Abstract
The volatile and odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) were analyzed by gas chromatography–mass spectrometry–olfactometry (GC– MS–O). The volatile compounds were extracted by the simultaneous distillation–extraction (SDE) method, then separated and identified by GC–MS. Odor-active compounds in the SDE extract were characterized by GC–MS–O. A total of 68 volatile compounds were found, including 23 aldehydes, 10 alcohols, nine ketones, 17 N- or S-containing compounds and aromatics, three acids, three alkanes, and three esters. Of these, 31 odor-active compounds were detected and identified. Trimethylamine (fishy), octanal (grassy, leafy, green), (E)-2-octenal (roast, fatty), 1-octen-3-ol (fishy, fatty, mushroom, grassy), 2-ethyl-1-hexanethiol (cooked fish), (E,E)-2,4-octadienal (cooked meat, sweet), 2-acetylthiazole (meaty, roast, nutty, sulfur), 2-acetylpyrrole (nutty, walnut, bread) were identified as the key odorants in the cooked meat of farmed obscure puffer based on posterior intensity and time-intensity methods.
Keywords: Gas chromatography-mass spectrometry, Gas chromatography-olfactometry, Intensity method, Obscure puffer (Takifugu obscurus), Volatile compounds
1. Introduction
Farmed puffer fish, especially Takifugu obscurus, are becoming increasingly popular among domestic and foreign consumers mainly due to the absence of tetrodotoxin in the meat, distinctive aroma, desirable taste, abundant collagen, and high nutritional value. Previous studies on puffer fish mainly focused on their aquaculture and biological property [1], toxicity [2], and nutrition evaluation [3,4]. Very few studies on the flavor of puffer fish were reported, although the flavor of many fish and seafoods has been researched. Milo and Grosch [5] investigated the odor defects in boiled cod (Gadus morhua) and boiled trout (Salmo fario); Varlet et al [6] compared the odor-active volatile compounds of fresh and smoked salmon; Senger-Emonnot et al [7] determined the odor-active aroma compounds in sea fig (Microcosmus sulcatus); and Selli et al [8] characterized the most odor-active compounds in cooked rainbow trout (Oncorhynchus mykiss) eliciting an off-odor.
The progress in the studies on flavor of fish and seafood should largely be attributed to the development of the research techniques, including isolation methods of flavor compounds and the separation, detection, and identification of odorants from volatile mixtures. Several methods used for isolating volatile compounds from food extracts were evaluated by Schultz et al [9]. Commonly, distillation and solvent extraction methods are considered to yield the near-complete flavor of food extracts. The simultaneous distillation–extraction (SDE) method has been widely used in flavor research and applied to the isolation of volatile compounds of some meats such as boiled beef and roasted beef [10].
Gas chromatography–olfactometry (GC–O) is a valuable technology which combines the separation capability of GC with the sensitivity of the human nose, which could provide an understanding of the important volatiles in a product and their relative contribution to odor and flavor attributes [11]. Since GC–O was proposed by Fuller et al [12] in 1964, several GC–O methods have been developed and widely applied to flavor research, including charm analysis, aroma extraction–dilution analysis, the detection frequency method, the posterior intensity method, and the time-intensity method. The characters of these methods and their uses in food flavor are reviewed.
The objective of this study was to characterize odor-active compounds in cooked meat of farmed obscure puffer (T. obscurus). The volatile compounds in the cooked meat were extracted by the SDE method using dichloromethane as extraction solvent. The odor-active compounds in the extract were analyzed by GC–mass spectrometry (MS)–O and quantitatively determined by GC–MS. Based on the posterior intensity and time-intensity methods of GC–O, the key compounds that are responsible for the odor of cooked meat were estimated.
2. Materials and methods
2.1. Sample preparation and reagents
The farmed obscure puffers (T. obscurus) were obtained from Zhongyang Co., Ltd (Jiangsu Province, China) in December 2011. After being caught, the fish were manually slaughtered by an experienced cook according to Nuisanceless Takifugu obscurus Safety Processing Operation Specification (DB32/ T543-2002) and Product of Geographical Indication: Nantong Yangtze puffer (farmed) (GB/T22655-2008), and then transported under ice in insulated polystyrene boxes to the research laboratory. The dorsal muscle was manually removed from nine fish (average mass: 350.0 ± 3.6 g), filleted, and then steamed at 100°C for 20 minutes to guarantee that the meat was fully cooked. After cooling to ambient temperature in an ice bath, the meat was ground. The ground meat was termed the cooked meat of farmed obscure puffer and used for sensory evaluation and extraction of volatile compounds.
C7–C30 n-alkanes were purchased from Sigma-Aldrich Chemical Co. (USA). 2,4,6-Trimethylpyridine (TMP; purity, 99%) was purchased from Sigma-Aldrich Trading Co., Ltd. (Shanghai, China). Dichloromethane, anhydrous sodium sulfate, and sodium chloride (analytical grade) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
2.2. Extraction of volatile compounds
An SDE method was used for extracting volatile compounds from the cooked meat and performed in a Likens–Nickerson apparatus (Model S250; Anhui Youxin Electrical Equipment Ltd, China) using dichloromethane as the extraction solvent.
Before extracting, 150 g of the cooked meat was homogenized with 300 mL of deionized water containing 40 g sodium chloride and 50 μg of TMP used as an internal standard (IS) in a homogenizer (PT1200E; Shanghai Equl Equipment Ltd, China). The homogenate was placed into a 1000-mL round-bottomed flask which was coupled to one arm of the SDE apparatus. A 100-mL round-bottomed flask containing 50 mL of redistilled dichloromethane as the extraction solvent was attached to the other arm of the SDE apparatus. Before the SDE run, the system was purged for 3 minutes with a stream of nitrogen to reduce the oxidation of volatile compounds in the process of extraction. The sample in the sample flask was boiled with an electrothermal magnetic force mixer (Model ZNCL-G; Qiangqiang Equipment Shanghai Co., Ltd, China) and stirred. After dichloromethane in the solvent flask was heated to boiling, the temperature was maintained at 55°C using a water bath (W2-100S; Shensheng Technology Shanghai Co., Ltd, China). The steam was cooled by the circulation of ethanol at −5°C. Each extraction step was carried out for 3 hours following the reflux of the two arms.
