Abstract
The influence of medium composition and cultural conditions on simultaneous yield of mycelia, intracellular polysaccharide, adenosine, and mannitol by Cordyceps militaris CGMCC 2909 was investigated with desirability functions in this study. An optimization strategy based on the desirability function approach, together with response surface methodology (RSM) has been used to optimize medium composition, and the optimal medium was obtained via the desirability as follows: yeast extract 10.33 g/L, sucrose 27.24 g/L, KH2PO4 5.60 g/L and the optimal culture conditions are initial pH 6, 25°C, rotation speed 150 r/minute, inoculum size 4%(v/v), and medium capacity 40 mL/250 mL. Under these conditions, the yield of mycelia, intracellular polysaccharide, adenosine and mannitol reached 12.19 g/L, 0.6 g/L, 61.84 mg/L, and 1.38 g/L, respectively, and the D value was 0.77. Furthermore, the polysaccharides showed significant antitumor activities against HeLa and HepG2 in vitro in a dose-dependent manner in 72 hours. At a concentration of 1000 mg/mL, the inhibition rate of polysaccharides was 92.38% and 98.79%. The IC50 for HeLa and HepG2 were 70.91 μg/mL and 97.63 μg/mL, respectively.
Keywords: Antitumor activities, Cordyceps militaris, Desirability function, Optimization, Polysaccharides
1. Introduction
Medicinal mushrooms are an abundant source of a wide range of useful natural products with biological activities. Cordyceps militaris, one of the Chinese traditional medicinal mushrooms, is an entomopathogenic fungus belonging to the class Ascomycetes. It has been widely used as folk tonic food and an invigorant for centuries and its various pharmacological activities have attracted much attention [1]. This mushroom produces many kinds of active components such as adenosine, polysaccharides, and mannitol [2–8]. Adenosine has many pharmacological effects; it can be used as a cardioprotective and therapeutic agent for chronic heart failure [9], and it also could inhibit the release of neurotransmitters in the central nervous system [10]. Polysaccharides are considered to possess antiinflammatory, antioxidant, antitumor, antimetastatic, immunomodulatory, hypoglycemic, steroidogenic, and hypolipidemic effects [11–17]. Mannitol (cordycepic acid) has diuretic, antifree radical, and antitussive activities, etc. [18].
Since solid culture of fungi takes a longer time to yield the fruiting body with slight production of an active substance, submerged cultivation of fungi is considered as a promising alternative for obtaining useful and potent substances for use in the composition of nutraceuticals and functional foods [19]. How to shorten the fermentation time of a fungus and increase the production of fermentation is still a challenge. Mycelium and biological activity metabolites were mainly used as a single response to optimize submerged cultivation [20,21]; however, two or multiple responses have already been used in the process optimization [22–24]. There was not, however, a solution to the problem of simultaneously enhancing the production of multiple responses in C. militaris submerged cultivation. Response surface methodology (RSM) is a collection of statistical and mathematical techniques and has been proven to be an effective mean, which includes studying the response of the statistically designed combinations, estimating the coefficients by fitting in a mathematical model which fits best the experimental conditions, predicting the response of the fitted model, and checking the adequacy of the model.
In recent years, studies of antitumor activities of C. militaris have been of particular interest. In a study by Park et al [25], an aqueous extract of C. militaris could inhibit cell growth of human leukemia U937 cells, by morphological change and apoptotic cell death. In a study by Yoo et al [26], C. militaris extract could reduce angiogenic related gene expression and inhibit cell growth of B16 melanoma cells. Cordycepin and polysaccharides, as the main constituents of Cordyceps species, are detected to have cytotoxic and antitumor activity [27–30].
In this study, we investigated the medium composition and cultural conditions for the submerged culture of C. militaris 2909 to obtain optimal production of mycelia, polysaccharides, adenosine, and mannitol, using sequential statistical methods combined with desirability function at the same time. In addition, the growth inhibitory effect of C. militaris polysaccharides on HeLa and HepG2 cells was studied.
2. Materials and methods
2.1. Microorganism and seed culture
The strain of C. militaris (CGMCC 2909) was maintained and cultured on potato-dextrose-agar slants, incubated at 25°C for 7 days, and then used for seed culture inoculation. The seed culture medium consisted of the following components: sucrose 25 g/L, peptone 10 g/L, yeast extract powder 20 g/L, KH2PO4 3 g/L, MgSO4·7H2O 3 g/L, (NH4)2SO4 10 g/L, ZnCl2 0.01 g/L, and vitamin B1 0.24 g/L. The fermentation medium was based on the seed culture medium to monitor carbon and nitrogen sources, mineral elements, etc. The flask culture incubated in a 250 mL Erlenmeyer flask containing 100 mL of the medium after inoculation with 2% (v/v) of the seed culture at 25°C on a rotary shaker incubator at 150 rpm for 5 days.
2.2. Analytical methods
2.2.1. Determination of mycelia dry weight
Mycelia of C. militaris in the fermentation broth were centrifuged, the supernatant was filtered through a filter paper and washed twice with distilled water, transferred to a lyophilizer in vacuo, dried to a constant weight and recorded as the dry weight, and then homogenized to powders (80 mesh).
