Abstract
In this study, linear relationships between response and concentration were used to estimate the detection limit (DL) and quantification limit (QL) for five avermectins: emamectin, abamectin, doramectin, moxidectin, and ivermectin. Estimation of DL and QL was based on the standard deviation of residual and y-intercept of the regression line at low concentrations of avermectins, using the dispersive solid-phase extraction procedure. Avermectin extracts were analyzed using liquid chromatography tandem mass spectrometry. Based on the regression slope, DL and QL were higher at concentrations of 0.3–0.4 μg/kg and 1 μg/kg, respectively, for all avermectin compounds. Linearity assessment was performed by linear regression, which incorporated a regression model, outlier rejection, and evaluation of the assumption with a significant test. For all avermectins, there is a significant correlation between response and concentration in the range 1–15 μg/kg, and the y-intercept passes through origin (zero).
Keywords: Avermectins, Detection limit, Dispersive solid-phase extraction, Linear regression, Linearity, Quantification limit
1. Introduction
Anthelmintic avermectin is one of the veterinary medicine groups that are licensed to be used as antiparasites in cattle, sheep, goats, reindeer, pigs, horses, and dogs [1]. A report also mentioned that avermectins can be used for the treatment and control of sea lice in Atlantic salmon [2]. Avermectin compounds consist of ivermectin, doramectin, abamectin (ABA), moxidectin (MOX), and emamectin (EMA) having the chemical structure of lactone disaccharides (namely, macrocyclic lactone), which consists of 16-member cyclic lactones, a spirochetal moiety, a benzofuran ring, and a disaccharide unit [3]. Grant and Briggs [4], expressed concerns on the usage of ivermectin, due to the highly toxic and persistent nature in the chemotherapeutic control of sea lice infection [5], as it may have a toxicity effect on nontargeted organisms. They highlighted the urgency of collecting data on the bioavailability of ivermectin; due to the abovementioned reasons, ivermectin may be found in sensitive marine organisms and may have subsequent consequences in the consumers of these marine organisms.
Rapid methods have been preferred in surveillance studies due to the requirement of quickness and simplicity of application. Extraction method of avermectins that was developed based on the quick, easy, cheap, effective, rugged, and safe (QuEChERS) concept [6] was used successfully in pesticide analysis. The method of analysis involves extraction with a solvent and a chelating salt, dispersive solid-phase extraction with cleanup materials, and quantification by Gas Chromatography Mass Spectrometer (GCMS). Nowadays, the concept has been implemented in the analysis of veterinary drugs in various matrices. Kinsella et al [7,8] have modified the original method used for the determination of anthelmintic avermectins. Cleanup materials used were octadecyl (C18) and primary and secondary amine, which are able to remove matrix interference in bovine liver and milk, respectively.
In order to assess the fitness of purpose and reliability of QuEChERS method for the determination of avermectins in fish, a method validation was carried out as required by the International Standards Organization (ISO 17025). Performance parameters for the intended purpose were as follows: specificity, selectivity, detection limit (DL), quantification limit (QL), linearity, precision, accuracy, and ruggedness. This paper demonstrates the estimation of DL and QL using the linear regression method, and the linearity study was based on a statistical approach to the determination of avermectins in fish with dispersive solid-phase extraction using Liquid Chromatography Tandem Mass Spectrometer (LCMSMS).
There are several approaches to the determination of DL and QL, which depend on the analytical method, irrespective of whether it is noninstrumental or instrumental. According to the International Conference on the Harmonization of Technical Requirements (ICHQ2B) [9] guideline, there are three approaches, which are based on visual evaluation, signal-to-noise ratio, and the standard deviation of the response and slope of the calibration curve. The signal to noise can be calculated at least 10 times the width of the signal peak at half its height [10]. In this study, DL and QL were estimated based on the standard deviation; DL and QL may be expressed as DL = 3.3σ/S and QL = 10σ/S. Here, σ is the standard deviation of the response and S is the slope of the calibration curve. The DL and QL values estimated by these approaches can vary by a factor of 5–6 from the smallest to the largest estimated value [11].
