Background.
There is limited information on the value of short-term invasive and noninvasive monitoring in kidney transplant recipients (KTR) undergoing therapy for chronic active antibody-mediated rejection (cAMR).
Methods.
We describe response rates in patients with cAMR receiving pulse steroids/IVIG ± rituximab 3-mo after index biopsy.
Results.
The study included 82 consecutive KTR. Mean time from transplant to cAMR was 10 y. Mean peritubular capillaritis (ptc), glomerulitis (g), microvascular inflammation (MVI), C4d, and cg Banff scores were 1.1, 2.1, 3.2, 0.2, and 2, respectively. Mean estimated glomerular filtration rate (eGFR) and urine protein creatinine (UPC) ratio were 38 mL/min and 1.6 g/g, respectively. Thirty (37%) patients lost their allograft during the mean follow-up of 2.4 y. In patients treated with pulse steroids/IVIG (n = 41), response rates for eGFR, UPC, donor-specific antibodies (DSAs), and MVI were 27%, 49%, 7%, and 19%, respectively. In the pulse steroids/IVIG/rituximab group, response rates were 66%, 61%, 20%, and 69%, respectively. Univariate analysis identified response in eGFR (HR = 0.03; P = 0.001; 95% CI, 0.004-0.26), UPC (HR = 0.38; P = 0.01; 95% CI, 0.18-0.82), and DSA (HR = 0.11; P = 0.004; 95% CI, 0.02-0.49) as predictors of graft survival. Multivariate analysis only retained eGFR response (HR = 0.12; P = 0.01; 95% CI, 0.02-0.64).
Conclusions.
In cAMR, short-term response to treatment for kidney function and DSA was associated with graft survival, but the role of early surveillance biopsies needs further evaluation.
Introduction
Kidney transplant recipients (KTR) with active antibody-medicated rejection (AMR) are at increased risk of chronic active AMR (cAMR) and poor long-term patient and allograft outcomes.1-5 We have reported that KTR with cAMR have a graft half-life of 12 mo if left untreated.4 Treatment with pulse steroids and IVIGs was associated with enhanced graft half-life to 24 mo,4,6 whereas the addition of single dose of rituximab to pulse steroids/IVIG was associated with further improvement in graft survival to 70% at 4 y.6 Choi et al demonstrated that adding tocilizumab to the previous regimen was associated with 80% graft survival at 6 y.7 However, the optimal treatment strategy for cAMR is not known, and most approaches are based on expert opinion.8,9 We recently reported on the risk of infections associated with specific treatments of cAMR and determined that the treatment of cAMR includes pulse steroids, rituximab, IVIG, and increased baseline immunosuppression, but not rituximab alone, was associated with sixfold increased risk of pneumonia.10
Monitoring strategies play an important role in defining the safety and efficacy of cAMR therapy.6,10 Furthermore, they can help define surrogate markers of treatment response and graft survival. We have noted that although short-term donor-specific antibody (DSA) monitoring and surveillance biopsies help to guide therapy, the graft survival advantage only became apparent after 1 y.6 In this study, we present our experience with surveillance 3-mo biopsies, DSA, and kidney function after the treatment of cAMR. We define short-term immunopathological and functional response rates to treatment modalities with pulse steroids/IVIG and pulse steroids/IVIG/rituximab, in an attempt to determine predictive short-term responses for long-term graft survival.
