Abstract
Background
Maintaining blood pressure (BP) control over time may contribute to lower risk for cardiovascular disease (CVD) among individuals who are taking antihypertensive medication.
Methods
The Jackson Heart Study (JHS) enrolled 5,306 African-American adults ≥21 years of age and was used to determine the proportion of African Americans that maintain persistent BP control, identify factors associated with persistent BP control, and determine the association of persistent BP control with CVD events. This analysis included 1,604 participants who were taking antihypertensive medication at Visit 1 and had BP data at Visits 1 (2000–2004), 2 (2005–2008), and 3 (2009–2013). Persistent BP control was defined as systolic BP <140 mm Hg and diastolic BP <90 mm Hg at all three visits. CVD events were assessed from Visit 3 through December 31, 2016. Hazard ratios (HR) for the association of persistent BP control with CVD outcomes were adjusted for age, sex, systolic BP, smoking, diabetes, and total and high-density lipoprotein cholesterol at Visit 3.
Results
At Visit 1, 1,226 of 1,604 participants (76.4%) with hypertension had controlled BP. Overall, 48.9% of participants taking antihypertensive medication at Visit 1 had persistent BP control. After multivariable adjustment for demographic, socioeconomic, clinical, behavioral, and psychosocial factors, and access-to-care, participants were more likely to have persistent BP control if they were <65 years of age, women, had family income ≥$25,000 at each visit, and visited a health professional in the year prior to each visit. The multivariable adjusted HR (95% confidence interval) comparing participants with versus without persistent BP control was 0.71 (0.46–1.10) for CVD, 0.68 (0.34–1.34) for coronary heart disease, 0.65 (0.27–1.52) for stroke, and 0.55 (0.33–0.90) for heart failure.
Conclusion
Less than half of JHS participants taking antihypertensive medication had persistent BP control, putting them at increased risk for heart failure.
Introduction
Hypertension is a major modifiable risk factor for cardiovascular disease (CVD [1–3]. Antihypertensive medication has been shown to lower blood pressure (BP) and reduce the risk for CVD among adults with hypertension [4]. Among individuals taking antihypertensive medication, those with controlled BP have a lower risk for CVD events compared to their counterparts with uncontrolled BP [5, 6]. However, according to the US National Health and Nutrition Examination Survey (NHANES), only 65% of US adults taking antihypertensive medication had systolic BP (SBP) <140 mm Hg and diastolic BP (DBP) <90 mm Hg in 2017–2018 [7].
NHANES only provides cross-sectional estimates of BP control at a single time point [8, 9]. However, prior studies report that many people with BP control at a single time point do not have BP control when assessed at multiple visits [10], what we refer to in the current manuscript as persistent BP control. In a secondary analysis of the Antihypertensive Lipid-Lowering to prevent Heart Attack Trial (ALLHAT), only 20% of participants had controlled SBP at eight follow-up visits conducted over a 22 month period [10]. ALLHAT participants were treated with antihypertensive medication following a standardized protocol and few data on persistent BP control over time are available from population-based studies. Therefore, it is unclear what percentage of people with hypertension in the general population versus those in a clinical trial maintain persistent BP control over time.
Among US adults with hypertension, African Americans are less likely than whites to have controlled BP and have a higher risk for hypertension-related CVD including stroke and heart failure [6]. Studying persistent BP control among African-American adults has the potential to inform interventions to improve BP control and reduce racial disparities in CVD. Therefore, the purpose of this study was to determine the percentage of African-American adults with persistent BP control, identify factors associated with persistent BP control, and determine the association of persistent BP control with risk for CVD outcomes. To address these goals, we analyzed data from the Jackson Heart Study (JHS), a community-based cohort of African-American adults.
