 μmol NADPH oxidized/min/mg protein.
3.4. Phytochemical analysis
The extract was analyzed for its phytochemical constituents, such as alkaloids, flavonoids, carbohydrates, steroids, tannins, cardiac glycosides, and proteins using standard procedures of analysis. Preliminary phytochemical analysis of DME revealed the presence of alkaloids, flavonoids, tannins, proteins, carbohydrates, and the absence of cardiac glycosides and steroids (Table 2).
Table 2.
Phytochemical analysis of the extract of Dolichos biflorus. Preliminary analysis of methanolic extract of Dolichos biflorus seeds showed the presence of alkaloids, flavonoids, tannins, proteins, and carbohydrates, and the absence of steroids and glycosides.
| Test | Observation |
|---|---|
| Alkaloids | |
| Mayer’s test | ++ |
| Wagner’s test | ++ |
| Modified Dragendorff’s test | ++ |
| Flavonoids | |
| Ferric chloride test | + |
| Sodium hydroxide solution test | + |
| Mineral acid | + |
| Steroids | |
| Liebermann–Burchard test | − |
| Carbohydrates | |
| Fehling’s test | + |
| Benedict’s test | + |
| Molisch’s test | + |
| Selivanoff’s test | ++ |
| Protein | |
| Biuret test | + |
| Cardiac glycosides | |
| Keller–Kiliani test | − |
| Tannins | |
| Ferric chloride test | + |
4. Discussion
Inflammation is the natural protective response of the body to the tissue injury caused by different agents, and forms the basis of many physiological and pathological processes. An imbalance between the two signals that initiate and shut the process of inflammation, leaves inflammation unchecked, resulting in cellular and tissue damage. Diet and nutrition have an important role in preventing common non-communicable diseases, including inflammatory disorders. The present study aims to evaluate the anti-inflammatory and free radical scavenging activity of DME in a carrageenan-induced rat model.
Carrageenan-induced inflammation is a useful model to detect the action of anti-inflammatory agents [26]. The carrageenan-induced paw edema model in rat is known to be sensitive to COX inhibitors and has been used to evaluate the effect of NSAIDs, which primarily inhibit COX involved in PG synthesis [27]. The administration of DME showed 73% inhibition of paw swelling compared to the standard drug Voveran, which showed 70%. Thus the present results indicate the efficacy of D. biflorus as an efficient therapeutic agent in acute anti-inflammatory conditions.
Acute phase proteins are synthesized in the liver in response to inflammation. They regulate the level of inflammatory mediators in response to demand and opsonize foreign particles. The plasma protein ceruloplasmin is a powerful free radical scavenger that oxidizes iron from the ferrous to ferric state. Ceruloplasmin level increases under conditions leading to the generation of oxygen products such as the superoxide radicals and hydroperoxide. In the DME treated rats the level of ceruloplasmin was reduced compared to carrageenan-induced rats. The decreased level of ceruloplasmin by DME showed a protective response to a decrease in circulating unbound Fe2+, which may act as an inhibitor of further free radical-induced lipid peroxidation.
Mononuclear cells are the main producer of the lipid mediators, thus indicating the cellular interdependence during the inflammatory process. COX and LOX are two important enzymes that catalyze the formation of mediators involved in the inflammatory process. Inhibitors of COX are the mainstays of current therapy aimed to modulate pain and inflammation, and to control fever. Currently available NSAIDs act by inhibiting the activity of COX enzymes that convert the arachidonic acid to PGH2 [28]. PG plays an important role in promoting the signs and symptoms of inflammation [29]. In the current study, the carrageenan-induced rats showed an increased activity of COX, whereas the DME administration significantly decreased the COX activity. Based on these reports it is inferred that the inhibitory effect of DME (50 mg/kg body weight) on carrageenan-induced inflammation in rats in the present study may be due to inhibition of the enzyme COX leading to inhibition of PG synthesis. Treatment with DME is shown to inhibit COX as comparable to Voveran.
