Abstract
Escherichia coli contains the CspA family, consisting of nine proteins (CspA to CspI), in which CspA, CspB, and CspG have been shown to be cold shock inducible and CspD has been shown to be stationary-phase inducible. The cspI gene is located at 35.2 min on the E. coli chromosome map, and CspI shows 70, 70, and 79% identity to CspA, CspB, and CspG, respectively. Analyses of cspI-lacZ fusion constructs and the cspI mRNA revealed that cspI is cold shock inducible. The 5′-untranslated region of the cspI mRNA consists of 145 bases and causes a negative effect on cspI expression at 37°C. The cspI mRNA was very unstable at 37°C but was stabilized upon cold shock. Analyses of the CspI protein on two-dimensional gel electrophoresis revealed that CspI production is maximal at or below 15°C. Taking these results together, E. coli possesses a total of four cold shock-inducible proteins in the CspA family. Interestingly, the optimal temperature ranges for their induction are different: CspA induction occurs over the broadest temperature range (30 to 10°C), CspI induction occurs over the narrowest and lowest temperature range (15 to 10°C), and CspB and CspG occurs at temperatures between the above extremes (20 to 10°C).
When Escherichia coli cells grown at 37°C are transferred to low temperatures such as 15°C, a set of proteins called cold shock proteins are transiently induced at very high levels during a growth lag period called the acclimation phase (16, 17, 30). Among them, CspA has been identified as a major cold shock protein, consisting of 70 amino acid residues. It has two RNA binding motifs, RNP1 and RNP2 (10, 16), and forms a β-barrel structure (7, 24, 27). CspA was revealed to cooperatively bind to RNAs and single-stranded DNAs and is considered to function as an RNA chaperone, which may prevent the formation of secondary structures of mRNAs for efficient translation at low temperatures (15).
The cspA promoter is highly active at 37°C, although CspA protein is hardly detected at this temperature (5, 22), indicating that cspA expression at low temperatures is regulated posttranscriptionally. It should be mentioned, however, that the AT-rich sequence immediately upstream of the −35 region of the cspA promoter functions as a UP element to enhance cspA transcription (9, 22). The cspA mRNA is extremely unstable at 37°C but is dramatically stabilized upon cold shock (3, 5, 8, 22). The cspA mRNA possesses an unusually long 5′ untranslated region (5′-UTR) consisting of 159 bases (29). Mutation analyses have shown that the 5′-UTR of the cspA mRNA plays a crucial role in its cold shock inducibility (5, 22). In addition, translation initiation of the cspA mRNA appears to be very efficient at low temperature in comparison with mRNAs of non-cold-shock proteins (22). Thus, the cspA expression is regulated in a complex manner, that is, at the levels of transcription, mRNA stability and translation efficiency (3, 5, 8, 9, 22).
cspA induction occurs transiently during the acclimation phase upon cold shock, as mentioned above. At the end of the acclimation phase, cspA expression is repressed to a new basal level (10). It has been proposed that the cold box sequence, which is located in the 5′-UTR, and a factor which might bind to the cold box to repress the cspA expression at the level of transcription are responsible for autoregulation of cspA expression (6, 14).
Six additional cspA homologues in E. coli, cspB to cspG, have been identified by means of Southern analysis with cspA as a probe (cspB and cspC [19]), isolation of multicopy suppressors of a chromosome partition mutant (cspC and cspE [35]) and cold shock induction (cspG [23]). Of these, cspA, cspB, and cspG are cold shock inducible (10, 19, 23) and cspC and cspE are expressed at both high and low temperatures (35). cspD is located upstream of clpA (11) and is induced during stationary phase and upon nutrition starvation (34). cspF is closely linked to cspB (33); however, its function is not known. cspA, cspB, and cspG mRNAs share a highly conserved, long 5′-UTR sequence (19, 23, 29), suggesting that the expression of cspB and cspG is regulated in a similar way to that of cspA. It should be noted, however, that CspB and CspG are induced in a narrower range of low temperature than is CspA (4). cspA is dispensable for growth at either 37 or 15°C, and the production of CspB and CspG increased in a cspA deletion strain, suggesting that they may have similar functions (2).
Upon completion of E. coli genome sequencing, two more CspA homologues, designated CspH and CspI (33), were found. They show the highest similarity to CspF and CspG, respectively (33). Although the primary amino acid sequence of CspI shows high identity not only to CspG (79%) but also to CspA (70%) and CspB (70%) (33), the region corresponding to the highly conserved 5′-UTR of cspA, cspB, and cspG is less highly conserved in cspI.
