Abstract
Intestinal anastomotic tissue follows a similar pattern of healing that is seen in all tissues with characteristic inflammatory, proliferative, and remodeling phases. Several aspects of intestinal healing are distinct from other tissues, however, including its time course and interaction with the environment of the gastrointestinal tract. As the anastomosis progresses through each stage, initial inflammatory cells are replaced by collagen-producing fibroblasts that generate the anastomosis’ strength. A complex network of cell-to-cell signaling mediates this process through the release of cytokines and growth factors including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF). Interventions based on these signaling pathways have been shown to improve anastomotic strength in animals, though methods for improving anastomotic healing in human patients remain unclear. Given the risks associated with anastomotic failure in patients, there is value in monitoring inflammatory markers and cytokines that can indicate the presence of a leak.
Keywords: Anastomotic healing, growth factors, collagen remodeling, anastomotic leak
Stages of Anastomotic Healing
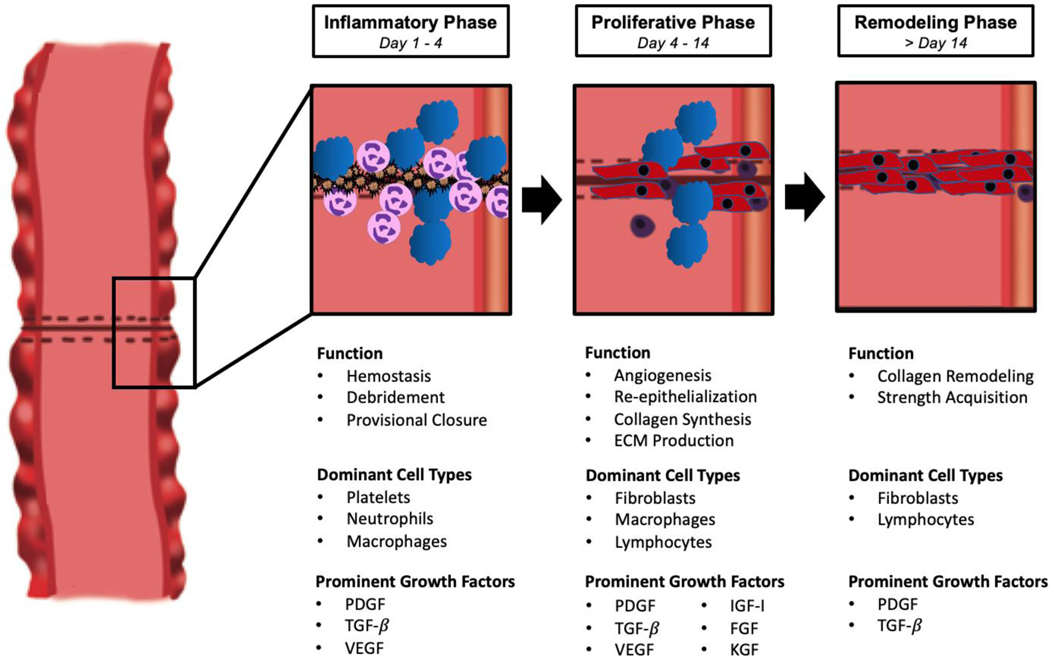
Anastomotic healing following intestinal surgery consists of a well-characterized series of events beginning with hemostasis and culminating in collagen remodeling and strength acquisition. Apposition of the serosal edges of divided bowel initiates a biological sequence that largely mimics healing in other parts of the body. While much of our knowledge regarding the involved cell types, cytokines, and growth factors in anastomotic healing comes from the study of cutaneous wound healing, anastomotic healing has multiple unique attributes. Despite these nuances in anastomotic healing, intestinal tissue follows the same broad stages of healing as the skin that will be addressed below: inflammation, proliferation, and remodeling (Figure 1).1
Figure 1:
Stages of anastomotic healing with associated functions, dominant cell types, and prominent growth factors.
Inflammatory Phase
The inflammatory phase of wound healing, also referred to as the exudative or “lag” phase, begins with hemostasis from platelet aggregation and the creation of fibrin clots.2 These clots act to both halt any bleeding and to create a temporary scaffold for the deposition of initial matrix proteins. In addition to forming these fibrin plugs, platelets congregating at the site of a mucosal injury release multiple cytokines and stored chemoattractant growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor β (TGF-β). This cytokine-rich environment at the site of injury induces increased permeability of the surrounding small vasculature, leading to the migration of inflammatory cells to the healing tissue. Following platelet aggregation, neutrophils and then macrophages are the earliest inflammatory cells to migrate to the site of injury. These cell types remain dominant at the healing anastomosis throughout the inflammatory phase, performing multiple functions including the phagocytosis of potential pathogens and the release of further growth factors to stimulate wound healing.3
Proliferative Phase
Following the inflammatory phase, healing intestinal tissue enters a proliferative stage in which fibroblasts are the dominant cell type present. In this phase of healing, fibroblasts’ main function is to deposit collagen in the wound base, replacing the initial temporary fibrin clot with granulation tissue.1 Fibroblasts are initially attracted to the cut edge of the bowel due to the release of chemotactic factors by macrophages including PDGF, TGF-β, and fibroblast growth factor (FGF). In contrast to the collagen composition of mature bowel, which is approximately 20% type III collagen, this early granulation tissue formed by fibroblasts contains upwards of 30–40% type III collagen and requires remodeling in the next phase of healing.1 Fibroblasts begin to appear in the late inflammatory stage and are the predominant cell type in the wound bed by day four, with the proliferation phase generally lasting 14 days. As fibroblasts accumulate, the previously dominant inflammatory cells including neutrophils and macrophages decrease in number and are replaced by lymphocytes.4 In addition to collagen deposition, this phase is characterized by angiogenesis and re-epithelialization of the bowel’s mucosal surface, each stimulated by specific growth factors discussed below. Smooth muscle cells and myofibroblasts appear late in this phase as the anastomosis acquires strength, contracting to decrease the size of the eventual scar.