MATERIALS AND METHODS
Study Population
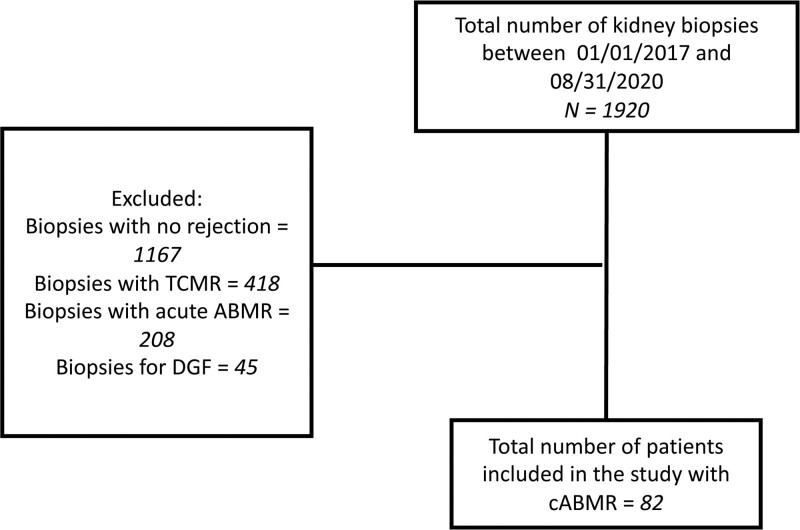
The study was approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison. Total number of biopsies performed during the study period (January 1, 2017, and August 31, 2020) was 1920; 1167 patients had no rejection, 467 had T-cell–mediated rejection (TCMR), 208 had acute AMR, and 82 had cAMR. There were 45 biopsies performed for delayed graft function. We included all KTR who had a first episode of biopsy-proven cAMR with at least one protocol follow-up biopsy (Figure 1). No patient received rejection treatment before their inclusion episode of biopsy-proven rejection. Patients who had rejection on initial biopsy but had no follow-up protocol biopsy were excluded from the study. Patients with any histologic component of TCMR, including t score >0 were also excluded. Patients with only active AMR with cg score = 0 were excluded from the study. Furthermore, patients with evidence of BK nephropathy or glomerulonephritis were excluded from the study. Patients with primary graft dysfunction (defined as needing chronic dialysis within 3-mo posttransplant or graft nephrectomy) were also excluded. Recipients of multi-organ transplants such as simultaneous liver and kidney, simultaneous pancreas and kidney, and simultaneous heart and kidney, were excluded. All biopsies were reevaluated to conform to Banff’s 2017 criteria.11 Death-censored kidney allograft failure was defined as patient return to dialysis or retransplant.
Figure 1.
Flowsheet. AMR, antibody-mediated rejection; cAMR, chronic active AMR; DGF, delayed graft function; TCMR, T-cell–mediated rejection.
Data Collection
We analyzed data on age, gender, race, retransplant status, the cause of end-stage kidney disease, type of transplant, induction immunosuppression, maintenance immunosuppression, the reason for the biopsy, donor-specific antibodies (DSA) at the time of biopsy, histology of the first and follow-up protocol biopsy, baseline creatinine, and estimated glomerular filtration rate (eGFR), creatinine and eGFR at time of rejection, creatinine, and eGFR after treatment, the treatment received, graft loss, and patient death.