Here we demonstrate that cspI is another cold shock gene, as judged by lacZ expression of both the transcriptional and the translational cspI-lacZ fusions and by CspI production, determined by two-dimensional gel electrophoresis. As shown for cspA, the cspI mRNA was dramatically stabilized upon cold shock and the overproduction of the cspI 5′-UTR caused derepression of cspA, cspB, cspG, and cspI. In addition, the cspI gene was induced in the lowest temperature range (15 to 10°C), suggesting that CspI may play an important physiological role in growth at very low temperature.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
E. coli JM83 (32) and AR134 (MC4100 pcnB80) (13) were used. Plasmid pUC19 (32) was used for cloning. Plasmids pRS414 and pRS415 (28) were used for construction of lacZ fusions. Plasmid pCspA-LacZ, in which the cspA upstream region and the first 13 codons of cspA were fused translationally to lacZ on the pRS414 vector, as described previously (19), was used as a control.
Luria-Bertani (LB) and M9 media, supplemented with 0.4% glucose, 0.4% Casamino Acids, and 50 μg/ml of thiamine, were used for bacterial growth. When necessary, ampicillin was added at a final concentration of 50 μg/ml.
General techniques.
DNA cloning was carried out by the method described previously (26). PCR amplification was carried out as specified in the manufacturer’s instruction manual (Boehringer) with 30 cycles of amplification steps each of 1 min at 95°C, 2 min at 50°C, and 2 min at 72°C. Restriction enzymes and DNA modification enzymes were purchased from Boehringer, Gibco BRL, and New England Biolabs.
Plasmid construction.
cspI was amplified by PCR with synthetic oligonucleotide primers, 8188 (5′-aagaattcAACATTTACATCGCGGAA-3′) and 8187 (5′-ttgggatCCTCAAAGCGCCACTA-3′), where the 5′ tails are shown in lowercase type and the EcoRI and BamHI sites, respectively, are underlined. Genomic DNA from prototype strain W3110 (18) was used as a template. The PCR fragment was directly cloned into the SmaI site of pUC19, yielding pNWI2. To construct a plasmid that can express only the 5′-UTR of cspI but not its coding region, pNWI2 was digested with StyI and SacI, blunt ended with T4 DNA polymerase, and then self-ligated, yielding pNWI6.
To construct the transcriptional and translational cspI-lacZ fusions, PCR was first carried out with primers 8188 (see above) and 8010 (5′-gggggatccGGGTTAAACCATTTCACT-3′), where the 5′ tail is shown in lowercase type and the BamHI site is underlined. The PCR fragment was digested with EcoRI and BamHI and then cloned into pRS414 for the construction of a translational fusion and into pRS415 for the construction of a transcriptional fusion, yielding pNWI3 and pNWI4, respectively. For the deletion construct of the entire 5′ untranslated region of the cspI-lacZ fusion, PCR was carried out with primers 8188 (see above) and 8404 (5′-ggggatcCAGAACACCATTAACGC-3′), where the 5′ tail is shown in lowercase type and the BamHI site is underlined. The PCR fragment was cloned into pRS415 in a similar way to that described above, yielding pNWI5. All the constructs were confirmed by DNA sequencing with Sequenase version 2.0 (Amersham).
Assay for β-galactosidase activity.
The cspI-lacZ fusion constructs were introduced into strain AR134. Cells were grown in LB or M9 medium at 37°C to mid-log phase and then transferred to 15°C. Portions of culture were taken immediately before the temperature downshift (0 h) and at 1, 2, 3, and 5 h after the temperature downshift. β-Galactosidase activity was measured as described by Miller (21). The assay was done at least in duplicate at each time point.
Isolation of RNA and primer extension.
Strain JM83 was grown in LB medium at 37°C to mid-log phase and then transferred to 15°C. RNA was extracted from a 1.5-ml culture by the hot-phenol method described previously (1). Primer 8272 (5′-CCAAAACCTTTTTCAGGG-3′) for detection of cspI and primer 4593 (5′-ACATAGTGTATTACCTTTAA-3′) for detection of cspA were labeled with [γ-32P]ATP (>5,000 Ci/mmol; DuPont-New England Nuclear) by using T4 polynucleotide kinase (Gibco BRL). Primer extension was carried out with 5 μg of RNA at 42°C for 1 h in a final volume of 10 μl, which contained 50 mM Tris-HCl (pH 8.5), 8 mM MgCl2, 30 mM KCl, 1 mM dithiothreitol, 0.4 pmol of 32P-labeled primer, 0.5 mM dATP, 0.5 mM dGTP, 0.5 mM dCTP, 0.5 mM dTTP, 10 U of RNase inhibitor (Boehringer Mannheim), and 6.25 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim). Primer extension products were analyzed on a 6% polyacrylamide gel under denaturing conditions.
For measurement of the stability of mRNA at 15°C, rifampin was added 1 h after the temperature downshift to 15°C at a final concentration of 200 μg/ml to stop transcription. For measurement of the stability of mRNA at 37°C, the culture was shifted to 15°C for 30 min to accumulate mRNAs, a 5-ml sample was taken and mixed with 5 ml of the medium (which was prewarmed at 60°C) in a glass flask kept at 37°C, rifampin was simultaneously added to a final concentration of 200 μg/ml, a 1.5-ml sample was taken at each time point, and RNA extraction and primer extension assays were done as described above. Primer extension products were quantitated by using a phosphorimager (Bio-Rad).