Remodeling Phase
The final and longest stage of anastomotic healing, remodeling, begins at approximately 14 days and continues for several weeks as the wound reorganizes into permanent scar tissue. Inflammatory cells that predominated in earlier stages of healing decrease further in number as the wound matures and, as in the proliferative phase, fibroblasts continue to be the main cell type present. The ratio of type I to type III collagen normalizes in the remodeling stage as matrix metalloproteinases (MMPs) work to remove type III collagen from granulation tissue and replace it with type I; following remodeling and maturation of the healing tissue, type I collagen represents 68% of total collagen content in the intestinal submucosa compared to 80% in healing dermis.1, 5 This more durable collagen mixture reorganizes into tight bundles while myofibroblasts contract to strengthen the healing wound. New vascular beds that began to form in the proliferative stage reorganize into mature networks as well, and after several weeks or months the anastomotic tissue stabilizes. Despite the time required for strength acquisition, healed wounds and anastomoses only partially regain the tensile strength of uninjured tissue.6
Differences in Anastomotic and Skin Healing
The main tenets of wound healing are conserved throughout the body as different tissues demonstrate the same stages of healing. While cutaneous wounds remain a focus for much of the literature on this topic, there have been an increasing number of studies focusing specifically on intestinal healing. Within the broader framework of wound healing established in dermatologic studies, there are several important characteristics distinct to anastomotic healing (Table 1).
Table 1.
Major differences between skin and anastomotic healing.
| Skin Wound | Intestinal Anastomosis | |
|---|---|---|
| Time to Full Strength Acquisition | Gradual, up to 6 Months | Rapid, approximately 1 Month |
| Strength Layers | Dermis | Submucosa and Serosa |
| Collagen Synthesis | ||
| Collagen-Producing Cells | Fibroblasts | Fibroblasts and Smooth Muscle Cells |
| Main Collagen Subtypes (%) | Type I (80%), Type 111 (20%) | Type I (68%), Type III (20%), Type V (12%) |
| Collagenase Activity | Minimal | Increased in inflammatory phase (Colon > Small Bowel) |
| Wound Environment | ||
| pH | Stable | Wide range based on location in GI tract |
| Microbiome | Aerobic skin flora, infections generally treated topically. Rare instances of bacteremia. | Gut microbiome with aerobic and anaerobic bacteria. Potential for intra-peritoneal infection in setting of anastomotic leak |
| Mechanical Stress | Potential tension with movement depending on location. Minimal shear stress | Persistent shear stress from intraluminal contents, peristalsis |
| Perfusion | Remains constant | Splanchnic perfusion decreased in setting of hypovolemia |
Perhaps the most important difference between the healing in the skin and the intestine is the time course of strength acquisition. While healing in the skin progresses gradually over several months, anastomotic tissue reaches near maximum strength within the first post-operative month.1 Interestingly, strength acquisition is not uniform across the gastrointestinal tract as the rate of collagen synthesis and remodeling in an ileal anastomosis is quicker than in a colonic anastomosis in a rat model.7 Beyond gaining strength more rapidly, ileal and other small bowel anastomoses also attain near normal strength by four weeks post-operatively while colonic anastomoses acquire only 75% of normal tissue strength at four months.1, 8 These differences in healing between the small and large intestine may be at least partially due to increased collagenase activity in the colon, which causes a temporary decrease in anastomotic strength in the first three days post-operatively. This transient effect is more pronounced in the colon than the small intestine and may partially underlie differences in strength between the two tissue types. Clinically, this disparity in collagenase activity may also underpin the higher rate of anastomotic leak seen in colonic anastomoses compared to those in the small bowel, particularly in the descending colon.9,10
The relatively rapid growth seen in anastomotic healing compared to cutaneous healing is essential due to the environment in the gastrointestinal tract. As the anastomosis gains strength and re-epithelializes, it is subject to continuous shear stress from intraluminal contents and peristalsis, both of which are not present in a cutaneous wound. Compounding this added stress, the healing bowel can experience relative ischemia as splanchnic blood flow is restricted in the setting of post-operative hypovolemia whereas the skin receives a relatively constant level of perfusion even in early hypovolemia.6
The gastrointestinal tract is also home to a broader and more diverse microbiome than the skin including anaerobic bacteria, which likely plays a role in the rate and efficacy of anastomotic healing.1 This has been shown in animal models, with germ-free rats having notably lower bursting pressures at seven days after colectomy compared to conventional rats; ex-germ-free rats in this same study demonstrated significantly improved anastomotic strength after inoculation with a conventional microbiome but not after mono-contamination with Escherichia coli, confirming the beneficial effect of a diverse microbiome.11 In humans, environmental factors including diet and antibiotic use can predispose patients to anastomotic leak mainly by altering the composition of the microbiome.12 The promotion of a diverse and healthy microbiome may therefore be able to improve surgical outcomes. This has been shown in mice as prehabilitation with a low-fat diet decreases leak rates by preventing overgrowth of pathogenic bacteria.13
The composition of the intestinal wall represents a final difference between the two types of healing. The ratio of various collagen types differs in the bowel due to the presence of type V collagen, which is largely absent in the skin but makes up 12% of collagen in mature bowel.6 Pericellular type V collagen can be detected in colonic anastomoses in rats as early as post-operative day two and contributes to the basement membrane of regenerating capillary walls.14 The increased number of layers within the intestinal wall likely adds strength as well; in addition to its mucosal, submucosal, and muscular layers, the intestine includes an outer serosal layer that is not present in the skin. Expression of type I and type III collagen genes is seen in both the submucosal and serosal layers beginning on post-operative day one, suggesting that serosal healing plays an early role in strength acquisition.15 The overall arc of wound healing remains the same in the bowel as in the skin, but these intestine-specific nuances are important to consider in patients undergoing intra-abdominal surgery.
