Background
Pyruvic acid concentration is a Allium spp. pungency. This study was initiated to accurately measure the background pyruvic acid levels in Romanian Allium spp. From the pungency point of view, all analyzed plant varieties in this study are considered low pungent cultivars based on the enzymatically produced pyruvate level (between 42 μmol/g and 222 μmol/g fresh wt). Chromatographic analysis was carried out for the different varieties of the most popular fresh leaves (Allium cepa var. “Diamant”, Allium cepa var. “Rubiniu”, and Allium ursinum L.) in order to identify the sulfur compounds. The thin layer chromatography analysis led to the identification of allicin, with Rf = 0.377–0.47, as an important sulfur compound. The gas chromatography-mass spectrometry analysis of the leaves’ extracts detected disulfides as the major sulfur compounds. Principal component analysis was performed to establish the differences in plant composition. These studies suggest the potential good uses of the fresh leaves of Romanian Allium spp. as condiment, ingredient, or preservative in the food industry.
Keywords: Allium cepa, Allium ursinum L., Bioactive compounds, Leaves, Thin layer chromatography
1. Introduction
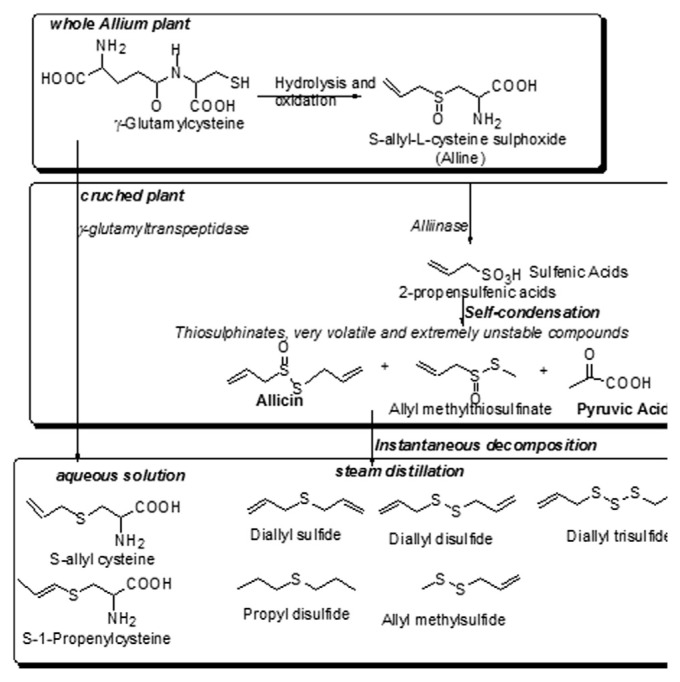
Allium is the most representative genus of the Liliaceae family, which includes 700 species of widely distributed bulbous perennials and biennials [1,2], and is an economically important genus because of the huge quantities of onions consumed. Nowadays, the Allium spp. plants are considered the most important vegetables consumed fresh or in different dishes in Europe, Asia, and America [3]. In Romania, over the past years, significant changes have taken place in the vegetables market. According to the statistical data compiled by the Ministry of Agriculture, in 2010, the production of dried onions was estimated at 362.3 thousand tons, placing it in third place [4]. Allium spp. plants are used as common foods and as agents for treatment of many diseases [5] because they contain phytonutrients. In fact, the edible parts of Allium spp. plants are used for the treatment and prevention of a large number of diseases (e.g., coronary heart disease [6–8], cancer [9,10], obesity, diabetes, disturbances of the gastrointestinal tract, hypercholesterolemia, and inflammatory diseases [11–13]), because they contain volatile sulfur-containing compounds, which have a distinct flavor and are responsible for the pungent odor. A special class of biologically active organo-sulfuric compounds, S-alk(en)yl-l-cysteine sulfoxides (such as alliin and γ-glutamylcysteines), dominates onion and wild garlic flavor. In the intact cells, the sulfoxides are found in the cytoplasm and in the vacuole. Disruption of plant material results in the hydrolysis of alk(en)yl cysteine sulfoxides, thus creating volatile alk(en)yl-thiosulfinates such as allicin and lipid-soluble sulfur compounds (such as diallyl sulfide, diallyl disulfide), which are the principal flavor compounds (Fig. 1).
