Abstract
Black yeasts are a phylogenetically diverse group of ascomycetous fungi that may exist in both unicellular and mycelial morphs. This group of fungi contains numerous commercially significant species as well as others whose precise roles are unknown, such as endolichenic species. There is currently a paucity of data about endolichenic black yeast species. To bridge this gap, we surveyed China’s Inner Mongolia Autonomous Region in July 2019. Several fungal species associated with diverse lichens were isolated during this survey. Among these were two isolates of a previously unknown species of oleaginous black yeast from Mycosphaerellales. Analyses of morphological and molecular data revealed that these two isolates were closely related to Xenodevriesia strelitziicola (Xenodevriesiaceae), although with significant differences. As a result, we established the genus Melanodevriesia gen. nov. to describe this previously unknown species, Melanodevriesia melanelixiae sp. nov. In addition, we used Transmission Electron Microscopy to visualise the intracellular oil bodies metabolised by this fungus in its unicellular state. The black yeast species identified in this study may have a wide range of commercial applications. More research is needed to determine the chemical composition of the microbial oil synthesized by this fungus and whether it has commercial value.
Citation: Chang R, Cao W, Wang Y, Li S, Li X, Bose T, Si HL (2022). Melanodevriesia, a new genus of endolichenic oleaginous black yeast recovered from the Inner Mongolia Region of China. Fungal Systematics and Evolution 9: 1–9. doi: 10.3114/fuse.2022.09.01
Keywords: Endolichenic fungi, intracellular oil bodies, Mycosphaerellales, new taxon, Xenodevriesiaceae
INTRODUCTION
Fungi and algae (or cyanobacteria) form a symbiotic relationship known as lichen (Lutzoni & Miadlikowska 2009). Ascomycota makes up the bulk of lichenised fungi, whereas the remaining fungi are from the Basidiomycota (He & Zhang 2012). In addition to these symbiotic fungi, lichen thalli also house a variety of other fungi such as endolichenic fungi (Kellogg & Raja 2017). The ecological role of these non-symbiotic fungi is still largely unknown (Singh et al. 2017). It is estimated that more than 18 000 endolichenic fungi colonise lichen thalli (Nash 2008); this includes a group of fungi often referred to as “black yeasts” (also known as “black fungi”) (Cañete-Gibas & Wiederhold 2018).
Black yeasts are melanised, non-lichenised and dematiaceous fungi that can concurrently exist in both unicellular and mycelial forms (Zalar et al. 1999). The group is phylogenetically diverse, although it mostly consists of fungi from Ascomycota (Selbmann et al. 2014b). Most of these black yeasts are from the classes Dothideomycetes and Eurotiomycetes (Egidi et al. 2014, Selbmann et al. 2014a). Black yeasts from Dothideomycetes concentrate in the order Mycosphaerellales (Abdollahzadeh et al. 2020), whereas in Eurotiomycetes they exclusively represent Chaetothyriales (Selbmann et al. 2005, Isola et al. 2016, Selbmann et al. 2014b, Sun et al. 2020). Melanisation and meristematic growth amongst these fungi evolved in response to extreme environments, in which they thrive (de Hoog 1993, Haase et al. 1999, Prenafeta-Boldú et al. 2006), such as high temperature, UV radiation, toxic chemicals, oligotrophic environments and many more (Jacobson 2000, Langfelder et al. 2003, Lian et al. 2005, Selbmann et al. 2005, Dadachova et al. 2007, Dadachova & Casadevall 2008, Zhao et al. 2010).
Apart from melanin, black yeasts also metabolise various other compounds that allow them to thrive in these extreme habitats such as betaine, carotenoids, mycosporines, trehalose and polyalcohols (Moreno et al. 2018). Furthermore, while growing on a carbohydrate-rich substrate, some black yeast species accumulate microbial oils (Lamers et al. 2016). Single-cell oils or microbial oils are intracellularly stored lipids produced by a variety of oleaginous microorganisms, such as fungi, bacteria, and algae (Li et al. 2008, Bellou et al. 2016). Single-cell oils are composed of triacylglycerols (TAGs), free fatty acids, polar lipids, sterols, hydrocarbons, and pigments (Ratledge 2004). Microbial oils are preferred over plant- and animal-derived oils because they can be readily scaled up through the application of biotechnology. Furthermore, seasonal fluctuations, geographic location, harvest time, and transportation, which are obstacles in the production of plant and animal oils, do not influence on the production of single-cell oil (Ward & Singh 2005, Thiru et al. 2011).
Oleaginous yeasts are a favoured source of microbial oils because they may accumulate more lipids than other microorganisms. Furthermore, the oil synthesised by bacteria is stored on the external membrane, making it difficult to extract, whereas those produced intracellularly by algae and yeasts have a high concentration of unsaturated fatty acids (Vasconcelos et al. 2019). So far, oleaginous yeast such as Yarrowia lipolytica, Rhodotorula glutinis, Cryptococcus curvatus, and Lipomyces starkeyi have all been widely studied (Qiao et al. 2017).