2.2.2. Measurements of polysaccharides
Dried mycelia powders were extracted three times with distilled water for 3.5 hours in an 80°C water bath (mycelia/distilled water ratio, 1:5), and then centrifuged at 7000g for 10 minutes. The amount of polysaccharides was then determined by a phenol-sulfuric acid method.
2.2.3. Measurement of adenosine by high performance liquid chromatography
An amount of 0.1 g dried mycelia powders was extracted with 5 mL distilled water for 3 hours in a 45°C water bath, and then centrifuged at 12,000g for 10 minutes. The supernatant was filtered with a 0.45 μm membrane, and the filtrate was analyzed by high performance liquid chromatography. A Kromasil C18 column (4.6 mm × 250 mm, 5 μm particle size) (Eliter Company, Liaoning, China) was used. The mobile phase was 10 mM KH2PO4, which was dissolved in methanol/distilled water(15:85) and the mobile phase was driven by a double pump (model: Waters 150, Millipore, Bedford, USA). Elution was performed at a flow rate of 1 mL/minute with a column temperature of 30°C. The UV wavelength of 260 nm was monitored by a tunable absorbance detector (model: Waters 486, Millipore). A 20 μL syringe was used for injection.
2.2.4. Determination of mannitol
Dried mycelia powders (0.1 g) were extracted with 5 mL distilled water for 3 hours in a 45°C water bath, and then centrifuged at 8000g for 10 minutes. The supernatant was diluted 50 times. The amount of mannitol was then determined by the spectrophotometric method [31].
2.2.5. Desirability
In the desirability function approach, the multicriteria problem is reduced to a single criterion problem of D optimization. In the present study, theyield ofmycelia, polysaccharides, adenosine, and mannitol were considered as four quality variables. The higher yields of the four variables were sought, which include the most desirable (ymax) and the undesirable values (ymin). The measured properties of each predicted response y are transformed to a dimensionless desirability value d. The scale of value d ranges between 0 and 1, and becomes more desirable as the corresponding response value increases. The desirability function contributed by the response is defined as follows:
| (1) |
where d is the desirability. The desirability represents the closeness of a response (y) to its most desirable value (ymax), d = 1; if a response is below a minimum acceptable value (ymin), d = 0; when a response does not reach the most desirable value, d lies between 0 and 1. According to the preliminary experiments, ymax and ymin for four responses are shown in Table 1.
Table 1.
Response target, ymax, ymin and relative emphasis of Cordyceps militaris.
| Response | Polysaccharide (g/L) | Yield of mycelia (g/L) | Adenosine (mg/L) | Mannitol (g/L) |
|---|---|---|---|---|
| y min | 0.05 | 1 | 5 | 0 |
| y max | 1 | 15 | 100 | 2 |
| w i | 0.3 | 0.3 | 0.35 | 0.05 |
For the four response system in this study, the overall desirability (D) where each single function could be equally defined is:
| (2) |
where di represents the desirability function computed for response yi (i = 1,2,3, ...), and wi is relative emphasis, which reflects the difference in the importance assigned to different responses. In Eq. (1), wi satisfies 0 < wi < 1 and w1 + w2 + ...+ wm = 1. In the expression of the function D, wi is a comparative scale for allotting importance to each di.
Finally, associated D, as the mixture of four responses (yield of mycelia, intracellular polysaccharide, adenosine, and mannitol), is calculated from the above definition, and selected as a criterion for the optimization medium composition and cultural conditions.
2.3. Experiment designs and statistical analysis
2.3.1. Selection of significant variables by Plackett-Burman design
In this study, for selection of the most significant medium composition affecting the value of desirability (D), a total of 10 variables, including eight nutritional factors (X1/peptone, X2/yeast extract powder, X3/sucrose, X4/MgSO4·7H2O, X5/KH2PO4, X6/ZnCl2, X7/vitamin B1, X8/(NH4)2SO4) and two dummy or unassigned variables (X9, X10) were tested and identified by the Plackett-Burman design experiment. The principal effects of each variable onthevalueofdesirability (D) were represented at the high level and the low level. The experimental design with the variables, symbol code, and experimental level of the variables are shown in Tables 2, w and 3. The significance (p value) of the effect of each variable was determined by the Student t test.
Table 2.
Experimental variables at different levels used for the value of desirability using Plackett-Burman design.
| Variables | Units | Symbol code | Experimental values | |
|---|---|---|---|---|
|
| ||||
| Low (−) | High (+) | |||
| Peptone | g/L | X1 | 5 | 15 |
| Yeast extract powder | g/L | X2 | 5 | 15 |
| Sucrose | g/L | X3 | 10 | 30 |
| MgSO4·7H2O | g/L | X4 | 1 | 3 |
| KH2PO4 | g/L | X5 | 1 | 3 |
| ZnCl2 | g/L | X6 | 0.011 | 0.034 |
| Vitamin B1 | g/L | X7 | 0.050 | 0.150 |
| (NH4)2SO4 | g/L | X8 | 7 | 21 |
| Unassigned variable | – | X9 | – | – |
| Unassigned variable | – | X10 | – | – |
Table 3.