Linearity of a procedure is its ability to obtain results that are directly or indirectly proportional to the concentration of a compound in a sample within a given range [12]. The most common method used to assess linearity during validation is the ordinary least squares method [12–14]. In evaluating the regression line [15], the following assumptions were made in this study: all errors occur in the y-direction, the y-direction errors are normally distributed, and the variation in the y-direction errors is the same for all values of the x-axis. The line was calculated by minimizing the sums of the squares of the distances between the standard points and the line in the y-direction. Visual checking of the residual plot of y-direction may be used to minimize the distance; possible outlier data were indicated at acceptable variation ± t(0.95, np − 2).sres, where the t-value is taken at the desired confidence level and (n − 2) degrees of freedom [15]. Any outlier data were rejected, and the regression model was recalculated. The final estimated linearity model need to be verified using the lack-of-fit test, to confirm that the selected regression and linearity are the correct ones [9].
The main objective of this study is to estimate the DL, QL, and linearity based on a statistical approach, to evaluate the fitness of the dispersive solid-phase extraction procedure with a new clean up material in the determination of avermectins in fish. This method can then be implemented as a routine analysis in the laboratory for future national surveillance.
2. Materials and methods
2.1. Preparation of standard
Reference materials of high purity were purchased from Dr. Ehrenstofer (Augsburg, Germany). Purity of emamectin benzoate was 90% and that of ABA, doramectin, MOX, ivermectin, and selamectin (SEL) was 96%.Standard stock solutions of 1 mg/mL in acetonitrile (High Pressure Liquid Chromatography, HPLC grade) were prepared for each reference material and stored at −20°C. An intermediate standard of 1 μg/mL was prepared by mixing external standards of emamectin benzoate, ABA, doramectin, MOX, and ivermectin, while an internal standard was prepared using SEL separately at a concentration of 1 μg/mL; all standards were stored at −20°C. The working external standard of 0.1 μg/mL was freshly prepared before use.
2.2. Extraction procedure
Barramundi fish samples were obtained from the cage water system in PulauAman, Pulau Pinang, Malaysia, and were confirmed to be free from the targeted compounds, using LC/MS/MS. Fish muscles were collected and homogenized before storing in polypropylene bags at −20°C. Prior to analysis, 10.0 g subsamples were weighed and added to polypropylene placed in 50 mL centrifuge tubes. Extraction procedures are based on the modification of QuEChERS methodology [7,8].
SEL (50 μL) was spiked into the sample as an internal standard. After leaving the sample for 15 minutes in a dark area, 10 mL acetonitrile was added to it and homogenized for 30 seconds. Homogenizer probes were rinsed into another centrifuge tube containing 3 mL acetonitrile. A chelating agent containing 4 g MgSO4 and 1 g NaCl was added, and the remaining salts were removed after rinsing with acetonitrile. Samples were shaken vigorously for 30 seconds and centrifuged for 10 minutes at 2862g at 4°C. All supernatants were transferred to centrifuge tubes containing clean up materials (0.25 g primary and secondary amine, 0.25 g C18, and 1.5 g MgSO4) for the dispersive solid-phase extraction procedure. Samples were vortexed for 30 seconds, centrifuged for 5 minutes at 2862g at 4°C, and dried under nitrogen at 50–55°C. Samples were reconstituted with acetonitrile–water (1:1, v/v), vortexed, and slowly filtered through a syringe PVDF filter (pore size 0.22 μm) into a vial.
2.3. Instrumentation LC/MS/MS
Extracted samples were subjected to chromatographic analysis using Finnigan surveyor MS pump plus (Thermo Fisher Scientific, Massachusetts, USA), and separation was carried out using a reversed-phase Hypersil Gold column (50 mm × 2.1 mm, particle size 3 μm, from Thermo Electron Corporation, MA, USA). Separation of analyte as retention time in chromatogram was achieved with a mobile phase at eluent A combination of 0.01% acetic acid in water and acetonitrile (9:1, v/v) and eluent B combination of 5mM ammonium formate in methanol (HPLC grade), and acetonitrile (75:25, v/v) at a flow rate of 200 μL/minute. Pump gradient program elution started at 80% eluent A for 1 minute and linearly increased for 0.5 minute at 5% eluent A. The composition was held for 6.5 minutes before re-equilibration with 80% eluent A for 5 minutes. Injection volume was 15 μL, and the column compartment was maintained at 40°C.