The majority of black yeast research in China is focused on species that cause human diseases, such as Exophiala asiatica, Aureobasidium spp., and others (Li et al. 2009, Wang et al. 2019). The knowledge on endolichenic black yeast species from China and globally is currently scarce. In an attempt to overcome this gap, we surveyed China’s Inner Mongolia Autonomous Region in July 2019. Several fungal species associated with diverse lichens were isolated during this survey. Among them were two isolates of a previously unknown species of black yeast from the order Mycosphaerellales. In this study, we described this black yeast species using both morphological and genetic data. In addition, we used transmission electron microscopy to visualize the intracellular oil bodies associated with this newly discovered species.
MATERIALS AND METHODS
Collections of lichens
Several Melanelixia subargentifera thalli were collected in July 2019 from Mt. Qingyangcheng, Balin Right Banner, Chifeng City, Inner Mongolia Autonomous Region (14 98.8m a.s.l., 44°13′45"N, 118°44′57"E). An individual lichen thallus was scraped off the substrate and kept separately in paper bags. Fungal isolations were made from lichen thalli in the laboratory.
Isolation of fungi from lichen thalli
An individual lichen thallus was cleaned with tap water and then repeatedly rinsed with sterile deionised water. The upper cortex of the thallus was scraped off using a Leica Zoom 2000 dissecting microscope. Pieces of medullary tissues were put on the surface of potato dextrose agar medium (PDA; 46 g PDA powder (Qingdao Hope Bio-Technology Co., Ltd., Shandong, China), and 1 L distilled water, pH 5.6 ± 0.2) amended with 0.05 % streptomycin (Cao et al. 2002). All Petri dishes were incubated at 25 °C for 14 d. Mycelia emerging from medullary tissues were sub-cultured onto new PDA plates.
DNA extraction, amplification and sequencing
Using the modified CTAB technique (Doyle & Doyle 1990), genomic DNA was extracted from 14-d-old fungal cultures growing on PDA. For all fungal isolates, the complete internal transcribed spacer (ITS) and partial nuclear large subunit ribosomal DNA (LSU) regions were amplified using primers ITS1/ITS4 (White et al. 1990) and LR0R/LR5 (Vilgalys & Hester 1990), respectively.
Each 50 μL of PCR amplification reaction included 19 μL of PCR grade water, 25 μL of 1-5TM 2× High-Fidelity Master Mix (Tsingke Biotech Co., China), 2 μL of each primer (10 μM), and 1 μL DNA template. For both gene regions, PCR amplifications were conducted with an initial denaturation at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 56 °C for 1 min, 72 °C for 1 min; and final extension at 72 °C for 10 min. Positive amplifications were verified using agarose gel electrophoresis and stained using ethidium bromide. Sangon Biotech Company (Shanghai, China) cleaned and sequenced the PCR products.
The BLAST algorithm (Altschul et al. 1990) available through NCBI GenBank was used for the preliminary identification of the fungal DNA sequences. All DNA sequences generated in this study were deposited in NCBI GenBank’s nucleotide database (Table 1).
Table 1.
GenBank accession numbers of selected taxa from Mycosphaerellales used for phylogenetic analyses. The new species is shown in boldface.
| Species | Strain/Voucher | LSU | ITS |
|---|---|---|---|
| Batcheloromyces alistairii | CPC 18251 | JX556237 | JX556227 |
| Batcheloromyces leucadendri | CPC 18277 | JF499852 | JF499832 |
| Batcheloromyces proteae | CBS 110696 | EU019247 | JF746163 |
| Capnodium coffeae | CBS 147.52 | GU214400 | MH856967 |
| Capnodium coffeicola | MFLUCC15-206 | KU358920 | KU358921 |
| Cladosporium cladosporioides | CBS 129108 | MH876646 | MH865207 |
| Cladosporium herbarum | CBS 129088 | MH876640 | MH865203 |
| Cladosporium myrtacearum | CBS 126349 | MH875385 | MH863925 |
| Cladosporium phyllactiniicola | CBS 126354 | MH875390 | MH863930 |
| Cladosporium pseudocladosporioides | CBS 125993 | MH875333 | MH863872 |
| Cladosporium scabrellum | CBS 126358 | MH875394 | MH863934 |
| Cladosporium tenuissimum | CBS 125995 | MH876286 | MH864840 |
| Cladosporium varians | CBS 126361 | MH875397 | MH863937 |
| Devriesia shelburniensis | CBS 115876 | KF442544 | KF442505 |
| Juncomyces californiensis | CPC 37989 | MT373351 | NR_170828 |
| Lecanosticta pini | CBS 871.95 | GQ852598 | GU214663 |
| Leptoxyphium madagascariense | CBS 124766 | MH874923 | MH863407 |