Immunosuppression
Patients undergoing kidney transplants at our center receive induction immunosuppression with either a T-celldepleting agent (anti-thymocyte globulin or alemtuzumab) or a nondepleting agent (basiliximab) based on immunological risk factors. Patients typically received a triple immunosuppressive regimen for maintenance immunosuppression, including a calcineurin inhibitor (usually tacrolimus), anti-proliferative agent (mycophenolate mofetil or mycophenolic acid), and steroids. Low-immunological-risk patients receiving T-cell–depleting induction are eligible for early steroid withdrawal. The dose- and drug-level targets were adjusted based on the patient’s clinical characteristics, including immunological risk, infections, malignancies, and rejections as previously described.12
Kidney Allograft Biopsies
Kidney allograft biopsies were performed for cause due to an unexplained rise in serum creatinine or a significant increase in urine protein to creatinine (UPC) ratio. In addition, protocol biopsies were performed at months 3 and 12 for all patients with pretransplant DSA and in patients who develop de novo DSA or have a substantial rise in DSA. Additionally, patients treated for rejection undergo follow-up biopsy approximately 3 mo after initial biopsy, per protocol. The biopsy was sometimes delayed for logistical reasons and was sometimes performed earlier due to concerns about poor response. For the purpose of this study, acute kidney injury was defined as an absolute increase in serum creatinine level of ≥0.3 mg/dL within 48 h or ≥50% increase in serum creatinine level occurring over 1 to 7 d or the presence of oliguria for more than 6 h, per current kidney disease improving global outcomes guideline recommendation.13 The biopsy sample was declared adequate if there were ≥10 glomeruli with at least 2 arteries, per Banff criteria.14
Treatment of cAMR
At our center, patients with cAMR receive a treatment regimen that begins with dexamethasone 100 mg by intravenous infusion on day 1, followed by 50 mg on day 2 and then a gradual taper of prednisone from 180 mg to 10 mg daily over a month. The steroid pulse is augmented by IVIG 500 mg/kg every other week for 3 doses. A single rituximab 375 mg/m2 dose is added in younger patients with high disease activity (microvascular inflammation) (MVI score), and favorable history of infections/malignancies.6 Typically, for patients with cAMR, baseline immunosuppression was increased to a target tacrolimus trough level of 8–10 ng/mL and full dose of mycophenolate. To avoid selection bias, patients who received steroids and IVIG were not compared with patients who received steroids, IVIG, and rituximab. Rather, we simply describe response rates to each treatment strategy.
Immunopathology and Kidney Function Response to Treatment
Histologic response to treatment was assessed on 3-mo follow-up biopsies. Patients were considered MVI (g + ptc) responders if 3-mo MVI score was 0. Baseline eGFR was determined by assessing the eGFR within the past 3 mo before the rejection episode.
Our laboratory uses chronic kidney disease epidemiology collaboration equation for eGFR calculation. eGFR at the time of the first biopsy was used as the nadir eGFR. eGFR at the time of second biopsy was used to determine kidney function response to treatment. Patients were considered responders if 3-mo eGFR returned to within 10% of baseline. Patients were considered proteinuria responders if 3-mo UPC ratio declined by 25% from the index biopsy. Patients were considered DSA responders if 3-mo DSA MFI declined by 50% or greater from index biopsy.
Statistical Analysis
Data are reported as mean ± SD or percentages. We did not compare data between the two treatment groups to avoid selection bias. However, we examined changes between index biopsy and surveillance biopsy timepoints within each group. Univariate and multivariate Cox regression analyses were performed in the entire cohort to determine the risk factors associated with death censored kidney allograft loss. Variables were included in the multivariate analysis if the P value in univariate analysis was less than 0.05. Kaplan–Meier survival analyses were conducted to display graft survival. P values <0.05 were considered statistically significant. All analyses were performed using the MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016).
RESULTS
Baseline Characteristics
There were 82 patients with cAMR who met the study inclusion criteria. Baseline characteristics of the cohort can be found in Table 1. In the patients who received steroids/IVIG, the mean age at the time of transplant was 42 ± 14 y, 58.5% were male, and the majority were non-Hispanic White (78%). In this group, 46% of patients received anti-thymocyte globulin, 39% received basiliximab, and 15% received alemtuzumab as an induction agent. The mean time from the transplant to the biopsy with the diagnosis of cAMR was 10.5 ± 6.6 y. Mean follow-up from cAMR was 2 ± 1 y. Mean time between the first and follow-up biopsy was 5 ± 4.6 mo.
TABLE 1.