Fig. 1.
Enzymatically produced sulfur compounds and pyruvic acid in Allium species.
These compounds are responsible for the characteristic smell and taste of Allium species plants [14] as well as most of their biological properties [5]. The main thiosulfinate compound present in onions and wild garlic extracts is allicin, also called diallyl thiosulfinate [15]. For Allium spp., pungency is very important, and the determination of pyruvate, which is formed as a stable primary compound from the enzymatic decomposition of each of the flavor precursors, is a good method for pungency assessment in onion and wild garlic [16,17] (Fig. 1). The health benefits of the bioactive compounds from Allium spp. plants have been demonstrated [18–20], so some people commonly consume these plants in an uncooked state. The aim of this study was to identify and quantify the sulfur compounds from Romanian Allium spp. leaves, as an important constituent of traditional foods. In order to assess the pungency level, thiosulfinate concentration, chromatographic identification of sulfur compounds, and antioxidant activity of three Romanian Allium spp. varieties (white onion, red onion, and wild garlic leaves) were analyzed.
2. Materials and methods
2.1. Plant samples
The most popular varieties of Romanian fresh onion leaves [Allium cepa L. var. “Diamant” (white onion), A. cepa var. “Rubiniu” (red onion)] from early hybrid groups were kindly supplied by the Research and Development Society for Vegetables Buzău, Romania. Fresh Allium ursinum L. leaves were collected from the forests of Bacău City in March 2011. All plant samples were free of external damages, hand selected, and chopped in the mortar before analysis.
2.2. Chemicals and materials
Vanillin and silica gel 60 F254 plates were supplied by Merck (Darmstadt, Germany). Toluene, glacial acetic acid, dichloromethane, and methanol were purchased from Lach-Ner s.r.o (Brno, Czech Republic). In addition, 0.0125% 2,4-dinitro-phenyl hydrazine (DNPH; with 95% purity), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and ethyl acetate were obtained from Sigma-Aldrich (Steinheim, Germany). Ultrapure water (TKA Smart 2 Pure UV/UF system; Niederelbert, Renania-Palatinat, Germany) was used for the experiments.
2.3. Thin layer chromatography identification of volatile sulfur compounds
The TLC (thin layer chromatography) pattern of various extracts can vary according to the extraction methods. The Wagner and Bladt method [21] was adopted to obtain the dichloromethane extracts of fresh samples of A. cepa var. “Diamant” (white onion leaves), A. cepa var “Rubiniu” (red onion leaves), and A. ursinum (wild garlic leaves), and spotted on Silica gel 60 F254 plates. After impregnation in the developing system with toluene/ethyl acetate (10:3), the silica gel plates were dried at 105°C and immersed in the detection system solvent (1 g vanillin to 100 mL glacial acid reagent). Sulfur compounds were visible as yellow, green, and brownish spots, and identified using retention factor (Rf) values.
2.4. Volatile sulfur compounds extraction
Fresh leaves (20 g; A. cepa var. “Rubiniu” and A. ursinum L.) were subjected to hydrodistillation using the Clenvenger apparatus. The fresh hydrodistillates were immediately analyzed by gas chromatography-mass spectrometry (GC-MS), and the antioxidant activity was determined.
2.5. GC-MS
Analysis was run on a Thermo Scientific ITQ-900 system (Trace GC Ultra/ITQ 900, Thermo, Milano, Italy) coupled with an ionic trap and fitted with a TG-XLB-MS capillary column with 0.25 μm film thickness, 30 m length, and 0.25 mm inner diameter, using helium as gas carrier with a flow rate of 1 mL/minute. The operating conditions were as follows: injector port temperature 250°C, split ratio 20:20, detector temperature 270°C, and program temperature from 40°C to 100°C with 4°C/minute, from 100°C to 150°C with 4°C/minute and from 150°C to 270°C with 10°C/minute.
The MS conditions were as follows: ionization voltage 70 eV, ion source temperature 200°C, scan range m/z 50–450. The qualitative identification of the sulfur compounds was based on computer matching with Wiley9 library and by comparison with data in the literature.