| Melanodevriesia melanelixia sp.nov. | CGMCC3.20308 | MW528742 | MW528736 |
| CGMCC3.20309 | MW580586 | MW580587 | |
| Meristemomyces frigidus | CCFEE5457 | GU250389 | KF309967 |
| Microcyclosporella mali | CBS 126135 | MH875501 | MH864044 |
| Microxiphium theae | CBS 202.30 | MH866561 | MH855113 |
| Montagnula cylindrospora | UTHSC-DI16-208 | LN907351 | LT796834 |
| Muriphila oklahomaensis | CCF5751 | LR736041 | LR736040 |
| Mycosphaerelloides madeirae | CBS 116066 | KX286989 | AY853188 |
| Neocatenulostroma germanicum | CBS 539.88 | EU019253 | MH862143 |
| Neocatenulostroma microsporum | CBS 110890 | EU019255 | AY260097 |
| Neodevriesia cladophorae | OUCMBI110119 | KU578114 | KP269029 |
| Neodevriesia grateloupiae | OUCMBI101249 | KU578120 | KU578118 |
| Neodevriesia modesta | CCFEE5672 | KF310026 | KF309984 |
| Neodevriesia simplex | CCFEE5681 | KF310027 | KF309985 |
| Neodevriesia strelitziae | CBS 122379 | GU301810 | MH863206 |
| Paramycosphaerella watsoniae | CPC 37392 | MN567653 | MN562146 |
| Paradevriesia compacta | CBS 118294 | NG_059089 | NR_144955 |
| Paradevriesia pseudoamericana | CPC 16174 | GU570544 | GU570527 |
| Paradevriesia americana | CBS 117726 | NG_059077 | NR_159866 |
| Phyllachora pomigena | CBS 195.33 | MH866862 | MH855411 |
| Polychaeton citri | CBS 116435 | GU214469 | GU214649 |
| Pseudotaeniolina globosa | CBS 109889 | MH874434 | MH862844 |
| Ramularia acris | CBS 109794 | KX287010 | KX287311 |
| Ramularia acroptili | CBS 120253 | EU019257 | EU019257 |
| Ramularia helminthiae | CPC 11504 | KX287183 | KX287481 |
| Ramularia lethalis | CPC 25910 | KX287174 | KX287472 |
| Ramularia tovarae | CBS 113305 | KJ504764 | KJ504807 |
| Stenella araguata | CBS 105.75 | EU019250 | MH860897 |
| Teratosphaeria dimorpha | CPC 14132 | FJ493215 | FJ023537 |
| Teratosphaeria ovata | CPC 14632 | FJ493218 | FJ023538 |
| Teratosphaeria profusa | CPC 12821 | FJ493220 | FJ493196 |
| Xenodevriesia strelitziicola | CBS 122480 | NG_059085 | MH863214 |
| X1045 | GU214635 | GU214635 | |
| Xenopenidiella nigrescens | DOC356 | KU216335 | KT833169 |
| Xenoramularia arxii | CBS 342.49 | NG_058254 | KX287552 |
| Xenoteratosphaeria jonkershoekensis | CBS 122897 | MH874777 | MH863253 |
Sequence alignment and phylogenetic analyses
During the preliminary identification of the ITS and LSU sequence data, two of our isolates appeared as a potentially new taxon, closely linked to Xenodevriesia and Paradevriesia (Mycosphaerellales). As a result, two separate datasets for the ITS and LSU gene regions were constructed for phylogenetic analyses. The sequences of the supposedly new taxon identified in this study were included in this data set, as well as selected taxa from the order Mycosphaerellales retrieved from GenBank. For phylogenetic taxon sampling, the neighbour-joining trees generated during BLAST searches and previously published phylogenetic by Crous et al. (2020) were used. Both datasets were aligned separately with MAFFT v. 7 (Katoh et al. 2019) and manually adjusted with MEGA v. 10.2.0 (Kumar et al. 2018).
Phylogenetic analyses of single-gene and concatenated datasets were done using maximum likelihood (ML), Bayesian inference (BI) and maximum parsimony (MP) approaches. Software required for ML and BI analyses were accessed through the CIPRES Science Gateway platform (https://www.phylo.org) (Miller et al. 2010). The best models of nucleotide substitution were determined by using jModelTest v. 2.1.6 (Darriba et al. 2012). RAxML v. 8.2.12 was used for ML analyses with GTR+GAMMA as the substitution model and 1 000 bootstrap replications (Stamatakis et al. 2008). For BI analyses, MrBayes v. 3.2.7 (Ronquist & Huelsenbeck 2003) with four MCMC chains were run from a random starting tree for 5 M generations with the stop value set at 0.01, the temperature set at 0.2, with trees sampled every 100 generations. We discarded 25 % of trees sampled as burn-in and the remaining trees (37 500) were used to construct majority rule consensus trees. The MP analyses were performed using MEGA v. 10.2.0 with 1 000 bootstrap replicates, gaps were treated as a fifth state character. The phylogenetic trees from the ML, MP and BI analyses were viewed using FigTree v. 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). All the alignments and phylogenetic trees were submitted to TreeBASE under accession number 28863.