5-LOX is a key enzyme involved in the synthesis of leukotrienes from arachidonic acid, an important mediator of inflammation [30]. On cell stimulation, elevated Ca2+ levels and activated signal transduction cascades activate 5-LOX and lead to the release of arachidonic acid from phospholipids by phospholipase A2. In the present study, the administration of DME showed a decrease in the activity of 5-LOX as compared to the carrageenan-induced rats. 15-Hydroxyeicosatetraenoic acid is a predominant amino acid metabolite produced by the enzyme 15-LOX and is believed to play a significant role in inflammation. The results showed that there was a significantly (p < 0.05) decreased activity of 15-LOX in DME-treated rats when compared with the carrageenan-induced rats. Thus the decreased 5-LOX and 15-LOX activity by DME suggests that inhibition of leukotriene synthesis may be another mechanism through which DME mediates the anti-inflammatory action [31]. The overall anti-inflammatory activity of the DME was pronounced when compared to the effect of the standard drug Voveran.
MPO is an enzyme stored in azurophilic granules of polymorphonuclear neutrophils and macrophages, released into extracellular fluid in the setting of inflammatory process. MPO produces hypochlorous acid from hydrogen peroxide (H2O2) and chloride anion during the neutrophil’s respiratory burst. In the present study the carrageenan-induced rats showed an increased activity of MPO in the paw tissue [32] whereas treatment with DME significantly decreased the elevated MPO activity in the monocytes, an indicator of neutrophil in inflamed paws, suggesting that inhibition of neutrophil infiltration may be another mechanism by which the extract achieves its anti-inflammatory effect compared carrageenan and voveran treated group.
Nitric oxide is an important chemical mediator generated by endothelial cells, macrophages, and neurons that is involved in the regulation of various physiological processes. Excess concentration of NO is implicated in the cytotoxic effects observed in various disorders such as AIDS, cancer, Alzheimer’s, and arthritis [33]. In the current study, the level of NOS in the monocytes was significantly increased in the carrageenan-induced groups, whereas it was decreased in the DME-treated group.
ROS are associated with the inflammatory response and frequently contribute to the tissue damaging effects of inflammatory reactions [34,35]. Reactive free radicals may damage cells by initiation of lipid peroxidation that causes profound alteration in the structural integrity and functions of cell membranes. MDA is the major reactive aldehyde, resulting from the peroxidation of biological membranes [36], and is an end product of the oxidation and decomposition of polyunsaturated fatty acids containing three or more double bonds. The increased concentration of lipid peroxidation product, measured as MDA is most widely used to assess the extent of inflammation [37]. The increased levels of MDA in the carrageenan-induced rats indicate increased lipid peroxidation during inflammation that contributes to the increased swelling of the paw. On treatment with DME, a significant decrease in the MDA levels were observed, which indicate the efficiency of DME in inhibiting lipid peroxidation.
The antioxidants that can scavenge ROS are expected to improve these conditions. The body has an effective mechanism to prevent and neutralize the free radical—induced damage. This is accomplished by a set of endogenous antioxidant enzymes, such as catalase, SOD, GPx, and GRd [38]. Catalase is a hemeprotein, localized in the peroxisomes or the microperoxisomes. The DME treatment significantly enhanced the catalase activity in the study by the decomposition of H2O2 to water and oxygen and thus protecting the cell from oxidative damage by H2O2 and OH–. The carrageenan-induced rats showed a decrease in the activity of catalase. SOD is a metalloprotein and is the first enzyme involved in the antioxidant defense by lowering the steady-state level of O2−. DME administration significantly decreased the activity of SOD compared to carrageenan-induced rats.
GPx is a selenoenzyme, two-thirds of which (in liver) is present in the cytosol and one-third in the mitochondria. It catalyzes the reaction of hydroperoxides with reduced glutathione to form glutathione disulphide (GSSG) and the reduction product of the hydroperoxide [39]. GSH is a major nonprotein thiol in living organisms that plays a central role in coordinating the body’s antioxidant defense processes. GRd reduces GSSG to the sulfhydryl form GSH, which is an important cellular antioxidant. For every mole of oxidized GSSG, one mole of NADPH is required to reduce GSSG to GSH. The free radicals formed as a result of respiratory burst in inflammation contribute to the swelling of paw, and are effectively scavenged by the combined action of GPx, GRd, and GSH. In the present study, it was found that DME treatment significantly increased the activity of GPx and GRd and the levels of GSH when compared with the carrageenan-induced rats. The effect of DME was more pronounced than the standard drug Voveran.