Growth Factors and Cell Signaling
The progression from each stage of healing to the next relies on a complex network of cell-to-cell signaling mediated in large part by growth factors and cytokines. This signaling begins early in the inflammatory stage of healing as platelets adhere to each other. In addition to providing hemostasis, platelets store multiple growth factors in pre-formed granules that are released in response to platelet aggregation. Essential growth factors in this step include PDGF, TGF-β, and VEGF, each of which plays a key role throughout healing and can be released by multiple inflammatory cell types.
Platelet-Derived Growth Factor (PDGF)
PDGF plays a particularly important role early in the inflammatory phase as it stimulates the release of TGF-β from macrophages while also increasing levels of VEGF and insulin-like growth factor I (IGF-I).16 Despite the central role that PDGF plays in signaling during the inflammatory phase, there is minimal evidence showing a causal link between its presence and improved anastomotic strength. Some indirect evidence actually shows that suppression of PDGF signaling improves anastomotic bursting pressure and hydroxyproline content in a rat model of intestinal healing. In a single study of rats treated with dexamethasone, those who were given post-operative intravenous trapidil, a competitive PDGF antagonist, showed improved anastomotic bursting pressure and hydroxyproline content; no difference was demonstrated in non-steroid treated rats, however.17 It is difficult to draw definitive conclusions about PDGF suppression based on this model, however, due to the multiple other functions of trapidil including blockage of thromboxane A2 and prostacyclin synthesis.
Transforming Growth Factor-β (TGF-β)
Prior animal studies on the role of TGF-β have similarly conflicting evidence for the role of this growth factor in early anastomotic strength acquisition. Increased TGF-β signaling via adenoviral gene transfer in rats with healing colonic anastomoses increases bursting pressure on post-operative day six; interestingly, increased bursting pressure was only demonstrated when TGF was administered on postoperative day three whereas no benefit was observed with administration on post-operative day zero.18 Other findings have disputed this benefit, however, as suppression of TGF-β signaling with the intraperitoneal administration of TGF-β neutralizing antibodies in rats with healing small bowel anastomoses was shown to improve mucosal growth and revascularization by post-operative day five.19 These divergent responses to TGF-β modulation may be attributable to differential TGF-β expression in colonic and small intestine anastomoses. Compared to normal intestinal tissue, colonic anastomoses in rats have significantly lower levels of TGF-β expression on post-operative days one and seven, whereas expression in the small intestine remains at baseline.20 The lack of clarity on the role of TGF-β in anastomotic healing is likely indicative of the complex nature of cell signaling during tissue healing, and further study is warranted.
Vascular Endothelial Growth Factor (VEGF)
With the introduction of new cell types in the proliferative phase, signaling pathways emerge that are essential in achieving the goals of neo-angiogenesis, mucosal regeneration, and formation of the extracellular matrix (ECM). Among these, revascularization through neo-angiogenesis plays a critical role in the progression of anastomotic healing by increasing oxygen supply and nutrient delivery to the wound bed. The key growth factors underlying neo-angiogenesis are VEGF and FGF. While VEGF also stimulates fibroblast migration in the proliferative phase, its primary role is to promote endothelial cell migration and proliferation, leading to an angiogenic effect in the healing anastomosis.16 VEGF administration in animal models has been shown to increase colonic anastomotic strength as early as post-operative day four.21 Gene therapy with recombinant VEGF in an opossum model of esophagogastrectomy similarly shown that increased expression of VEGF has a beneficial effect on anastomotic healing.22 This evidence for the central role of VEGF has been strengthened by studying animal models of bevacizumab administration, a VEGF inhibitor; in rabbits treated with bevacizumab, bursting pressure of intestinal anastomoses significantly decreases compared to controls.23 While similar study in rats found no deleterious effects from bevacizumab administration, the majority of evidence from animal models suggests that the pro- angiogenic effects of VEGF are essential in anastomotic strengthening.24
Keratinocyte Growth Factor (KGF) and Insulin-Like Growth Factor-I (IGF-I)
Mucosal regeneration occurs simultaneous to neo-angiogenesis during the early proliferative phase via keratinocyte growth factor (KGF). The role of KGF in wound healing has primarily been studied in cutaneous wounds, though there is some evidence for its role in intestinal healing. Intraperitoneal delivery of KGF in rats with left-sided colon anastomoses significantly thickens the mucosal layer of the anastomosis, reduces inflammation, and increases bursting pressure on post-operative days two, four, and seven.25 The same group also showed that IGF-I had a similar effect as KGF, though co-administration did not lead to further acceleration of anastomotic healing.26 Further studies have showed that exogenous IGF-I administration improves anastomotic strength and healing in animals treated with a variety of immunosuppressive medications including 5-fluorouracil (5-FU), corticosteroids, and mycophenolate.27–29 Taken together, these studies suggest that this positive effect from IGF-I may act to counteract the negative affect of immunosuppression via increased mucosal production.