Baseline characteristics
| Baseline characteristics | Steroids/IVIG (N = 41) | Steroids/IVIG/Rituximab (N = 41) |
|---|---|---|
| Mean age at time of transplant ± SD, y | 42 ± 14 | 41 ± 14 |
| Male, n (%) | 24 (58.5) | 27 (66) |
| White, n (%) | 32 (78) | 34 (83) |
| Diabetic ESRD, n (%) | 4 (10) | 8 (19.5) |
| Living donor txp, n (%) | 12 (29) | 12 (29) |
| History of failed allograft, n (%) | 11 (27) | 11 (27) |
| Antithymocyte globulin induction, n (%) | 19 (46) | 21 (51) |
| Alemtuzumab induction, n (%) | 6 (15) | 3 (7) |
| Basiliximab induction, n (%) | 16 (39) | 17 (41) |
| Mean time from transplant to biopsy ± SD, y | 10.5 ± 6.6 | 9.5 ± 8 |
| Mean follow-up from transplant ± SD, y | 12.5 ± 6.5 | 12 ± 8 |
| Mean follow-up from biopsy ± SD, y | 2 ± 1 | 3 ± 1 |
| Time between two biopsies ± SD, mo | 5 ± 4.6 | 4 ± 2 |
ESRD, End-Stage Renal Disease; txp, transplantation.
In patients who received steroids/IVIG/rituximab, the mean age at transplant was 41 ± 14 y, 66% were male, and the majority were non-Hispanic White (83%). In this group, 51% of patients received antithymocyte globulin, 41% received basiliximab, and 7% received alemtuzumab as an induction agent. Mean time from the transplant to the biopsy with the diagnosis of cAMR was 9.5 ± 8 y. Mean follow-up from cAMR was 3 ± 1 y. Mean time between the first and follow-up biopsy was 4 ± 2 mo.
Changes in Histopathology
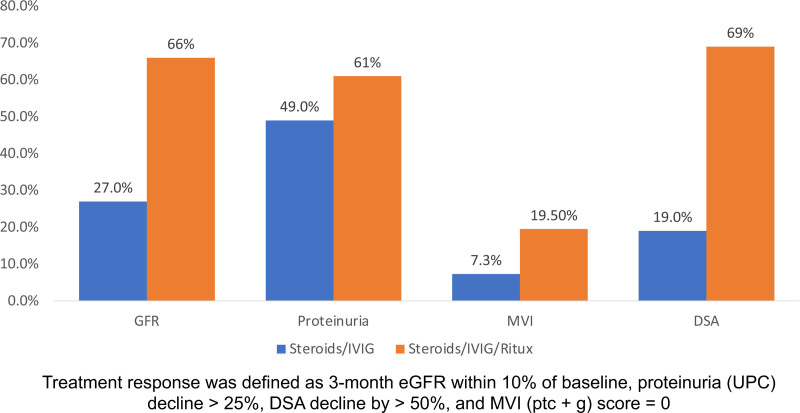
Pathology Banff scores on index and surveillance biopsies are displayed in (Table 2). Transplant glomerulopathy (cg score) was moderately severe in both groups (cg 2 ± 1 and 1.8 ± 0.8, respectively). Both g and MVI scores improved significantly regardless of the treatment received. There was also a decline in peritubular capillaritis (ptc) scores, although the change did not reach statistical significance. Tubular atrophy (ct scores) was significantly worse after receiving pulse steroids/IVIG/rituximab. There was no other significant change between index and surveillance biopsies. A positive response for MVI was noted in 7.3% and 19.5% of patients in the pulse steroids/IVIG and pulse steroids/IVIG/rituximab groups, respectively (Figure 2).
TABLE 2.