After extraction, the SDE extract was dried by slowly adding 3 g of anhydrous sodium sulfate with swirling which was maintained at −18°C overnight. The extract was filtered through a folded filter paper (Whatman No. 2) containing defatted cotton (1 × 1 cm2) with favorable hydrophilic properties. The filtrate (70 mL) was then collected and concentrated to 2 mL in an Older-show fractionation apparatus, and then to exactly 0.5 mL under a gentle stream of nitrogen.
To increase the concentration of volatile compounds in the extract, five extracts (each 0.5 mL) obtained by the same SDE procedures were pooled and then concentrated to 1 mL under a gentle steam of nitrogen. The final SDE extract was stored at −80°C for GC–MS–O and GC analysis.
2.3. GC–MS–O analysis
The GC–MS–O system consisted of a 6890 GC equipped with a 5973 mass-selective detector (Agilent Inc., USA), and a sniffing port ODP2 (Gerstel, Baltimore, MD, USA) supplied with humidified air at 40°C. Approximately 2 μL of each SDE extract was injected in splitless mode into a capillary column (HP-INNOWax column; 60 m length × 0.25 mm i.d. × 0.25-μm film thickness; Agilent Inc., USA). The GC effluent was split (at a 1:1 ratio) between the mass detector and the sniffing port. The temperature of the injector was set at 250°C. The oven temperature was programmed from 40°C for 5 minutes, increasing at 4°C/minute to 230°C with a final hold for 20 minutes. A solvent delay of 3 minutes was selected. The flow rate of carrier gas helium (purity, 99.999%) was 1.0 mL/ minute.
The MS conditions were as follows: detector interface temperature, 250°C; ion source temperature, 230°C; ionization energy, 70 eV; mass range, 20–300 a.m.u.; electron multiplier voltage, 1698 V; and scan rate, 1.81/second.
The GC–O analysis was performed by two assessors with 3 years’ experience, one male (aged 26) and one female (aged 25). An intensity method was used to measure the odor intensity of the compounds detected by smelling and recording the odor descriptions. The perceived odor intensity was evaluated and recorded using the degree of 1 (very weak odor intensity), 2 (moderate intensity), 3 (strong intensity), and 4 (very strong intensity). Each panelist repeated the sniffing test three times. Overall, six GC–O experiments were conducted. Only when the two assessors smelled the compound more than two times, the compound was considered effective, and only the descriptions belonging to the same odor attribute were retained.
2.4. Identification of volatile compounds
Identification of compounds was confirmed by comparing their retention indices (RIs) and mass spectra of the in-house database (Wiley6/NIST 2005). The RIs of the compounds were determined by running n-alkanes under the same chromatographic conditions and calculated according to the following equation [13]:
| (1) |
where Rt(i) is the retention time of each targeted compound (i), Rt(n) and Rt(n + 1) are retention times of n-alkanes eluting directly before and after the targeted compound (i) under the same chromatographic conditions. The n is the carbon number of n-alkane.
2.5. GC analysis and determination of odor activity value
The GC was used for determining the concentrations of odor-active compounds in SDE extract. The GC system consisted of a Thermo Fisher chromatograph equipped with a flame ionization detector (FID). For running GC, all chromatographic conditions including injection volume (2 μL) of the SDE extract, chromatographic column, etc., were the same as that for the GC–MS–O analysis except FID at 250°C. Quantitative data for an identified compound (i) were obtained by the IS method without considering the calibration factor, that is, F = 1.00 for all compounds. The total amount of substance groups was the result of the addition of approximate concentrations of all identified volatile compounds. Concentrations of positively identified compounds were determined using GC response factors and total ion areas for each compound relative to the IS are calculated as follows:
| (2) |
where the amount ratio is peak area of each identified compound (i) divided by peak area of the IS, that is, TMP [14].
3. Results and discussion
3.1. Volatile composition in the cooked meat of farmed obscure puffer
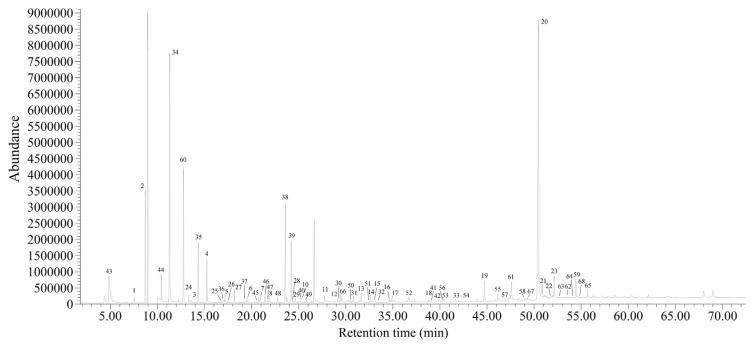
The total ion chromatogram of volatile compounds in the SDE extract from the cooked meat of farmed obscure puffer was obtained by GC–MS and is shown in Fig. 1. By comparing their RI values and spectra, a total of 68 volatile compounds were identified in the extract. The examination of their structures revealed considerable variation in the 68 compounds. They were classified into seven classes by their general properties and chemical structures, including aldehydes (n = 23), alcohols (n = 10), ketones (n = 9), N- or S-containing compounds and aromatics (n = 17), acids (n = 3), alkanes (n = 3), esters (n = 3), as shown in Table 1. It was found that the aldehydes were the most dominant chemical class in cooked obscure puffer meat, accounting for 49.03% of the total volatile compounds based on the ratio of their ion peak area to total ion peak area. These aldehydes are widespread, as they have already been found in many other fish species and other seafood products, such as rainbow trout (O. mykiss) [8], turbot [15], scallop [16], sardine [17], cod [5], salmon [6], and Baltic herring (Clupea harengus membras) [18]. The majority of aldehydes, which have been reported as being green plant-like, grassy, dark chocolate, malty, fatty, sweet floral, apple-like, melon-like, nutty, and fruity, are present in various fresh fish and shellfish at various concentrations [19,20].
