Abstract
The prevalence of diabetes mellitus (DM) has dramatically increased in the past decade. Furthermore, increasing evidence from research shows that oxidative stress (OS) plays a crucial role in the pathogenesis of diabetes and in its complications. A search for ways to reduce oxidative damage has become the focus of interest for the majority of scientists. In this study, we determined the radical scavenging activity of single green tea constituents by using an on-line high performance liquid chromatography (HPLC)–2,2-diphenyl-1-picrylhydrazyl (DPPH) method and evaluated the antioxidant effects on type 2 diabetic patients by performing a double-blind, placebo-controlled study. Epigallocatechin gallate was identified as the most potent antioxidant, contributing approximately 50% of the total antioxidant capacity of green tea extract. We also found a statistically significant decrement of lipid peroxidation markers in patients treated with green tea extract after 9 months or after 18 months of follow-up. Overall, these findings are attractive for diabetic patients, helping them to keep a high level of performance and well-being, which ultimately may delay the time of disability and reduce mortality.
Keywords: Antioxidant, Diabetes mellitus, Green tea extract, HPLC, Oxidative stress
1. Introduction
Diabetes mellitus (DM) is undoubtedly one of the most challenging health problems in the 21st century. The world prevalence of DM in 2012 was 8.3%, with an estimated number of 371 million carriers, and by 2030, this number may reach 552 million carriers. Half of individuals with diabetes are unaware they have the disease [1]. Type 2 DM is the most prevalent form of this disease, comprising 90% of people with diabetes around the world, and is largely the result of excess body weight and physical inactivity. The relative risk of death due to vascular complications is at least double that in patients with DM than in their peers without DM [2]. People with diabetes are at high risk of major health problems earlier in life than those without diabetes. Such a lifelong chronic disease sooner or later adversely affects the emotional status and quality of life [3,4]. There is no doubt that oxidative stress (OS), in addition to the abnormally high glucose concentration, plays a crucial role in the pathogenesis of DM and its complications. There are many studies demonstrating an inseparable link between OS and DM [5–9]. The mechanisms by which hyperglycemia can generate reactive oxygen and nitrogen species (RONS) were thoroughly explored by Jay et al [7] and later by Bandeira et al [5]. Increase in mitochondrial RONS generation contributes to the establishment and maintenance of OS, leading to insulin resistance and alterations of the vasculature, kidneys, neurons, retina, and pancreatic beta cells [6]. This increased RONS generation causes the activation of the main pathways, described by Giacco and Brownlee [9], which is involved in the pathogenesis of DM complications. Patients with diabetes not only have increased levels of RONS (circulating markers of free radical-induced damage), but also reduced antioxidant defenses. These may be due to a decrease in endogenous antioxidants, as well as a depletion of dietary antioxidants and the other essential components, iron overload and/or increased production of RONS, the presence of toxins which produce such species, or the excessive activation of natural producing systems of RONS [5,7]. Therefore, all of these parts are very important for scientists searching for ways to slow down the development of diabetic complications.
In response to the increasingly growing popularity and a greater demand for medicinal plants, we decided to evaluate the antioxidant properties of one of the most ancient and popular, a plant green tea (Camellia sinensis L.) extract (Extract Camellia sinensis (ECs)) for therapeutic use. Green tea is very rich in polyphenolic constituents (most of which are flavonoids) which have high antiinflammatory, antioxidant, and antimutagenic properties in various biological systems [10]. Polyphenolic compounds are well known to be responsible for the antioxidant properties in many plants [11,12]. A major class of green tea flavonoids is the catechins (the main one being epigallocatechin-3-gallate), which play a key role as antioxidants in prevention and treatment of many diseases [10,13,14]. Green tea also contains flavonols (e.g., quercetin, kaempferol, myricetin), phenolic acids (e.g., gallic and chlorogenic acids), proanthocyanidins (prodelphinidin), xanthic bases (caffeine, theophylline), polysaccharides, essential amino acids (e.g., glycine, serine, valine, leucine, threonine, and characteristic amino acid theanine), also vitamins (B, C, E), and minerals and trace elements (calcium, magnesium, manganese, copper, zinc, selenium, potassium) [13,15]. Although the mechanism is not completely clear, there is no doubt that ECs have beneficial effect on OS. Green tea polyphenols can be direct antioxidants by scavenging reactive oxygen species or chelating transition metals [10,16]. Alternatively, they may act indirectly by upregulating phase II antioxidant enzymes [17].