Other Growth Factors
A host of other growth factors and cell signaling pathways also play a role during the proliferative phase. Fibroblast growth factor, which increases through the proliferative phase in conjunction with multiplying fibroblasts, has been shown to improve healing in multiple types of anastomoses; prior animal models demonstrating this effect include a rat model of esophageal anastomosis with direct FGF application by gelatin film, as well as a dog model of pancreaticojejunostomy with local FGF administration using hydrogel microspheres.30, 31 Epidermal growth factor (EGF) and human growth hormone (hGH) have each been shown to improve anastomotic healing and bursting pressure as well, specifically in rats treated with steroids or other immunologic agents.32 Heparin-binding EGF-like growth factor (HB-EGF) has likewise been shown by one group to improve anastomotic healing in mice with direct application; importantly, HB-EGF knockout mice in the same study were shown to have decreased anastomotic strength, suggesting a critical role for HB-EGF.33 While the exact mechanisms underlying the impact of many of these growth factors remain undefined, these recent studies have helped to develop a basic understanding of the signaling pathways that guide anastomotic healing.
Tissue Remodeling
Collagen Synthesis and Degradation
Fibroblasts are the most common cell type present in the wound bed during the proliferative phase, carrying out several functions including recreation of the ECM and collagen deposition in granulation tissue. During the proliferative phase, high levels of IL-1β and PDGF stimulate the activation of myofibroblasts, a form of activated fibroblast that participates in wound contracture.34 The number of fibroblasts peaks during the proliferative phase and then gradually decreases as collagen concentration and wound breaking strength increase.1 This reduction in fibroblast number is in part due to the actions of TGF-β1 and FGF, which induce apoptosis of granulation tissue fibroblasts.35 With this decline in fibroblast function, wound healing transitions from collagen production to remodeling.
Collagen content in colonic anastomoses has been shown to peak at six days post-operatively in a rat model, with collagen deposition slowing after day six. Notably, this model showed that collagen content in the healing anastomosis was tenfold higher than in healing skin wounds.36 Small bowel anastomoses may reach peak collagen content significantly sooner with one animal model showing a tenfold maximum increase in collagen activity in ileal anastomoses by day four; this response to wounding in the small intestine was both faster and stronger than in colonic anastomoses.7 Increased collagen content alone is not necessarily indicative of anastomotic strength however; strength is derived from the reorganization of collagen, and inhibition of cross-linking has been shown to decrease anastomotic strength without altering overall collagen content.37
In all intestinal tissues, organization and remodeling of the immature collagen matrix deposited by fibroblasts is largely mediated by MMPs that degrade the ECM. This family of proteins has over 20 distinct subtypes in humans divided into subgroups based on which ECM components each enzyme breaks down (i.e. collagenases, gelatinases).38 Effective remodeling depends on a balance of activity between MMPs and tissue inhibitors of metalloproteinases (TIMPs), allowing for the recycling of collagen and creation of a mature scar. Various MMPs and TIMPs are highly expressed at different points in wound healing, with MMP-3, MMP-8, MMP-9, TIMP-2, and total MMP activity shown to peak early and decrease over time while MMP-2 and TIMP-1 gradually increase.38 Expression varies by tissue type as well, with MMP expression patterns varying considerably between ileum and colon samples; zymography from rat tissue extracts shows bands likely corresponding to pro-MMP-2 and active MMP-2 that are only found in the ileum, while different pro-MMP-1 subtypes are expressed in each tissue. 39
Remodeling of the anastomosis is dependent on MMPs, but over-expression of these enzymes early in anastomotic healing can lead to unbalanced collagen degradation resulting in anastomotic dehiscence. Pre-operative ischemia, which is a risk factor for anastomotic dehiscence, induces more widespread MMP and TIMP-1 expression in peri-anastomotic colonic tissue in a rabbit model during the first several days post-operatively. This overexpression drew an indirect association between MMP activity and anastomotic failure, which has been further bolstered by evidence that microbiome-stimulated activation of MMP-9 contributes to anastomotic leak as well.40 Consistent with these findings, inhibition of MMP activity significantly improves bursting strength in rat anastomoses.41 Different factors within the anastomotic microenvironment may then influence rates of dehiscence by altering MMP activity. In normal healing tissue, these effects are likely attenuated by inhibition of MMP activity by TIMPs and other growth factors present in early healing such as TGF-β, which acts to suppress MMP-9 function.42
Interventions to Improve Anastomotic Strength and Healing
Failure of anastomotic healing and leakage of intraluminal contents can have devastating consequences for patients undergoing intra-abdominal surgery. Given the high risks associated with anastomotic leak, there have been many studies on interventions to improve anastomotic healing in animal models. Due to the central role that MMPs play in collagen remodeling, they have been a frequent target for inhibition in these studies. Specific, systemic inhibition of MMP-8, MMP-9, and MMP-12 has been shown to improve bursting strength in colonic anastomoses in rats, with similar benefits seen with non-selective inhibition in other rat models.41, 43–45 Local inhibition using sutures coated with doxycycline, an MMP inhibitor, have demonstrated improved anastomotic strength as well.46
Direct application of several growth factors discussed in the previous section have been studied as well. VEGF gene therapy or administration in particular seems to improve anastomotic healing, with animal models of esophagogastrostomy and colonic anastomosis both demonstrating this beneficial effect likely due to accelerated angiogenesis.21, 22, 47 In animal models using immunosuppressive medications such as corticosteroids or 5-FU there is a benefit from systemic IGF-I administration as well.27–29 This improvement in immunosuppressed mice correlates with evidence that IGF-1 coated sutures improve anastomotic strength and hydroxyproline content in a rat model of colitis.48 The positive effect of growth factor administration in immunosuppressed animal models is of particular interest considering the wide variety of patients on immune-modulating therapy who require intestinal surgery.