Fig. 1.
Total ion chromatogram of volatile compounds in the simultaneous distillation–extraction extract from cooked meat of farmed obscure puffer (Takifugu obscurus) by gas chromatography–mass spectrometry.
Table 1.
Volatile compounds identified in the simultaneous distillation–extraction extract from cooked meat of farmed obscure puffer (Takifugu obscurus) by gas chromatography–mass spectrometry.
| No. | RI | Compound | Methods of identification |
|---|---|---|---|
| Aldehydes (23) | |||
| 1 | 906 | Propanal | MS, RI |
| 2 | 945 | 2-Methylpropanal | MS, RI |
| 3 | 1057 | (E)-2-Butenal | MS, RI |
| 4 | 1094 | Hexanal | MS, RI |
| 5 | 1137 | (E)-2-Pentenal | MS, RI |
| 6 | 1189 | Heptanal | MS, RI |
| 7 | 1221 | (E)-2-Hexenal | MS, RI |
| 8 | 1243 | (Z)-4-Heptenal | MS, RI |
| 9 | 1289 | Octanal | MS, RI |
| 10 | 1324 | (E)-2-Heptenal | MS, RI |
| 11 | 1391 | Nonanal | MS, RI |
| 12 | 1429 | (E)-2-Octenal | MS, RI |
| 13 | 1494 | 2,4-Heptadienal | MS, RI |
| 14 | 1528 | Benzaldehyde | MS, RI |
| 15 | 1536 | (E)-2-Nonenal | MS, RI |
| 16 | 1587 | 2,6-Nonadienal | MS, RI |
| 17 | 1591 | (E,E)-2,4-Octadienal | MS, RI |
| 18 | 1718 | 4-Ethylbenzaldehyde | MS, RI |
| 19 | 1923 | Tetradecanal | MS |
| 20 | 2144 | Hexadecanal | MS |
| 21 | 2170 | cis-11-Hexadecenal | MS |
| 22 | 2208 | (Z)-14-Methyl-8-hexadecenal | MS |
| 23 | >2300 | (Z)-13-Octadecenal | MS |
| Alcohols (10) | |||
| 24 | 1053 | 2-Methyl-3-buten-2-ol | MS, RI |
| 25 | 1130 | 1,3-Butanediol | MS, RI |
| 26 | 1144 | 1-Butanol | MS, RI |
| 27 | 1159 | 1-Penten-3-ol | MS, RI |
| 28 | 1302 | 2-Penten-1-ol | MS, RI |
| 29 | 1311 | 3-Methyl-2-buten-1-ol | MS, RI |
| 30 | 1437 | 1-Octen-3-ol | MS, RI |
| 31 | 1477 | 2-Ethyl-1-hexanol | MS, RI |
| 32 | 1544 | 1-Octanol | MS, RI |
| 33 | 1875 | (E)-2-Tetradecen-1-ol | MS |
| Ketones (9) | |||
| 34 | 1008 | 2,3-Butanedione | MS, RI |
| 35 | 1074 | 2,3-Pentanedione | MS, RI |
| 36 | 1133 | 3-Penten-2-one | MS, RI |
| 37 | 1185 | 2-Heptanone | MS, RI |
| 38 | 1285 | 3-Hydroxy-2-butanone | MS, RI |
| 39 | 1299 | 1-Hydroxy-2-propanone | MS, RI |
| 40 | 1321 | 2,3-Octanedione | MS, RI |
| 41 | 1723 | 3-Undecen-2-one | MS, RI |
| 42 | 1853 | (5Z)-6,10-Dimethyl-5, 9-undecadien-2-one | MS |
| N- or S-containing compounds and aromatics (17) | |||
| 43 | 808 | Trimethylamine | MS, RI |
| 44 | 986 | 2-Ethylfuran | MS, RI |
| 45 | 1218 | Pyrazine | MS, RI |
| 46 | 1233 | 2-Pentylfuran | MS, RI |
| 47 | 1239 | (Dimethylamino)-acetonitrile | MS |
| 48 | 1265 | 2-Methylpyrazine | MS, RI |
| 49 | 1326 | N,N-dimethylformamide | MS, RI |
| 50 | 1473 | 2-Ethyl-1-hexanethiol | MS |
| 51 | 1525 | 4-Ethyl-5-methylthiazole | MS |
| 52 | 1652 | 2-Acetylthiazole | MS, RI |
| 53 | 1773 | Dibutylformamide | MS |
| 54 | 1894 | 2,5-Dimethyl-3-acetylthiophene | MS |
| 55 | 1975 | 2-Acetylpyrrole | MS, RI |
| 56 | 1871 | 2-Methylnaphthalene | MS, RI |
| 57 | 2004 | Phenol | MS, RI |
| 58 | 2061 | 3-Dodecenyl-2,5-furandione | MS |
| 59 | 2300 | 2,4-Bis(1,1-dimethylethyl)-phenol | MS |
| Alkanes (3) | |||
| 60 | 1040 | Chloroform | MS, RI |
| 61 | 2076 | 1-Nonadecene | MS, RI |
| 62 | 2271 | 1,7,11-Trimethyl-4-(1-methylethyl)-cyclotetradecane | MS |
| Esters (3) | |||
| 63 | 2214 | Hexadecanoic acid methyl ester | MS |
| 64 | 2290 | Isobutyl phthalate diisobutyl ester | MS |
| 65 | >2300 | Dibutyl phthalate | MS |
| Acids (3) | |||
| 66 | 1441 | Acetic acid | MS, RI |
| 67 | 2094 | Tetradecanoic acid | MS, RI |
| 68 | >2300 | n-Hexadecanoic acid | MS, RI |
When only MS or RI is available for identification of a compound, it is considered as an attempt of identification. Means of identification: MS, mass spectrum (identified by the mass spectra of the compounds); RI, retention index (when the RI of the compound identified corresponds to the RI in the literature).
N- or S-containing compounds and aromatics were the second largest volatile group in cooked obscure puffer meat, accounting for 7.58%. Because of their low sensory thresholds, the N- or S-containing compounds are important fraction of aroma in numerous fish species.