The aim of this study was to identify the most active as antioxidant ECs components and to evaluate the radical scavenging activity of separate ECs active constituents using an on-line high performance liquid chromatography (HPLC)–2,2-diphenyl-1-picrylhydrazyl radicals (DDPH) method. The antioxidant effects of ECs on type 2 diabetic patients were also evaluated, by measuring the total antioxidant status (TAS), activity of antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)] and the amount of biomarkers for OS (lipid hydroperoxides (LOOH), malondialdehyde (MDA), and 4-hydroxy-2-nonenal (HNE)].
Until now, there were no such clinical studies to evaluate natural antioxidants, such as green tea extract, and effects on the development of DM complications by reducing OS in Europe. Moreover, the postcolumn HPLC–DPPH method for determination and evaluation of the antioxidant activity of the separated active constituents of ECs was used for the first time.
2. Materials and methods
2.1. HPLC method and chemicals
Chemicals of analytical purity were used. Trolox [(R)-6-methoxy-2,5,7,8-tetramethylchroman-2-carboxylic acid] was received from Acros Organics (New York, USA), ethanol (rectified spirit 96.3%) was received from Stumbras (Kaunas, Lithuania), and sodium citrate and citric acid were received from Fluka Chemie (Buchs, Switzerland). Methanol and acetonitrile of HPLC gradient grade, as well as the commercial standards of catechins, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, ethyl acetate, glacial acetic acid and the rest of the chemicals were received from Sigma-Aldrich Chemie (Steinheim, Germany). Ultrapure water from the Milli-Q system (Millipore, Bedford, MA, USA) with conductivity of 18.2 MQ was used in all experiments.
Chromatographic separation was performed by the method of Saito et al [18] and was slightly modified as follows: compounds were separated on an ACE C-18 column (250 × 4.6 mm, 2.65 μm) with a precolumn (Advanced Chromatography Technologies, Aberdeen, Scotland) at 25°C; water/acetonitrile/methanol/ethyl acetate/glacial acetic acid (89:6:1:3:1 v/v/v/v/v) was used as eluent A and acetonitrile as eluent B. The mobile phase was delivered at 1.0 mL/minute in a linear gradient mode: 0–10 minutes, 100% A and 0% B; 30 minutes, 85% A and 15% B; 40–45 minutes, 20% A and 80% B; 46 minutes, 100% A and 0% B.
The postcolumn HPLC-DPPH method described by Raudonis et al [19] was used to evaluate the contribution of individual constituents to antioxidant activity in ECs. A reference antioxidant Trolox (chemical analog of vitamin E for polar-solvent-soluble systems) was used for standardization of antioxidant activity.
The DPPH radical scavenging activities of ECs active constituents were calculated from the standard curve of Trolox at concentrations of 0.02 mg/mL, 0.04 mg/mL, 0.06 mg/mL, 0.08 mg/mL, 0.1 mg/mL, and 0.12 mg/mL. The Trolox equivalent antioxidant capacity (TEAC) was calculated for individual constituents of ECs and expressed as micromoles of Trolox equivalent (TE) per 100 g of dry extract (μmol TE/100 g).
2.2. Study design and participants
The randomized, double-blind, placebo-controlled study was conducted in Endocrinology Clinic, Hospital of Lithuanian University of Health Sciences Kaunas Clinics, Lithuania. The experimental protocol used in this study was approved by the Lithuanian Bioethics Committee, State Data Protection Inspectorate (Number BE-2-5, 15 April 2009). Written, informed, and voluntary consent was obtained from all participants. All participants were outpatients of the Endocrinology Clinic, Hospital of Lithuanian University of Health Sciences Kaunas Clinics, Kaunas, Lithuania. Individuals diagnosed with type 2 DM (treated with insulin, metformin or a combination of both) aged from 35 years to 80 years and followed up for diabetic retinopathy, nephropathy, or neuropathy, were enrolled into the study. They were not deprived of taking their regular prescribed medications, but were advised to abstain from other dietary supplements rich in antioxidants.