Twelve-trial Plackett-Burman design matrix for eight variables with experimental values along with the observed and predicted value of desirability.
| Run | Experimental values | Value of D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | ||
| 1 | 15 | 5 | 30 | 1 | 1 | 0.011 | 0.150 | 21 | (+) | (−) | 0.32 |
| 2 | 15 | 15 | 10 | 3 | 1 | 0.011 | 0.050 | 21 | (+) | (+) | 0.37 |
| 3 | 5 | 15 | 30 | 1 | 3 | 0.011 | 0.050 | 7 | (+) | (+) | 0.50 |
| 4 | 15 | 5 | 30 | 3 | 1 | 0.034 | 0.050 | 7 | (−) | (+) | 0.36 |
| 5 | 15 | 15 | 10 | 3 | 3 | 0.011 | 0.150 | 7 | (−) | (−) | 0.51 |
| 6 | 15 | 15 | 30 | 1 | 3 | 0.034 | 0.050 | 21 | (−) | (−) | 0.53 |
| 7 | 5 | 15 | 30 | 3 | 1 | 0.034 | 0.150 | 7 | (+) | (−) | 0.46 |
| 8 | 5 | 5 | 30 | 3 | 3 | 0.011 | 0.150 | 21 | (−) | (+) | 0.38 |
| 9 | 5 | 5 | 10 | 3 | 3 | 0.034 | 0.050 | 21 | (+) | (−) | 0.37 |
| 10 | 15 | 5 | 10 | 1 | 3 | 0.034 | 0.150 | 7 | (+) | (+) | 0.41 |
| 11 | 5 | 15 | 10 | 1 | 1 | 0.034 | 0.150 | 21 | (−) | (+) | 0.38 |
| 12 | 5 | 5 | 10 | 1 | 1 | 0.011 | 0.050 | 7 | (−) | (−) | 0.30 |
2.3.2. RSM, the Box-Behnken design
The Box-Behnken design (BBD) was conducted to find the optimum level of the most significant variables [yeast extract powder (X2), sucrose (X3), KH2PO4 (X5)] identified by the Plackett-Burman design and to study their interactions. Each effective variable in the design was studied at three different levels (coded as −1, 0 and +1), as is shown in Table 4. A total of 15 experiments were carried out and the whole experimental design with 12 factorial points and three center points is given in Table 6.
Table 4.
Experimental codes, ranges, and levels of the variables used for the Box-Behnken design (BBD).
| Variables | Units | Symbol code | Levels | ||
|---|---|---|---|---|---|
|
| |||||
| −1 | 0 | +1 | |||
| Yeast extract powder | g/L | X2 | 5 | 15 | 25 |
| Sucrose | g/L | X3 | 10 | 25 | 40 |
| KH2PO4 | g/L | X5 | 2 | 4 | 6 |
Table 6.
Experimental design and results of Box-Behnken design.
| Run | Code factor value | D | ||
|---|---|---|---|---|
|
| ||||
| Yeast extract powder | Sucrose | KH2PO4 | ||
| 1 | −1 | −1 | 0 | 0.38 |
| 2 | −1 | 1 | 0 | 0.52 |
| 3 | 1 | −1 | 0 | 0.50 |
| 4 | 1 | 1 | 0 | 0.35 |
| 5 | 0 | −1 | −1 | 0.42 |
| 6 | 0 | −1 | 1 | 0.46 |
| 7 | 0 | 1 | −1 | 0.44 |
| 8 | 0 | 1 | 1 | 0.57 |
| 9 | −1 | 0 | −1 | 0.58 |
| 10 | 1 | 0 | −1 | 0.59 |
| 11 | −1 | 0 | 1 | 0.74 |
| 12 | 1 | 0 | 1 | 0.58 |
| 13 | 0 | 0 | 0 | 0.76 |
| 14 | 0 | 0 | 0 | 0.70 |
| 15 | 0 | 0 | 0 | 0.75 |
The value of desirability (D) was taken as response (Y) and the experimental results were fitted with a multiple regression analysis, which was explained by a quadratic polynomial equation:
where Y is the predicted response, Xi and Xj are independent variables which influence the response variable Y, β0 is the offset term, βi is the linear coefficient, βii is the quadratic coefficient, and βij is the interaction coefficient.
The fitness of the second-order polynomial model was expressed via the regression coefficient R2 and the detailed analysis of variance (ANOVA) was conducted in a coded level of variables to determine the effects of individual variables. SAS software (version 8.0 by SAS Institute Inc., Cary, NC, USA) and OriginPro 7.5 software (Origin Lab Corp., Northampton, USA) were used for the regression analysis and graphical analysis of the experimental data.
2.4. Cultural conditions
2.4.1. Effect of initial pH on the D value
The investigated initial pH values of fermentation broth included 3, 4, 5, 6, 7, and 8.
2.4.2. Effect of culture time on the D value
The tested culture time was every 24 hours until the 7th day.