2.6. Determination of pyruvic acid
The enzymatically produced pyruvic acid content was estimated using the dinitrophenyl hydrazine (DNPH) reagent [17,22] and was calculated using the equation:
| [1] |
where PT is the total pyruvic acid (μmol/g fresh wt) and PC is the control level of endogenous pyruvic acid (μmol/g fresh wt). For a total pyruvic acid determination (PT), around 1 g of the selected plants was homogenized for 2 minutes in an ultrasound bath with distilled water. The homogenate was allowed to stand for 15 minutes at room temperature, and then quantitatively filtrated through a Whatman cellulose filter paper. Endogenous pyruvic acid was also measured in conjunction with the total pyruvic acid. This background amount of pyruvic acid is subtracted from the total pyruvic acid to obtain the enzymatically produced pyruvic acid. A certain amount of pyruvic acid exists endogenously in these plants. Pyruvic acid is a major product formed via glycolysis and other metabolic processes. The heat provided in the microwave step denatures the enzyme, alliinase, and therefore stops the reaction. The control level of endogenous pyruvic acid (Pc) was determined after the microwave treatment (Microwave oven Panasonic NN-E202W model; power to 1000 W) of the plant sample during 30 seconds to deactivate alliinase, wherein 1 g of the microwave-treated plant was ultrasound homogenized for 2 minutes in distilled water and allowed to stand at room temperature for 15 minutes, and then filtrated. For each type of filtrate, 2 mL clarified filtrate was taken, and to this 1.0 mL of 0.0125% DNPH [prepared in 2 N HCl (hydrochloric acid)] was added. The reaction mixture was placed in a water bath at 37°C for 15 minutes. After removing the samples from the water bath, 5.0 mL of 0.6 N NaOH was added. The absorbance was recorded at 515 nm. An extended standard curve was constructed for pyruvic acid (ranging from 0.05 μmol/mL to 1 μmol/mL), and the pyruvic acid levels in samples were determined by directly comparing the absorbance rates with those on the curve.
2.7. DPPH assay
Antioxidant activity was measured by the free radical DPPH as previously described [2,23,24]. The samples (0.5 mL) were mixed with 1 mL of 0.0035% of DPPH solution in methanol, and 95% MeOH was added to a final volume of 4 mL. The absorbance of the resulting solutions and the blank (with the same chemicals, except sample) was recorded after 1 hour at room temperature. The disappearance of DPPH was measured spectrophotometrically at 515 nm. The results, expressed as a percentage, were calculated using the following equation:
| [2] |
where A0 is the absorbance of the control reaction (containing all reagents except the extract) and A1 is the absorbance of the analyzed extract.
2.8. Statistical analysis
The experimental results were analyzed using principal component analysis (PCA) with full cross-validation. PCA constitutes the most basic statistical method of all multivariate data analysis, and involves decomposing one “data matrix” into a structural part (model) and a “noise” part (error). The main purpose of all multivariate data analyses is to decompose the data in order to detect and model “hidden phenomena”. PCA was assessed using the Unscrambler X 10.1 software version from CAMO Software AS (Oslo, Norway). PCA was used to evaluate the experimental results for pyruvic acid, thiosulfinate concentrations, and antioxidant activity for all the studied plant species.