The status of type 2 diabetes and its complications, OS parameters, and biochemical measurements were evaluated at the baseline. The baseline measurements were repeated after 9 months and 18 months of receiving preparations. During the first 9 months, patients received capsules twice a day, and during the second 9 months, they received capsules three times a day.
2.3. Study preparations
All patients were randomly allocated to receive either green tea extract or placebo capsules. Placebo capsules were made from microcrystalline cellulose, a material indifferent to disease (joint-stock company, Sanitas, Kaunas, Lithuania). An ECs capsule contains 200 mg standardized extract of C. sinensis L. leaves, adjusted to 70% polyphenols (Sanitas). Dry Green tea extract for the production of capsules was received from Taiyo GmbH (Filderstadt, Germany). The origin of raw material was China.
2.4. Biochemical measurements
All measurements of plasma OS parameters were performed in the Laboratory of Biochemistry, Riga Stradins University, Riga, Latvia. Determination of antioxidative enzymes SOD [20], CAT [21], selenium dependent GPx [22], and TAS [23] were performed on an automatic chemical analyzer “Dayton RX” based on spectrophotometer methods according to the instructions provided by Randox Laboratories and using its own kits (Randox Laboratories Limited, Crumlin, UK). The degree of lipid peroxidation was evaluated by quantitative determination of LOOH [24] using a commercial diagnostic kit (Northwest LSS Inc., Washington, USA) and concentration of MDA + HNE [25] using an LPO Microplate Based Assay Kit, Cat. Number FR22 (Oxford Biomedical Research, Michigan, USA) on a microplate absorbance reader (Tecan, Grodig, Austria). The manuals of standard methods with reagents from Sigma-Aldrich (Missouri, USA) were used to determine the concentration of selenium [26] and vitamin E [27]. The control serums Seronorm TE Serum level I (Cat. Number 201 405) and vitamin A/E level II (Cat. Number 007.002.018) used for quality control of selenium and vitamin E were received from Sero AS (Billingstad, Norway) and Eurotrol (Ede, Netherlands), respectively.
2.5. Statistical analysis
Data were analyzed using SSPS version 20.0 (SPSS Inc., Chicago, IL, USA) for Windows and Microsoft Excel 2010 (Microsoft, Redmond, WA, USA). Data are expressed as mean ± standard deviation (S.D.). Nonparametric tests were used because of the small number of variables. The comparisons between the two groups (placebo and ECs) were made by the Mann-Whitney U test at each time point. The comparisons between three different measurements were made using Friedman and Wilcoxon tests. The results were considered statistically significant at p < 0.05.
3. Results and discussion
3.1. Antioxidant activity of individual constituents of ECs
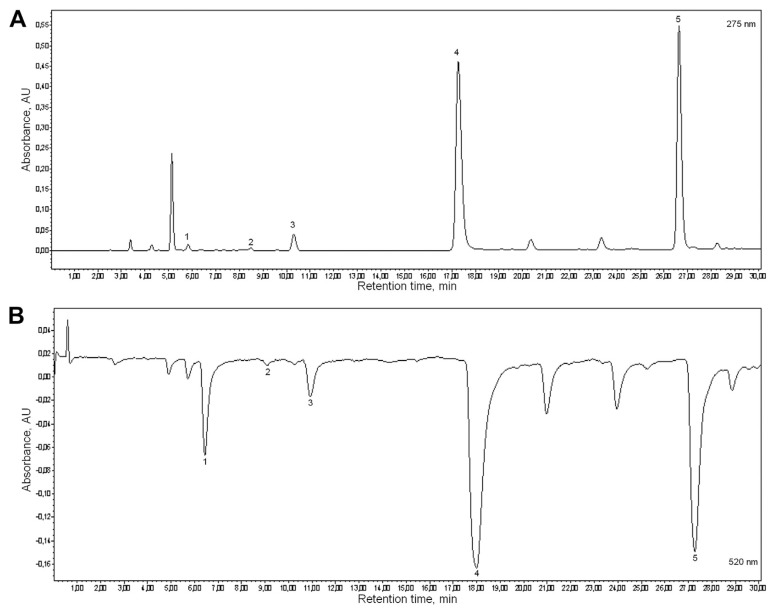
The antioxidant activity of ECs mainly depends on catechins and their ability to scavenge free radicals [10,16]. In this research, we evaluated the antioxidant activity of the main green tea catechins, which are in the highest amount in the extract, (catechin, epicatechin, epigallocatechin, epicatechin-3-gallate, and epigallocatechin-3-gallate) and their contribution to the total antioxidant activity of ECs. All experiments were performed in triplicate. An example of the chromatograms of ECs obtained by performing evaluation of active compounds of ECs by the on-line HPLC-DDPH method is presented in Fig. 1. The DDPH scavenging activity of all constituents was expressed as TEs. All catechins, except catechin (which is in smaller quantity in extract), possessed a good DPPH radical scavenging activity. Epigallocatechin-3-gallate presented the highest DPPH scavenging activity (23.41 ± 5.73 μmol TE/100 g), followed by epicatechin-3-gallate (16.36 ± 3.84 μmol TE/100 g), epigallocatechin (4.06 ± 0.24 μmol TE/100 g), epicatechin (1.57 ± 020 μmol