2.4.3. Effect of culture temperature on the D value
The tested culture temperatures included 20°C, 25°C, 30°C, 35°C, and 40°C.
2.5. Antitumor activities of polysaccharides in vitro
2.5.1. Extraction of crude water-soluble polysaccharides
The dry mycelia powder (60 meshes) of C. militaris CNCC2909 was recirculated by 95% ethanol overnight, and dried at room temperature.
The residues were extracted twice with distilled water at a ratio of 1:20 (residue:distilled water) at 85°C in a water bath for 3.5 hours, and then centrifuged at 8000g for 10 minutes. The supernatants, collected and concentrated to 1/4 total volume of supernatants, were frozen for 24 hours, melted slowly at room temperature, and centrifuged at 10,000g to remove the precipitates for several times, until there was no precipitation. The supernatants were extracted by the Sevag method to remove the protein [32]. The solutions were dialyzed against water for 3 days, and concentrated to 1/5 total volume of solution. The crude polysaccharides were obtained by lyophilizing in vacuo; the weight was also estimated.
2.5.2. Cell culture and modified tetrazolium salt (MTT) assay
The human hepatocellular carcinoma HepG2 cells and HeLa cells were cultured on a Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum, 100 U/mL penicillin, and streptomycin at 37°C in humidified atmosphere with 5% CO2. The cells were resuspended in medium of 1 × 105 cells/mL, and were incubated in 96-well plates containing 100 μL culture suspensions. After adherence for 24 hours, the cells of each well were treated with different concentrations of polysaccharides, and control groups were treated with culture medium. After 72 hours, cell viability was tested with the MTT assay [33].
3. Results and discussion
3.1. Screening of critical variables
A total of eight variables in the value of D were observed in the Plackett-Burman design (Table 4) when analyzing the effects of optimizing culture variables on the value of D. The estimated effect, regression coefficient, and corresponding t and p values for the values of desirability in eight variable Plackett-Burman design experiments are shown in Table 5. Model terms evidencing values of p > F < 0.05 were considered to play a critical role in enhancing the value of D, and hence were selected for response surface optimization. Yeast extract powder was considered to be the most significant factor on the value of D, with a probability value of 0.017, followed by K2HPO4 (0.020), and sucrose (0.046). All of the three significant variables (yeast extract powder, K2HPO4, and sucrose) exerted positive effects on the value of D, and the optimum levels of them were further analyzed by the Box-Behnken design. The coefficient of determination (R2) = 0.9997.
Table 5.
Results of statistical analysis for the Plackett-Burman design.
| Variables | Degree of freedom | Sum of squares | Mean square | t | F | p > F |
|---|---|---|---|---|---|---|
| Peptone | 1 | 0.000986 | 0.000986 | 6.89 | 47.41804 | 0.091809 |
| Yeast extract powder | 1 | 0.030643 | 0.030643 | 38.38 | 1473.005 | 0.016584 |
| Sucrose | 1 | 0.003917 | 0.003917 | 13.72 | 188.2801 | 0.046314 |
| MgSO4·7H2O | 1 | 0.000024 | 0.000024 | 1.08 | 1.157667 | 0.476719 |
| KH2PO4 | 1 | 0.021303 | 0.021303 | 32.00 | 1024 | 0.019888 |
| ZnCl2 | 1 | 0.001117 | 0.001117 | 7.33 | 53.71591 | 0.086329 |
| Vitamin B1 | 1 | 0.000095 | 0.000095 | 2.14 | 4.57635 | 0.278378 |
| (NH4)2SO4 | 1 | 0.00254 | 0.00254 | −11.05 | 122.1165 | 0.057453 |
| X9 | 1 | 0.000034 | 0.000034 | −1.28 | 1.634514 | 0.422575 |
| X10 | 1 | 0.000654 | 0.000654 | −5.61 | 31.44512 | 0.112347 |
| Model | 10 | 0.061315 | 0.006131 | 294.7349 | 0.045302 | |
| Error | 1 | 0.000021 | 0.000021 | |||
| Total | 11 | 0.061335 |
3.2. Optimization of screened variables using RSM (BBD)
The BBD was used to optimize the levels of significant variables (yeast extract powder, K2HPO4, and sucrose) and the effects of their interactions on the value of D. The corresponding results obtained by BBD are presented in Table 6.
The second-order polynomial equation provided a mathematical model, as a function to describe the relation between variables and the response. The regression equation was expressed as:
in which Y represents the value of D, and X2 is yeast extract powder, X3 is sucrose, and X5 is KH2PO4.
The ANOVA analysis of the optimization results indicated the response surface model terms, which are listed in Table 7. The coefficient of determination (R2) for the model was 0.9772, which could explain the 97.72% variability in the data of the model. The F value of the model was 23.83413 and the p value was 0.001377, which indicated that the experimental data fitted well with the quadratic model. The Student t test and p value were used in the significance of each coefficient from Table 8. The significant variable in the model was X5 with a p value of 0.021076 (p < 0.05). , X2X3, and were determined to have more significant effects than the others, for which the p values were 0.008475, 0.008082, and 0.0001 (p < 0.05). These results indicated that interactions between yeast extract powder and sucrose were significant model terms for the value of D.