Protein-labeling experiment.
Cells were grown in M9 medium supplemented with glucose, 19 amino acids (no methionine), and thiamine at 37°C, and then transferred to an indicated temperature. Cells were labeled with [35S]methionine (1092 Ci/mmol; Amersham) for 30 min at lower temperatures and then chased for 5 min by adding nonradioactive methionine to a final concentration of 0.2 M. Cell lysates were prepared and processed by two-dimensional gel electrophoresis as described previously (31).
RESULTS AND DISCUSSION
Sequence comparison among CspA, CspB, CspG, and CspI.
The E. coli genome-sequencing project has revealed the ninth member of the CspA family, which we designated CspI (33). CspI shows the highest identity to CspG (79%) (Fig. 1A), and on the phylogenetic tree it belongs to the same group as CspA, CspB, and CspG, all of which are cold shock inducible as described previously (33). The three-dimensional structure of CspA has been determined. It consists of five antiparallel β-strands forming a β-barrel structure with two β-sheets (7, 24, 27). CspI contains well-conserved hydrophobic residues including V9, I21, V30, V32, and V51 (Fig. 1A), which form a hydrophobic core in CspA (7, 24, 27). In addition, two RNA binding motifs, RNP1 and RNP2, are well conserved in CspI (Fig. 1A). These facts suggest that CspI may form a conformation similar to that of CspA and may also bind to RNA and single-stranded DNA, as CspA does (15).
FIG. 1.
Sequence comparison of four cold shock-inducible members of the E. coli CspA family. (A) Amino acid sequence alignments of CspI (database accession no. AE000252), CspG (AE000201), CspB (AE000252), and CspA (AE000433). Residues identical to CspI are shown as dots. The residues forming the hydrophobic core in the β-barrel structure are indicated by solid circles above the sequences. The RNA binding motifs, RNP1 and RNP2, are boxed. Their amino acid sequence homologies are shown on the right, with CspI set at 100%. (B) Sequence alignment of the promoter, 5′-UTR, and the first 13 codon nucleotides of cspI, cspG, cspB, and cspA. Nucleotides identical to cspI are shown as dots. To maximize the alignment, some gaps have been introduced; these are indicated by dashes. The transcription start sites are in bold letters and are marked as +1. The translation start codon ATGs are also in bold letters and are underlined. The most homologous sequences (UP element, -35 region, -10 region, cold box, upstream sequence, Shine-Dalgarno [SD] sequence, and downstream box) are boxed and labeled above the boxes.
Cold shock-inducible expression of cspI.
The cspI gene is located at 35.2 min on the E. coli chromosome and transcribed counterclockwise. To determine the regulation of cspI expression, the 387-bp DNA fragment containing the 346-bp upstream sequence of the cspI coding region and the region for the first 13 codons of cspI was amplified and cloned, to translationally and transcriptionally fuse to lacZ on pRS414 and pRS415, yielding pNWI3 and pNWI4, respectively. These fusion constructs were introduced into AR134, and the β-galactosidase activity was measured. AR134 is a pcnB mutant (13), which keeps pBR322 derivatives at low copy number (20) to minimize any positive or negative effects of the multicopy plasmids on cspI-lacZ expression.
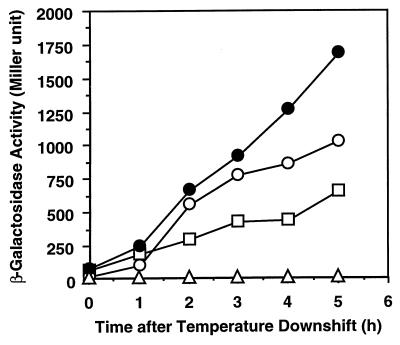
β-Galactosidase activities of both fusion constructs were very low at 37°C at mid-log phase (zero time point in Fig. 2). Since CspI shows the highest identity to cold shock-inducible CspG, we analyzed the effect of temperature downshift on cspI-lacZ expression. After the temperature downshift from 37 to 15°C, the β-galactosidase activities of both the transcriptional and the translational fusion constructs dramatically increased and were both approximately twofold higher than that of the cspA-lacZ translational fusion, as shown in Fig. 2, indicating that cspI is a cold shock-inducible gene like cspA, cspB, and cspG.
FIG. 2.
Cold shock induction of β-galactosidase activity. Strain AR134, harboring various plasmids, was grown to mid-log phase at 37°C in LB medium containing ampicillin (50 μg/ml) and then transferred to 15°C. Samples were taken at 0, 1, 2, 3, and 5 h after the temperature downshift, and β-galactosidase activity was measured. The assay was carried out at least in duplicate at each time point. Symbols: ○, pNWI3; ●, pNWI4; □, pCspA-LacZ; ▵, pRS414.
