Abstract
With stroke being the leading global cause of disability in adults, the use of clinical rating scales in stroke patients is important not only for diagnostic and therapeutic purposes but also for prognostic and care implications. Scales that quantify neurological disability can be particularly useful for assessing and guiding decisions in acute management and rehabilitative treatment. We analyzed and discussed some of the main rating scales most used in stroke in clinical practice, which measure both acute neurological deficit and functional outcome in stroke. In acute stroke, it is that in most cases, the scales evaluate a neurological deficit attributable to an alteration of the anterior and not posterior circulation and most of them assess a moderate stroke rather than a mild or severe one. In a rehabilitation treatment, they are sometimes too simplified; thus, the patient can reach a near-normal score and can have significant cognitive deficits that can affect both the possibility of communication and the reliability of responses. A patient with autonomy in the activities of daily living may not be completely autonomous. In future, the use of composite rating scales could be useful for a detailed measurement of neurological deficits in acute stroke and better assess the efficacy of a treatment and functional outcome.
Keywords: Acute neurological deficit, acute stroke, anterior circulation infarcts, functional outcome, posterior circulation infarcts, stroke scale
Introduction
A clinical assessment scale should have several characteristics, in addition to being appropriate for such a measure, it should be reliable, valid, efficient in terms of speed of administration and reactivity to clinical changes [Figure 1].[1] Reliability is a measure of consistency in the score and it is derived derives from the reproducibility of the same score by the same evaluator (intraobserver agreement) and between markers (interobserver agreement), and if all elements inside a scale measure the similar value, it is an additional degree of reliability, frequently referred to as internal uniformity. This is very important in stroke clinical trials, where numerous stroke survivors can be evaluated by various research centers around the world. Although the intrinsic validity of a scale is inherent to the scale, the reliability of the valuation can be changed. Many approaches are used in large-scale trials to improve assessment consistency, including exercise in the use of scales, certification tests, and the use of homogeneous protocols. Kappa statistics are frequently used in the biomedical literature to assess agreement in reliability. The kappa coefficient is derived from the agreement between the examiners. This statistic measures higher observed agreement than chance and can range from −1 to 1. A value of 0 indicates statistical independence and a value of 1 indicates agreement among examiners. Conventionally, a kappa >0.6 is considered an adequate chord value to validate the use of a scale.[2] Increasingly sophisticated analyses, such as Bland–Altman, are used to assess reliability.[3] Other important factors for clinical scales are efficacy, with easy and quick administration, and the ability to detect changes over time, particularly important for a high-incidence condition and prevalence, such as stroke.
Figure 1.
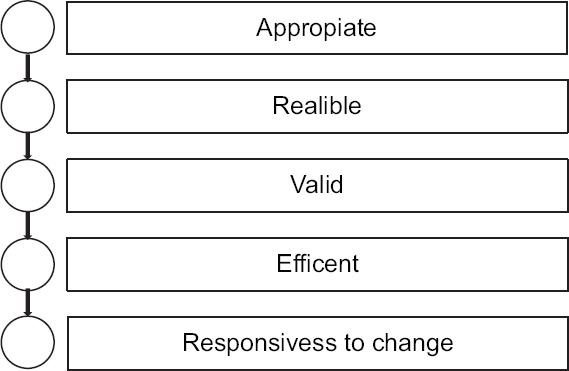
Characteristics of a clinical scale of evaluation
As stroke is the principal cause of disability in adults,[4,5] using clinical rating scales in stroke patients is important not only for diagnostic and therapeutic purposes but also for prognostic and care implications. The main problems affecting the outcome of stroke and which are susceptible to measurement are acute disability (e.g., hemiparesis or aphasia), functional outcome, i.e., the loss of capacity to make certain tasks (e.g., feed or walking), or loss of the capacity to perform normal functions and activities (e.g., occupation or hobbies) and quality of life. Scales that amount to the disability can be particularly useful for triaging and guiding critical treatment choices. The National Institutes of Health Stroke Scale (NIHSS) is a validated scale for acute stroke patients arriving in the emergency department, for the evolutionary monitoring of neurologic deficits in the hospitalized patient, and is predictive of outcomes at long term.[6,7] Sometimes, the acute neurological deficit assessment scales, represented with various numerical scores for each subgroup of the scale, are arbitrary and may not adequately reflect the impact these neurological deficits have on the patient. For example, on the NIHSS, moderate aphasia receives 2 points and it is the same score that is also attributed to the sensory hemysindrome. For these reasons, over the years, there has been a general tendency to assess a patient's level of disability using different types of functional outcome assessments rather than relying solely on scores derived from a detailed neurological examination. The measures of functional outcome assessment scales, such as the modified Rankin scale (mRS) and the Barthel index (BI), are generally much simpler to administer and are much less graded than scales based on a neurological deficit. These scales are usually used to evaluate disability outcomes in poststroke patients and can be used in rehabilitation treatment. A problem, however, with the assessments of these outcome scales is that they are sometimes too simplified and that the patient can reach an almost normal score and have significant cognitive deficits that can affect both the possibility of communication and the reliability of the answers. A patient may be able to perform the activities of daily living and not be completely autonomous.