Other interventions from previous studies have had mixed results. Non-steroidal anti-inflammatory drugs (NSAIDs) have been proposed as a novel intervention to limit inflammation and subsequently promote healing, and one rat model did show that NSAIDs can limit collagen degradation in colonic anastomoses.49 Other animal models have shown no effect on anastomotic strength from NSAIDs, however, and their clinical impact seems to be minimal.50 Other systemic interventions are less well-studied. Preparation with glutamine and synbiotics was shown to increase bursting pressure in a rat model, while platelet rich plasma and a superoxide dismutase mimic improved bursting pressure in rats receiving 5-FU and chemoradiotherapy, respectively.51–53
Previous systematic reviews have compared various less well-studied agents, though almost exclusively in animal models. In a 2016 meta-analysis of animal studies by Nerstrom et al. hyperbaric oxygen was the only identified beneficial intervention, improving bursting pressure in rat models of anastomotic ischemia; granulocyte macrophage-colony stimulating factor showed no benefit in chemotherapeutic models, and there was inadequate data to evaluate any other agents in the metaanalysis.54 A separate meta-analysis from Oines et al. in 2014 identified several other agents that increased bursting pressure in animal models including iloprost, tacrolimus, erythropoietin, hGH, and IGF-I. This group again pointed to the benefit of MMP inhibition as well. Only one agent in this meta-analysis, aprotinin, was used in human patients, and it showed no impact on the rate of anastomotic leak in a cohort of 113 patients.55
Monitoring of Anastomotic Healing
Anastomotic dehiscence leads to a broad spectrum of post-operative complications, ranging from subclinical leaks to life-threatening sepsis. Given the potential for devastating complications, there have been many studies aimed at predicting or diagnosing anastomotic leak early in its course. Classical indicators of anastomotic leak, such as altered vital signs, are often non-specific with exceedingly small positive predictive value for a leak.56 More promising data has emerged from studying markers in either serum or perianastomotic peritoneal fluid. These diverse indicators are generally inflammatory markers, cytokines, or metabolites that indirectly indicate inflammation or ischemia at the healing anastomosis.
Inflammatory markers and Cytokines
The most well-studied biological markers of anastomotic leak after intestinal surgery have been inflammatory markers such as C-reactive protein (CRP), calprotectin, and procalcitonin. CRP and calprotectin are elevated in the setting of intestinal inflammation while procalcitonin is a marker for bacterial infection, both of which are prominent features of anastomotic leak.57, 58 Their utility in the detection of anastomotic leak is best as a screening test with high negative predictive value (NPV) for normal values. Serum CRP levels, for example, have been shown to have a NPV of 89–97% in colorectal surgery patients when using a cutoff value between 135 and 180 mg/L on post-operative day four.59, 60 Procalcitonin and calprotectin serum levels have been shown to be similarly useful, though the best NPV is observed when using these markers in combination with CRP.61, 62 Combining serum CRP levels with intraperitoneal MMP-9 levels was also shown to increase its predictive value.63 Given these repeated findings, normal values for serum inflammatory markers may have a role in guiding early discharge after colorectal surgery. It is important to note, however, that these markers tend to be less accurate in predicting leak following laparoscopic surgery when compared to open surgery.64 As minimally invasive techniques continue to proliferate, the usefulness of measuring inflammatory markers to rule out leak may become less clear.
Cytokines involved in immune cell activation have also been identified as markers for anastomotic leak, both in serum and intraperitoneal fluid. In particular, TNF-α, IL-1β, IL-6, IL-8, and IL-10 have been frequently studied in this capacity. Individually, elevation of each of these cytokines is nonspecific for anastomotic leak, but combining them into models may help screen patients for dehiscence.65 This is particularly true in the early post-operative period; IL-6, IL-10, and TNF-α have all been observed to be higher on post-operative day 1 in the intraperitoneal fluid of patients with anastomotic leak.63, 66, 67 Interestingly, patients with early anastomotic leak have elevated levels of these intraperitoneal cytokines as well as IL-1β and IL-8 during the first five post-operative days, yet those with leaks later in their course do not have similar early cytokine responses.68 These findings suggest that the utility of intraperitoneal cytokines for detecting leak is highest immediately post-operatively and then diminishes.
Acid/Base Status & Metabolites
In addition to measuring serum markers, clinicians can use output from drains placed at the time of surgery to surveil the anastomotic environment. Peritoneal fluid output from either traditional surgical drains or smaller microdialysis catheters have been used with success in a variety of studies to characterize changes seen in anastomotic leak. Fluid pH and the concentration of glucose metabolites have shown the best efficacy in predicting leak, likely due to the effect of ischemia on carbohydrate metabolism.