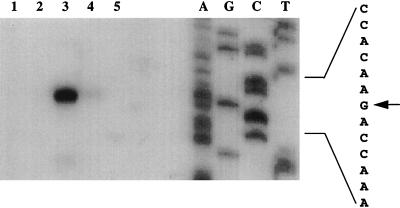
Although the expression of cspA, cspB, cspG, and cspI is cold shock inducible and the primary amino acid sequence of CspI is highly homologous to those of CspA, CspB, and CspG, a putative 5′-UTR sequence for cspI does not show high similarity to those for cspA, cspB, and cspG, suggesting that cspI might be regulated somewhat differently. To identify the 5′-UTR of cspI, the transcription start site of cspI was determined by primer extension analysis with primer 8272, which is specific for cspI. This primer corresponds to the complementary strand for cspI codons 14 through 19. Total RNA of strain JM83 was extracted from cells grown at 37°C and from cells grown for 0.5 and 3 h after the temperature downshift to 15°C. Primer extension products of cspI from pre-cold-shocked cells were hardly detected (Fig. 3, lane 2). However, at 0.5 h after the temperature downshift, the amounts of primer extension products dramatically increased (lane 3), indicating that the amount of cspI mRNA greatly increased upon cold shock. At 3 h after the temperature downshift, the amount of cspI mRNA was reduced to a new basal level (lane 4), which is slightly higher than that at 37°C. The pattern of cold shock induction of the cspI mRNA is very similar to those of the cspA, cspB, and cspG mRNAs (4, 23), indicating that cspI is also transiently induced upon cold shock. The cspI mRNA was not induced during stationary phase at 37°C (lane 5).
FIG. 3.
Primer extension analysis of the cspI mRNA. Strain JM83 was grown in LB medium at 37°C. RNA extraction and primer extension analysis were carried out as described in Materials and Methods. The primer corresponds to the complementary strand for codons 14 to 19 of cspI. Lanes: 1, without RNA; 2, with RNA extracted from exponentially growing cells at 37°C; 3, with RNA extracted from cells at 0.5 h after the temperature downshift to 15°C; 4, with RNA extracted from cells at 3 h after the shift; 5, with RNA extracted from stationary-phase cells at 37°C. Primer extension products were analyzed on a denatured polyacrylamide gel together with a sequencing ladder. The sequence is shown at the right; the arrow indicates the transcription start site.
On the basis of the primer extension experiment described above, the possible transcription start site and the deduced promoter region, the -35 and -10 sequences (TTGCTA and GTTAAT, respectively) are identified as shown in Fig. 1B. The promoter sequence of cspI is very similar to those of cspA, cspB, and cspG. Upstream of the -35 region of cspA, cspB, and cspG is an AT-rich region called the UP element (25), which is believed to play an important role in maintaining the high promoter activity of cspA at both 37 and 15°C (22). The UP element also exists in the cspI gene and is likely to play an important role in cspI transcription at low temperature.
The primer extension experiment (Fig. 3) also reveals that the cspI mRNA contains a long 5′-UTR consisting of 145 bases, which is comparable to the 5′-UTRs of cspA (159 bases), cspB (161 bases), and cspG (156 bases). Although the sequence of the 5′-UTR of cspI is not highly homologous to those of cspA, cspB, and cspG, the 5′-UTR of cspI still contains a well-conserved motif, termed the cold box, which is believed to be involved in autoregulation at the end of the acclimation phase (6, 14, see below). Farther downstream in the 5′-UTR, there is a 12-base conserved sequence designated the upstream sequence, which may be involved in the translation efficiency of cspA (36). It should be noted that cspA, cspB, and cspG all have a downstream box downstream of the translation initiation codon, which has been shown to play an important role in cold shock induction at the level of translation (22). As shown in Fig. 1B, cspI has exactly the same downstream box as cspB and cspG, suggesting that the cspI downstream box also plays an essential role in translation at low temperature.
cspI mRNA stabilization upon cold shock.
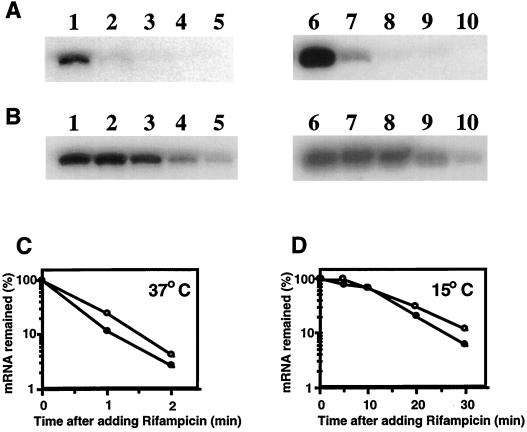
Based on the analysis of the cspI-lacZ fusion constructs, cspI expression seems to be regulated by transcription and/or mRNA stability (Fig. 2). It has been shown that mRNA stability plays a critical role in the cold inducibility of cspA and that the stability of mRNA is regulated by its long 5′-UTR (3, 5, 8). Therefore, we examined the stability of the cspI mRNA at both 37 and 15°C by primer extension analyses. As shown in Fig. 4, the cspI mRNA was very unstable at 37°C, with a half-life of approximately 30 s, somewhat more stable than the cspA mRNA (half-life, approximately 20 s). However, at 1 h after the temperature downshift, the cspI mRNA and the cspA mRNA were stabilized with half-lives of 14 and 12 min, respectively. These results suggest that mRNA stability also plays a major role in the cold shock induction of cspI.