3.2. Odor-active compounds by GC–O posterior intensity and time-intensity methods
The aromagram of odor-active compounds in the SDE extract was obtained using the GC–O posterior intensity method. The odor-active compounds were identified by comparing the RIs of odor signals sniffed by assessors with the RIs of volatile compounds shown in Table 1. As a result, a total of 31 odor-active compounds in the extract were detected and their odor descriptions are shown in Table 2. They were widely distributed over the six classes of volatile compounds mentioned in the “Volatile Composition in the Cooked Meat of Farmed Obscure Puffer” section, that is, aldehydes, alcohols, ketones, N- or S-containing compounds and aromatics, esters, and acids.
Table 2.
Characteristic odor-active compounds in cooked meat of farmed obscure puffer (Takifugu obscurus) by GC-O (n = 6).
| Peak N. | RI | Compound | Odor intensity | Odor description | Sniffing | Concentration |
|---|---|---|---|---|---|---|
|
|
|
|||||
| Period (min) | (ng/g) | |||||
| 4 | 1094 | Hexanal | 1 | Fishy, grassy, leafy, green | 0.14 | 192.7 ± 2.3 |
| 9 | 1289 | Octanal | 3 | Grassy, leafy, green | 0.26 | 36.74 ± 0.60 |
| 10 | 1243 | (Z)-4-Heptenal | 2 | Fishy, grassy, leafy, green | 0.2 | 38.38 ± 0.91 |
| 15 | 1536 | (E)-2-Nonenal | 1 | Fishy, fatty, cucumber, green | 0.23 | 21.81 ± 1.10 |
| 27 | 1159 | 1-Penten-3-ol | 1 | Fishy, grassy | 0.2 | 48.03 ± 1.54 |
| 30 | 1437 | 1-Octen-3-ol | 4 | Fishy, fatty, mushroom, grassy | 0.25 | 50.77 ± 1.59 |
| 43 | 808 | Trimethylamine | 4 | Fishy | 0.1 | 273.85 ± 4.13 |
| 46 | 1233 | 2-Pentylfuran | 1 | Fishy, grassy | 0.13 | 30.40 ± 0.47 |
| 2 | 945 | 2-Methylpropanal | 3 | Nutty, malty, burnt | 0.17 | 11.31 ± 0.31 |
| 6 | 1189 | Heptanal | 1 | Nutty, burnt | 0.07 | 27.55 ± 0.33 |
| 12 | 1429 | (E)-2-Octenal | 3 | Roast, fatty | 0.24 | 23.50 ± 0.89 |
| 16 | 1587 | 2,6-Nonadienal | 3 | Roast, waxy | 0.04 | 17.16 ± 1.32 |
| 34 | 1008 | 2,3-Butanedione | 1 | Sweet, caramel | 0.13 | 1227.58 ± 22.92 |
| 35 | 1074 | 2,3-Pentanedione | 1 | Sweet, caramel, malty, buttery | 0.17 | 233.13 ± 4.17 |
| 44 | 986 | 2-Ethylfuran | 1 | Sweet | 0.04 | 166.31 ± 2.04 |
| 55 | 1975 | 2-Acetylpyrrole | 4 | Nutty, walnut, bread | 0.08 | 46.92 ± 3.29 |
| 7 | 1324 | (E)-2-Heptenal | 3 | Raw fish | 0.19 | 6.74 ± 0.35 |
| 17 | 1591 | (E,E)-2,4-Octadienal | 3 | Cooked meat, sweet | 0.23 | 7.22 ± 0.99 |
| 31 | 1477 | 2-Ethyl-1-hexanol | 3 | Raw fish, green | 0.11 | 32.17 ± 1.16 |
| 49 | 1326 | N,N-dimethylformamide | 3 | Raw fish | 0.14 | 23.01 ± 1.18 |
| 50 | 1473 | 2-Ethyl-1-hexanethiol | 4 | Cooked fish | 0.1 | 7.34 ± 0.46 |
| 52 | 1652 | 2-Acetylthiazole | 4 | Meaty, roast, nutty, sulfur | 0.33 | 45.39 ± 0.59 |
| 53 | 1773 | Dibutylformamide | 4 | Crab meat | 0.08 | 33.68 ± 3.03 |
| 13 | 1495 | 2,4-Heptadienal | 3 | Fatty, hay, fishy | 0.19 | 10.02 ± 0.51 |
| 20 | 2144 | Hexadecanal | 2 | Fatty | 0.12 | 4439.58 ± 47.09 |
| 23 | >2300 | (Z)-13-Octadecenal | 1 | Fatty | 0.18 | 141.93 ± 3.58 |
| 68 | >2300 | n-Hexadecanoic acid | 1 | Fatty | 0.12 | 1491.32 ± 25.08 |
| 11 | 1391 | Nonanal | 3 | Geranium, plastic, marine | 0.04 | 50.05 ± 1.22 |
| 14 | 1531 | Benzaldehyde | 1 | Bitter almond, woody, burnt | 0.01 | 52.63 ± 0.96 |
| 59 | 2300 | 2,4-Bis(1,1-dimethylethyl)-phenol | 1 | Paint | 0.15 | 127.58 ± 5.20 |
| 66 | 1441 | Acetic acid | 3 | Sour | 0.16 | 47.62 ± 0.14 |
1 = very weak odor intensity; 2 = moderate intensity; 3 = strong intensity; and 4 = very strong intensity.
GC–O = gas chromatography–olfactometry; RI = retention index.
3.2.1. Odor-active aldehydes
Odor-active aldehydes are important contributors to the flavor of cooked meat of farmed obscure puffer. A total of 15 odor-active aldehydes were detected and identified from the SDE extract. In the 15 aldehydes, 14 belong to the aliphatic aldehydes ranging between C4 and C16 chain length and the benzaldehyde falls into the class of aromatic aldehydes. They all contributed to the overall perceived sensation of the cooked meat.