Changes in histopathology
| Pulse steroids/IVIG, mean ± SD | Pulse steroids/IVIG/Rituximab, mean ± SD | |||||
|---|---|---|---|---|---|---|
| Banff score | Index biopsy | Surveillance biopsy | P | Index biopsy | Surveillance biopsy | P |
| i | 0.1 ± 0.4 | 0.05 ± 0.3 | 0.5 | 0.05 ± 0.2 | 0.05 ± 0.3 | 1 |
| t | 0 | 0 | N/A | 0 | 0 | N/A |
| v | 0 | 0 | N/A | 0 | 0 | N/A |
| ptc | 1.1 ± 1 | 0.8 ± 0.7 | 0.1 | 1.2 ± 1 | 1 ± 0.8 | 0.2 |
| g | 2.1 ± 1 | 1.7 ± 1 | 0.04 | 2.1 ± 1 | 1.5 ± 1 | 0.01 |
| mvi | 3.2 ± 1.3 | 2.5 ± 1.5 | 0.02 | 3.3 ± 1 | 2.3 ± 1.5 | 0.01 |
| C4d | 0.2 ± 0.7 | 0.1 ± 0.5 | 0.4 | 0.2 ± 0.7 | 0.3 ± 0.7 | 0.4 |
| ah | 1.4 ± 1.2 | 1.2 ± 1.3 | 0.4 | 1.2 ± 1.3 | 1.3 ± 1.3 | 0.7 |
| ci | 1.6 ± 1 | 1.7 ± 1 | 0.6 | 1 ± 0.6 | 1.2 ± 0.7 | 0.2 |
| ct | 1.5 ± 1 | 1.7 ± 1 | 0.4 | 1 ± 0.5 | 1.3 ± 0.6 | 0.01 |
| cv | 1.1 ± 1 | 1.2 ± 1 | 0.6 | 0.7 ± 0.7 | 0.9 ± 0.8 | 0.2 |
| cg | 2 ± 1 | 1.6 ± 1 | 0.07 | 1.8 ± 0.8 | 1.5 ± 1.1 | 0.2 |
| ci+ct+cv+cg | 6.3 ± 2.6 | 6.2 ± 2.5 | 0.8 | 4.5 ± 1.8 | 4.8 ± 2 | 1 |
ah, arteriolar hyalinosis; cg, glomerular basement membrane double contours; ci, interstitial fibrosis; ct, tubular atrophy; cv, vascular fibrous intimal thickening; g, glomerulitis; i, interstitial inflammation; mvi, microvascular inflammation; N/A, not applicable; ptc, peritubular capillaritis; t, tubulitis; v, intimal arteritis.
Figure 2.
Three-month response rates to prescriptions in cAMR. cAMR, chronic active antibody-mediated rejection; DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; MVI, microvascular inflammation; UPC, urine-protein creatinine ratio.
Changes in Kidney Function and DSA
Kidney function parameters (serum creatinine, eGFR, and UPC) and circulating HLA DSA levels are displayed in Table 3. For patients who received steroids/IVIG, overall changes in serum creatinine, eGFR, and proteinuria were not statistically significant. Similarly, changes in DSA did not reach statistical significance. According to our predefined criteria, eGFR, UPC, and DSA response rates were at 27%, 49%, and 19%, respectively (Figure 2). In the steroids/IVIG/rituximab group, UPC and DSA to class I HLA declined significantly. Positive response for eGFR, UPC, and DSA occurred in 66%, 61%, and 69%, respectively (Figure 2).
TABLE 3.
Changes in kidney function and DSA
| Pulse steroids/IVIG | Pulse steroids/IVIG/Rituximab | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Baseline | Index biopsy | Surveillance biopsy | P a | Baseline | Index biopsy | Surveillance biopsy | P a |
| Kidney function | ||||||||
| Creatinine (mg/dL) | 1.6 ± 0.5 | 2 ± 0.7 | 2.2 ± 1 | 0.2 | 1.4 ± 0.4 | 1.6 ± 0.5 | 1.8 ± 0.4 | 0.08 |
| eGFR (mL/min) | 47 ± 16 | 36 ± 13 | 35 ± 14 | 0.7 | 51.5 ± 14 | 40 ± 12.5 | 42 ± 13 | 0.4 |
| UPC (g/g) | 0.6 ± 0.6 | 1.8 ± 1.4 | 1.7 ± 1.6 | 0.8 | 0.5 ± 0.4 | 1.3 ± 1.4 | 0.7 ± 0.8 | 0.02 |
| HLA DSA | ||||||||
| Class I DSA (MFI) | N/A | 2063 ± 6200 | 1215 ± 3402 | 0.4 | N/A | 1577 ± 1911 | 739 ± 1096 | 0.04 |
| Class II DSA (MFI) | 4034 ± 6930 | 3173 ± 7665 | 0.5 | 5386 ± 7896 | 2895 ± 7543 | 0.1 | ||
| Class I + II DSA (MFI) | 8237 ± 17 373 | 4327 ± 8707 | 0.2 | 5953 ± 12 216 | 3518 ± 14 473 | 0.3 | ||
aBetween index and surveillance biopsy.
DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; MFI, mean fluorescence intensity; N/A, not applicable; UPC, urine-protein creatinine ratio.
Graft Outcomes
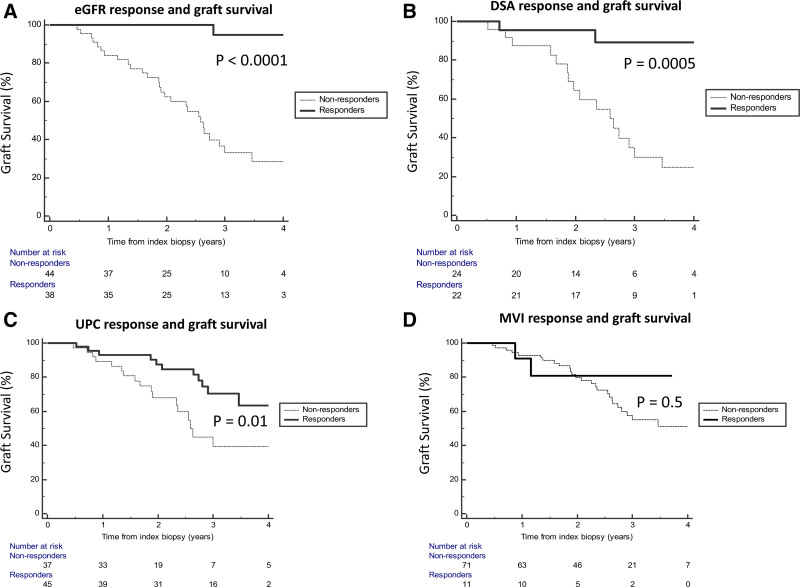
Next, we examined whether short-term response rates in kidney function, DSA, and pathology could predict long-term graft survival. We first addressed this question with Kaplan-Meier survival analyses and determined that a positive response for eGFR, DSA, and UPC was associated with better death-censored graft survival (P < 0.0001, P = 0.0005 and P = 0.01, respectively, Figure 3). Interestingly, MVI response was not associated with improved death-censored graft survival (P = 0.5).
Figure 3.
Short-term response in kidney function and DSA associated with graft survival. DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; MVI, microvascular inflammation; UPC, urine-protein creatinine ratio.
Next, we conducted univariable and multivariate Cox regression analyses adjusted for baseline donor, recipient, and transplant characteristics, to confirm whether these associations were independent predictors of outcomes (Table 4). In univariable analyses, rituximab use (HR = 0.13; P = 0.0001; 95% CI, 0.05-0.34) and a response in eGFR (HR = 0.03; P = 0.001; 95% CI, 0.004-0.26), UPC (HR = 0.38; P = 0.01; 95% CI, 0.18-0.82), and DSA (HR = 0.11; P = 0.004; 95% CI, 0.02-0.49) were associated with improved death-censored graft survival. However, on multivariate analysis, only eGFR response was retained (HR = 0.12; P = 0.01; 95% CI, 0.02-0.64). Interestingly, changes in MVI were not associated with graft survival (HR = 0.86; P = 0.2; 95% CI, 0.69-1.09).
TABLE 4.