TE/100 g), and finally catechin (0.15 ± 0.02 μmol TE/100 g). Approved domination of the free radical scavenging activity of the catechin fraction against the other phenolic green tea compounds in the medical product by such a method was established for the first time. Our results are consistent with the findings by Nanjo et al [28] in the past century, who reported that the orthotrihydroxyl group in the B ring (in epigallocatechin and epigallocatechin-3-gallate) and the galloyl moiety at the 3 position of flavan-3-ol skeleton (in epicatechin-3-gallate and epigallocatechin-3-gallate) are the most important in scavenging ability on the DPPH radical. All subsequent research confirmed that epigallocatechin-3-gallate (the most abundant of the catechins) exhibit the highest radical scavenging activity and antioxidant activity of ECs [16]. Meanwhile, the data of epigallocatechin and epicatechin-3-gallate antioxidant activity are contradictory. Our research data, according to the antioxidant activity, shows that catechins can be arranged as follows: epigallocatechin-3-gallate > epicatechin-3-gallate > epigallocatechin > epicatechin > catechin. The same succession was also described by Higdon and Frei [29].
Fig. 1.
(A) High performance liquid chromatography (HPLC); and (B) UV–2,2-diphenyl-1-picrylhydrazyl (DPPH) chromatograms of the Extract Camellia sinensis. Peaks: 1. epigallocatechin, 2. catechin, 3. epicatechin, 4. epigallocatechin-3-gallate, and 5. epicatechin-3-gallate.
3.2. Antioxidant effects of ECs on diabetic patients
ECs and its main constituents, catechins, undoubtedly have antioxidant properties that were also confirmed by our study. We wanted to evaluate whether natural antioxidants present in Camellia sinensis L. leaves extract are capable of protecting or reducing oxidative damage in diabetic patients with complications. Because the OS plays a crucial role in the pathogenesis of DM and its complications [5–9] and there are a large number of previous studies demonstrating beneficial effects of ECs on OS [10,13,17], we expected to receive significant evidence of a reduction in the amount of lipid peroxidation markers and/or an increase in activity of antioxidant enzymes.
Currently, the results are from 45 patients who finished the study (20 patients in the ECs group and 25 in the placebo group). The age and duration of DM were similar in both groups. The mean age was 62.18 ± 10.37 years and the mean duration of DM was 11.76 ± 11.4 years. All patients were over 60 years with a long duration of diabetes and more progressive diabetic complications. The mean level of glycated hemoglobin was 7.81 ± 1.64% and there were no significant changes of glycated hemoglobin during the study in both groups. In the diabetic state, hyperglycemia per se generates RONS production [5,30]. Additionally, factors such as obesity, smoking, sedentary lifestyles, hypertension, a long duration of DM, and age may contribute to the establishment and maintenance of OS, leading to the aggravation of type 2 diabetes. In our study, most of the patients were obese. The mean body mass index was 35.23 ± 4.98 and 34.98 ± 6.44, respectively, in ECs and placebo groups. Waist circumference was greater in the ECs and placebo groups compared to hip circumference, in 47.4% and 39.1% of patients, respectively. An increase in the waist–hip ratio also incident to DM and demonstrates the greater risk of lifestyle related diseases. Other factors (such as hypertension and smoking) that can influence RONS production were not of great importance. Only 8.9% of patients (3 patients in the ECs and 1 in the placebo group) reported that they smoked, whereas the rest of the respondents did not smoke or had quit smoking when DM was diagnosed. Hypertension, which occurred in 65% of patients and 80% of patients in the ECs and placebo groups, respectively, was well controlled by antihypertensive drugs.