3. Results and discussion
3.1. TLC identification
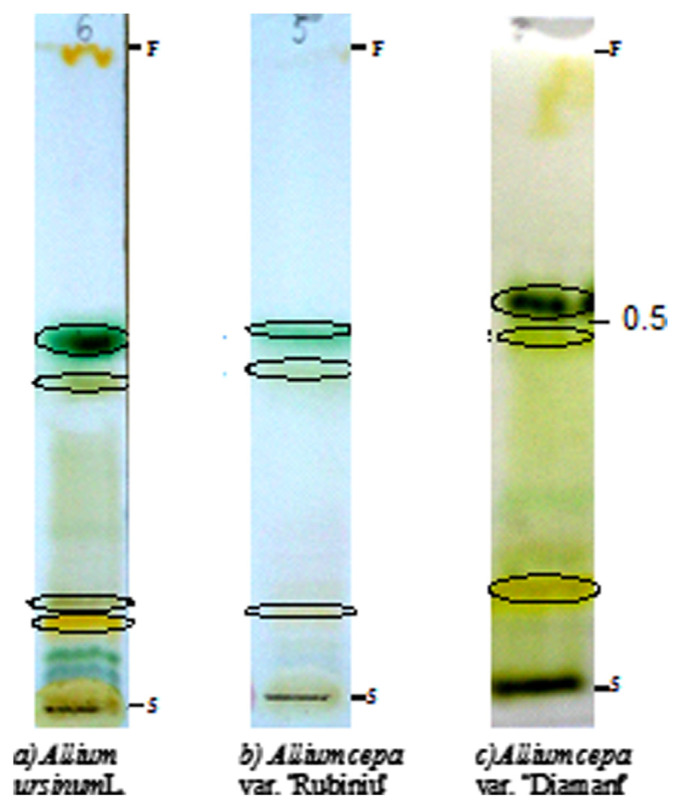
The TLC method was applied for identification of sulfur compounds. Fresh extracts showed brown or dark yellow zones, with these colors being specific for thiosulfinate compounds. After treatment with the detection system (Fig. 2), A. ursinum (wild garlic leaves) mainly showed yellow-brown (Rf = 0.477–0.151), yellow, or dark yellow (Rf = 0.129) spots. Extracts of A. cepa var. “Rubiniu” (red onion leaves) showed brown and yellow-brown zones with Rf values of 0.488 and 0.213, respectively. Moreover, the extract of A. cepa var. “Diamant” is distinguishable by the characteristic yellow-brown and gray-yellow zones with Rf values of 0.377 and 0.209, respectively. The Rf values of 0.377–0.47 allowed the conclusion that the method is adequate to identify allicin. In agreement with other reports [21], all the leaf extracts also presented other compound-specific zones (dark green) for chlorophyll with an Rf range of 0.547–0.436, and ajoens or cepaenes with a low Rf range between 0.056 and 0.078. The brown zones at the solvent front are attributable to sulfides such as allyl disulfide. The brown zones from the starting line may be attributable to degradation products.
Fig. 2.
Thin layer chromatography chromatograms for dichloromethane extracts of the fresh leaves (brown zones at the solvent front—sulfides; brown or dark yellow zones—thiosulfinates; dark green—chlorophyll).
3.2. Volatile sulfur compounds analysis
A. cepa var. “Rubiniu” and A. ursinum L. fresh hydrodistillate were analyzed to identify the sulfur compounds. The identified sulfur compounds and their mass spectral data are listed in Table 1 [25–27] (A. cepa var. “Rubiniu” hydrodistillate) and Table 2 [27] (A. ursinum L. hydrodistillate), respectively.
Table 1.
Sulfur compounds identified in Allium cepa var. “Rubiniu” hydrodistillate.
| No. | Sulfur compound | Retention time, tr (min) | Mass spectral data (relative abundance, %) | Probability, % |
|---|---|---|---|---|
| 1. | Allyl methyl disulfidea,b | 10.71 | 120 (M*, 100), 105 (1.6), 80 (9.3), 79 (17.4), 73 (8.2), 71 (7.4), 64 (12.9) | 70 |
| 2. | Methyl propyl disulfidea,c | 17.35 | 122 (90), 80 (M*, 100), 41(45), 43(38), 45(25), 64 (15) | 48.64 |
| 3. | 2.4-Dimethyl thiophenea,b | 18.21 | 111(M*, 100), 97 (40), 112(78) | 74,7 |
| 4. | Dipropyl disulfidea,c | 23.05 | 150 (37), 108 (24), 43(M*, 100), 41 (22), 27 (15) | 43.5 |
| 5. | 1.5-Dithiocanea | 25.17 | 148 (60), 106 (M*, 100), 41 (35), 45 (30), 46 (20), 47 (14), 73 (20), 74 (14) | 56.2 |
Identified by mass spectral data in the Wiley date base library stored in the gas chromatography-mass spectrometry system.
Identified by mass spectral data compared with data reported in the literature [27].
Table 2.