Colony morphology and light microscopy
The two isolates (CGMCC3.20308 and CGMCC3.20309) of the potentially new taxon from Mycosphaerellales were used for recording culture morphology and microscopic structures. For this purpose, both isolates were sub-cultured onto PDA and oatmeal agar (OA; 30 g oatmeal, 15 g agar, 1 L distilled water, pH 7.2 ± 0.2). All the Petri plates were incubated at 25 °C for 40 d. Microscopic morphological characters such as hyphae, conidia, and conidiophores were photographed and measured (n =50 / structure) using a Leica DFC495 camera attached to a Leica DM6 microscope. ImageJ was used for measuring the taxonomically relevant structures (Collins 2007).
The ex-holotype cultures were deposited in Beijing, China General Microbiological Culture Collection Center (CGMCC). The type specimen was deposited in the Institute of Microbiology’s (HMAS) Fungarium in Beijing, China.
Electron microscopy for visualising intracellular oil bodies
For visualising intracellular oil bodies using transmission electron microscopy (TEM), isolates of the unknown fungus were sub-cultured onto PDA for 14 d. Thereafter, the yeast-like cells were fixed using 2.5 % glutaraldehyde at 4 °C for 2–3 h (Brisson et al. 1996). The fixed cells were rinsed repeatedly using 0.1 M phosphate buffer saline (PBS; pH 7.2). Cells were post-fixed using 1 % osmium tetroxide for 1.5 h in darkness. These post-fixed cells were rinsed twice with PBS followed by ultrapure water (three to four times). The cells were gradually dehydrated with 50, 70, 80, and 90 % ethanol, then 90 % acetone and absolute acetone. The dehydrated tissues were embedded in Epon 812 and sliced into 70 nm ultra-thin sections using a Leica UC7 ultra-microtome. Sections were stained using 2 % uranyl acetate for 15 min followed by lead citrate for 8 min (Reynolds 1963). Stained sections were dried under infrared light for 10 min. The structure of oil bodies in the cells were observed using a Hitachi HT-7800 transmission electron microscope at 80 kV.
RESULTS
Phylogenetic analyses
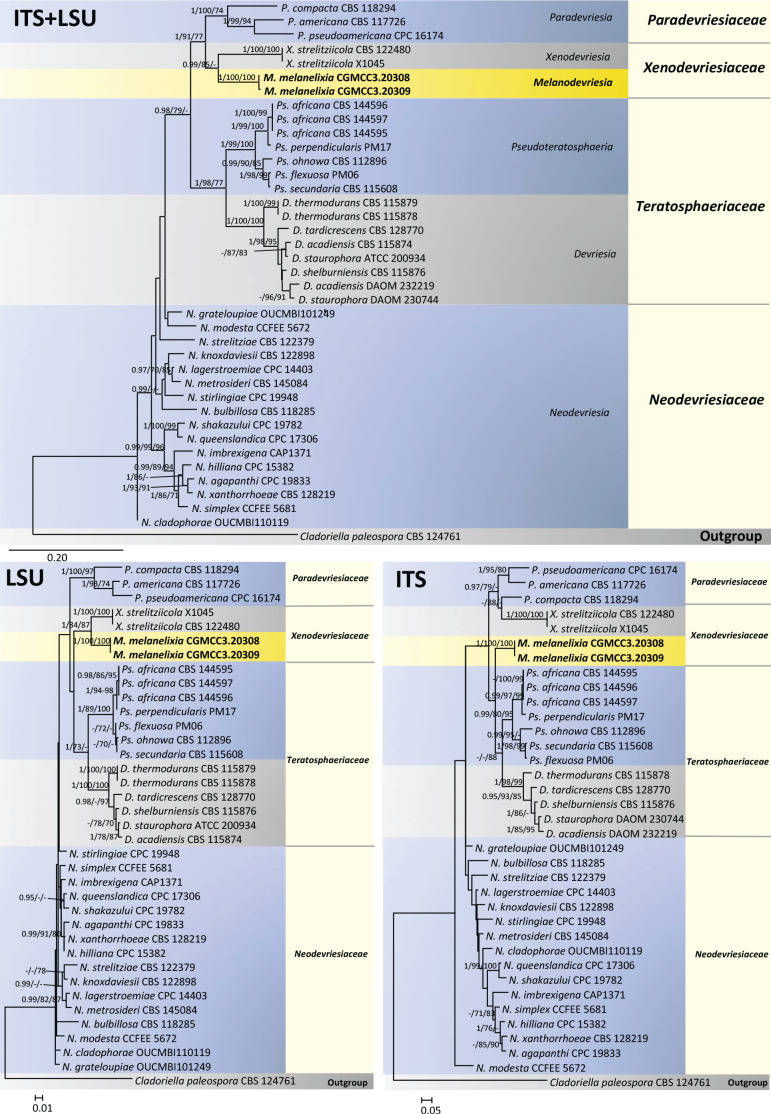
In the phylogeny of selected taxa from the Mycosphaerellales, Cladosporiales and Capnodiales, the ML tree topologies were largely consistent between the datasets (Fig. 1). However, compared to the LSU and concatenated ITS+LSU phylogeny, the placement of the novel species differed in the ITS phylogeny. In both the LSU and ITS+LSU phylogenies the new species is sister to Xenodevriesia strelitziicola in the Xenodevriesiaceae. However, posterior probability and maximum-likelihood bootstrap values supporting this clustering were highly significant for the LSU tree only (Fig. 1). In the ITS tree, the new species emerged as a basal lineage to a clade that included species of Neodevriesia, Paradevriesia, and X. strelitziicola with poor statistical support. The parsimony analyses did not provide any support for the associations in the ITS+LSU and ITS phylogenies, but moderate support in the LSU phylogeny (Fig. 1). The strange placement of our isolates in the ITS phylogeny could be an artefact of the divergent ITS sequences spanning different families used in the analysis.