All investigated OS parameters of both groups and at different time points of the study are shown in Table 1. The amounts of MDA, HNE, and LOOH were increased in the beginning of the study. In response to the increased activity of lipid peroxidation, we did not observe an effective compensatory increase in indicators of the antioxidant defense system. This confirms the hypothesis that RONS generation and lipid peroxidation processes are accelerated in diabetic patients. Moreover, although the amount of antioxidant enzymes were within established reference ranges, a decrease in the content of selenium, which is essential for the activity of GPx [31], was observed at the beginning of study.
Table 1.
Comparison of the investigated parameters between groups at baseline, after 9 months, and after 18 months.
| Group | At baseline (I) | After 9 mo (II) | After 18 mo (III) | p | |
|---|---|---|---|---|---|
| SOD (U/g Hb) | ECs | 1360.75 ± 140.9 | 1450.05 ± 170.6 | 1410.75 ± 131,6 | I–II p = 0.022 |
| Placebo | 1406.20 ± 139.5 | 1487.24 ± 128.8 | 1464.04 ± 143.0 | I–II p = 0.010, I–III p = 0.042 | |
| p | NS | NS | NS | ||
| CAT (k/g Hb) | ECs | 229.05 ± 74.1 | 219.11 ± 61.5 | 237.82 ± 65.3 | |
| Placebo | 211.75 ± 60.1 | 242.48 ± 68.5 | 206.04 ± 79.4 | II–III p = 0.017 | |
| p | NS | NS | NS | ||
| GPx (U/L) | ECs | 6971.05 ± 2006.5 | 7586.80 ± 2865.9 | 6561.19 ± 1560.0 | |
| Placebo | 6398.28 ± 1964.3 | 7311.70 ± 2024.7 | 6787.78 ± 1670.7 | I–II p = 0.045 | |
| p | NS | NS | NS | ||
| Vitamin E (μg/mL) | ECs | 13.41 ± 4.3 | 13.58 ± 4.7 | 13.96 ± 5.2 | |
| Placebo | 15.81 ± 4.4 | 13.18 ± 3.9 | 12.03 ± 3.3 | I–II p = 0.004, II–III p = 0.037, I–III p < 0.001 | |
| p | NS | NS | NS | ||
| TAS (mmol/L) | ECs | 1.64 ± 0.16 | 1.67 ± 0.18 | 1.66 ± 0.12 | |
| Placebo | 1.54 ± 0.17 | 1.67 ± 0.19 | 1.60 ± 0.17 | ||
| p | NS | NS | NS | ||
| Se (μg/L) | ECs | 78.50 ± 16.76 | |||
| Placebo | 77.52 ± 15.79 | ||||
| p | NS | ||||
| MDA (μM) | ECs | 1.81 ± 0.66 | 1.51 ± 0.40 | 1.62 ± 0.80 | I–II p = 0.022, I–III p = 0.047 |
| Placebo | 1.84 ± 1.28 | 1.68 ± 0.51 | 1.89 ± 1.20 | ||
| p | NS | NS | NS | ||
| HNE (μM) | ECs | 4.36 ± 3.46 | 4.58 ± 3.22 | 4.54 ± 1.95 | |
| Placebo | 6.51 ± 5.00 | 5.69 ± 2.64 | 5.01 ± 3.14 | ||
| p | NS | NS | NS | ||
| MDA ± HNE (μM) | ECs | 5.99 ± 3.73 | 6.21 ± 3.10 | 6.16 ± 2.29 | |
| Placebo | 7.77 ± 5.24 | 7.37 ± 2.65 | 6.92 ± 3.62 | ||
| p | NS | NS | NS | ||
| LOOH (μM) | ECs | 7.80 ± 3.7 | 5.88 ± 2.9 | 7.30 ± 5.6 | |
| Placebo | 8.58 ± 5.4 | 6.95 ± 3.4 | 7.26 ± 4.2 | ||
| p | NS | NS | NS |
CAT = catalase; ECs = Extract Camellia sinensis; GPx = glutathione peroxidase; HNE = 4-hydroxy-2-nonenal; LOOH = lipid hydroperoxides; MDA = malondialdehyde; Se = Selenium; SOD = superoxide dismutase; TAS = total antioxidant status.