2-Methylpropanal (RI 945) with the molecular formula C4H8O was the smallest odor-active aldehyde among the identified aldehydes. However, 2-methylpropanal has not been reported in any other fish species, and it was the compound contributing to the odor of fish sauce. It had a strong posterior odor intensity and was described as having nutty, malty, burnt odors [21], although its content occurring in the cooked meat of farmed obscure puffer was only approximately 11 ng/g. 2-Methylpropanal was a thermally produced volatile aldehyde and generated through Strecker degradation from valine–sugar reactions [22] in the steam-cooking process.
Hexanal (C6H12O, RI 1094) occurred in cooked meat of farmed obscure puffer at a high concentration (192.7 ng/g) and contributed fishy, grassy, leafy, and green odors to its total flavor. Hexanal may be responsible for the aldehydic aroma note and was also detected with a distinct coarse, plant-like odor in immediately harvested finfish [23]. It has been also reported that hexanal is mainly derived from the oxidation of linoleic acid and provides green and fatty character to fish and other seafoods [24].
Odor-active C7 aldehydes detected in the SDE extract were heptanal (C7H14O, RI 1189), (Z)-4-heptenal (C7H12O, RI 1243), (E)-2-heptenal (C7H12O, RI 1324), and 2,4-heptadienal (C7H10O, RI 1495). Heptanal was described as having nutty and burnt odors, (Z)-4-heptenal as having fishy, grassy, leafy, and green odors, (E)-2-heptenal as having raw fish odor, and 2,4-heptadienal as having fatty, hay, and fishy odor. It has been proved that (Z)-4-heptenal is commonly found in cooked, stored seafood [25] and produced by the water-mediated retro-aldol condensation of (E,Z)-2,6-nonadienal (RI 1587) [26]. 2,4-Heptadienal is derived from autoxidation of long-chain polyunsaturated n–3 fatty acid, docosahexaenoic acid.
Three odor-active C8 aldehydes found in the extract were octanal (C8H16O, RI 1289), (E)-2-octenal (C8H14O, RI 1429), and (E,E)-2,4-octadienal (C8H12O, RI 1591). Octanal, (E)-2-octenal, and (E,E)-2,4-octadienal mainly contributed a fresh plant-like odor, a fatty odor, and a cooked meat odor, respectively, which were also detected in freshwater fish tench [27].
Nonanal (C9H18O, RI 1391), (E)-2-nonenal (C9H16O, RI 1536) and 2,6-nonadienal (C9H14O, RI 1587) are C9 odor-active aldehydes found in the extract. Nonanal provides geranium, plastic, marine odors to the flavor of cooked meat of farmed obscure puffer. (E)-2-nonenal provides fishy, cucumber, fatty, green odors. It has been reported that (E)-2-nonenal is a lipid peroxidation product derived from oxidized n–6 poly-unsaturated fatty acids. It could be considered as the off-flavor compound, which was described as having earthy and wet earth odors [8]. 2,6-Nonadienal is derived from autoxidation of lipids once the fish dies [26] and provides roast, waxy odors. These C9 odor-active aldehydes are common in freshwater fish [28].
Hexadecanal (C16H32O, RI 2144) contributes to fatty odor. Its content is the highest among all odor-active compounds, reaching 4439.58 ng/g in cooked meat of farmed obscure puffer. However, with a high-sensory threshold value, hexadecanal always has a small contribution to food flavor. (Z)-13-octadecenal (C18H34O) contributes fatty odor similar to hexadecanal.
Benzaldehyde (C7H6O, RI 1531), the only odor-active aromatic aldehyde, is formed by oxidation of benzyl alcohol catalyzed by dehydrogenases [29]. It could be speculated that this compound could contribute to the flavor of fish as it has a low threshold value. Benzaldehyde, with an almond-like odor, was also previously identified in fresh-baked sockeye salmon and fish sauce.
3.2.2. Odor-active alcohols
Three odor-active alcohols were identified from the SDE extract, which partly contributed to the flavor of the cooked meat of farmed obscure puffer.
1-Penten-3-ol (C5H10O, RI 1159) contributed to fishy, grassy odors and was generated from oxidation of PUFAs catalyzed by lipoxygenase or hydroperoxidase. It was found in refrigerated sardine (Sardinops melanostica) and identified as the compound that provides a major contribution to the paint-like and chemical-like odors [28].
1-Octen-3-ol (C8C16O, RI 1437) provided fishy, fatty, mushroom, grassy odors, and was mainly derived from enzymes involved in the biosynthesis by a lipoxygenase and a hydroperoxide lyase [23]. In particular, 1-octen-3-ol is an important contributor to off-flavors due to its low-odor threshold and it is also increased during frozen storage [30].
2-Ethyl-1-hexanol (C8H18O, RI 1477) was described as having raw fish, green odors. This alcohol was also detected in raw oyster [31], cooked catfish [32], sardine [33], and fresh and smoked salmon [6]. Interestingly, 2-ethyl-1-hexanol was also identified in fried bacon and fried pork loin, providing a wet and earthy odor [34].
3.2.3. Odor-active ketones
2,3-Butanedione (C4H6O2, RI 1008) was the second most abundant odor-active compound (1227.58 ng/g), and contributed sweet, caramel odors. 2,3-Butanedione was reported to contribute an intense buttery and desirable aroma in crustaceans [35] and was previously found to be in high amounts in shrimp paste [36]. As previously stated, 2,3-pentanedione (C5H8O2, RI 1074) was detected with sweet, caramel, malty, and buttery odors in the cooked meat of farmed obscure puffer. It had been reported that 2,3-pentanedione was detected with a buttery and caramel odor in cooked fillet of an European catfish (Silurus glanis) [37] and a fruity odor in salted-dried white herring [16]. Thermal degradation, oxidation of fat, degradation of amino acids, and the Maillard reaction were possible mechanisms for the formation of ketones.