Variables associated with death-censored graft loss
| Variables | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
| HR | P | 95% CI | HR | P | 95% CI | |
| Age >55 at txp | 1.01 | 0.97 | 0.41–2.49 | |||
| Male | 1.17 | 0.68 | 0.53–2.60 | |||
| White | 0.67 | 0.36 | 0.28–1.58 | |||
| History of failed transplant | 0.85 | 0.73 | 0.34–2.12 | |||
| DM as cause of ESRD | 0.51 | 0.27 | 0.15–1.71 | |||
| Living donor transplant | 1.76 | 0.13 | 0.83–3.74 | |||
| Depleting Induction | 1.38 | 0.39 | 0.65–2.94 | |||
| DSA present at biopsy | 1.18 | 0.66 | 0.55–2.55 | |||
| Chronicity score >8 | 11.91 | 0.0001 | 5.38–26.33 | 1.54 | 0.48 | 0.45–5.25 |
| eGFR response, yes/no | 0.03 | 0.001 | 0.004–0.26 | 0.12 | 0.013 | 0.02–0.64 |
| DSA response, yes/no | 0.11 | 0.004 | 0.026–0.49 | 1.28 | 0.78 | 0.21–7.77 |
| UPC response, yes/no | 0.38 | 0.01 | 0.18–0.82 | 1.02 | 0.96 | 0.32–3.20 |
| MVI response, yes/no | 0.65 | 0.55 | 0.15–2.75 | |||
| C4d response, yes/no | 1.61 | 0.45 | 0.42–6.08 | |||
| Change in MVI between two biopsies | 0.86 | 0.2 | 0.69–1.09 | |||
| Rituximab use | 0.13 | 0.0001 | 0.05–0.34 | 0.27 | 0.10 | 0.05–1.29 |
CI, confidence interval; DM, diabetes mellitus; DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; ESRD, End-Stage Renal Disease; HR, hazard ratio; MVI, microvascular inflammation; txp, transplantation; UPC, urine-protein creatinine ratio.
DISCUSSION
Optimal monitoring strategies are needed to address cAMR, a leading cause of late graft failure after kidney transplantation. We examined the value of standard 3-mo invasive and noninvasive monitoring protocols after the diagnosis of cAMR. Our findings suggest that a return of eGFR within 10% of baseline is the best predictor of graft survival. A decline in DSA >50% and an improvement in proteinuria >25% were also associated with improved graft survival, but multivariate analyses only retained eGFR response as the independent predictor of outcomes. Interestingly, despite significantly improved MVI scores on follow-up biopsies, MVI response did not appear to predict graft survival, calling into question the value of short-term protocol biopsies after cAMR.
Currently, there is no consensus on how patients with cAMR should be monitored after treatment or if early response to treatment is associated with favorable graft outcomes. In a study by Redfield et al, cAMR was associated with poor graft outcomes, especially if untreated.4 The authors called for earlier monitoring strategies and improved treatment approaches. In that study, patients with DSA MFI >2500, chronicity score >8/12, serum creatinine >3 mg/dL, and UPC >1 were at greater risk of graft failure.4 However, these measurements did not consider baseline values or short-term response to therapy. In our study, we found that eGFR response 3-mo after cAMR treatment to be the single most important factor in predicting long-term graft survival. This is consistent with previous reports demonstrating that in patients with acute rejection, a return of serum creatinine or eGFR to baseline is associated with better graft outcomes.15,16 Although improvements in proteinuria and DSA were also associated with enhanced graft survival in Kaplan-Meier and univariable Cox regression analyses, their independent role was not confirmed in multivariable studies. Nevertheless, previous reports have suggested that a decline in DSA by 50% is associated with improved graft survival.17 More studies are needed to determine whether the resolution of proteinuria after cAMR is independently associated with improved outcomes. Attenuation of microcirculation inflammation in response to treatment failed to predict long-term graft outcomes. Consistent with this observation, we have recently demonstrated that changes in disease activity may be delayed in refractory AMR,18 suggesting that overall, the degree of microcirculation inflammation on surveillance biopsies may be a poor predictor of short- or long-term graft survival in patients with cAMR. The ongoing Clazakizumab for the Treatment of Chronic Active Antibody Mediated Rejection in KTR (IMAGINE) trial (NCT03744910) may shed light on the treatment strategies for cAMR.