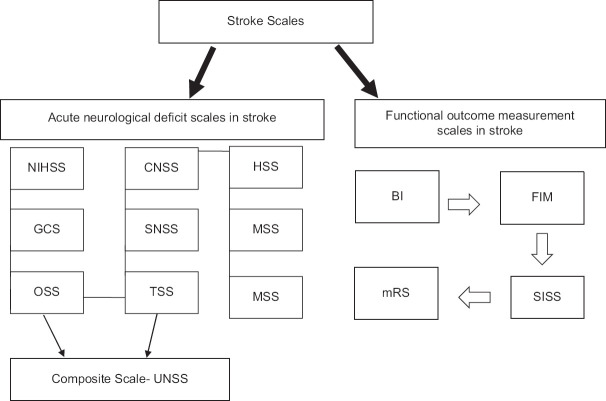
In this review, we will analyze and discuss some of the main grading scales most used in clinical practice to assess both acute and poststroke neurological failures in stroke patients [Figure 2].
Figure 2.
Schematic representation of the stroke scales
Methods
We used online databases (PubMed, Embase, Cochrane Library, and reference lists) for articles published until December 15, 2021, using the keywords “stroke scales,” “acute neurological deficit,” “clinometric property,” functional outcome measurement,” and “rating scale.” Secondary searches included articles cited in sources identified by the previous search. In the research work, we included randomized control trials, open trials, case series, and case reports.
Acute Neurological Deficit Scales in Stroke
National Institutes of Health Stroke Scale
The NIHSS score is used to assess the severity of a stroke, particularly before and after thrombolytic treatment,[6,7,8,9,10,11] with an administration time of approximately 10–15 min.[7] The NIHSS is an 11-item score scale, which provides a numerical measure of some of the signs of a standard neurological examination.[7,10] The scale evaluates the level of consciousness, extraocular activity, visual fields, facial muscle activity, limb strength, sensory activity, coordination (ataxia), language production and understanding (aphasia), joint speech (dysarthria), and neglect.[10] The use of this scale seems to be reliable in telemedicine and to be useful for remote evaluation.[12] The NIHSS showed inter-examiner reliability and validity in terms of prospective clinical research and predictively of poststroke outcome.[7,8,10] One measure of the validity of the NIHSS score is its relationship with infarcted volumes (concomitant validity), using both computed tomography and nuclear magnetic resonance imaging.[7,8] Physicians can use this scale for initial assessment, providing quick and accurate assessments of stroke-related neurological deficits that can easily be communicated to other doctors, ultimately saving valuable time in patient triage and treatment. The NIHSS is a sensitive score scale used to monitor the neurological deterioration of patients with acute stroke, although the Glasgow Coma Scale (GCS) is used in the presence of a coma.[7]
The prognostic value of this scale can help plan a patient's rehabilitation, even starting from the day of admission.[7] More than 80% of patients whose score at admission is 5 or lower will be discharged, while those with scores between 6 and 13 usually will require rehabilitation, and those with scores of 14 or higher often will require intensive rehabilitation.[11] Consideration of some limitations of the NIHSS guarantees us a more appropriate use. For example, the score scale does not include a detailed assessment of the cranial nerves, and relatively low scores may occur in patients with vertebrobasilar arterial system infarction, such as brain stem or cerebellum infarcts.[11,12] A cerebellar stroke or Wallenberg syndrome (lateral medulla) can have total NIHSS scores as low as 2–4 points, but these strokes can be disabling and even life-threatening. In fact, previous clinical studies have shown that the NIHSS has a good correlation with stroke in the territory of the middle cerebral artery (anterior circulation) while underestimating the clinical severity of a posterior circulation infarction.[11] For example, neurological deficits attributable to a posterior circulation stroke, such as diplopia, dysphagia, hypo-anacusis, and ataxic gait, are not scored. However, in the context of anterior circulation strokes, it should be borne in mind that the same does not evaluate the paralysis of the hand (parietal hand) from a heart attack affecting the paracentral lobule.[13] Furthermore, the NIHSS gives no indication in identifying the actual cause of the neurological deficit.