Table 7.
ANOVA results of the value of D from Box-Behnken design.
| Source | Sum of squares | Degrees of freedom | Mean square | F | p > F |
|---|---|---|---|---|---|
| Model | 0.249543 | 9 | 0.027727 | 23.83413 | 0.001377 |
| Error | 0.005817 | 5 | 0.001163 | ||
| Total | 0.25536 | 14 |
R2 = 0.9772.
Table 8.
Regression analysis of the Box-Behnken design experiments.
| Source | Estimate | Standard error | t | p > |t| |
|---|---|---|---|---|
| X 2 | −0.025 | −0.012059 | 2.07316 | 0.092866 |
| X 3 | 0.015 | 0.012059 | 1.243896 | 0.268673 |
| X 5 | 0.04 | 0.012059 | 3.317057 | 0.021076 |
| X2 × X2 | −0.074583 | 0.01775 | −4.20183 | 0.008475 |
| X2 × X3 | −0.0725 | 0.017054 | −4.25124 | 0.008082 |
| X2 × X5 | −0.0425 | 0.017054 | −2.49211 | 0.055019 |
| X3 × X3 | −0.224583 | 0.01775 | −12.6524 | 0.0001 |
| X3 × X5 | 0.0225 | 0.017054 | 1.319351 | 0.244236 |
| X5 × X5 | −0.039583 | 0.01775 | −2.23002 | 0.076163 |
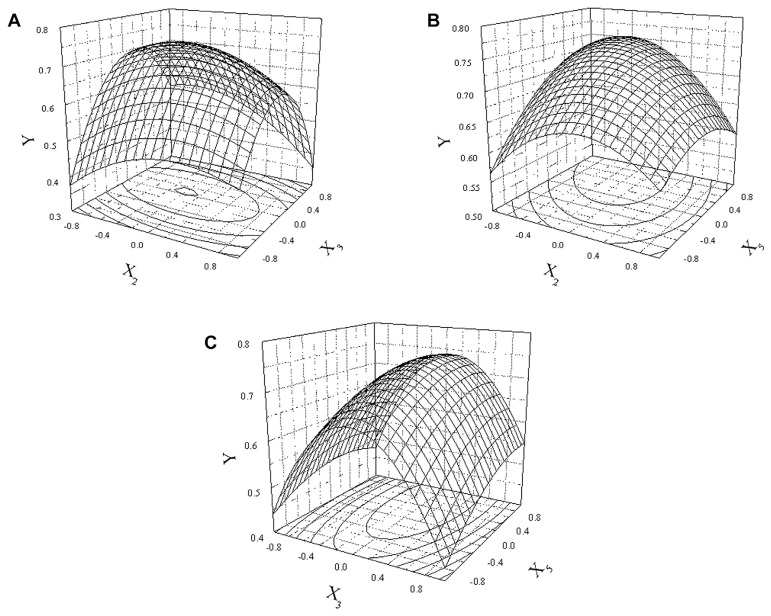
The three-dimensional response surface plots and the corresponding contour plots were used to optimize the levels of all variables for the value of D, which were constructed by plotting the value of D on the Z-axis against any two independent variables and discussing the interaction between them (Figs. 1–3). From the response surface plots and contour plots, the value of D was found to present a maximum in the tested range. Based on the aforementioned equation and the response surface plots, the optimum levels of three variables were as follows: yeast extract powder was 10.33 g/L, sucrose was 27.24 g/L, and KH2PO4 was 5.60 g/L. The maximum value of D, which could be obtained by the model, was 0.76. The optimal medium including yeast extract 10.33 g/L, sucrose 27.24 g/L, KH2PO4 5.60 g/L, peptone 5 g/L, MgSO4·7H2O 1 g/L, ZnCl2 0.011 g/L, vitamin B1 0.05 g/L, and (NH4)2SO4 21 g/L were employed.
Fig. 1.
Response surface and contour plots showing the mutual effects between candidate variables and desirability value. (A) Response surface plots for the value of D showing the interaction between yeast extract powder and sucrose; (B) response surface plots for the value of D showing the interaction between sucrose and KH2PO4; and (C) response surface plots for the value of D showing the interaction between yeast extract powder and KH2PO4.
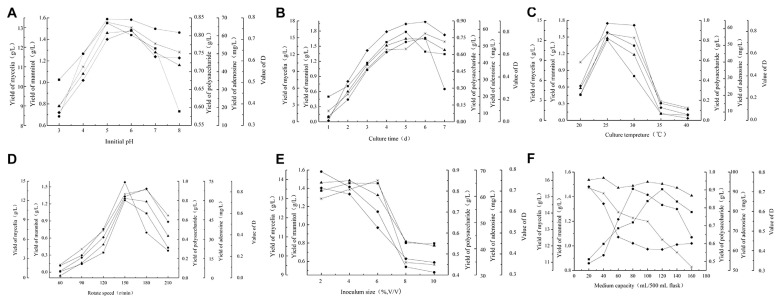
Fig. 2.