Mass spectrometry analysis was performed using Finnigan TSQ Quantum Discovery triple quadrupole mass spectrometry (Thermo Fisher Scientific). The mass spectrometer was operated using electrospray ionization in positive mode (ESI+); the optimum parameters for ion source were the following: spray voltage 4500 V, sheath gas pressure 35 Arb, auxiliary gas pressure 8 Arb, capillary temperature 300°C, and collision cell pressure 1.5 mTorr. Data acquisition was carried out with scanning-type selected reaction monitoring with two product ions being monitored for quantification and qualification of each external standard, while the internal standard SEL was monitored at ion m/z 608.2.
2.4. Analytical curve
Detection of the lowest concentration of avermectins was carried out by LC/MS/MS using a spiked blank sample in the concentration range of 0.1–0.5 μg/kg. DL and QL for each avermectin were estimated based on the standard deviation of ratio response and slope. Values of ratio response and slope were obtained from the calibration curve or regression line [9]. Two types of standard deviations were used in estimating DL and QL: residual standard deviation of a regression line (sres) and standard deviation of y-intercept (syo), which can be calculated using function LINEST in Microsoft Office Excel.
Data were collected from the regression model at six points, for five avermectins in a spiked blank sample at various concentrations ranging from 0.4 μg/kg to 1.2 μg/kg. A response ratio between response of target compound and response of internal standard SEL were calculated against the concentration for each batches. The mean response ratio for three batches was calculated using Microsoft Office Excel (2007) spreadsheet; regression line response ratios were plotted against concentration.
2.5. Linearity and working range
Linearity was demonstrated by analyzing the spiked sample over the whole range from QL to 15 times of QL, to accommodate the routine analysis. According to the ICHQ2B guideline [9], six concentration levels were used in this study. Extraction was carried out on three different days with freshly prepared standard solution for measurement independence [13]. The ordinary least square regression calculated consisted of correlation coefficient, y-intercept, slope, residual, and respective variances. The linearity was assessed by visual evaluation of a plot of the difference response ratio versus the respective concentration level. It is a good indicator of the deviation in relation to the linearity assumption.
3. Results and discussion
3.1. DL and QL
In the concentration range of 0.1–0.5 μg/kg, all avermectins compounds were reliably detected at 0.4 μg/kg, but were not necessarily quantitated [16]. Results of the regression model for five avermectins in a spiked blank sample at six concentrations (from 0.4 μg/kg to 1.2 μg/kg) are shown in Table 1.
Table 1.
Regression line parameters for avermectin compounds.
| Compound | R 2 | Slope | y-Intercept | s yo | s res |
|---|---|---|---|---|---|
| EMA | 0.9331 | 3.3008 | −0.6184 | 0.2407 | 0.4051 |
| ABA | 0.9354 | 0.2079 | −0.0352 | 0.0131 | 0.0198 |
| DOR | 0.9407 | 0.3094 | −0.0280 | 0.0187 | 0.0281 |
| MOX | 0.9500 | 0.1897 | −0.0088 | 0.0118 | 0.0199 |
| IVE | 0.9221 | 0.9004 | −0.1888 | 0.0630 | 0.0948 |
ABA = abamectin; DOR = doramectin; EMA = emamectin; IVE = ivermectin; MOX = moxidectin; sres = residual standard deviation of a regression line; syo = standard deviation of y-intercept.
Two standard deviations, sres and syo obtained from Table 1, were used in estimating DL and QL, based on formula from ICH guideline (1996) [9] and the results are shown in Table 2. The results showed that DL and QL estimated based on the syo values were lower than those estimated based on the sres values in the regression line. This finding is also supported by Ribani et al [12] that the sres value showed a greater value limit compared to other approaches that used signal to noise and by syo of the regression line. Therefore, DL was estimated by sres due to the reliability of avermectin detection in spiked samples at concentrations of 0.4 μg/kg. In addition, sres values represent random experimental errors in the measurement of y, which are the differences between the experimental y-values and the fitted y-values [15].
Table 2.