Vitamin C is another antioxidant that alleviates the effect of oxidative stress. The activities of these antioxidants are increased by proinflammatory cytokines. Vitamin C is a strong antioxidant and its level was increased upon DME treatment compared to the carrageenan-induced rat. From these results it is clear that the increased levels of vitamin C spares the oxidation of vitamin E, which is again a strong membrane antioxidant. This sparing effect of vitamin C quenches the increased generation of free radicals in DME administered rats, which confers protection during inflammation. Thus the results indicate that the administration of DME to carrageenan-induced rats counteracts the increased levels of lipid peroxide products. This decreased lipid peroxidation is associated with increased activities of antioxidant enzymes—catalase, SOD, GPx, and GRd—and antioxidants such as GSH and vitamin C. Thus the study indicates the protective effect of D. biflorus in carrageenan-induced lipid peroxidation, which may be due to its antioxidant effect.
Preliminary phytochemical analysis of DME revealed the presence of alkaloids, flavonoids, tannins, proteins, and carbohydrates, and the absence of cardiac glycosides and steroids. Thus the phytochemical screening also supports the anti-inflammatory and antioxidant effects of the methanolic extract. The qualitative analysis of DME (Table 2) showed higher content of alkaloids. The high content of alkaloids in DME may contribute to the antioxidant and anti-inflammatory activity of the extract. Further studies are in progress to isolate the active component in the extract and to evaluate its molecular mechanism of anti-inflammatory activity and the pathways involved in inflammation.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–73. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 3. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hajime Sano. Treatment of rheumatic diseases: current status and future prospective. Topics: 1. Steroid/nonsteroidal anti-inflammatory drugs; 2. Nonsteroidal anti-inflammatory drugs (COX-2 selective inhibitors) Nihon Naika Gakkai Zasshi. 2011;100:2888–901. doi: 10.2169/naika.100.2888. [In Japanese] [DOI] [PubMed] [Google Scholar]
- 5. Los M, Mozoluk M, Ferrari D, et al. Activation and caspase mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–88. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goud BK, Devi O, Bhavna N, et al. Nutritional antioxidants and lipid profile in newly diagnosed rheumatoid arthritis patients. Internat Med J Malaysia. 2012;11:1. [Google Scholar]
- 7. Sivaraj A, Vinothkumar P, Sathiyaraj K, et al. Hepatoprotective potential of Andrographis paniculata aqueous leaf extract on ethanol induced liver toxicity in albino rats. J Appl Pharm Sci. 2011;01(06):204–8. [Google Scholar]
- 8. Garimella TS, Jolly CI, Narayanan S. In vitro studies on antilithiatic activity of seeds of Dolichos biflorus Linn. and rhizomes of Bergenia ligulata Wall. Phytother Res. 2001;15:351–5. doi: 10.1002/ptr.833. [DOI] [PubMed] [Google Scholar]
- 9. Laskar S, Bhattarcharyya UK, Sinhababu A, et al. Antihepatotoxic activity of kulthi (Dolichos biflorus) seed in rats. Fitoterapia. 1998;69:401–2. [Google Scholar]
- 10. Muthu AK, Sethupathy S, Manavalan R, et al. Hypolipidemic effect of methanolic extract of Dolichos biflorus Linn. In high fat diet fed rats. Indian J Exp Biol. 2005;43:522–5. [PubMed] [Google Scholar]
- 11. Pant MC, Uddin I, Bhardwaj UR, et al. Blood sugar and total cholesterol lowering effect of Glucine soja (Sieb. and Zucc.), Mucuna pruriens (D.C.) and Dolichos biflorus (Linn.) seed diets in normal fasting albino rats. Indian J Med Res. 1968;56:1808–12. [PubMed] [Google Scholar]