Effect of different cultural conditions on Cordyceps militaris cultivation. (A) Effect of initial pH on C. militaris cultivation; (B) effects of initial pH on C. militaris cultivation; (C) effects of culture time on C. militaris cultivation; (D) effects of rotate speed on C. militaris cultivation; (E) effects of inoculum size on C. militaris cultivation; (F) effects of medium capacity on C. militaris cultivation. (*) yield of mycelia; (●) yield of mannitol; (■) yield of polysaccharide; (◆) yield of adenosine; (▲) value of D.
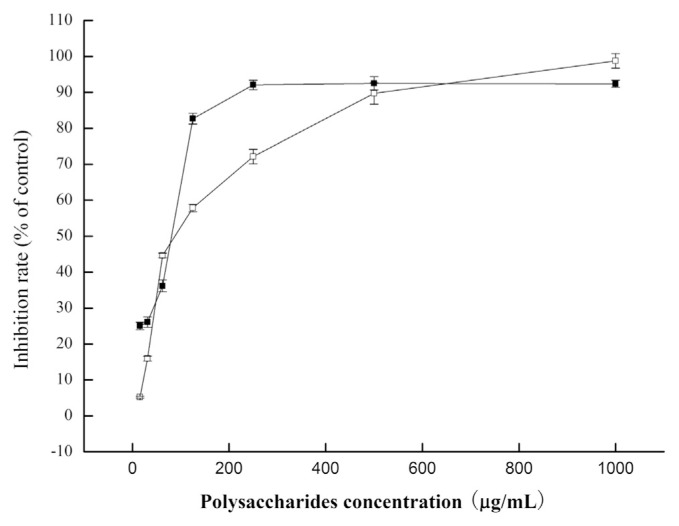
Fig. 3.
Inhibition of Cordyceps militaris mycelium polysaccharides at different concentrations against HeLa and HepG2 cells in 72 hours. (■) HeLa; (□) HepG2.
In order to validate the predicted results of the statistical model, experiments of the optimum medium composition were used to perform the highest point of the model and the obtained value of D was 0.73. This showed that the model is valid to simulate and predict the value of D.
3.3. Optimization of cultural conditions
3.3.1. Effect of initial pH on the D value
The initial pH of the medium usually affects the fungal cell shape, structure, cell membrane function, mycelium growth, and the metabolite biosynthesis. The effects of initial pH on the maximal D value are shown in Fig. 2A. When the initial pH increased from 3 to 5, mycelia yields, intracellular polysaccharide, and mannitol production increased rapidly, and when the initial pH increased from 5 to 8, the yields decreased gradually. Adenosine production increased until the initial pH was 6, and then decreased. The highest productions of mycelia, polysaccharide, mannitol, and adenosine were 13.28 g/L, 0.84 g/L, 1.59 g/L, and 63.16 mg/L. The highest value of D was 0.74 when the initial pH was 6.
3.3.2. Effect of culture time on the D value
The harvest time affects the production capacity. As shown in Fig. 2B, the yield of the mycelia, mannitol, and adenosine increased significantly until the 6th day with maximal values of 15.75 g/L, 1.66 g/L, and 64.51 mg/L, and then decreased slightly, which might be due to the mycelia autolysis and metabolite decomposition.
Polysaccharide production reached the highest value of 0.80 g/L on Day 5. The maximal associated D value reached 0.74 on Days 5 and 6 of fermentation in flask culture.
3.3.3. Effect of culture temperature on the D value
The culture temperature is one of the most important factors for the fungus growth, and influences fungus growth rate and the metabolic process. C. militaris was incubated in optimum medium at various temperatures from 20°C to 40°C in Fig. 2C. The highest mycelia, polysaccharide, mannitol, and adenosine production were 13.01 g/L, 0.80 g/L, 1.58 g/L, and 63.15 mg/L at 25°C, respectively, and the maximal associated D value was 0.74 at 25°C. This result indicated that the low or high temperatures were not suitable for the mycelia growth and metabolite biosynthesis.
3.3.4. Effect of rotate speed on the D value
To investigate the effects of the rotate speed on the flask culture, C. militaris was cultivated in optimum medium at 60–210 r/minute. Maximal polysaccharide and mannitol production of 0.80 g/L and 1.58 g/L, respectively, were obtained at the rotate speed of 150 r/minute. Maximum mycelia (13.71 g/L) and adenosine (69.03 mg/L) production were obtained at 180 r/minute. The optimal associated D value was 0.74 when the rotate speed was 150 r/minute in the shake-flask.
3.3.5. Effect of inoculum size on the D value
Inoculum size was significant for fungi fermentation processes. Therefore, different inoculum sizes ranging from 2% to 10% (v/v) were investigated to ensure the fermentation processes. As shown in Fig. 2E, the maximal values of mannitol and adenosine production were 1.58 g/L and 63.16 mg/L at an inoculum size of 2% and declined immediately. Mycelia and polysaccharide production were both near optimal values at inoculum sizes of 2~6%, and decreased remarkably outside this range. The highest D value reached 0.75 when the inoculum size was 4%.