Fig. 1.
Maximum likelihood trees were constructed using single gene (ITS and LSU) and concatenated (ITS+LSU) datasets. In the ITS+LSU and LSU trees, both isolates of Melanodevriesia melanelixiae sp. nov. formed a monophyletic clade and were sisters to Xenodevriesia strelitziicola. However, this clustering was highly significant for the LSU tree only. In the ITS tree, M. melanelixia emerged as a basal diverging taxon within a clade that includes species of Neodevriesia, Paradevriesia, and Xenodevriesia strelitziicola, but with poor statistical support. The numbers on the branches are statistical support values, Bootstrap values (< 75 %) from maximum likelihood and maximum parsimony analyses, respectively. Thickened branches indicate the posterior probability values ≥ 0.90.
The tree topologies from both the LSU and ITS+LSU datasets, as well as the accompanying statistical support values, revealed that our two isolates of the previously undescribed species represents a new genus. Below, we establish the new genus Melanodevriesia to accommodate this unknown species as Melanodevriesia melanelixiae sp. nov.
Taxonomy
Melanodevriesia H.L. Si, W.Q. Cao, & T. Bose, gen. nov. MycoBank MB 839404.
Etymology: The name refers to the black colony formed by the fungus when growing on PDA and OA.
Slow-growing colonies on PDA and OA are black to brownish black in colour. The fungus grows in a yeast-like unicellular state on PDA, producing pseudohyphae by continuous budding. These yeast-like cells have several conspicuous intracellular oil bodies. The thallus on OA and other oligotrophic media is made up of septate straight or corrugated branching hyphae.
Type species: Melanodevriesia melanelixiae H.L. Si, W.Q. Cao & T. Bose
Notes: Melanodevriesia is currently a monotypic genus that includes M. melanelixiae, which is described below. Despite being a sister genus of Xenodevriesia (Xenodevriesiaceae), Melanodevriesia has distinct morphological characteristics. Melanodevriesia has two thallus morphologies: yeast-like and mycelial, both of which are black to brownish black in colour, but Xenodevriesia possesses a brown mycelial thallus (Crous et al. 2019). Melanodevriesia produces chlamydospores which are lacking in Xenodevriesia.
Melanodevriesia melanelixiae H.L. Si, W.Q. Cao, T. Bose, sp. nov. MycoBank MB 840429. Figs 2, 3.
Fig. 2.
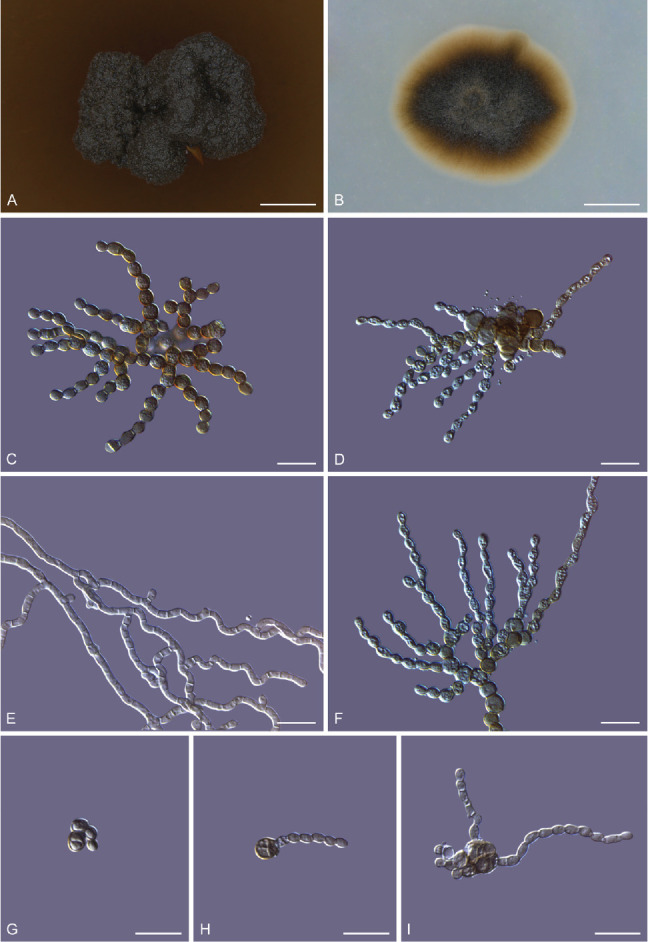
Morphology of Melanodevriesia melanelixiae sp. nov. (ex-type CGMCC3.20309). Colony morphology on potato dextrose agar (A) and oatmeal agar (B). C, D. Microscopic structures of 14-d-old culture growing on PDA medium with yeast-like unicellular morph forming pseudohyphae through budding. E. Straight and corrugated septate hyphae produced by the mycelial state of the fungus. F. A cluster of monilioid chlamydospores. G–I. Single chlamydospores germinating into unicellular cells that multiply through budding, forming a multicellular structure from which pseudohyphae emerge. Scale bars: A, B = 2 mm; C–I = 10 μm.