In the absence of adequate oxygen supply, anaerobic metabolism depends on glycolysis to break down glucose with fermentation of the resultant pyruvate, producing lactic acid. Decreased oxygen delivery due to ischemia at the site of a leak should then logically lead to increased fermentation and decreased pH as the concentration of lactic acid increases. In a recent prospective study of 753 patients undergoing rectal surgery, the pH of drain effluent was shown to be a reliable marker of anastomotic leak; using a pH cutoff of 6.798 led to a remarkable high sensitivity and specificity of 98.7% and 94.7%, respectively, for anastomotic leak.69 This group did not evaluate concentrations of glucose metabolites, but this evidence for pH as a marker corresponds well with previous findings regarding peritoneal concentrations of glucose, lactate, and pyruvate in anastomotic leak. Numerous studies have shown that peritoneal lactate increases in the setting of leak, though with somewhat low predictive values.70, 71 Lactate to pyruvate ratio, which increases in the setting of ischemia, is similarly elevated in the setting of anastomotic leak.66, 72
Footnotes
Disclosure: The author reports no potential conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26:131–136. [DOI] [PubMed] [Google Scholar]
- 2.Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008;13:3532–3548. [DOI] [PubMed] [Google Scholar]
- 3.Lam A, Fleischer B, Alverdy J. The Biology of Anastomotic Healing-the Unknown Overwhelms the Known. J Gastrointest Surg. 2020;24:2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. [DOI] [PubMed] [Google Scholar]
- 6.Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin North Am. 1997;77:549–573. [DOI] [PubMed] [Google Scholar]
- 7.Martens MF, Hendriks T. Postoperative changes in collagen synthesis in intestinal anastomoses of the rat: differences between small and large bowel. Gut. 1991;32:1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mast BA. Healing in other tissues. Surg Clin North Am. 1997;77:529–547. [DOI] [PubMed] [Google Scholar]
- 9.Telem DA, Sur M, Tabrizian P, et al. Diagnosis of gastrointestinal anastomotic dehiscence after hospital discharge: Impact on patient management and outcome. Surgery. 2010;147:127–133. [DOI] [PubMed] [Google Scholar]
- 10.Veyrie N, Ata T, Muscari F, et al. Anastomotic leakage after elective right versus left colectomy for cancer: prevalence and independent risk factors. J Am Coll Surg. 2007;205:785–793. [DOI] [PubMed] [Google Scholar]
- 11.Okada M, Bothin C, Kanazawa K, Midtvedt T. Experimental study of the influence of intestinal flora on the healing of intestinal anastomoses. Br J Surg. 1999;86:961–965. [DOI] [PubMed] [Google Scholar]
- 12.Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg. 2018;105:e131–e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyoju SK, Adriaansens C, Wienholts K, et al. Low-fat/high-fibre diet prehabilitation improves anastomotic healing via the microbiome: an experimental model. Br J Surg. 2020;107:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasken P, Lehto M, Renvall S. Fibronectin, laminin, and collagen types I, III, IV and V in the healing rat colon anastomosis. Ann Chir Gynaecol. 1990;79:65–71. [PubMed] [Google Scholar]
- 15.Braskén P. Healing of experimental colon anastomosis. Eur J Surg Suppl. 1991:1–51. [PubMed] [Google Scholar]
- 16.Rijcken E, Sachs L, Fuchs T, Spiegel HU, Neumann PA. Growth factors and gastrointestinal anastomotic healing. J Surg Res. 2014;187:202–210. [DOI] [PubMed] [Google Scholar]
- 17.Colak T, Nayci A, Polat G, et al. Effects of trapidil on the healing of colonic anastomoses in an experimental rat model. ANZ J Surg. 2003;73:916–921. [DOI] [PubMed] [Google Scholar]
- 18.Migaly J, Lieberman J, Long W, Fisher C, Rolandelli RH. Effect of adenoviral-mediated transfer of transforming growth factor-beta1 on colonic anastomotic healing. Dis Colon Rectum. 2004;47:1699–1705. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda H, Motohiro T, Nakai K, et al. Negative effect of transforming growth factor-beta-1 on intestinal anastomotic tissue regeneration. Eur Surg Res. 2001;33:388–394. [DOI] [PubMed] [Google Scholar]
- 20.Zubaidi A, Buie WD, Hart DA, Sigalet D. Temporal expression of cytokines in rat cutaneous, fascial, and intestinal wounds: a comparative study. Dig Dis Sci. 2010;55:1581–1588. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M, Tanaka E, Imaizumi T, et al. Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. Eur Surg Res. 2009;42:249–257. [DOI] [PubMed] [Google Scholar]
- 22.Enestvedt CK, Hosack L, Hoppo T, et al. Recombinant vascular endothelial growth factor165 gene therapy improves anastomotic healing in an animal model of ischemic esophagogastrostomy. Dis Esophagus. 2012;25:456–464. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H, Yokoyama Y, Uehara K, et al. The effects of bevacizumab on intestinal anastomotic healing in rabbits. Surg Today. 2016;46:1456–1463. [DOI] [PubMed] [Google Scholar]