We found no statistically significant effects of green tea in patients with type 2 diabetes as compared with the placebo group either after 9 months or after 18 months of follow-up. However, there were some noticeable changes within the groups. The activity of SOD significantly increased in ECs group after 9 months, whereas the changes of other antioxidant enzymes were insignificant and numerically small. In the placebo group, a significant increment of SOD after 9 months and 18 months was observed, and a significant increment of GPx was observed after the first 9 months. Meanwhile, the activity of CAT significantly decreased after the second 9 months in the placebo group. These findings do not match with those previously described by Forester and Lambert [17]. These differences may be due to the total body damage by a long duration of diabetes and rapidly progressive diabetic complications, as well as longer lasting exhausting effects of OS. Also, we found a statistically significant decrement of content of vitamin E in the placebo group after both 9 months and 18 months. A trend of a slight increment of vitamin E, albeit insignificant and numerically small, was observed in the ECs group during the study. In agreement with other studies [10,32], these data showed a beneficial effect of ECs on oxidative damage by reducing the amount of lipid peroxidation markers. A decrement of LOOH and MDA was observed in the ECs group as compared with the placebo group; the changes in lipid peroxidation markers were not statistically significant. Because ECs have strong antioxidant properties, we expected to see more differences between the ECs group and the placebo group. Supposedly, it was influenced by the older age of patients, already existing significant diabetic complications, and the duration of the study, but further exploration needs to be done to evaluate the effects of green tea extracts, maybe in patients with early diabetic complications. However, our findings confirm the benefits of green tea extract when lipid peroxidation is increased and there is an insufficient natural antioxidant defense system.
ECs capsules used in this study were manufactured by Sanitas. Considering the manufacturer’s recommendations, two to three capsules of this dietary supplement should be taken daily, which is equivalent to 280–420 mg polyphenols. Most of the previous studies indicate antioxidant effects of green tea extract after consumption of 400–500 mg green tea polyphenols for 2 months, 3 months, or 6 months [17,33–35]. Although the manufacturer’s recommended dose is smaller than that most commonly used in trials, we decided to evaluate whether the lower dose used over a long time could cause antioxidant effects in diabetic patients. A 9 month study duration was chosen in order to evaluate the long term effects of ECs on the development of diabetic complications. According to the data, the concentration of ECs was not of great importance. However, there were some changes between the first 9 months and the second 9 months (when patients received capsules twice a day and 3 times a day, respectively), but these changes were insignificant both in the ECs group and the placebo group.
4. Conclusion
Epigallocatechin gallate was identified as the most potent antioxidant, with a TEAC value of 23.41 ± 5.73 μmol TE/100 g, contributing to approximately 50% of the total antioxidant capacity of green tea extract. The antioxidant properties of ECs catechins increase in the order: catechin < epicatechin < epigallocatechin < epicatechin-3-gallate < epigallocatechin-3-gallate.
The findings of this study confirm the beneficial influence of green tea extract on reducing the amount of MDA and LOOH, although significant changes between the ECs and placebo groups were not observed. The present findings are not beneficial in diabetic patients with more acute diabetic complications and further study in different circumstances needs to be performed. Nevertheless, the findings are attractive for diabetic patients, especially with a shorter duration of DM, helping them to keep a high level of performance and well-being, which ultimately may delay the time of disability and reduce mortality.
Acknowledgments
The authors would like to thank the Department of Analytical and Toxicological Chemistry of the Lithuanian University of Health Sciences for using HPLC. This research is a part of an international “Eureka” E! 3695 project “Creation of the methodology for effects of natural antioxidants on the development of diabetes mellitus complications” (“Antioksdiabet”). This project has been established with financial support from the European Social Fund Agency, Lithuania, in the “Human Resource Development Action Program”, project Number VP1-3.1-SMM-06-V-01-003. We would also like to thank the Laboratory of Biochemistry, Riga Stradins University, Latvia for performing biochemical tests of blood plasma and the residents of the Endocrinological Clinic, Hospital of Lithuanian University of Health Sciences Kaunas Clinics, Lithuania for their help. The provision of ECs and placebo capsules from the joint-stock company Sanitas is also appreciated.