Fig. 3.
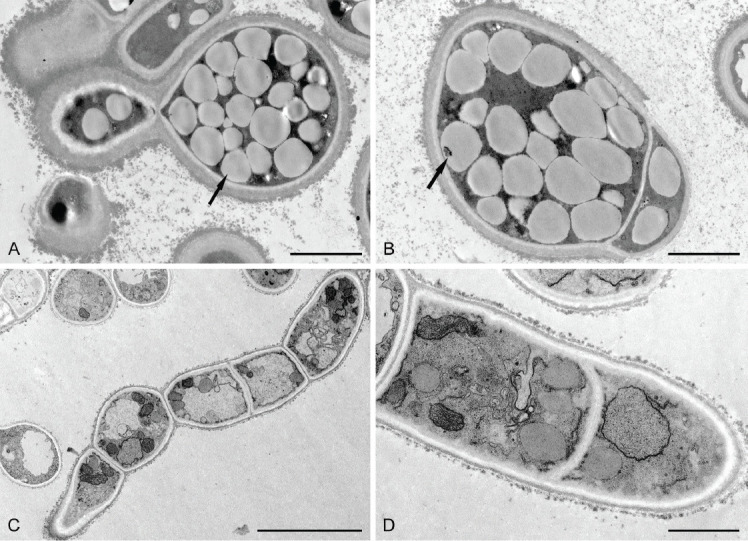
Transmission electron microscopic images of pseudohyphae and mycelium of Melanodevriesia melanelixiae sp. nov. (ex-type CGMCC3.20309). A, B. Budding yeast-like unicellular cell with thick cell walls. Multiple intracellular oil bodies concealing the cell organelles (indicated with arrows). C, D. Septate hyphae with a thin cell wall that is devoid of intracellular oil bodies. Due to the lack of intracellular oil bodies, various cell organelles are visible. Scale bars: A, B = 2 μm; C = 10 μm; D = 1 μm.
Etymology: The name is derived from the lichen Melanelixia subargentifera, from which both isolates of this fungus were obtained.
This fungus can exist in both a yeast-like and a mycelial state. The yeast-like thallus produces pseudohyphae through budding. These pseudohyphae are branched, septate, constricted at the septa, composed of oval to urceiform cells, hyaline to brown in colour, smooth-walled, guttulate, measuring 1.4–3 × 2.3–4.6 μm (Fig. 2). In the mycelial state, hyphae grow into the substrate. Hyphae branched, septate, smooth-walled, smooth or corrugated, cylindrical, hyaline to pale brown in colour, measuring 1.3–2 μm wide (Fig. 2). Chlamydospores spherical to ovoid in shape, solitary often monilioid forming radiating clusters, smooth-walled, pale brown to dark brown in colour, usually aseptate, rarely septate, guttulate, measuring 2.8–4.2 × 2.8–4.8 μm (Fig. 2). Chlamydospores geminate into yeast-like unicellular conidia that are globose to sub-globose in shape, pale brown to dark brown in colour, thick-walled, measuring 4–7.3 × 3.6–6.2 μm (Fig. 2). These unicellular cells multiply through budding (Fig. 2) forming multicellular structures from which pseudohyphae emerge randomly (Fig. 2). No sexual reproductive structures were observed.
Culture characteristics: After 12 wk on PDA, the surfaces of the colonies were dark brown to black with the reverse dull brown in colour, erumpent, hollow, with irregular margins, rarely with a few aerial mycelia. After a few weeks after subculturing, the colony stains the PDA brown. Colonies slow-growing, reaching 3.1 ± 0.1mm diam after incubating at 25 °C for 12 wk (Fig. 2).
After 8 wk on OA, the colonies are round to oval in shape, with smooth margins, surface taupe brown to olive-brown with the reverse taupe brown in colour. Colonies are slow-growing on OA yet faster than on PDA, reaching 5.42 ± 0.2 mm diam after incubating at 25 °C for 8 wk (Fig. 2).
Intracellular oil bodies: The TEM of yeast-like cells grown on PDA revealed thick cell walls with many inconspicuous oil bodies concealing the other cell organelles. Hyphae grown on OA lacked thick cell walls and intracellular oil bodies (Fig. 3).