- 24.Pavlidis ET, Ballas KD, Symeonidis NG, et al. The effect of bevacizumab on colon anastomotic healing in rats. Int J Colorectal Dis. 2010;25:1465–1473. [DOI] [PubMed] [Google Scholar]
- 25.Egger B, Tolmos J, Procaccino F, et al. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. Am J Surg. 1998;176:18–24. [DOI] [PubMed] [Google Scholar]
- 26.Egger B, Inglin R, Zeeh J, Dirsch O, Huang Y, Büchler MW. Insulin-like growth factor I and truncated keratinocyte growth factor accelerate healing of left-sided colonic anastomoses. Br J Surg. 2001;88:90–98. [DOI] [PubMed] [Google Scholar]
- 27.Inglin RA, Baumann G, Wagner OJ, Candinas D, Egger B. Insulin-like growth factor I improves aspects of mycophenolate mofetil-impaired anastomotic healing in an experimental model. Br J Surg. 2008;95:793–798. [DOI] [PubMed] [Google Scholar]
- 28.Mantzoros I, Kanellos I, Angelopoulos S, et al. The effect of insulin-like growth factor I on healing of colonic anastomoses in cortisone-treated rats. Dis Colon Rectum. 2006;49:1431–1438. [DOI] [PubMed] [Google Scholar]
- 29.Zacharakis E, Demetriades H, Pramateftakis MG, et al. Effect of IGF-I on healing of colonic anastomoses in rats under 5-FU treatment. J Surg Res. 2008;144:138–144. [DOI] [PubMed] [Google Scholar]
- 30.Katsuno A, Aimoto T, Uchida E, Tabata Y, Miyamoto M, Tajiri T. The controlled release of basic fibroblast growth factor promotes a rapid healing of pancreaticojejunal anastomosis with potent angiogenesis and accelerates apoptosis in granulation tissue. J Surg Res. 2011;167:166172. [DOI] [PubMed] [Google Scholar]
- 31.Fedakar-Senyucel M, Bingol-Kologlu M, Vargun R, et al. The effects of local and sustained release of fibroblast growth factor on wound healing in esophageal anastomoses. J Pediatr Surg. 2008;43:290–295. [DOI] [PubMed] [Google Scholar]
- 32.Sakallioglu AE, Yagmurlu A, Dindar H, Hasirci N, Renda N, Deveci MS. Sustained local application of low-dose epidermal growth factor on steroid-inhibited colonic wound healing. J Pediatr Surg. 2004;39:591–595. [DOI] [PubMed] [Google Scholar]
- 33.Radulescu A, Zhang HY, Chen CL, et al. Heparin-binding EGF-like growth factor promotes intestinal anastomotic healing. J Surg Res. 2011;171:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham MF, Drucker DE, Diegelmann RF, Elson CO. Collagen synthesis by human intestinal smooth muscle cells in culture. Gastroenterology. 1987;92:400–405. [DOI] [PubMed] [Google Scholar]
- 35.Akasaka Y, Ono I, Kamiya T, et al. The mechanisms underlying fibroblast apoptosis regulated by growth factors during wound healing. J Pathol. 2010;221:285–299. [DOI] [PubMed] [Google Scholar]
- 36.Oxlund H, Christensen H, Seyer-Hansen M, Andreassen TT. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res. 1996;66:25–30. [DOI] [PubMed] [Google Scholar]
- 37.van Doorn K, de Man B, Hendriks T. The effects of lathyrogens on intestinal anastomoses in the rat. Exp Mol Pathol. 1990;52:37–45. [DOI] [PubMed] [Google Scholar]
- 38.Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen. 2003;11:268274. [DOI] [PubMed] [Google Scholar]
- 39.Seifert WF, Wobbes T, Hendriks T. Divergent patterns of matrix metalloproteinase activity during wound healing in ileum and colon of rats. Gut. 1996;39:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shogan BD, Belogortseva N, Luong PM, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015;7:286ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krarup PM, Eld M, Jorgensen LN, Hansen MB, Ågren MS. Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon. Int J Colorectal Dis. 2017;32:1277–1284. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappaB site. Biochem J. 2004;381:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krarup PM, Eld M, Heinemeier K, Jorgensen LN, Hansen MB, Ågren MS. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int J Colorectal Dis. 2013;28:1151–1159. [DOI] [PubMed] [Google Scholar]
- 44.Ågren MS, Andersen TL, Andersen L, et al. Nonselective matrix metalloproteinase but not tumor necrosis factor-α inhibition effectively preserves the early critical colon anastomotic integrity. Int J Colorectal Dis. 2011;26:329–337. [DOI] [PubMed] [Google Scholar]
- 45.Rehn M, Krarup PM, Christensen LH, Seidelin JB, Ågren MS, Syk I. GM6001 Increases Anastomotic Leakage following Colonic Obstruction Possibly by Impeding Epithelialization. Surg Infect (Larchmt). 2015;16:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasternak B, Rehn M, Andersen L, et al. Doxycycline-coated sutures improve mechanical strength of intestinal anastomoses. Int J Colorectal Dis. 2008;23:271–276. [DOI] [PubMed] [Google Scholar]
- 47.Enestvedt CK, Hosack L, Winn SR, et al. VEGF gene therapy augments localized angiogenesis and promotes anastomotic wound healing: a pilot study in a clinically relevant animal model. J Gastrointest Surg. 2008;12:1762–1770; discussion 1771–1762. [DOI] [PubMed] [Google Scholar]
- 48.Rijcken E, Fuchs T, Sachs L, Kersting CM, Bruewer M, Krieglstein CF. Insulin-like growth factor 1-coated sutures improve anastomotic healing in an experimental model of colitis. Br J Surg. 2010;97:258–265. [DOI] [PubMed] [Google Scholar]