Methods
Study population
The JHS was designed to determine the reasons for the high prevalence of CVD in African-Americans and identify approaches for reducing this risk [11]. Between 2000 and 2004, 5,306 non-institutionalized African-American adults ≥21 years of age were enrolled into the JHS. Participants were recruited from the three counties (Hinds, Madison, and Rankin) that comprise the Jackson, Mississippi metropolitan area. To date, there have been three JHS visits including baseline (Visit 1) from 2000–2004, Visit 2 from 2005–2008, and Visit 3 from 2009–2013. We restricted the analysis of the proportion of participants with persistent BP control and factors associated with persistent BP control to those who were taking antihypertensive medication at Visit 1 and had complete data on SBP and DBP at Visit 1, Visit 2, and Visit 3 (S1 Fig in S1 File). Antihypertensive medication use was defined by self-report and confirmed by a review of prescription pill bottles that was conducted by study staff. After applying these criteria, 1,604 participants were included in the analysis. For the analysis examining the association of persistent BP control with CVD events following Visit 3, we further excluded participants with a history of CVD at Visit 1, those who did not consent to follow-up for CVD events and those who had a CVD outcome between Visits 1 and 3. Overall, 1,151 participants were included in the analysis of persistent BP control and CVD events. The JHS was approved by the institutional review boards of the University of Mississippi Medical Center, Jackson State University, and Tougaloo College, and all participants provided written informed consent at each visit.
Persistent BP control
At each study visit, BP was measured by trained staff following a standardized protocol while participants were seated and after a five-minute rest. Participants’ right arms were fitted with an appropriately-sized cuff and two BP measurements were taken, with a one minute interval separating the measurements. Staff used a random-zero sphygmomanometer (Hawksley and Sons, Ltd, London, UK) to measure BP at Visits 1 and 2, and a semi-automated oscillometric device (Omron HEM-907XL, Omron Healthcare Inc., Lake Forest, IL) at Visit 3. BP measurements performed using the random-zero sphygmomanometer were calibrated to the oscillometric device after the completion of a BP comparability study [12]. The mean BP from each visit was used in the analyses. At each visit, controlled BP was defined as SBP <140 mm Hg and DBP <90 mm Hg. Persistent BP control was defined as having controlled BP at all three study visits.
Baseline factors
Demographic factors included age, sex, education level and marital status. The number of antihypertensive medication classes taken at baseline was included as a clinical factor. Cigarette smoking at baseline was included as a behavioral factor, and we included health insurance status at baseline as a measure of access to healthcare. We included weekly stress and depressive symptoms at baseline as psychosocial factors. S1 Table in S1 File lists the definitions of and the methods used to assess baseline factors.
Time-varying factors
Several factors were available at multiple JHS visits (S1 Table in S1 File). Annual family income was included as a socioeconomic factor. Body mass index (BMI) and measures of glycemic control were included as clinical factors. Behavioral factors included adherence to antihypertensive medication, alcohol consumption, and physical activity. Participant access to healthcare included annual healthcare visits and difficulty accessing healthcare. Anger expression and daily discrimination were included as psychosocial factors. For each study visit, we categorized participants as having ideal or non-ideal levels of each of these factors (see S1 Table in S1 File). We then categorized participants as maintaining ideal levels of each factor if they were in the ideal category at all visits at which they were assessed.
Incident CVD events
The primary CVD outcome was a composite of coronary heart disease (CHD: i.e., myocardial infarction, fatal CHD, or a cardiac procedure), stroke, and heart failure. CHD, stroke and heart failure were investigated individually as secondary CVD outcomes. CVD events were identified by annual telephone follow-up interviews, hospitalization surveillance, and death certificate review. Possible events were then adjudicated by trained abstractors. A detailed description of the JHS follow-up and CVD event adjudication process has been published previously [13].