Sulfur compounds identified in hydrodistillate from wild garlic leaves.
| No. | Compounds name | Retention time, tR (min) | Mass spectral date (relative abundance), % |
|---|---|---|---|
| 1. | Dimethyl trisulfide (DMTS)a,b | 23.47 | 126 (M*, 100); 79 (42), 47 (30), 46 (20), 64 (20), 80 (16), 128 (14) |
| 2. | cis-Methyl propenyl disulfide | 19.79 | 120 (M*, 100); 72 (56), 75 (39), 80 (28), 87 (12) |
| 3. | Dimethyl disulfide (DMS)b | 11.51 | 94 (M*, 100); 79(64), 45(68), 47(28), 61(20) |
| 4. | 1.3-Dithianec | 19.45 | 119.8 (M*, 100); 86.9 (34), 72.9 (50), 63.8 (38) |
| 5. | trans-Methyl-propenyl sulfideb | 7.69 | 73 (M*, 100); 88 (98), 45 (M*, 100), 39 (50), 47 (30), 72 (17) |
| 6. | Methyl propyl disulfideb | 17.49 | 122 (70); 80 (M*, 100), 43 (69), 41(52), 45 (40), 47 (20), 64 (15), 94 (10) |
Identified by mass spectral data in the Wiley date base library stored in the gas chromatography-mass spectrometry system.
Identified by mass spectral data compared with data reported in the literature [27].
Detected only in Allium ursinum L. hydrodistillate.
The identified components are disulfides with different radicals (allyl, methyl, and propyl) that are specific for Allium varieties leaves, and they are mainly presented in hydrodistillates. This aspect is very important because these compounds can be produced during the cooking process [14].
In comparison, in the A. ursinum L. hydrodistillate, based on mass spectrum analysis and mass fragmentation at m/z 119.8, 1.3-dithiane was identified (Table 2).
3.3. Pyruvic acid, thiosulfinate, and antioxidant activity from Allium spp. leaves
The pyruvic acid that universally exists in the plant tissue and is an indicator of pungency in onion plants [17] was determined. Estimation of the pungency level, which is an important attribute, enables the classification of onion leaves and bulbs. Pungency was quantitatively determined by measuring the enzymatically produced pyruvic acid. All the analyzed plant samples can be considered low pungent cultivars because of the concentration of enzymatically produced pyruvic acid (42.6–222.422 μmol/g fresh wt). Calculation for thiosulfinate compounds is based on ½ mol thiosulfinate for each mole of pyruvic acid produced. The antioxidant activity of some Allium species is attributed to sulfur-containing constituents and their precursors [2]. Because of its stability (in radical form) and the simplicity of the assay, the DPPH radical (2,2-diphenyl-1-pic-rylhydrazyl) is one of the most commonly used substrates for the fast evaluation of antioxidant activity [23].
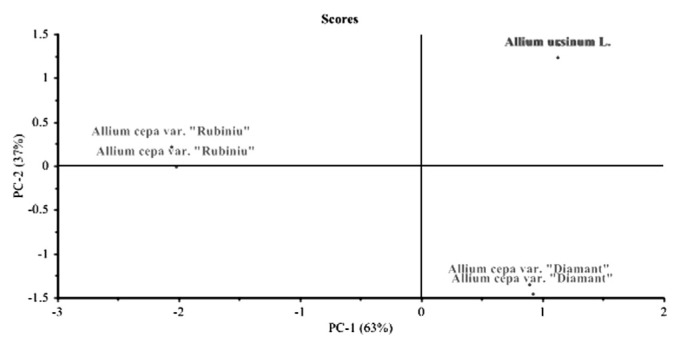
Statistical analysis indicates that two PCs account for 99.89% of the data variation. All three variants of Allium were very well defined as different plant types owing to their different chemical properties.
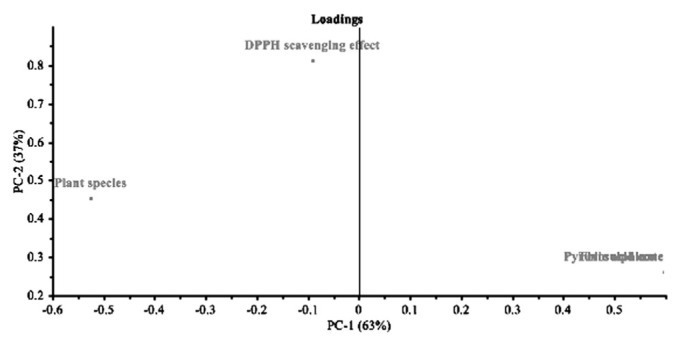
PC1 was given by the pyruvic acid and thiosulfinate concentrations found in fresh plants. Furthermore, pyruvic acid and thiosulfinate concentrations were very well correlated to each other, but negatively correlated with plant species (Figs. 3 and 4).