Glasgow coma scale
The GCS score is widely used to assess the severity of trauma in the acute phase and, in any case, evaluates the patient's level of consciousness and/or the severity of the coma. In hospitals, it is also used to monitor the level of consciousness of patients in intensive care units.[14] The final score ranges from a minimum of 3 points (severe coma) to a maximum of 15 (full consciousness).[7,8,9,15] The GCS score is considered an imperfect predictor of the subject's productivity and degree of independence.[7] The limitations of GCS relate to the inability to provide verbal scores and eye-opening in patients who have large facial lesions or are intubated. To overcome this problem concerning the motor response of the scale, a higher score ranging from 1 to 6 is used as a more universally applicable indicator of the state of consciousness; moreover, in particular, for the absence of verbal responses in intubated patients, other scales such as Swedish Reaction Level Scale[8] are used. Finally, another validated scale used in polytrauma is the Injury Severity Score.[8]
Other Rating Scales
Other scales have been validated to better measure neurological deficits in acute stroke. These include:
Scandinavian Neurological Stroke Scale (SNSS) assesses infarction of the anterior circulation more adequately (including walking) than the posterior one[8]
Orgogozo Stroke Scale (OSS), not widely used, also evaluates infarcts of the anterior circulation, has a very high interobserver reliability, and provides a more detailed assessment of motor deficit[8]
Canadian Neurological Stroke Scale, which is simple to administer and with good interobserver reliability, significantly assesses anterior circulation infarcts[8,9]
Toronto Stroke Scale, a scale which is more complex and more difficult to learn to the point of requiring neurological training, requires a long administration time (10–20 min) and evaluates infarcts not only of the anterior circulation[8]
Hemispheric Stroke Scale is a scale with a complexity in the assessment, with more difficulties in learning, which requires a long administration time (from 15 to 30 min) and assesses cerebral infarcts in various territories[6,8]
Mathew Stroke Scale (MSS), is a scale with poor inter-observer agreement. It takes about 15 minutes for its administration and difficulty in learning by the observer. It can identify stroke in both the anterior and posterior circulation[8]
European Stroke Scale evaluates anterior circulation infarcts and is similar to the NIHSS except that the level of consciousness and gait are additionally assessed. This scale is not widely used but has high inter- and intraobserver reliability and requires neurological training such as the Scandinavian Stroke Scale, the Orgogozo Scale, and the Canadian Neurological Scale.[8,9]
Unified Neurological Stroke Scale (Composite Scale)
It is a composite scale derived from a combination of the OSS and the SNSS.[16] The scale demonstrated good interobserver agreement and reliability but must be administered by a specialist and takes approximately 15 min. Another disadvantage is the fact that it is not widely used and has not been validated in several clinical studies.[3]
Functional Outcome Measurement Scales in Stroke
Barthel index
BI is a scale that measures 10 key aspects of activity related to self-care and patient mobility.[6,7,8,17] The normal score is 100, and lower scores indicate various degrees of dependence.[8] BI is determined by observing patients in a series of activities, while some items are aimed to assess bowel and bladder continence. The intraobserver and interobserver reliabilities of this scale are quite high.[6,7,8] BI measurements were observed to be similar whether obtained from telephone interviews or direct interviews, making this scale a potentially useful tool when study patients are unable to be evaluated directly in follow-ups. Although, in patients with cognitive dysfunction, severe disease, and over 75 years of age, self-reported scores may be less accurate than direct measurements.[6]
BI has moderate validity in agreement agreed with the volume of infarct volume,[8] but it has been demonstrated that as significant shown to have important predictive value.[3] For example, 6 months after acute stroke, patients with scores from 0 to 40 had died or were living in long-term care facilities, while patients with scores of 81–100 lived in their own home. In addition, stroke patients with scores above 60 after rehabilitation could be active in their homes and communities, have more social interactions, and be happier with life in general than those with scores of 60 or less.[6,7,8] We have seen how BI evaluates different activities of daily living and specific physiological deficits, but many aspects of independence, such as cognitive level, language, visual function, emotional disability, and pain, are not evaluated. For example, a patient with severe aphasia may be totally normal in all items of BI but may not be able to be autonomous outdoor without the support of another person or ask for help if needed.