Statistical analysis
We estimated summary statistics for participants with and without persistent BP control, separately. We calculated the proportion of participants with persistent BP control, overall and by levels of baseline and time-varying factors. We used two Poisson regression models with robust variance estimates to calculate risk ratios (RR) and 95% confidence intervals (CI) for the association of baseline and time-varying factors with persistent BP control. Model 1 included adjustment for age and sex and each baseline and time-varying factor listed above one at a time. Model 2 included all baseline and time-varying factors. Multiple imputation with chained equations was applied to impute missing values (S2 Table in S1 File). Imputation models were regression based and used all analysis variables to impute missing data. A total of 10 imputed datasets were created and results were pooled to obtain valid standard error estimates. All calculations were done for the overall included population and for those with controlled BP at baseline.
We calculated incidence rates and hazard ratios (HR) with 95% CIs for CVD, CHD, stroke, and heart failure among participants with and without persistent BP control. For the calculation of incidence rates and HRs, participants were followed from the date of their Visit 3 examination through the date of their first CVD event with censoring occurring for those who remained event free on the date of their last contact with the JHS, date of death, or December 31, 2016, whichever occurred first. Two models with progressive adjustment were used to calculate HRs. Model 1 included adjustment for age and sex. Model 2 included adjustment for age, sex, SBP, smoking, diabetes, total cholesterol, and high-density lipoprotein (HDL) cholesterol measured at Visit 3. These variables are included in the Pooled Cohort Risk Equations which is used to assess 10-year CVD risk [14] and we adjusted for these variables due to their robust association with CVD event risk [14]. In a secondary analysis, we calculated the incidence rates and HRs for CVD, CHD, stroke, and heart failure comparing participants with persistent BP control across all three study visits to participants with controlled BP at Visit 3 but without persistent BP control. Statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC) and STATA Version 16.1 (StataCorp, College Station, TX).
Results
At Visit 1, 76.4% (n = 1,226 of 1,604) of participants included in the current analysis had controlled BP. Among those with controlled BP at Visit 1, 64.0% had persistent BP control (i.e., controlled BP at all three visits). Overall, 785 of 1,604 (48.9%) participants had persistent BP control over a median follow up time of 8.0 years (25th-75th percentile: 7.4–8.3 years). Characteristics of participants with and without persistent BP control are presented in Table 1.
Table 1. Baseline characteristics of Jackson Heart Study participants taking antihypertensive medication by persistent blood pressure control status.
| Characteristics | Persistent blood pressure control | |
|---|---|---|
| Yes n = 785 | No n = 819 | |
| Demographic | ||
| Age in years, mean (SD) | 57.1 (9.8) | 60.6 (10.2) |
| Men, % | 28.8 | 31.9 |
| Income <$25,000 per year, % | 32.4 | 41.3 |
| Less than high school education, % | 14.7 | 22.1 |
| Married, % | 57.7 | 54.9 |
| Clinical factors | ||
| Systolic blood pressure, mean (SD) | 122 (10) | 138 (16) |
| Diastolic blood pressure, mean (SD) | 74.1 (7.6) | 77.9 (9.4) |
| Number of antihypertensive medication classes, % | ||
| 1 | 27.0 | 30.2 |
| 2 | 45.2 | 40.2 |
| 3 | 19.4 | 19.8 |
| ≥4 | 8.4 | 9.9 |
| Body mass index < 25 kg/m2, % | 6.9 | 8.7 |
| Ideal glycemic control, % | 31.3 | 30.9 |
| Behavioral factors | ||
| Adherence to antihypertensive medication, % | 78.7 | 71.2 |
| Cigarette smoking, no, % | 91.6 | 91.9 |
| Alcohol consumption, no, % | 59.0 | 62.3 |
| Ideal physical activity, % | 20.3 | 17.3 |
| Access to health care | ||
| Health insurance, % | 91.1 | 89.0 |
| Healthcare visit in the past year, % | 86.1 | 84.8 |
| Difficulty in obtaining health services, % | 27.1 | 27.2 |
| Psychosocial factors | ||
| Stress, % | ||
| Low | 35.1 | 31.9 |
| Moderate | 31.9 | 34.6 |
| High | 33.0 | 33.5 |
| Depressive symptoms, % | 19.0 | 23.4 |
| Anger-in, % | ||
| Low | 37.0 | 34.6 |
| Moderate | 36.2 | 34.4 |
| High | 26.8 | 31.0 |
| Anger-out, % | ||
| Low | 32.2 | 30.4 |
| Moderate | 40.7 | 37.9 |
| High | 27.1 | 31.8 |
| Daily discrimination, % | ||
| Quartile 1 (low) | 26.0 | 27.5 |
| Quartile 2 | 21.0 | 21.7 |
| Quartile 3 | 28.1 | 25.8 |
| Quartile 4 (high) | 24.9 | 25.0 |
SD = standard deviation.