Fig. 3.
Scores plot of the analyzed Allium fresh plant extracts.
Fig. 4.
Loading plot of the variables used to describe Allium fresh plant extracts.
PC2, which explains almost 37% of the data variation, was given by the DPPH scavenging activities of the plant hydrodistillates. DPPH scavenging activity was uncorrelated with the other variables. We observed that all three types of plants were very different from each other according to their pyruvic acid content, thiosulfinate concentrations, and DPPH scavenging activity.
A. ursinum L. and A. cepa var. “Diamant” have similar concentrations of pyruvic acid and thiosulfinate, but were different in terms of antioxidant activity. We observed that A. ursinum L. leaves (with % RSC = 32.41 ± 0.777) presented the highest value, whereas that of A. cepa var. “Diamant” showed the weakest antioxidant activity (with % RSC = 19.63 ± 0.535). A. cepa var. “Rubiniu” presented an antioxidant activity of % RSC = 28.01 ± 0.815. A. ursinum L. has the highest amounts for all analyzed parameters.
A. cepa var. “Rubiniu” contains the lowest amounts of pyruvic acid and thiosulfinate concentrations. These differences can be attributed to the natural qualitative and quantitative variability in the raw material. Therefore, A. ursinum L. leaves are a natural source of good protection against the oxidative damage that occurs in our body; unfortunately, they are available in fresh form only during spring.
Outliers have not been detected. The Hotellings T2 statistic, one of the most powerful outlier diagnostics used in multivariate analysis problems, did not detect outliers at the 5% significance level. Furthermore, the explained variance in validation follows the general pattern, indicating the absence of outliers.
4. Conclusion
TLC analysis allowed the identification of allicin, the main thiosulfinate compound that is formed by the action of the enzyme alliinase on alliin. Pyruvic acid determination is an indirect measure of total thiosulfinate present in disrupted plant tissue; however, it is lacking in specificity and cannot provide any differentiation of the individual alk(en)yl thiosulfinates. Further chromatographic and biological studies are needed to identify the constituents and precisely evaluate their biological activities and mechanisms. There seems to be an urgent need to validate available research data using evidence-based methodology to reach decisive conclusions. Our study may be considered a new report on the identification and characterization of bioactive compounds in fresh leaves of Romanian Allium spp. as natural and important food sources, especially during the spring season. PCA analysis allowed us to validate the experimental results and to distinguish the Allium plant species based on their chemical composition.
Acknowledgments
This work has benefited from the financial and technical support of the project RE-SPIA (695/09.04.2010, SMIS code 11377), financed by REGIO (Regional Operational Programme 2007–2013) and implemented by the Faculty of Food Science and Engineering, “Dunarea de Jos” University of Galati, Galat ‚i, Romania.
Funding Statement
This work has benefited from the financial and technical support of the project RE-SPIA (695/09.04.2010, SMIS code 11377), financed by REGIO (Regional Operational Programme 2007–2013) and implemented by the Faculty of Food Science and Engineering, “Dunarea de Jos” University of Galati, Galat ‚i, Romania.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1. Najjaa H, Neffati M, Zouari S, et al. Essential oil composition and antibacterial activity of different extracts of Allium roseum L., a North African endemic species. C R Chim. 2007;10:820–6. [Google Scholar]
- 2. Tepe B, Sokmen M, Akpulat Askin H, et al. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005;92:89–92. [Google Scholar]
- 3. Djurdjevic L, Dinic A, Pavlovic P, et al. Allelopathic potential of Allium ursinum L. Biochem Syst Ecol. 2004;32:533–44. [Google Scholar]
- 4.FRD Center Market reportReport. FRD Center Market Entry Services. Demo report of the Romanian agribusiness market. Apr, 2011. [accessed 04, 06,11]. Available at: http://www.market-entry.ro.