3.2.4. N- or S-containing compounds and aromatics
Trimethylamine (C12H18O4, RI 808) was one of prominent members in the N-containing compounds group. It is generally believed to be produced by microbial metabolism in the presence of the precursor trimethylamine oxide. Trimethylamine is reported in large amounts in seafood, and it is described as having an ammonia-like and fishy odor [38]. Furan could be formed by the Maillard reaction [39], but 2-pentylfuran (C9H14O, RI 1233) was reported to be derived by oxidation of n–6 PUFAs [40] and was reported for the first time as a seafood volatile in turbot (Scophthalmus maximus) [15]; however, its positive or negative contribution to food aroma is debatable [41]. The formation of 2-ethylfuran (C6H8O, RI 986) in oxidized n–3 PUFAs in fish can be explained by the same mechanism postulated previously for 2-pentylfuran formed in oxidized n–6 PUFAs. Pyrazines are products formed between sugars and amino acids under suitable conditions [42]. High temperature generally promotes the rate of formation of the Maillard reaction products. With regard to naphthalene compounds, 2-methylnaphthalene (C11H10, RI 1495) could be produced from the degradation of plant materials by micro-organisms and accumulated in the fish tissues as environmental contaminants that provide a plastic and earthy note [39]. As previously stated, this compound was detected with an earthy and grilled odor in cooked mussel [43] and with a moss and plastic odor in smoked salmon [6].
3.2.5. Esters, acids, and alkanes
Esters might be products of esterification of the alcohols with carboxylic acids that are formed by microbial degradation of lipids. Esters are considered to be the most important constituent of aroma in fruits, responsible for various fruity and floral notes [44]. Hexadecanoic acid methyl ester (C17H34O2, RI 2214) was detected in farmed obscure puffer meat. This compound is especially important for a pleasant violet and green note and is also detected in cooked rainbow trout [8]. Three acids were identified and quantified in farmed obscure puffer meat. Among them, n-hexadecanoic acid (C16H32O2, RI 2300) showed the highest concentration (1491.32 ng/g). Acetic acid (C4H7ClO4S, RI 1441) was present in low concentration (47.62 ng/g). Because acetic acid has a lower threshold, it might have a larger contribution to the flavor.
Alkanes have relatively high thresholds, meaning they may have little contribution to the flavor. In this study, three alkanes were determined, which possibly originated from Styrofoam boxes used for storing fish after being caught [45].
In summary, the Maillard reaction, Strecker degradation, thermal degradation, degradation of amino acids, and oxidation of fatty acids products could strongly affect the overall aroma of farmed obscure puffer meat.
3.2.6. Aroma-active odorants obtained from intensity method
The intensity method was performed to further categorize the 31 odor-active compounds perceived in farmed obscure puffer meat by GC–MS–O, according to their odor potency by two of the most sensitive and experienced panelists selected. Based on their odor characteristics, these 31 compounds can be categorized into five groups. The first group was represented by odorants (4, 9, 10, 15, 27, 30, 43, and 46) with fishy, grassy, leafy, green notes. The second group consisted of odorants with burnt, sweet, caramel, nutty, and roast notes (2, 6, 12, 16, 34, 35, 44, and 55). Compounds with meaty, cooked meat, raw fish notes (7, 17, 31, 49, 50, 52, and 53) comprised the third group of odorants. The fourth group was represented by fatty and fish oil odors (13, 20, 23, and 68). The fifth group consisted of the remaining odorants with widely varying aroma properties.
As shown in Table 2, trimethylamine, 1-octen-3-ol, 2-ethyl-1-hexanethiol, 2-acetylthiazole, and 2-acetylpyrrole had the highest odor intensity based on the posterior intensity method. These six compounds may contribute more actively to the overall aroma of farmed obscure puffer meat. Based on the time-intensity method, octanal, (E)-2-octenal, 1-octen-3-ol, (E)-2-nonenal, (E,E)-2,4-octadienal, and 2-acetylthiazole had longer sustained period. On the basis of the two olfactometric intensity methods, the results indicated that trime-thylamine (fishy), octanal (grassy, leafy, green), (E)-2-octenal (roast, fatty), 1-octen-3-ol (fishy, fatty, mushroom, grassy), 2-ethyl-1-hexanethiol (cooked fish), (E,E)-2,4-octadienal (cooked meat, sweet), 2-acetylthiazole (meaty, roast, nutty, sulfur), and 2-acetylpyrrole (nutty, walnut, bread) were identified as the key odorants in the cooked meat of farmed obscure puffer.
Trimethylamine is widely present in seafoods. Rochat et al used olfactometric analysis and found that trimethylamine was a key odorant in shrimps [46]. Octanal and (E)-2-octenal were the two important odor compounds in Sciaenops ocellatus [47]. 1-Octen-3-ol was a characteristic odorant in S. ocellatus, Lateolabrax japonicus, and Pseudosciaena crocea, respectively. (E,E)-2,4-octadienal was one of the key odorants in cooked mussels [48]. However, 2-acetylthiazole, 2-ethyl-1-hexanethiol, and 2-acetylpyrrole have not been reported in any other fish species. It was reported that 2-acetylthiazole was a characteristic odorant in crustaceans [49]. 2-Ethyl-1-hexanethiol was only detected in heartwood of sandalwood tree [49] and white truffle [50]. 2-Acetylpyrrole was most common in plants and plant products, such as soy paste [51].
4. Conclusions
In summary, a total of 68 volatile compounds including 23 aldehydes, 10 alcohols, nine ketones, 17 N- or S-containing compounds and aromatics, three acids, three alkanes, and three esters were identified in cooked meat of farmed obscure puffer (T. obscurus) by GC–MS. The predominant volatile compounds were aldehydes, N- or S-containing compounds and aromatics, and alcohols. Of these, 31 odor-active compounds were identified by GC–MS–O. The results showed that trimethylamine (fishy), octanal (grassy, leafy, green), (E)-2-octenal (roast, fatty), 1-octen-3-ol (fishy, fatty, mushroom, grassy), 2-ethyl-1-hexanethiol (cooked fish), (E,E)-2,4-octadienal (cooked meat, sweet), 2-acetylthiazole (meaty, roast, nutty, sulfur), 2-acetylpyrrole (nutty, walnut, bread) were identified as the key odorants in the cooked meat of farmed obscure puffer based on posterior intensity and time-intensity methods.