Modified Rankin scale
It is another scale for assessing residual disability in stroke patients.[6,17] The modified version, or mRS, is categorically defined with 5 different degrees: 0 indicates no symptoms and 5 indicates severe disability. Scores are assigned based on the amount of assistance needed by the patient to achieve various levels of autonomy, and a shift of one point on this scale is clinically important. Its validity in assessing residual disability in stroke patients and interobserver and intraobserver reliability have been well documented. The mRS correlates modestly with infarct volumes[7,8] which is similar to the results for BI.
mRS offers a quick and easy valuation of a patient's stroke outcomes, activities, and contribution in a social setting. Items regarding cognitive function, language, visual function, and pain are not assessed directly but are included in the mRS score. This is often considered a disadvantage as in some cases a relatively small cerebral infarct or mild neurological deficit can cause severe disability (such as a visual field disorder in a truck driver), while a major cerebral infarction can sometimes cause mild disabilities in others (for example, a cerebellar infarction in a sedentary person), and especially the presence of neuropsychological alterations can influence the patient's recovery and perception of disability.
Functional Independence Measure
The Functional Independence Measure (FIM) is a measure of disability, used in addition to BI and mRS in functional outcome studies of stroke.[8,17,18] It is a scale of 18 items divided into 6 subgroups and which evaluates 2 dimensions: (1) physical characteristics (nutrition, personal cleanliness, ability to bathe, dress, use of the bathroom, bladder and rectal control, and ability to move and walk) and (2) cognitive characteristics (communication, social interactions, problem-solving, and memory). Regarding the 18 items, each of them is evaluated with a scale having a score from 1 (completely dependent) to 7 (completely independent). It has high interobserver agreement even when administered by telephone.[8,18] The advantages of FIM over mRS, GOS, and BI consist in a more detailed evaluation of the patient's functional abilities, however, it is more difficult to learn.[18]
Stroke Impact Scale
The Stroke Impact Scale (SIS) is a scale developed more for patients and caregivers than for research studies and is used to measure the quality of life in stroke survivors with high intraobserver reliability.[7,19] There are several versions of the SIS, and further refinements are still in progress. The latest version of the SIS (SIS-16) evaluates more disability levels 1–3 months after stroke. Like the BI, the SIS-16 reported greater sensitivity for detecting different levels of disability. One of the main limitations of the SIS is the necessity for self-reporting or the use of a proxy.[19.20] Moreover, it is a limitation in aphasic patients and in patients who have a denial of their deficit or disease. Furthermore, proxy responses have been reported to differ from direct patient responses and sometimes overestimate the severity of clinical conditions.[7,19] This rating scale is currently not widely used.[7]
Discussion
Better knowledge in the use of clinical stroke rating scales is essential for evaluating stroke patients in both the acute and rehabilitation phases. These scales range from relatively simple and quick measurements to very detailed assessments that take time. The evaluation scales should have demonstrated inter- and intraobserver reliability, be authorized for their use, be responsive to significant clinical changes, and finally, be easy to learn and administer. Many of these scales have been designed primarily to evaluate anterior circulation infarcts and are almost all calibrated mainly on motor function even if they often do not evaluate walking. A problem in using the scales, in acute, is that minor neurological deficits can give the same score as major neurological deficits, and that most of them value a medium-severity stroke more than a mild or severe stroke. Currently, in the acute phase of the stroke, the NIHSS is the preferred scale given its diffusion, high reliability, and rapidity of administration.[10,17]
Conclusion
In future, the use of composite rating scales,[6,16] often used in pain,[21] and derived from scores of different scales, seems to be useful for a detailed measurement of neurological deficits in stroke, and therefore, it demonstrates greater effectiveness of a treatment as well as a more detailed description of the functional outcome. Moreover, a greater development of these composite assessment scales could offer a good balance between a detailed global assessment of the neurological deficit and the ease of use.
Conflicts of interest
None declared.
Ethical approval
This manuscript does not consist of human subject research and therefore is not under the jurisdiction of an Institutional Review Board.
Funding
None.
Acknowledgment
Antonio Siniscalchi serves as a national member for the Italian National Society of Neurovascular Disease (SINV).