- 12. Pattanaik N, Singh AV, Pandey RS, et al. Toxicology and free radicals scavenging property of Tamra bhasma. Indian J Clin Biochem. 2003;18:181–9. doi: 10.1007/BF02867385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rao M, Rao PNP, Kamath R, et al. Reduction of cisplastin induced nephrotoxicity by cistone, a polyherbal Ayurvedic reparation, in C57BL/6J mice bearing B16F1 melanoma without reducing its antitumor activity. J Ethnopharmacol. 1999;68:77–81. doi: 10.1016/s0378-8741(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 14. Winter CA, Risley EA, Nusa GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 15. Radhika A, Jacob SS, Sudhakaran PR. Influence of oxidatively modified LDL on monocyte macrophage differentiation. Mol Cell Biochem. 2007;305:133–43. doi: 10.1007/s11010-007-9536-0. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu T, Kondo K, Hayaishi O. Role of prostaglandin endoperoxidase in serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981;26:271–6. doi: 10.1016/0003-9861(81)90091-6. [DOI] [PubMed] [Google Scholar]
- 17. Axelrod B, Cheesebrough TM, Laakso S. Lipoxygenase from soybeans. Methods Enzymol. 1981;71:441–53. [Google Scholar]
- 18. Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Clin Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 19.Salter M, Knowles RG. Assay of NOS activity by the measurements of conversation of oxyhemoglobin to methemoglobin by NO. In: Titheradge MA, editor. Methods in molecular biology nitric oxide protocol. Totowa: Humana Press; 1997. pp. 61–5. [DOI] [PubMed] [Google Scholar]
- 20. Sindhu G, Ratheesh M, Shyni GL, et al. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 21. David M, Richard JS. Glutathione reductase. Methods Enz Anal. 1983;3:258–65. [Google Scholar]
- 22. Ravin HA. Improved colorimetric enzymatic ceruloplasmin assay. J Lab Clin Med. 1961;85:161–8. [PubMed] [Google Scholar]
- 23. Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–73. [PubMed] [Google Scholar]
- 24.Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3rd ed. London: Chapman and Hall; 1998. p. 279. [Google Scholar]
- 25.Trease GE, Evans WC. A text book of pharmacognosy. London: Academic Press; 1989. pp. 22–40. [Google Scholar]
- 26. Di Rosa M, Giround JP, Willoughy DA. Studies of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 27. Crunkhorn, Meacock SER. Mediators of the inflammation induced in the rat paw by carrageenan. BR J Pharmacol. 1971;42:392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for the aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 29.Vane JR, Botting RM. Lipid mediators. Vol. 220. New York: Academic Press; 1994. Biological properties of cyclooxygenase products; pp. 61–97. [Google Scholar]
- 30. Chen M, Lam BK, Kanaoka Y, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–42. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathis S, Jala VR, Haribabu B. Role of leukotriene B4 receptors in rheumatoid arthritis. Autoimmun Rev. 2007;7:12–7. doi: 10.1016/j.autrev.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haegens A, Vernooy JH, Heeringa P, et al. Myeloperoxidase modulates lung epithelial responses to proinflammatory agents. Eur Respir J. 2008;31:252–60. doi: 10.1183/09031936.00029307. [DOI] [PubMed] [Google Scholar]
- 33. Tinker AC, Wallace AV. Selective inhibitors of inducible NOS potential agents for the treatment of inflammatory diseases? Curr Top Med Chem. 2006;6:77–92. doi: 10.2174/156802606775270297. [DOI] [PubMed] [Google Scholar]
- 34. Pawliczak R. The role of radical oxygen species in airway inflammation. Pol Merkuriusz Lekarski. 2003;14:493–6. [In Polish] [PubMed] [Google Scholar]
- 35. Cuzzocrea S, McDonald MC, Filipe HM, et al. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur J Pharmacol. 2000;390:209–22. doi: 10.1016/s0014-2999(99)00910-3. [DOI] [PubMed] [Google Scholar]
- 36. Pincemail J, Defraigne JO, Limet R. Oxidative stress in clinical situations - fact or fiction? Eur J Anaesthesiol. 1996;13:219–34. doi: 10.1046/j.1365-2346.1996.00979.x. [DOI] [PubMed] [Google Scholar]
- 37. Ahmad F, Khan RA, Rasheed S. The study of analgesic and anti-inflammatory activity of Lactuca scariola and Artemisia absinthium. J Islamic Acad Sci. 1992;5:111–4. [Google Scholar]
- 38. Uday Bandyopadhyay, Dipak Das, Ranajit Banerjee K. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci. 1999;77:658–65. [Google Scholar]
- 39. Fridovich I. Superoxide dismutase. Ann Res Biochem. 1975;44:147–59. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]