Acknowledgments
This work was supported by Shanghai Engineering Research Center of Aquatic-Product Processing and Preservation (11DZ2280300), the Leading Academic Discipline Project of Shanghai Municipal Education Commission (Project No. J50704).
Funding Statement
This work was supported by Shanghai Engineering Research Center of Aquatic-Product Processing and Preservation (11DZ2280300), the Leading Academic Discipline Project of Shanghai Municipal Education Commission (Project No. J50704).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1. Yang Z, Chen Y. Salinity tolerance of embryos of obscure puffer Takifugu obscurus. Aquaculture. 2006;253:393–7. [Google Scholar]
- 2. Yang G, Xu J, Liang S, et al. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon. 2010;56:324–9. doi: 10.1016/j.toxicon.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 3. Tao N-P, Wang L-Y, Gong X, et al. Comparison of nutritional composition of farmed Pufferfish muscles among Fugu obscurus, Fugu flavidus and Fugu rubripes. J Food Compost Anal. 2012;28:40–5. [Google Scholar]
- 4. Gu S, Zhao C. Comparison of nutrient components in the muscle of wild and artificially reared Takifugu obscurus. Agric Sci Technol. 2008;9:118–20. [Google Scholar]
- 5. Milo C, Grosch W. Changes in the odorants of boiled salmon and cod as affected by the storage of the raw material. J Agric Food Chem. 1996;44:2366–71. [Google Scholar]
- 6. Varlet V, Knockaert C, Prost C, et al. Comparison of odor-active volatile compounds of fresh and smoked salmon. J Agric Food Chem. 2006;54:3391–401. doi: 10.1021/jf053001p. [DOI] [PubMed] [Google Scholar]
- 7. Senger-Emonnot P, Rochard S, Pellegrin F, et al. Odour active aroma compounds of sea fig (Microcosmus sulcatus) Food Chem. 2006;97:465–71. [Google Scholar]
- 8. Selli S, Rannou C, Prost C, et al. Characterization of aroma-active compounds in rainbow trout (Oncorhynchus mykiss) eliciting an off-odor. J Agric Food Chem. 2006;54:9496–502. doi: 10.1021/jf0619582. [DOI] [PubMed] [Google Scholar]
- 9. Schultz TH, Flath RA, Mon TR, et al. Isolation of volatile components from a model system. J Agric Food Chem. 1977;25:446–9. [Google Scholar]
- 10. MacLeod G, Coppock BM. A comparison of the chemical composition of boiled and roasted aromas of heated beef. J Agric Food Chem. 1977;25:113–7. [Google Scholar]
- 11. Delahunty CM, Eyres G, Dufour JP. Gas chromatography–olfactometry. J Sep Sci. 2006;29:2107–25. doi: 10.1002/jssc.200500509. [DOI] [PubMed] [Google Scholar]
- 12. Fuller GH, Steltenkamp R, Tisserand G. The gas chromatograph with human sensor: perfumer model. Ann N Y Acad Sci. 1964;116:711–24. doi: 10.1111/j.1749-6632.1964.tb45106.x. [DOI] [PubMed] [Google Scholar]
- 13. Van den Dool H, Dec Kratz P. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A. 1963;11:463–71. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 14. Yu H-Z, Chen S-S. Identification of characteristic aroma-active compounds in steamed mangrove crab (Scylla serrata) Food Res Int. 2010;43:2081–6. [Google Scholar]
- 15. Prost C, Serot T, Demaimay M. Identification of the most potent odorants in wild and farmed cooked turbot (Scophtalamus maximus L.) J Agric Food Chem. 1998;46:3214–9. [Google Scholar]
- 16. Chung HY, Yeung CW, Kim J-S, et al. Static headspace analysis-olfactometry (SHA-O) of odor impact components in salted-dried white herring (Ilisha elongata) Food Chem. 2007;104:842–51. [Google Scholar]
- 17. Prost C, Hallier A, Cardinal M, et al. Effect of storage time on raw sardine (Sardina pilchardus) flavor and aroma quality. J Food Sci. 2004;69:S198–204. [Google Scholar]
- 18. Sérot T, Gandemer G, Demaimay M. Lipid and fatty acid compositions of muscle from farmed and wild adult turbot. Aquac Int. 1998;6:331–43. [Google Scholar]
- 19.Alasalvar C, Quantick P, Grigor J. Aroma compounds of fresh and stored mackerel (Scomber scombrus) In: Shahidi F, Cadwallader KR, editors. Flavor and lipid chemistry of seafoods. USA, Washington DC: ACS Publications; 1997. pp. 39–54. [Google Scholar]
- 20. Alasalvar C, Taylor KA, Shahidi F. Comparison of volatiles of cultured and wild sea bream (Sparus aurata) during storage in ice by dynamic headspace analysis/gas chromatography-mass spectrometry. J Agric Food Chem. 2005;53:2616–22. doi: 10.1021/jf0483826. [DOI] [PubMed] [Google Scholar]
- 21. Fukami K, Ishiyama S, Yaguramaki H, et al. Identification of distinctive volatile compounds in fish sauce. J Agric Food Chem. 2002;50:5412–6. doi: 10.1021/jf020405y. [DOI] [PubMed] [Google Scholar]
- 22. Buttery RG, Parker FD, Teranishi R, et al. Volatile components of alfalfa leaf-cutter bee cells. J Agric Food Chem. 1981;29:955–8. [Google Scholar]
- 23. Josephson DB, Lindsay RC, Stuiber DA. Identification of compounds characterizing the aroma of fresh whitefish (Coregonus clupeaformis) J Agric Food Chem. 1983;31:326–30. [Google Scholar]
- 24. Caprino F, Moretti VM, Bellagamba F, et al. Fatty acid composition and volatile compounds of caviar from farmed white sturgeon (Acipenser transmontanus) Anal Chim Acta. 2008;617:139–47. doi: 10.1016/j.aca.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 25. McGill AS, Hardy R, Burt J, et al. Hept-cis-4-enal and its contribution to the off-flavour in cold stored cod. J Sci Food Agric. 1974;25:1477–89. [Google Scholar]