3.3.6. Effect of medium capacity on the D value
Medium capacity is a significant factor in the fermentation process, which is related to oxygen supply in flasks. It may influence mycelia growth and bioactive metabolites biosynthesis. Fig. 2F showed the effects of different medium capacities ranging from 20~140 mL in a 250 mL shake-flask on the D value. The highest mycelia and adenosine productions were 15.53 g/L and 92.57 mg/L, respectively, when the medium capacity was 20 mL. Mycelia production declined rapidly following the increase of medium capacity, but there was no remarkable difference in the medium capacity ranging from 60–160 mL for adenosine production. The maximal mannitol and polysaccharide productions were 1.46 g/L and 0.90 g/L, respectively, in the medium capacity of 80 mL and 120 mL. Although there were various different effects on mycelia and various metabolites production, the associated D value ranged from 0.68 to 0.77 and the highest D value was 0.77, when the medium capacity was 40 mL.
From the above results, the optimal culture conditions for associate D value were set as follows: the initial pH 6, 25°C, 150 r/minute, inoculum size 4% (v/v), medium capacity 40 mL/250 mL. Under these conditions, the obtained value of D in the best medium was 0.77: mycelia, polysaccharide, adenosine, and mannitol production were 15.20 g/L, 0.60 g/L, 83.93 g/L, and 0.92 g/L, respectively.
3.3.7. Growth inhibition of C. militaris polysaccharides on HeLa and HepG2
As shown in Fig. 3, C. militaris polysaccharides showed significantly higher inhibition effect against HeLa and HepG2 in vitro, while the inhibition ability was dose-dependent. The maximal inhibition rate of polysaccharides against HeLa and HepG2 were 92.38% and 98.79%, respectively, when the concentration was 1000 μg/mL in 72 hours.
The IC50 for HeLa was 70.91 μg/mL (Table 9), much lower than HepG2 (97.63 μg/mL), when the HeLa was incubated for 72 hours, and this result showed that the inhibition ability against HeLa was much better than against HepG2.
Table 9.
The IC50 of Cordyceps militaris mycelium polysaccharides for HeLa and HepG2 in 72 hours.
| Cell line | IC50 (μg/mL) |
|---|---|
| HeLa | 70.91 |
| HepG2 | 97.63 |
4. Conclusion
In this study, we used desirability functions to optimize multiobjective products, including mycelia, polysaccharide, adenosine, and mannitol of C. militaris, which could be a potential means to optimize the fermentation of other microorganisms. The mycelia, polysaccharide, adenosine, and mannitol production were 15.20 g/L, 0.60 g/L, 83.93 mg/L, and 0.92 g/L, respectively, at the culture conditions: medium capacity 100 mL/250 mL, inoculation size 4% (v/v) of the seed culture, 25°C on a rotary shaker incubator at 150 rpm for 5 days. After a series of optimization processes, compared with the basal conditions, the productions of mycelia 13.52 g/L, polysaccharide 0.493 g/L, adenosine 75.54 mg/L, and mannitol 0.826 g/L were enhanced by 12.42%, 21.70%, 11.11%, and 11.38%, respectively. These results clearly confirmed that desirability functions and RSM are effective tools for mathematical modeling and factor analysis of the optimization process. The yield of C. militaris CGMCC 2909 fermentation improved compared with existing publications, which is significant for mass fermentation of C. militaris. There is great potential for the work of C. militaris, which could save materials, energy, reduce the cost, and so on. In addition, the inhibitory effect of polysaccharide in C. militaris against HeLa and HepG2 was detected by MTT. The inhibition against HeLa was much better than that against HepG2. When cells were incubated with polysaccharides of 1000 μg/mL for 72 hours, complete inhibition against HeLa and HepG2 could be reached. Therefore, intracellular polysaccharides may be used as potential medication in cancer treatment.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Zhu SJ, Pan J, Zhao B, et al. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J Ethnopharmacol. 2013;149:713–9. doi: 10.1016/j.jep.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 2. Wang M, Meng X, Yang R, et al. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int J Biol Macromol. 2013;59:178–83. doi: 10.1016/j.ijbiomac.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 3. Smiderle FR, Sassaki GL, Van Griensven LJ, et al. Isolation and chemical characterization of a glucogalactomannan of the medicinal mushroom Cordyceps militaris. Carbohydr Polym. 2013;97:74–80. doi: 10.1016/j.carbpol.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 4. Shin S, Lee S, Kwon J, et al. Cordycepin suppresses expression of diabetes regulating genes by inhibition of lipopolysaccharide-induced inflammation in macrophages. Immune Netw. 2009;9:98–105. doi: 10.4110/in.2009.9.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509–19. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 6. Ling JY, Sun YJ, Zhang H, et al. Measurement of cordycepin and adenosine in stroma of Cordyceps sp. by capillary zone electrophoresis (CZE) J Biosci Bioeng. 2002;94:371–4. doi: 10.1263/jbb.94.371. [DOI] [PubMed] [Google Scholar]