Typus: China, Inner Mongolia Autonomous Region, Chifeng, Balin Right Banner, Mt. Qingyangcheng, 44°13′46″N, 118°44′57″E, 1 498.8 m alt, isolated from the medullary tissue of Melanelixia subargentifera, 7 Jul. 2019, H.L. Si (holotype HMAS 350275; ex-type culture CGMCC3.20308).
Notes: Melanodevriesia melanelixiae differs from X. strelitziicola in that it contains at least two thallus morphologies and chlamydospores. Besides this, we did not observe any sexual reproductive structures (Crous et al. 2009, 2019).
DISCUSSION
In the present study, two isolates of a black yeast species were isolated from two separate thalli of Melanelixia subargentifera collected at the same coordinates. Analyses of morphological and molecular data revealed that these two isolates represent an undescribed genus. As a result, we established Melanodevriesia gen. nov. to describe this fungus as Melanodevriesia melanelixiae sp. nov. The TEM images revealed that during the unicellular phase of its life cycle, this fungus accumulates multiple prominent intracellular oil bodies.
In our LSU and ITS+LSU phylogenies, Melanodevriesia melanelixiae sp. nov. emerged as a sister taxon of X. strelitziicola, a mycelial fungus isolated from a Strelitzia sp. in South Africa (Crous et al. 2009, 2019). This clustering, however, was only significant in the LSU tree. Future discoveries of new species from Xenodevriesiaceae and the availability of sequences from additional gene regions may aid in further delimiting this family.
Melanodevriesia melanelixiae sp. nov. was isolated from the medullary tissue of the lichen Melanelixia subargentifera. The slow growth and melanisation of this fungus, like that of other black yeasts, allow it to flourish in harsh conditions like the one where we collected our samples in China. We were unable to determine the particular ecological role of M. melanelixiae. However, we believe that this fungus increases the overall fitness of the lichen, allowing it to flourish in harsh environments. This is not an unreasonable hypothesis because Phaeotheca, an early-diverging capnodiaceous black yeast encapsulates the algae Trentepohlia when proliferating within the thallus of Racodium rupestre (Crous et al. 2009). This loose association of black yeast and algae might be the early stages of lichen development because the fungus increases the carbon supply to the algae (Gostinčar et al. 2012).
Transmission electron microscopy images of our newly discovered fungus, M. melanelixiae, revealed that in its yeast-like form, this organism accumulates a copious number of intracellular oil bodies. Similar to several other black yeast species, the microbial oil metabolised by M. melanelixiae might have a wide range of commercial applications. However, more research is needed to determine the chemical composition of the microbial oil metabolised by M. melanelixiae and if this fungus can be commercially exploited for the production of microbial oils.
Acknowledgments
This study was funded by The National Natural Science Foundation of China (Project No.: 31600100). The authors would like to thank Prof. Lisong Wang of the Kunming Institute of Botany, The Chinese Academy of Sciences and Prof. Zuntian Zhao of Shandong Normal University for their assistance in identifying lichen specimens.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
REFERENCES
- Abdollahzadeh J, Groenewald JZ, Coetzee M, et al. (2020). Evolution of lifestyles in Capnodiales. Studies in Mycology 95: 381–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Bellou S, Triantaphyllidou I-E, Aggeli D, et al. (2016). Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Current Opinion in Biotechnology 37: 24–35. [DOI] [PubMed] [Google Scholar]
- Brisson A, Gharibian S, Eagen R, et al. (1996). Localization and characterization of the melanin granules produced by the sap-staining fungus Ophiostoma piceae. Material und Organismen 30: 23–32. [Google Scholar]
- Cañete-Gibas CF, Wiederhold NP. (2018). The black yeasts: an update on species identification and diagnosis. Current Fungal Infection Reports 12: 59–65. [Google Scholar]
- Cao LX, You JL, Zhou SN. (2002) Endophytic fungi from Musa acuminata leaves and roots in South China. World Journal of Microbiology and Biotechnology 18: 169–171. [Google Scholar]
- Collins TJ. (2007). ImageJ for microscopy. Biotechniques 43: 25–30. [DOI] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, et al. (2009). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. (2019). New and interesting fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Chooi YH, et al. (2020). Fungal Planet description sheets: 1042–1111. Persoonia 44: 301–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E, Bryan RA, Huang X, et al. (2007). Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2: e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E, Casadevall A. (2008). Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Current Opinion in Microbiology 11: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog GS. (1993). Evolution of black yeasts: possible adaptation to the human host. Antonie van Leeuwenhoek 63: 105–109. [DOI] [PubMed] [Google Scholar]
- Doyle JJT, Doyle JL. (1990). Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Egidi E, de Hoog GS, Isola D, et al. (2014). Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Diversity 65: 127–165. [Google Scholar]