References
- 1.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: Clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201–11. doi: 10.2147/CIA.S32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraemer HC, Bloch DA. Kappa coefficients in epidemiology: An appraisal of a reappraisal. J Clin Epidemiol. 1988;41:959–68. doi: 10.1016/0895-4356(88)90032-7. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 4.Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, et al. Cerebral stroke injury: The role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol. 2014;25:131–7. doi: 10.1515/jbcpp-2013-0121. [DOI] [PubMed] [Google Scholar]
- 5.Siniscalchi A, Iannacchero R, Anticoli S, Pezzella FR, De Sarro G, Gallelli L. Anti-inflammatory strategies in stroke: A potential therapeutic target. Curr Vasc Pharmacol. 2016;14:98–105. doi: 10.2174/1570161113666150923111329. [DOI] [PubMed] [Google Scholar]
- 6.De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993;24:1178–81. doi: 10.1161/01.str.24.8.1178. [DOI] [PubMed] [Google Scholar]
- 7.Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–12. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- 8.Herndon RM, editor. Handbook of Neurologic Rating Scales. 2nd edition. New York: Demos Medical Publishing; 2006. [Google Scholar]
- 9.Ghandehari K. Challenging comparison of stroke scales. J Res Med Sci. 2013;18:906–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Pezzella FR, Picconi O, De Luca A, Lyden PD, Fiorelli M. Development of the Italian version of the National Institutes of Health Stroke Scale: It-NIHSS. Stroke. 2009;40:2557–9. doi: 10.1161/STROKEAHA.108.534495. [DOI] [PubMed] [Google Scholar]
- 11.Siniscalchi A, Sztajzel R, Malferrari G, Gallelli L. The National Institutes of Health Stroke Scale: Its role in patients with posterior circulation stroke. Hosp Top. 2017;95:79–81. doi: 10.1080/00185868.2017.1322888. [DOI] [PubMed] [Google Scholar]
- 12.Pezzella FR, Pozzessere C, Siniscalchi A, Gallelli L, Anticoli S. The cloud stroke unit: 24-hour acute stroke expertise-on-demand. Hosp Top. 2013;91:81–6. doi: 10.1080/00185868.2013.848160. [DOI] [PubMed] [Google Scholar]
- 13.Siniscalchi A, Lochner P, Perrotta P, Rizzuto S, De Sarro G, Gallelli L. Isolated hand palsy in National Institutes of Health Stroke Scale (NIHSS): Is it useful? West J Emerg Med. 2018;19:524–6. doi: 10.5811/westjem.2018.2.37654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta R, trainee GP, Chinthapalli K consultant neurologist. Glasgow coma scale explained. BMJ. 2019;365:l1296. doi: 10.1136/bmj.l1296. [DOI] [PubMed] [Google Scholar]
- 15.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 2014;13:844–54. doi: 10.1016/S1474-4422(14)70120-6. [DOI] [PubMed] [Google Scholar]
- 16.Edwards DF, Chen YW, Diringer MN. Unified Neurological Stroke Scale is valid in ischemic and hemorrhagic stroke. Stroke. 1995;26:1852–8. doi: 10.1161/01.str.26.10.1852. [DOI] [PubMed] [Google Scholar]
- 17.Alijanpour S, Mostafazdeh-Bora M, Ahmadi Ahangar A. Different Stroke Scales; which scale or scales should be used? Caspian J Intern Med. 2021;12:1–21. doi: 10.22088/cjim.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bräutigam K, Flemming A, Schulz H, Dassen T. How reliable is the functional independence measure (FMI)? Pflege. 2002;15:131–6. doi: 10.1024/1012-5302.15.3.131. [DOI] [PubMed] [Google Scholar]
- 19.Edwards B, O’Connell B. Internal consistency and validity of the Stroke Impact Scale 2.0 (SIS 2.0) and SIS-16 in an Australian sample. Qual Life Res. 2003;12:1127–35. doi: 10.1023/a:1026109920478. [DOI] [PubMed] [Google Scholar]
- 20.Mulder M, Nijland R. Stroke Impact Scale. J Physiother. 2016;62:117. doi: 10.1016/j.jphys.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Silva NE, Trindade PH, Oliveira AR, Taffarel MO, Moreira MA, Denadai R, et al. Validation of the Unesp-Botucatu composite scale to assess acute postoperative abdominal pain in sheep (USAPS) PLoS One. 2020;15:e0239622. doi: 10.1371/journal.pone.0239622. [DOI] [PMC free article] [PubMed] [Google Scholar]