Estimation of DL and QL for avermectins based on linear regression.
| Compound | Estimated DL (μg/kg) | Estimated QL (μg/kg) | ||
|---|---|---|---|---|
|
|
|
|||
| s yo | s res | s yo | s res | |
| EMA | 0.22 | 0.40 | 0.73 | 1.23 |
| ABA | 0.19 | 0.31 | 0.63 | 0.95 |
| DOR | 0.18 | 0.30 | 0.60 | 0.91 |
| MOX | 0.19 | 0.35 | 0.62 | 1.05 |
| IVE | 0.21 | 0.35 | 0.70 | 1.05 |
ABA = abamectin; DL = detection limit; DOR = doramectin; EMA = emamectin; IVE = ivermectin; MOX = moxidectin; QL = quantification limit; sres = residual standard deviation of a regression line; syo = standard deviation of y-intercept.
As the values of QL estimated based on sres varied (Table 2), 10 blank samples were spiked at 1 μg/kg for each compound after rounding up and down the estimated QL to facilitate spiking in the sample. The result showed that precision, expressed in terms of the relative standard deviation, for all compounds was in the range of 7–19%, which is lower than that suggested by the Horwitz equation (relative standard deviation of 45%) [17].
3.2. Linearity and working range
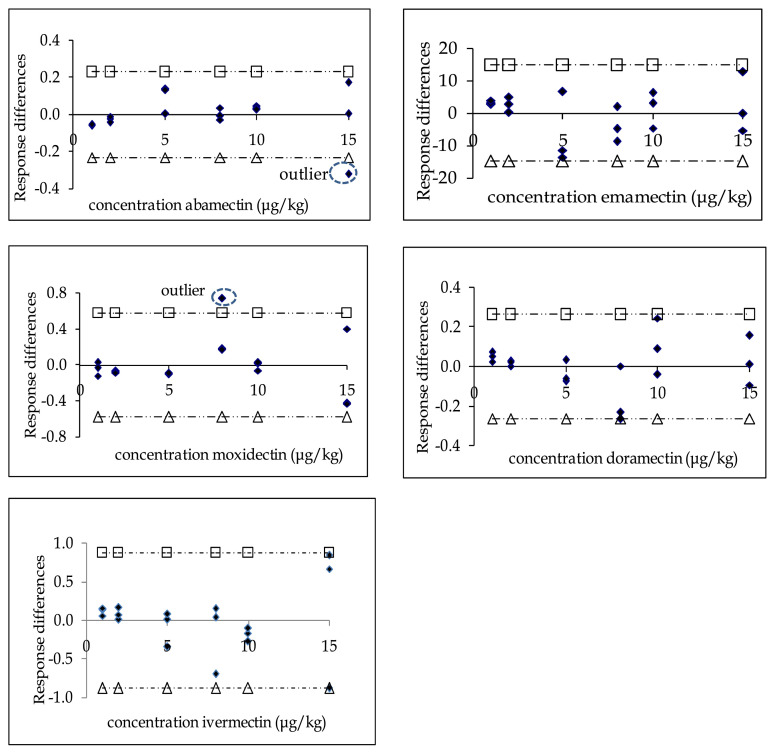
Results of random scatter of y-residual of all avermectins are shown in Fig. 1. As can be seen from the figure, two compounds, ABA and MOX, demonstrated outlier data. The outlier data were statistically rejected due to the values that fall outside the two horizontal dotted–dashed lines corresponding to ±t(0.95, np − 2).sres. The rejected values may be due to the relative error of this method. The outlier data for ABA and MOX were recalculated by the ordinary least square regression method for further evaluation. Both compounds were found to have no outlier rejection, and the estimated regression models were selected.
Fig. 1.
Random scatter of y-residual of avermectins. Symbol ◆ represents response differences, dotted–dashed △ represents −t(0.95, np − 2).sres , and dotted-dashed □ represents −t(0.95, np − 2).sres values.
Because the linearity of all avermectins was estimated, verification was carried out to determine whether the linearity and models are correct. The significant tests were calculated in a form of analysis of variance, and the results are shown in Table 3. At concentrations of 1–15 μg/kg, the regression and linearity for all compounds were accepted, where the value of Fisher ratio was higher than F critical at α = 0.05. The results proved that response values were significantly correlated to the compound concentrations. Although all compounds gave accepted regression model with linear correlation, not all compounds produced an R2 value closer to 1. Sanagi et al [11] also supported the finding that the correlation R2 is not necessarily based on the outcome of the linear relationship.