- 26. Josephson DB, Lindsay RC. Retro-aldol degradations of unsaturated aldehydes: role in the formation of c4-heptenal from t2,c6-nonadienal in fish, oyster and other flavors. J Am Oil Chem Soc. 1987;64:132–8. [Google Scholar]
- 27. Turchini GM, Moretti VM, Mentasti T, et al. Effects of dietary lipid source on fillet chemical composition, flavour volatile compounds and sensory characteristics in the freshwater fish tench (Tinca tinca L.) Food Chem. 2007;102:1144–55. [Google Scholar]
- 28. Shi WZ, Chen QY, Wang XC, et al. Research on predominant volatile compounds of grass carp meat. Adv Mater Res. 2013;781:1852–5. [Google Scholar]
- 29. Eriksson C. Aroma compounds derived from oxidized lipids. Biochemical and analytical aspects. J Agric Food Chem. 1975;23:126–8. [Google Scholar]
- 30. Iglesias J, Medina I, Bianchi F, et al. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography–mass spectrometry. Food Chem. 2009;115:1473–8. [Google Scholar]
- 31. Pennarun AL, Prost C, Demaimay M. Identification and origin of the character-impact compounds of raw oyster Crassostrea gigas. J Sci Food Agric. 2002;82:1652–60. [Google Scholar]
- 32. Grimm CC, Lloyd SW, Batista R, et al. Using microwave distillation-solid-phase microextraction–gas chromatography–mass spectrometry for analyzing fish tissue. J Chromatogr Sci. 2000;38:289–96. doi: 10.1093/chromsci/38.7.289. [DOI] [PubMed] [Google Scholar]
- 33. Chouinard P, Corneau L, Butler W, et al. Effect of dietary lipid source on conjugated linoleic acid concentrations in milk fat. J Dairy Sci. 2001;84:680–90. doi: 10.3168/jds.S0022-0302(01)74522-5. [DOI] [PubMed] [Google Scholar]
- 34. Timon ML, Carrapiso AI, Jurado A, et al. A study of the aroma of fried bacon and fried pork loin. J Sci Food Agric. 2004;84:825–31. [Google Scholar]
- 35. Tanchotikul U, Hsieh TCY. Volatile flavor components in crayfish waste. J Food Sci. 1989;54:1515–20. [Google Scholar]
- 36. Choi SH, Kato H. Volatile components of Sergia lucens and its fermented product. Agric Biol Chem. 1984;48:1479–86. [Google Scholar]
- 37. Hallier A, Prost C, Serot T. Influence of rearing conditions on the volatile compounds of cooked fillets of Silurus glanis (European catfish) J Agric Food Chem. 2005;53:7204–11. doi: 10.1021/jf050559o. [DOI] [PubMed] [Google Scholar]
- 38. Chen D-W, Zhang M. Determination of odour-active compounds in the cooked meat of Chinese mitten crab (Eriocheir sinensis) by solid-phase microextraction, gas chromatography-olfactometry and gas chromatography-mass spectrometry. J Food Drug Anal. 2010;18:290–6. [Google Scholar]
- 39. Chung HY, Yung IKS, Ma WCJ, et al. Analysis of volatile components in frozen and dried scallops (Patinopecten yessoensis) by gas chromatography/mass spectrometry. Food Res Int. 2002;35:43–53. [Google Scholar]
- 40. Frankel E. Volatile lipid oxidation products. Prog Lipid Res. 1983;22:1–33. doi: 10.1016/0163-7827(83)90002-4. [DOI] [PubMed] [Google Scholar]
- 41.Spurvey S, Pan B, Shahidi F. Flavour of shellfish. In: Shahidi F, editor. Flavor of meat, meat products and seafoods. 2nd ed. London, UK: Blackie Academic and Professional; 1998. pp. 159–96. [Google Scholar]
- 42. Sung W-C. Volatile constituents detected in smoke condensates from the combination of the smoking ingredients sucrose, black tea leaves, and bread flour. J Food Drug Anal. 2013;21:292–300. [Google Scholar]
- 43. Le Guen S, Prost C, Demaimay M. Critical comparison of three olfactometric methods for the identification of the most potent odorants in cooked mussels (Mytilus edulis) J Agric Food Chem. 2000;48:1307–14. doi: 10.1021/jf990745s. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Yang C, Li S, et al. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009;116:356–64. [Google Scholar]
- 45. Olafsdottir G, Jonsdottir R, Lauzon HL, et al. Characterization of volatile compounds in chilled cod (Gadus morhua) fillets by gas chromatography and detection of quality indicators by an electronic nose. J Agric Food Chem. 2005;53:10140–7. doi: 10.1021/jf0517804. [DOI] [PubMed] [Google Scholar]
- 46. Rochat S, Egger J, Chaintreau A. Strategy for the identification of key odorants: application to shrimp aroma. J Chromatogr A. 2009;1216:6424–32. doi: 10.1016/j.chroma.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 47. Chun-xia L, Li-ping W, Hong-hai W, et al. Investigation on the key odor compounds of three cage-farming fishes. Food Ferment Industries. 2010;10:056. [Google Scholar]
- 48.Baek H, Cadwallader KR. Character-impact aroma compounds of crustaceans. In: Shahidi F, Cadwallader KR, editors. Flavor and lipid chemistry of seafoods. USA, Washington DC: ACS Publications; 1997. pp. 85–94. [Google Scholar]
- 49. Misra BB, Das SS, Dey S. Volatile profiling from heartwood of East Indian sandalwood tree. J Pharm Res. 2013;7:299–303. [Google Scholar]
- 50. Gioacchini AM, Menotta M, Guescini M, et al. Geographical traceability of Italian white truffle (Tuber magnatum Pico) by the analysis of volatile organic compounds. Rapid Commun Mass Spectrom. 2008;22:3147–53. doi: 10.1002/rcm.3714. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Li X, Lo C-K, et al. Characterization of the volatile substances and aroma components from traditional soypaste. Molecules. 2010;15:3421–7. doi: 10.3390/molecules15053421. [DOI] [PMC free article] [PubMed] [Google Scholar]