Our report has the limitations of a single-center observational study with a small sample size. The lack of association between MVI with graft survival may be due to confounding parameters, biopsy sampling timing, and multiple operators involved. Although 50% of the cohort received rituximab, we did not compare treatment regimens. Rather, we simply described short-term immunopathological and functional response rates to pulse steroids/IVIG ± rituximab as a reference point for further studies and clinical trials. More importantly, our study indicates that in patients with cAMR, noninvasive monitoring may be a safe and effective approach to guide treatment and inform on long-term outcomes. Further studies are needed to determine whether enhanced multimodality utilizing noninvasive tests combining kidney function, DSA, donor-derived cell-free DNA, and peripheral blood gene expression profiling can aid in optimization of immunosuppression and graft survival in patients with cAMR.19,20
Footnotes
The authors declare no funding or conflicts of interest.
F.A. participated in design, data collection, analysis, and article preparation. S.P., M.J., N.G., V.M., E.Y., D.M., L.H., M.M., and W.Z. involved in analysis and editing. A.D. involved original idea, study concept, study design, analysis, article preparation, and editing.
REFERENCES
- 1.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. [DOI] [PubMed] [Google Scholar]
- 2.Dunn TB, Noreen H, Gillingham K, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011;11:2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amico P, Hönger G, Mayr M, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681–1688. [DOI] [PubMed] [Google Scholar]
- 4.Redfield RR, Ellis TM, Zhong W, et al. Current outcomes of chronic active antibody mediated rejection - a large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol. 2016;77:346–352. [DOI] [PubMed] [Google Scholar]
- 5.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. [DOI] [PubMed] [Google Scholar]
- 6.Parajuli S, Mandelbrot DA, Muth B, et al. Rituximab and monitoring strategies for late antibody-mediated rejection after kidney transplantation. Transplant Direct. 2017;3:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 8.Velidedeoglu E, Cavaillé-Coll MW, Bala S, et al. Summary of 2017 FDA public workshop: antibody-mediated rejection in kidney transplantation. Transplantation. 2018;102:e257–e264. [DOI] [PubMed] [Google Scholar]
- 9.Schinstock CA, Askar M, Bagnasco SM, et al. A 2020 Banff Antibody-mediated Injury Working Group examination of international practices for diagnosing antibody-mediated rejection in kidney transplantation—a cohort study. Transpl Int. 2021;34:488–498. [DOI] [PubMed] [Google Scholar]
- 10.Joachim E, Parajuli S, Swanson KJ, et al. Treatment of chronic active antibody-mediated rejection with pulse steroids, IVIG, with or without rituximab is associated with increased risk of pneumonia. Transplant Direct. 2021;7:e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active t cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parajuli S, Aziz F, Garg N, et al. Histopathological characteristics and causes of kidney graft failure in the current era of immunosuppression. World J Transplant. 2019;9:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. [DOI] [PubMed] [Google Scholar]
- 14.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. [DOI] [PubMed] [Google Scholar]
- 15.Clayton PA, McDonald SP, Russ GR, et al. Long-term outcomes after acute rejection in kidney transplant recipients: an ANZDATA analysis. J Am Soc Nephrol. 2019;30:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. [DOI] [PubMed] [Google Scholar]
- 17.Everly MJ, Everly JJ, Arend LJ, et al. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9:1063–1071. [DOI] [PubMed] [Google Scholar]
- 18.Degner KR, Parajuli S, Aziz F, et al. Modest improvements in refractory antibody-mediated rejection after prolonged treatment. Kidney Int Rep. 2021;6:1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akalin E, Weir MR, Bunnapradist S, et al. Clinical validation of an immune quiescence gene expression signature in kidney transplantation. Kidney360. 2021;2:1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Guo K, Heilman RL, et al. Combining blood gene expression and cellfree DNA to diagnose subclinical rejection in kidney transplant recipients. Clin J Am Soc Nephrol. 2021;16:1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]