Table 3.
Confirmation test of linearity for avermectins.
| Sources of variation | Sum of squares | Degrees of freedom | Mean squares | Fisher ratio | F crit a | Conclusion |
|---|---|---|---|---|---|---|
| EMA (1–15 μg/kg) | ||||||
| Regression | 14,358.467 | 1 | 14,358.467 | 314.276 | 4.747 | Regression accepted |
| Lack of fit | 232.250 | 4 | 58.062 | 1.271 | 3.259 | Linearity accepted |
| Pure error | 548.248 | 12 | 45.687 | |||
| Total | 15,138.965 | 17 | ||||
| ABA (1–15 μg/kg) | ||||||
| Regression | 12.285 | 1 | 12.285 | 5133.736 | 4.747 | Regression accepted |
| Lack of fit | 0.028 | 4 | 0.007 | 2.930 | 3.259 | Linearity accepted |
| Pure error | 0.029 | 12 | 0.002 | |||
| Total | 12.342 | 17 | ||||
| DOR (1–15 μg/kg) | ||||||
| Regression | 44.885 | 1 | 44.885 | 4398.502 | 4.747 | Regression accepted |
| Lack of fit | 0.125 | 4 | 0.031 | 3.057 | 3.259 | Linearity accepted |
| Pure error | 0.122 | 12 | 0.010 | |||
| Total | 45.132 | 17 | ||||
| MOX (1–15 μg/kg) | ||||||
| Regression | 23.855 | 1 | 23.855 | 605.673 | 4.747 | Regression accepted |
| Lack of fit | 0.145 | 4 | 0.036 | 0.923 | 3.259 | Linearity accepted |
| Pure error | 0.473 | 12 | 0.039 | |||
| Total | 24.473 | 17 | ||||
| IVE (1–15 μg/kg) | ||||||
| Regression | 286.407 | 1 | 286.407 | 1458.989 | 4.747 | Regression accepted |
| Lack of fit | 0.396 | 4 | 0.099 | 0.504 | 3.259 | Linearity accepted |
| Pure error | 2.356 | 12 | 0.196 | |||
| Total | 289.158 | 17 | ||||
ABA = abamectin; DOR = doramectin; EMA = emamectin; IVE = ivermectin; MOX = moxidectin.
Critical value of F distribution at α = 0.05.
As mentioned in the International Union of Pure and Applied Chemistry (IUPAC) guideline [18], a significance test to determine whether the intercept differs from zero and also whether the linear calibration curve passes through the origin needs to be performed. A further t test was performed, and results are shown in Table 4. All compounds gave a calculated two-tailed t-test lower than t test (tcrit), and it was confirmed that the y-intercept passes through zero. Therefore, a simpler calibration curve that includes zero can be applied for a routine analysis.
Table 4.
Summary of linear regression of avermectins.
| Compound | Linear range (μg/kg) | Calibration linear equation | R 2 | t cal | t crit a | Passes through origin |
|---|---|---|---|---|---|---|
| EMA | 1–15 | y = 5.8715x − 1.3775 | 0.9484 | 0.482 | 2.776 | Yes |
| ABA | 1–15 | y = 0.1885x − 0.0491 | 0.9954 | 1.905 | 2.776 | Yes |
| DOR | 1–15 | y = 0.3283x − 0.0496 | 0.9945 | 0.975 | 2.776 | Yes |
| MOX | 1–15 | y = 0.2344x + 0.0106 | 0.9747 | 0.041 | 2.776 | Yes |
| IVE | 1–15 | y = 0.8292x − 0.2033 | 0.9905 | 1.197 | 2.776 | Yes |
ABA = abamectin; DOR = doramectin; EMA = emamectin; IVE = ivermectin; MOX = moxidectin.
Critical value at n = 2 degrees of freedom and 95% confidence limit.
In conclusion, the statistical approach by assessing linear regression was reliable to validate estimated DL and QL. The working range was accepted the linearity for the routine analysis of avermectins. Therefore, this approach can be used for in-house validation procedure. Calculations were performed using Microsoft Office Excel (2007) spreadsheet and did not require any statistical program. The spreadsheet can also be used as a template for other analyses.