- Gostinčar C, Muggia L, Grube M. (2012). Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Frontiers in Microbiology 3: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase G, Sonntag L, Melzer-Krick B, et al. (1999). Phylogenetic inference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Studies in Mycology 43: 80–97. [Google Scholar]
- He Y, Zhang Z. (2012). Diversity of organism in the Usnea longissima lichen. African Journal of Microbiology Research 6: 4797–4804. [Google Scholar]
- Isola D, Zucconi L, Onofri S, et al. (2016). Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Diversity 76: 75–96. [Google Scholar]
- Jacobson ES. (2000). Pathogenic roles for fungal melanins. Clinical Microbiology Reviews 13: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg JJ, Raja HA. (2017). Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon. Phytochemistry Reviews 16: 271–293. [Google Scholar]
- Kumar S, Stecher G, Li M, et al. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers D, van Biezen N, Martens D, et al. (2016). Selection of oleaginous yeasts for fatty acid production. BMC Biotechnology 16: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder K, Streibel M, Jahn B, et al. (2003). Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genetics and Biology 38: 143–158. [DOI] [PubMed] [Google Scholar]
- Li DM, Li RY, de Hoog GS, et al. (2009). Exophiala asiatica, a new species from a fatal case in China. Medical Mycology 47: 101–109. [DOI] [PubMed] [Google Scholar]
- Li Y, Horsman M, Wu N, et al. (2008). Biofuels from microalgae. Biotechnology Progress 24: 815–820. [DOI] [PubMed] [Google Scholar]
- Lian T, Simmer MI, D’Souza CA, et al. (2005). Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Molecular Microbiology 55: 1452–1472. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Miadlikowska J. (2009). Lichens. Current Biology 19: R502–503. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer WT, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Institute of Electrical and Electronics Engineers, New Orleans, LA: 1–8. [Google Scholar]
- Moreno LF, Vicente VA, de Hoog S. (2018). Black yeasts in the omics era: Achievements and challenges. Medical Mycology 56: S32–S41. [DOI] [PubMed] [Google Scholar]
- Nash TH. (2008). Nutrients, elemental accumulation, and mineral cycling. Lichen Biology, Second Edition: 234–251. [Google Scholar]
- Prenafeta-Boldú FX, Summerbell R, de Hoog GS. (2006). Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard? FEMS Microbiology Reviews 30: 109–130. [DOI] [PubMed] [Google Scholar]
- Qiao K, Wasylenko TM, Zhou K, et al. (2017). Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nature Biotechnology 35: 173–177. [DOI] [PubMed] [Google Scholar]
- Ratledge C. (2004). Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 86: 807–815. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Selbmann L, de Hoog GS, Mazzaglia A, et al. (2005). Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Studies in Mycology 51: 1–32. [Google Scholar]
- Selbmann L, de Hoog GS, Zucconi L, et al. (2014a). Black yeasts in cold habitats. In: Cold-adapted yeasts: biodiversity, adaptation strategies and biotechnological significance (Buzzini P, Margesin R, eds). Springer Berlin Heidelberg, Berlin, Heidelberg: 173–189. [Google Scholar]
- Selbmann L, Isola D, Egidi E, et al. (2014b). Mountain tips as reservoirs for new rock-fungal entities: Saxomyces gen. nov. and four new species from the Alps. Fungal Diversity 65: 167–182. [Google Scholar]
- Singh BN, Upreti DK, Gupta VK, et al. (2017). Endolichenic fungi: A hidden reservoir of next generation biopharmaceuticals. Trends in Biotechnology 35: 808–813. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008). A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Sun W, Su L, Yang S, et al. (2020). Unveiling the hidden diversity of rock-inhabiting fungi: Chaetothyriales from China. Journal of Fungi 6: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiru M, Sankh S, Rangaswamy V. (2011). Process for biodiesel production from Cryptococcus curvatus. Bioresource Technology 102: 10436–10440. [DOI] [PubMed] [Google Scholar]
- Vasconcelos B, Teixeira JC, Dragone G, et al. (2019). Oleaginous yeasts for sustainable lipid production-from biodiesel to surf boards, a wide range of “green” applications. Applied Microbiology and Biotechnology 103: 3651–3667. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Danesi P, James TY, et al. (2019). Comparative pathogenicity of opportunistic black yeasts in Aureobasidium. Mycoses 62: 803–811. [DOI] [PubMed] [Google Scholar]
- Ward OP, Singh A. (2005). Omega-3/6 fatty acids: Alternative sources of production. Process Biochemistry 40: 3627–3652. [Google Scholar]
- Wheeler MH, Bell AA. (1988). Melanins and their importance in pathogenic fungi. Current Topics in Medical Mycology 2: 338–387. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee SB, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications (Innis MA, Gelfand DH, Sninsky JJ, et al. eds). Academic Press, New York: 315–322. [Google Scholar]
- Zalar P, de Hoog GS, Gunde-Cimerman N. (1999). Taxonomy of the endoconidial black yeast genera Phaeotheca and Hyphospora. Studies in Mycology 43: 49–56. [Google Scholar]
- Zhao J, Zeng J, de Hoog GS, et al. (2010). Isolation and identification of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microbial Ecology 60: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]