FIG. 4.
Analysis of mRNA stability. A culture of strain JM83 grown in LB medium at 37°C was shifted to 15°C. (A) For measurement of mRNA stability at 37°C, a culture preincubated at 15°C for 30 min was shifted back to 37°C. Rifampin was added to the culture to a final concentration of 200 μg/ml at the point of the temperature upshift. RNAs were extracted at 0 (lanes 1 and 6), 1 (lanes 2 and 7), 2 (lanes 3 and 8), 3 (lanes 4 and 9), and 5 (lanes 5 and 10) min after the addition of rifampin. (B) For measurement of the mRNA stability at 15°C, rifampin was added 1 h after the temperature downshift and RNAs were extracted at 0 (lanes 1 and 6), 5 (lanes 2 and 7), 10 (lanes 3 and 8), 20 (lanes 4 and 9), and 30 (lanes 5 and 10) min after the addition of rifampin. Primer extension was carried out with a primer for cspI (lanes 1 to 5) and with a primer for cspA (lanes 6 to 10) as described in Materials and Methods. (C and D) Graphical presentations based on the results obtained in panels A and B, respectively. The radioactivities of primer extension products were measured and plotted, with the product at time zero set to 100%. Symbols: ○, cspI; ●, cspA.
When the 5′-UTR of the cspA mRNA was deleted, cspA expression was observed even at 37°C, indicating that the 5′-UTR has a negative effect on cspA expression at 37°C (22). To elucidate the effect of cspI 5′-UTR on cspI expression, another transcriptional cspI-lacZ fusion construct, pNWI5, which does not contain the 5′-UTR, was prepared (see Materials and Methods). At 37°C, the β-galactosidase activity in the cells harboring pNWI5 was 980 U, which was much higher than that in the cells harboring pNWI4 (29 U), which contains the 5′-UTR. This indicates that the cspI 5′-UTR has a negative effect on its own gene expression at 37°C, as the cspA 5′-UTR does.
CspI induction at lower temperatures.
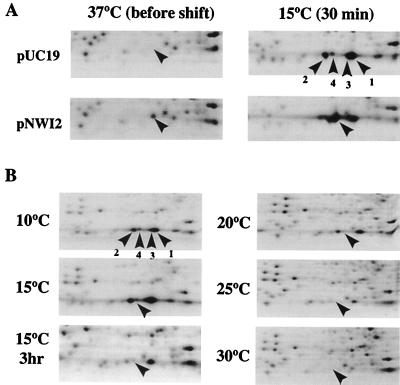
CspA, CspB, CspG, and CspI are all cold shock inducible. However, upon cold shock, the temperature dependence of CspA induction is broader while that of CspB and CspG is restricted to lower temperatures and to a narrower temperature range (4). To examine the optimal temperature for CspI induction, two-dimensional (2D) gel electrophoresis was carried out with cells labeled with [35S]methionine at different temperatures. For this purpose, we first determined the spot corresponding to CspI by using a plasmid which overproduces CspI. As shown in Fig. 5A, CspI migrated very close to CspB and its production was indeed induced upon cold shock.
FIG. 5.
Analysis of CspI protein by 2D gel electrophoresis. (A) Cultures of strain JM83 harboring pUC19 or pNWI2 were labeled with [35S]methionine at 37°C and labeled 30 min after the temperature downshift to 15°C. Total-cell extracts were analyzed by 2D gel electrophoresis, and autoradiograms were obtained. Only a portion corresponding to low-molecular-weight proteins is shown. (B) Cultures of strain JM83 were labeled with [35S]methionine 30 min after the temperature downshift from 37°C to 30, 25, 20, 15, and 10°C, and the one indicated by “15°C 3 hr” is a sample labeled at 3 h after the temperature downshift to 15°C. CspI is indicated by an arrowhead. The positions of CspA, CspB, and CspG are shown by labeled arrowheads in one of the panels in A and B: 1, CspA; 2, CspB; 3, CspG; and 4, CspI.
CspI production from the chromosomal cspI gene could not be detected at 37°C but became clearly detectable at 15°C (Fig. 5B), unambiguously demonstrating that cspI is a cold shock-inducible gene. Next, cells grown at 37°C to mid-log phase was transferred to different temperatures, i.e., 30, 25, 20, 15, and 10°C. At 30 min after the temperature shift, cells were labeled with [35S]methionine for 30 min and total cellular proteins were analyzed by 2D gel electrophoresis. In contrast to CspA, CspB, and CspG, CspI was induced only when the temperature was shifted to or below 15°C (Fig. 5B), indicating that CspI has the narrowest and lowest temperature range for its induction. CspI production was reduced to a lower level at 3 h after the temperature downshift, as can be seen from the gel (Fig. 5B), which is in good agreement with the results of the primer extension analysis as shown in Fig. 3. This indicates that CspI production is transiently induced during the acclimation phase upon cold shock, as for CspA, CspB, and CspG (4, 10, 23, 29).