- 7. Kim SW, Xu CP, Hwang HJ, et al. Production and characterization of exopolysaccharides from an enthomopathogenic fungus Cordyceps militaris NG3. Biotechnol Prog. 2003;19:428–35. doi: 10.1021/bp025644k. [DOI] [PubMed] [Google Scholar]
- 8. Hong IP, Nam SH, Sung GB, et al. Chemical components of Paecilomyces tenuipes (Peck) Samson. Mycobiology. 2007;35:215–8. doi: 10.4489/MYCO.2007.35.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitakaze M, Hori M. Adenosine therapy: a new approach to chronic heart failure. Expert Opin Investig Drugs. 2000;9:2519–35. doi: 10.1517/13543784.9.11.2519. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro JA. Purinergic inhibition of neurotransmitter release in the central nervous system. Pharmacol Toxicol. 1995;77:299–305. doi: 10.1111/j.1600-0773.1995.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 11. Zhang M, Cui SW, Cheung PCK, et al. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Tech. 2007;18:4–19. [Google Scholar]
- 12. Yang J, Zhang W, Shi P, et al. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathol Res Pract. 2005;201:745–50. doi: 10.1016/j.prp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13. Wen Y, Yan L, Chen C. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J Food Drug Anal. 2013;21:219–26. [Google Scholar]
- 14. Yu R, Song L, Zhao Y, et al. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia. 2004;75:465–72. doi: 10.1016/j.fitote.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15. Li SP, Zhao KJ, Ji ZN, et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–13. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 16. Kiho T, Yamane A, Hui J, et al. Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biol Pharm Bull. 1996;19:294–6. doi: 10.1248/bpb.19.294. [DOI] [PubMed] [Google Scholar]
- 17. Yang BK, Ha JY, Jeong SC, et al. Production of exo-polymers by submerged mycelial culture of Cordyceps militaris and its hypolipidemic effect. J Microbiol Biotechn. 2000;10:784–8. [Google Scholar]
- 18. Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–84. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 19. Shih I, Tsai K, Hsieh C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Eng J. 2007;33:193–201. [Google Scholar]
- 20. Masuda M, Urabe E, Honda H, et al. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb Tech. 2007;40:1199–205. [Google Scholar]
- 21. Mao X, Eksriwong T, Chauvatcharin S, et al. Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Process Biochem. 2005;40:1667–72. [Google Scholar]
- 22. Xiao J, Chen D, Wan W, et al. Enhanced simultaneous production of mycelia and intracellular polysaccharide in submerged cultivation of Cordyceps jiangxiensis using desirability functions. Process Biochem. 2006;41:1887–93. [Google Scholar]
- 23. Park JP, Kim SW, Hwang HJ, et al. Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett Appl Microbiol. 2001;33:76–81. doi: 10.1046/j.1472-765x.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- 24. Kim SW, Hwang HJ, Xu CP, et al. Optimization of submerged culture process for the production of mycelial biomass and exo-polysaccharides by Cordyceps militaris C738. J Appl Microbiol. 2003;94:120–6. doi: 10.1046/j.1365-2672.2003.01754.x. [DOI] [PubMed] [Google Scholar]
- 25. Park C, Hong SH, Lee JY, et al. Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis. Oncol Rep. 2005;13:1211–6. [PubMed] [Google Scholar]
- 26. Yoo HS, Shin JW, Cho JH, et al. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin. 2004;25:657–65. [PubMed] [Google Scholar]
- 27. Kodama EN, McCaffrey RP, Yusa K, et al. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochem Pharmacol. 2000;59:273–81. doi: 10.1016/s0006-2952(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 28. Chen JY, Lai YJ, Chiang SH, et al. Antitumor cell proliferation and antigenotoxic effects of sufu and koji granule, the Chinese traditional fermented product of soybeans. J Food Drug Anal. 2012;20:832–8. [Google Scholar]
- 29. Nakamura K, Yamaguchi Y, Kagota S, et al. Inhibitory effect of Cordyceps sinensis on spontaneous liver metastasis of Lewis lung carcinoma and B16 melanoma cells in syngeneic mice. Jpn J Pharmacol. 1999;79:335–41. doi: 10.1254/jjp.79.335. [DOI] [PubMed] [Google Scholar]
- 30. Ohmori T, Tamura K, Fukui K, et al. Isolation of galactosaminoglycan moiety (CO-N) from protein-bound polysaccharide of Cordyceps ophioglossoides and its effects against murine tumors. Chem Pharm Bull (Tokyo) 1989;37:1019–22. doi: 10.1248/cpb.37.1019. [DOI] [PubMed] [Google Scholar]
- 31. Zhang N, Liu Y, Lu JH, et al. Isolation, purification and bioactivities of polysaccharides from Irpex lacteus. Chem Res Chinese U. 2012;28:249–54. [Google Scholar]
- 32. Jiang YH, Jiang XL, Wang P, et al. The antitumor and antioxidative activities of polysaccharides isolated from Isaria farinosa B05. Microbiol Res. 2008;163:424–30. doi: 10.1016/j.micres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 33. Wu JY, Zhang QX, Leung PH. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine. 2007;14:43–9. doi: 10.1016/j.phymed.2005.11.005. [DOI] [PubMed] [Google Scholar]