- 5. Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 6. Gorinstein S, Jastrzebski Z, Namiesnik J, et al. The atherosclerotic heart disease and protecting properties of garlic: contemporary data. Mol Nutr Food Res. 2007;51:1365–81. doi: 10.1002/mnfr.200700064. [DOI] [PubMed] [Google Scholar]
- 7. Hiyasat B, Sabha D, Grotzinger K, et al. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology. 2009;83:197–204. doi: 10.1159/000196811. [DOI] [PubMed] [Google Scholar]
- 8. Siegel G, Malmsten M, Pietzsch J, et al. The effect of garlic on arteriosclerotic nanoplaque formation and size. Phytomedicine. 2004;11:24–35. doi: 10.1078/0944-7113-00377. [DOI] [PubMed] [Google Scholar]
- 9. Sengupta A, Ghosh S, Bhattacharjee S. Allium vegetables in cancer prevention: an overview. Asian Pac J Cancer Prev. 2004;5:237–45. [PubMed] [Google Scholar]
- 10. Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–81. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11. Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry. 2002;48:209–15. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 12. Kalayarasan S, Prabhu PN, Manikandan R, et al. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol. 2009;606:162–71. doi: 10.1016/j.ejphar.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi M, Shibamoto T. Chemical compositions and antioxidant/anti-inflammatory activities of steam distillate from freeze-dried onion (Allium cepa L.) sprout. J Agric Food Chem. 2008;56:10462–7. doi: 10.1021/jf801220b. [DOI] [PubMed] [Google Scholar]
- 14. Gođevac D, Vujisić L, Mojović M, et al. Evaluation of antioxidant capacity of Allium ursinum L. volatile oil and its effect on membrane fluidity. Food Chem. 2008;107:1692–700. [Google Scholar]
- 15. Kimbaris AC, Siatis NG, Daferera DJ, et al. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum) Ultrason Sonochem. 2006;13:54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Teare Ketter CA, Randle WM.Pungency assessment in onions. In: Karcher SJ, editor. Tested studies for laboratory teaching; Proceedings of the 19th Workshop/Conference of the Association for Biology Laboratory Education; Able; 1998; pp. 177–96. [Google Scholar]
- 17. Dhumal K, Datir S, Pandey R. Assessment of bulb pungency level in different Indian cultivars of onion (Allium cepa L.) Food Chem. 2007;100:1328–30. [Google Scholar]
- 18. Santas J, Carbó R, Gordon MH, et al. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008;107:1210–6. [Google Scholar]
- 19. Gennaro L, Leonardi C, Esposito F, et al. Flavonoid and carbohydrate contents in Tropea red onions: effects of homelike peeling and storage. J Agric Food Chem. 2002;50:1904–10. doi: 10.1021/jf011102r. [DOI] [PubMed] [Google Scholar]
- 20. Sellappan S, Akoh CC. Flavonoids and antioxidant capacity of Georgia-grown Vidalia onions. J Agric Food Chem. 2002;50:5338–42. doi: 10.1021/jf020333a. [DOI] [PubMed] [Google Scholar]
- 21.Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. 2nd ed. Heidelberg: Springer-Verlag; 1996. pp. 302–3. [Google Scholar]
- 22. Anthon GE, Barrett DM. Modified method for the determination of pyruvic acid with DNPH in the assessment of onion pungency. J Sci Food Agric. 2003;83:1210–3. [Google Scholar]
- 23. Bozin B, Mimica-Dukic N, Samojlik I, et al. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chem. 2008;111:925–9. [Google Scholar]
- 24. Mimica-Dukic N, Bozin B, Sokovic M, et al. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J Agric Food Chem. 2004;52:2485–9. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- 25. Abu-Lafi S, Dembicki JW, Goldshlag P, et al. The use of the ‘Cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the Allium sativum. J Food Compos Anal. 2004;17:235–45. [Google Scholar]
- 26. Yabuki Y, Mukaida Y, Saito Y, et al. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler) Food Chem. 2010;120:343–8. [Google Scholar]
- 27. Wu T, Wang X, Li D, et al. Emission of volatile organic sulfur compounds (VOSCs) during aerobic decomposition of food wastes. Atmos Environ. 2010;44:5065–71. [Google Scholar]