It is interesting that the rate of CspI synthesis is much lower than that of CspA (Fig. 5), although the β-galactosidase activity of the cspI-lacZ translational fusion construct is higher than that of the cspA-lacZ translational fusion construct (Fig. 2). In both fusion constructs, the first 13 codons of cspI or cspA were translationally fused to lacZ as mentioned above. Since transcription initiation and translation initiation from the fusion constructs are likely to occur in the same manner as those from the chromosomal copy of cspA and cspI, we examined any difference in protein stability between CspA and CspI. Their half-lives were measured by pulse-chase experiments 30 min after the temperature downshift and found to be longer than 5 h, although CspI was somewhat less stable than CspA (data not shown). The chasing in the 2D electrophoresis in Fig. 5 was only 5 min. Therefore, it is unlikely that the low production of CspI is due to protein stability. These results therefore suggest that the difference between CspA and CspI production at low temperature may be at the level of translation elongation.
Derepression by overexpression of the cspA or cspI 5′-UTR.
It has been reported that when the cspA 5′-UTR was overproduced during cold shock treatment, CspA and CspB expression were no longer transient and a high level of CspA and CspB expression was still observed after the acclimation phase (6, 14). This phenomenon, called derepression, was also observed when the region from +1 to +25 of the cspA 5′-UTR was overexpressed. Within this region, a highly conserved sequence, designated the cold box sequence, was found (Fig. 1B) (14). Conversely, deletion of the cold box region abolished the derepression effect of the cspA 5′-UTR (6). It has been proposed that a factor might bind to the cold box to repress the cspA expression at the level of transcription at the end of the acclimation phase (6, 14). The overproduction of the 5′-UTR containing the cold box is thus expected to sequester this factor to derepress cspA expression.
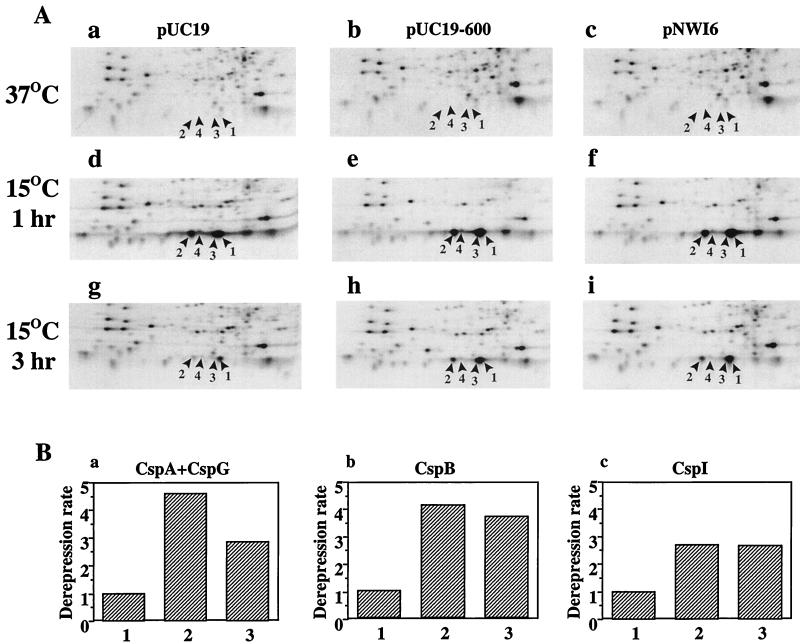
As mentioned above, the cspI 5′-UTR possesses a cold box sequence. To investigate whether the cspI cold box has a similar effect as that of cspA, we constructed a plasmid pNWI6 that is able to overexpress the cspI 5′-UTR from +1 to +113. By primer extension analysis, we confirmed that the cspI 5′-UTR was effectively overexpressed (data not shown). pUC19-600 that can overexpress the cspA 5′-UTR (14) was used as a positive control, and pUC19 was used as a negative control. Before and at 1 h after temperature downshift, the patterns of CspA, CspB, CspG, and CspI expression were essentially the same among cells harboring pUC19, pUC19-600, and pNWI6 (Fig. 6A, panels a to f). At 3 h after the temperature downshift, all four proteins were greatly repressed in cells harboring pUC19 (Fig. 6A, panel g) while production of all four proteins became derepressed in cells overexpressing either the cspA or the cspI 5′-UTR (Fig. 6A, panels h and i, respectively). As shown in Fig. 6B, overexpression of both the cspA and the cspI 5′-UTRs has a derepression effect, although the effect of the cspI 5′-UTR is weaker than that of the cspA 5′-UTR. These results suggest that the cspI 5′-UTR probably plays a role in the autoregulation of the cspI gene at the end of the acclimation phase.