- 49.Mastboom WJ, Hendriks T, van Elteren P, de Boer HH. The influence of NSAIDs on experimental intestinal anastomoses. Dis Colon Rectum. 1991;34:236–243. [DOI] [PubMed] [Google Scholar]
- 50.Ghiselli R, Lucarini G, Ortenzi M, et al. Anastomotic healing in a rat model of peritonitis after non-steroidal anti-inflammatory drug administration. Eur J Histochem. 2020;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapidis N, Tziouvaras C, Ioannidis O, Kalaitsidou I, Botsios D. The effect of glutamine and synbiotics on the healing of colonic anastomosis. Rev Esp Enferm Dig. 2014;106:255–262. [PubMed] [Google Scholar]
- 52.Gorur M, Sozutek A, Irkorucu O, Karakaya B. The influence of platelet-rich plasma (PRP) on colonic anastomosis healing impaired by intraperitoneal 5-flourouracil application. An experimental study. Acta Cir Bras. 2020;35:e202000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Wang Q, Luo J, et al. Superoxide Dismutase Mimic, MnTE-2-PyP Enhances Rectal Anastomotic Strength in Rats after Preoperative Chemoradiotherapy. Oxid Med Cell Longev. 2020;2020:3509859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nerstrøm M, Krarup PM, Jorgensen LN, Ågren MS. Therapeutic improvement of colonic anastomotic healing under complicated conditions: A systematic review. World J Gastrointest Surg. 2016;8:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oines MN, Krarup PM, Jorgensen LN, Agren MS. Pharmacological interventions for improved colonic anastomotic healing: a meta-analysis. World J Gastroenterol. 2014;20:12637–12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erb L, Hyman NH, Osler T. Abnormal vital signs are common after bowel resection and do not predict anastomotic leak. J Am Coll Surg. 2014;218:1195–1199. [DOI] [PubMed] [Google Scholar]
- 57.Hamade B, Huang DT. Procalcitonin: Where Are We Now? Crit Care Clin. 2020;36:23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayling RM, Kok K. Fecal Calprotectin. Adv Clin Chem. 2018;87:161–190. [DOI] [PubMed] [Google Scholar]
- 59.Messias BA, Botelho RV, Saad SS, Mocchetti ER, Turke KC, Waisberg J. Serum C-reactive protein is a useful marker to exclude anastomotic leakage after colorectal surgery. Sci Rep. 2020;10:1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256:245–250. [DOI] [PubMed] [Google Scholar]
- 61.Giaccaglia V, Salvi PF, Cunsolo GV, et al. Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care. 2014;29:528–532. [DOI] [PubMed] [Google Scholar]
- 62.Reisinger KW, Poeze M, Hulsewé KW, et al. Accurate prediction of anastomotic leakage after colorectal surgery using plasma markers for intestinal damage and inflammation. J Am Coll Surg. 2014;219:744–751. [DOI] [PubMed] [Google Scholar]
- 63.Sparreboom CL, Komen N, Rizopoulos D, et al. A multicentre cohort study of serum and peritoneal biomarkers to predict anastomotic leakage after rectal cancer resection. Colorectal Dis. 2020;22:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waterland P, Ng J, Jones A, et al. Using CRP to predict anastomotic leakage after open and laparoscopic colorectal surgery: is there a difference? Int J Colorectal Dis. 2016;31:861–868. [DOI] [PubMed] [Google Scholar]
- 65.Su’a BU, Mikaere HL, Rahiri JL, Bissett IB, Hill AG. Systematic review of the role of biomarkers in diagnosing anastomotic leakage following colorectal surgery. Br J Surg. 2017;104:503–512. [DOI] [PubMed] [Google Scholar]
- 66.Oikonomakis I, Jansson D, Hörer TM, Skoog P, Nilsson KF, Jansson K. Results of postoperative microdialysis intraperitoneal and at the anastomosis in patients developing anastomotic leakage after rectal cancer surgery. Scand J Gastroenterol. 2019;54:1261–1268. [DOI] [PubMed] [Google Scholar]
- 67.Sammour T, Singh PP, Zargar-Shoshtari K, Su’a B, Hill AG. Peritoneal Cytokine Levels Can Predict Anastomotic Leak on the First Postoperative Day. Dis Colon Rectum. 2016;59:551–556. [DOI] [PubMed] [Google Scholar]
- 68.Ellebæk MB, Baatrup G, Gjedsted J, Fristrup C, Qvist N. Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: a pilot study. Tech Coloproctol. 2014;18:1067–1074. [DOI] [PubMed] [Google Scholar]
- 69.Yang L, Huang XE, Xu L, et al. Acidic pelvic drainage as a predictive factor for anastomotic leakage after surgery for patients with rectal cancer. Asian Pac J Cancer Prev. 2013;14:54415447. [DOI] [PubMed] [Google Scholar]
- 70.Ellebæk MB, Daams F, Jansson K, et al. Peritoneal microdialysis as a tool for detecting anastomotic leakage in patients after left-side colon and rectal resection. A systematic review.Scand J Gastroenterol. 2018;53:1625–1632. [DOI] [PubMed] [Google Scholar]
- 71.Ellebaek MB, Rahr HB, Boye S, Fristrup C, Qvist N. Detection of early anastomotic leakage by intraperitoneal microdialysis after low anterior resection for rectal cancer: a prospective cohort study. Colorectal Dis. 2019;21:1387–1396. [DOI] [PubMed] [Google Scholar]
- 72.Hörer TM, Norgren L, Jansson K. Intraperitoneal glycerol levels and lactate/pyruvate ratio: early markers of postoperative complications. Scand J Gastroenterol. 2011;46:913–919. [DOI] [PubMed] [Google Scholar]