Funding Statement
This project has been established with financial support from the European Social Fund Agency, Lithuania, in the “Human Resource Development Action Program”, project Number VP1-3.1-SMM-06-V-01-003.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes Atlas. International Diabetes Federation Web. 2013. [accessed 25, 05, 13]. Available at: http://www.idf.org/diabetesatlas.
- 2.World Health Organization. Fact sheet no 312. World Health Organization Web. 2013. [accessed 25, 05, 13]. Available at: http://www.who.int/diabetes/en.
- 3. Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes: a systematic review from the European Depression in Diabetes (EDID) Research Consortium. Curr Diabetes Rev. 2009;5:112–9. doi: 10.2174/157339909788166828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vadstrup ES, Frolich A, Perrild H, et al. Health-related quality of life and self-related health in patients with type 2 diabetes: Effects of group-based rehabilitation versus individual counseling. Health Qual Life Outcomes. 2011;9:110. doi: 10.1186/1477-7525-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bandeira SM, Fonseca LJ, Guedes SG, et al. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14:3265–84. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashan N, Kovsan J, Kachko I, et al. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. http://www.ncbi.nlm.nih.gov/pubmed/191267542009;89:27-71. http://dx.doi.org/10.1152/physrev.00014.2008. [DOI] [PubMed]
- 7. Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 8. Pitocco D, Zaccardi F, Stasio E, et al. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud. 2010;7:15–25. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanwar J, Taskeen M, Mohammad I, et al. Recent advances on tea polyphenols. Front Biosci (Elite Ed) 2012;4:111–31. doi: 10.2741/363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen YL, Yan LP, Chen CS. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J Food Drug Anal. 2013;21:219–26. [Google Scholar]
- 12. Chiu YW, Lo HJ, Huang HY, et al. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 13. Sinija VR, Mishra HN. Green tea: Health benefits, review. J Nutr Environ Med. 2008;17(4):232–42. [Google Scholar]
- 14. Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chin Med. 2010;5:13–21. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forester SC, Lambert JD. Antioxidant effects of green tea. Mol Nutr Food Res. 2011;55:844–54. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito ST, Welzel A, Suyenaga ES, et al. A method for fast determination of epigallocatechin gallate (EGCG), epicatechin (EC), catechin (C) and caffeine (CAF) in green tea using HPLC. Cienc Tecnol Aliment. 2006;26:394–400. [Google Scholar]
- 19. Raudonis R, Jakstas V, Burdulis D, et al. Investigation of contribution of individual constituents to antioxidant activity in herbal drugs using postcolumn HPLC method. Medicina. 2009;45:382–94. [PubMed] [Google Scholar]
- 20. Wooliams JA, Wiener G, Anderson PH, et al. Variation in the activities of glutathione-peroxidase and superoxide-dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–6. [PubMed] [Google Scholar]
- 21. Aebi H. Catalase in vitro. Meth Enzymol. 1984;165:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 22. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158. [PubMed] [Google Scholar]
- 23. Miller NJ, Rice-Evans C, Davies MJ, et al. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–12. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 24.Nelson DL, Cox MM. Lehninger principals of biochemistry. 3rd ed. New York: Worth Publishers; 2000. [Google Scholar]
- 25. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid-peroxidation products - malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 26. Alfthan G. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Analytical Chimica Acta. 1984;165:187–94. [Google Scholar]
- 27. Thompson JN, Erdody P, Maxwell WB. Simultaneous fluorometric determinations of vitamins A and E in human serum and plasma. Biochem Med. 1973;8:403–14. [Google Scholar]
- 28. Nanjo F, Goto K, Seto R, et al. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 29. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 30. Alfadda AA, Sallaml RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–97. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coimbra S, Castro E, Rocha-Pereira P, et al. The effect of green tea in oxidative stress. Clin Nutr. 2006;25:790–6. doi: 10.1016/j.clnu.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 33. Yang CS, Lambert JD, Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83:11. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basu A, Du M, Sanchez K, et al. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011;27:206–13. doi: 10.1016/j.nut.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Q, Yang H, Wang J, et al. Effect of green tea on reward learning in healthy individuals: a randomized, double-blind, placebo-controlled pilot study. Nutr J. 2013;12:84. doi: 10.1186/1475-2891-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]