Acknowledgments
We thank Universiti Putra Malaysia; the National Public Health Laboratory, Ministry of Health; and the Food Safety and Quality Division, Ministry of Health, for their technical support, research facilities, and financial support.
Funding Statement
We thank Universiti Putra Malaysia; the National Public Health Laboratory, Ministry of Health; and the Food Safety and Quality Division, Ministry of Health, for their technical support, research facilities, and financial support.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1.Crosby NT. Determination of veterinary residues in food. England: Woodhead Publishing; 1991. [Google Scholar]
- 2. Stone J, Roy W, Sutherland I, et al. Safety and efficacy of emamectin benzoate administered in-feed to Atlantic salmon, Salmo salar L., smolts in freshwater, as a preventative treatment against infestations of sea lice, Lepeophtheirus salmonis (Krøyer) Aquaculture. 2002;210:21–34. [Google Scholar]
- 3.Botsoglou NA, Fletouris DJ. Drug residues in foods: pharmacology, food safety, and analysis. New York, NY: Marcel Dekker; 2001. [Google Scholar]
- 4. Grant A, Briggs AD. Use of ivermectin in marine fish farms: some concerns. Mar Pollut Bull. 1998;36:566–8. [Google Scholar]
- 5. Roth M, Richards R, Sommerville C. Current practices in the chemotherapeutic control of sea lice infestations in aquaculture: a review. J Fish Dis. 2006;16:1–26. [Google Scholar]
- 6.Anastassiades M, Scherbaum E, Bertsch D.Presented at the MGPR Symposium; Aix en Province, France; 2003. [Google Scholar]
- 7. Kinsella B, Lehotay SJ, Mastovska K, et al. New method for the analysis of flukicide and other anthelmintic residues in bovine milk and liver using liquid chromatography–tandem mass spectrometry. Anal Chim Acta. 2009;637:196–207. doi: 10.1016/j.aca.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 8. Kinsella B, Whelan M, Cantwell H, et al. A dual validation approach to detect anthelmintic residues in bovine liver over an extended concentration range. Talanta. 2010;83:14–24. doi: 10.1016/j.talanta.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 9.ICHQ2B. Validation of analytical procedures, methodology. International Conference on the Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) Q2B; 1996; [Google Scholar]
- 10. Chen YA, Hsu KY. Development of a LC–MS/MS-based method for determining metolazone concentrations in human plasma: application to a pharmacokinetic study. J Food Drug Anal. 2013;21:154–9. [Google Scholar]
- 11. Sanagi MM, Ling SL, Nasir Z, et al. Comparison of signal-to-noise, blank determination, and linear regression methods for the estimation of detection and quantification limits for volatile organic compounds by gas chromatography. J AOAC Int. 2009;92:1833–8. [PubMed] [Google Scholar]
- 12. Ribani M, Collins CH, Bottoli CBG. Validation of chromatographic methods: evaluation of detection and quantification limits in the determination of impurities in omeprazole. J Chromatogr A. 2007;1156:201–5. doi: 10.1016/j.chroma.2006.12.080. [DOI] [PubMed] [Google Scholar]
- 13. Sanagi MM, Nasir Z, Ling SL, et al. A practical approach for linearity assessment of calibration curves under the International Union of Pure and Applied Chemistry (IUPAC) guidelines for an in-house validation of method of analysis. J AOAC Int. 2010;93:1322–30. [PubMed] [Google Scholar]
- 14. de Souza SVC, Junqueira RG. A procedure to assess linearity by ordinary least squares method. Anal Chim Acta. 2005;552:25–35. [Google Scholar]
- 15.Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. London: Prentice Hall; 2005. [Google Scholar]
- 16. ISO. Standard ISO 11843-1. 2 Capability of detection. 1997 [Google Scholar]
- 17. Horwitz W, Albert R. The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int. 2006;89:1095–109. [PubMed] [Google Scholar]
- 18. Thompson M, Ellison SLR, Wood R. Harmonized guidelines for single-laboratory validation of methods of analysis. Pure Appl Chem. 2002;74:835–55. [Google Scholar]