FIG. 6.
Effect of cspI 5′-UTR overexpression on CspA, CspB, CspG, and CspI expression. (A) Cultures of strain JM83 harboring pUC19 (a, d, and g), pUC19-600 (b, e, and h), or pNWI6 (c, f, and i) were labeled with [35S]methionine at 37°C (a, b, and c), and at 1 h (d, e, and f) and 3 h (g, h, and i) after the temperature downshift to 15°C. Total-cell extracts were analyzed by 2D gel electrophoresis, and autoradiograms were obtained. Only a portion corresponding to low-molecular-weight proteins is shown. The cold shock proteins are indicated: 1, CspA; 2, CspB; 3, CspG; 4, CspI. (B) The amounts of the four proteins were quantitated with a phosphorimager, with ribosomal protein L11 as a reference. The amounts of the proteins at each time point upon cold shock are given as the ratio to the amount of the L11 spot. Since the CspA and CspG spots are not separated, we counted them together. The ratio of the amount of protein at 1 h upon cold shock to that at 3 h upon cold shock is its repression rate. The derepression rate is the ratio of repression rate in control cells to that in the 5′-UTR overexpression cells. Lanes: 1, control cells; 2, cells overproducing cspA 5′-UTR; 3, cells overproducing cspI 5′-UTR.
Concluding remarks.
E. coli has nine csp genes, cspA to cspI (33). Of these, cspA (10), cspB (19), cspG (23), and cspI (this study) are cold shock inducible. All these csp genes share several important features. (i) They all contain a UP element immediately upstream of the promoter, which contributes to maintain the high promoter activity even at low temperatures (9, 22). (ii) They all contain a long 5′-UTR in their mRNAs (159, 161, 156, and 145 bases for cspA, cspB, cspG, and cspI, respectively). As found for the cspA mRNA (22), these 5′-UTRs are believed to exert a negative effect on their expression at 37°C, while they cause a positive effect on their cold shock inducibility. (iii) They all have the cold box at the 5′-end region of their 5′-UTRs, which plays a role in autoregulation to repress their own gene expression at the end of the acclimation phase (6, 14). (iv) They all contain a downstream box downstream of the translation initiation codon, which plays an essential role in the cold shock induction by enhancing translation (22). Taken together, expression of all four csp genes appears to be regulated essentially in the same manner. It should be mentioned, however, that the optimal temperature ranges for the induction of these genes are different. CspI is induced at a relatively lower temperature range than the others.
How are the differences in the temperature range of induction achieved? It is unlikely that the difference in optimal temperature is caused at the level of transcription initiation, because the putative promoter region and the UP element are very well conserved among cspA, cspB, cspG, and cspI, as shown in Fig. 1B. It is also unlikely that it is caused at the level of translation initiation, because their Shine-Dalgarno sequences, downstream boxes, and coding regions are also quite well conserved (see Fig. 1B). In contrast, the nucleotide sequences of their 5′-UTRs are different, suggesting differences in their mRNA secondary structures and therefore in their stabilities. Thus, the 5′-UTRs of the csp genes exert a negative effect on their expression at 37°C, affecting their mRNA stabilities and translation initiation efficiencies (22, 36). These are likely to be differently modulated upon temperature downshift, depending upon the secondary structure of each mRNA. A larger temperature difference might be required for cspI expression, while a smaller temperature difference would be enough for cspA expression.
It is interesting that although the cspA gene was dispensable for cell growth at both high and low temperatures, the production of CspB and CspG significantly increased in a cspA deletion mutant (2). This indicates that the CspA function may be at least partially complemented by CspB and CspG. It has been reported that in Bacillus subtilis, which contains three csp genes, at least one of the three csp genes is required for cell growth (12). It seems likely that the functions of CspA, CspB, CspG, and CspI overlap. To examine this possibility, we are attempting to construct multiple-deletion strains including a quadruple-deletion mutant. This approach may reveal a possibility that CspA, CspB, CspG, and CspI each play their own specific roles in the cells.
ACKNOWLEDGMENTS
We thank R. M. Simons for plasmids. We also thank S. Phadtare for comments.
This work was supported by a grant (to M.I.) from the National Institutes of Health (GM19043).
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Bae W, Jones P G, Inouye M. CspA, the major cold-shock protein of Escherichia coli, negatively regulates its own gene expression. J Bacteriol. 1997;179:7081–7088. doi: 10.1128/jb.179.22.7081-7088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandi A, Pietroni P, Gualerzi C O, Pon C L. Post-transcriptional regulation of CspA expression in Escherichia coli. Mol Microbiol. 1996;19:231–240. doi: 10.1046/j.1365-2958.1996.362897.x. [DOI] [PubMed] [Google Scholar]
- 4.Etchegaray J P, Jones P G, Inouye M. Differential thermoregulation of two highly homologous cold-shock genes, cspA and cspB, of Escherichia coli. Genes Cells. 1996;1:171–178. doi: 10.1046/j.1365-2443.1996.d01-231.x. [DOI] [PubMed] [Google Scholar]
- 5.Fang L, Jiang W, Bae W, Inouye M. Promoter-independent cold-shock induction of cspA and its derepression at 37°C by mRNA stabilization. Mol Microbiol. 1997;23:355–364. doi: 10.1046/j.1365-2958.1997.2351592.x. [DOI] [PubMed] [Google Scholar]
- 6.Fang L, Hou Y, Inouye M. Role of the cold-box region in the 5′ untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli. J Bacteriol. 1998;180:90–95. doi: 10.1128/jb.180.1.90-95.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng W, Tejero R, Zimmerman D E, Inouye M, Montelione G T. Solution NMR structure and backbone dynamics of the major cold-shock protein (CspA) from Escherichia coli: evidence for conformational dynamics in the single-stranded RNA-binding site. Biochemistry. 1998;37:10881–10896. doi: 10.1021/bi980269j. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg D, Azar I, Oppenheim A B. Differential mRNA stability of the cspA gene in the cold-shock response of Escherichia coli. Mol Microbiol. 1996;19:241–248. doi: 10.1046/j.1365-2958.1996.363898.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg D, Azar I, Oppenheim A B, Brandi A, Pon C L, Gualerzi C O. Role of Escherichia coli cspA promoter sequence and translational apparatus adaptation in the cold shock response. Mol Gen Genet. 1997;256:282–290. doi: 10.1007/s004380050571. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein J, Pollitt N S, Inouye M. Major cold shock proteins of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman S, Clark W P, Maurizi M R. The ATP-dependent Clp protease of Escherichia coli: sequence of clpA and identification of a Clp-specific substrate. J Biol Chem. 1990;265:7886–7893. [PubMed] [Google Scholar]
- 12.Graumann P, Wendrich T M, Weber M H W, Schröder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 13.Harlocker S L, Rampersaud A, Yang W-P, Inouye M. Phenotypic revertant mutations of a new OmpR2 mutant (V203Q) of Escherichia coli lie in the envZ gene, which encodes in the OmpR kinase. J Bacteriol. 1993;175:1956–1960. doi: 10.1128/jb.175.7.1956-1960.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang W, Fang L, Inouye M. The role of 5′-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. J Bacteriol. 1996;178:4919–4925. doi: 10.1128/jb.178.16.4919-4925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Hou Y, Inouye M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- 16.Jones P G, Inouye M. The cold-shock response—a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones P G, VanBogelen R A, Neidhardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee S J, Xie A, Jiang W, Etchegaray J-P, Jones P G, Inouye M. Family of the major cold-shock protein, CspA (CS7.4) of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol Microbiol. 1994;11:833–839. doi: 10.1111/j.1365-2958.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 20.Lopilato J, Bortuer S, Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduced plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986;205:285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. A short course in bacterial genetics—a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 22.Mitta M, Fang L, Inouye M. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol Microbiol. 1997;26:321–335. doi: 10.1046/j.1365-2958.1997.5771943.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima K, Kanamaru K, Mizuno T, Horikoshi K. A novel member of the cspA family of genes that is induced by cold-shock in Escherichia coli. J Bacteriol. 1996;178:2994–2997. doi: 10.1128/jb.178.10.2994-2997.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newkirk K, Feng W, Jiang W, Tejero R, Emerson S D, Inouye M, Montelione G T. Solution NMR structure of the major cold shock protein (CspA) from Escherichia coli: identification of a binding epitope for DNA. Proc Natl Acad Sci USA. 1994;91:5114–5118. doi: 10.1073/pnas.91.11.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross W, Gosink K K, Salmon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Schindelin H, Jiang W, Inouye M, Heinemann U. Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc Natl Acad Sci USA. 1994;91:5119–5123. doi: 10.1073/pnas.91.11.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe H, Goldstein J, Yang M, Inouye M. Identification of the promoter region of the Escherichia coli major cold shock gene, cspA. J Bacteriol. 1992;174:3867–3873. doi: 10.1128/jb.174.12.3867-3873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thieringer H A, Jones P G, Inouye M. Cold shock and adaptation. BioEssays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.VanBogelen R A, Hutton M E, Neidhardt F C. Gene-protein database of Escherichia coli K-12: Edition 3. Electrophoresis. 1990;11:1131–1166. doi: 10.1002/elps.1150111205. [DOI] [PubMed] [Google Scholar]
- 32.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–255. doi: 10.1046/j.1365-2958.1998.00683.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka K, Inouye M. Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J Bacteriol. 1997;179:5126–5130. doi: 10.1128/jb.179.16.5126-5130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanaka K, Mitani T, Ogura T, Niki H, Hiraga S. Cloning, sequencing, and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol Microbiol. 1994;13:301–312. doi: 10.1111/j.1365-2958.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamanaka, K., M. Mitta, and M. Inouye. Unpublished data.