Stress scale tertile cut points: low (0–31), moderate (32–80), high (81–482)
Anger-in scale tertile cut points: low (8–11), moderate (12–14), high (15–28)
Anger-out scale tertile cut points: low (8–10), moderate (11–13), high (14–29)
Daily discrimination scale quartile cut points: quartile 1 (1.00–1.32), quartile 2 (1.33–1.76), quartile 3 (1.77–2.54), quartile 4 (2.55–7.00).
Prevalence of persistent BP control by participant characteristics
Participants who were <65 years of age, maintained an income ≥$25,000, had a high school education, were adherent to their antihypertensive medication across the three study visits, drank alcohol, and had visited a healthcare professional in the year before each study visit were more likely to have persistent BP control (Table 2). Each of these factors, except being adherent to antihypertensive medication and drinking alcohol, was associated with persistent BP control among participants with controlled BP at baseline (S3 Table in S1 File).
Table 2. Percentage of participants with persistent blood pressure control in sub-groups.
| Characteristics | Percentage with persistent BP control | p-value |
|---|---|---|
| Overall | 48.9 | |
| Demographic | ||
| Age | ||
| <65 years | 53.4 | <0.001 |
| ≥65 years | 38.8 | |
| Sex | ||
| Men | 46.4 | 0.19 |
| Women | 50.0 | |
| Maintained income ≥$25,000 per year* | ||
| No | 43.8 | <0.001 |
| Yes | 56.1 | |
| High school education | ||
| No | 38.9 | <0.001 |
| Yes | 51.2 | |
| Marital status | ||
| Married | 50.2 | 0.29 |
| Not married | 47.4 | |
| Clinical factors | ||
| Number of antihypertensive medication classes | ||
| 1 | 46.2 | 0.19 |
| 2 | 51.9 | |
| 3 | 48.4 | |
| ≥4 | 44.9 | |
| Maintained ideal body mass index* | ||
| No | 49.9 | 0.47 |
| Yes | 45.2 | |
| Maintained ideal glycemic control* | ||
| No | 49.7 | 0.69 |
| Yes | 51.9 | |
| Behavioral factors | ||
| Maintained adherence to antihypertensive medication* | ||
| No | 46.6 | 0.02 |
| Yes | 52.7 | |
| Cigarette smoking | ||
| No | 48.9 | 0.86 |
| Yes | 50.0 | |
| Maintained ideal alcohol consumption status* | ||
| No | 51.9 | 0.03 |
| Yes | 46.5 | |
| Maintained ideal physical activity* | ||
| No | 49.3 | 0.93 |
| Yes | 48.8 | |
| Access to health care | ||
| Health insurance | ||
| Uninsured | 43.8 | 0.18 |
| Insured | 49.5 | |
| Reported visiting a healthcare professional in the past year at each study visit* | ||
| No | 43.6 | 0.003 |
| Yes | 51.8 | |
| Maintained no difficulty in obtaining health services* | ||
| No | 47.8 | 0.21 |
| Yes | 51.0 | |
| Psychosocial factors | ||
| Stress | ||
| Low | 53.5 | 0.54 |
| Moderate | 49.0 | |
| High | 50.7 | |
| Depression | ||
| No depressive symptoms | 51.1 | 0.09 |
| Depressive symptoms | 44.6 | |
| Maintained ideal anger-in* | ||
| No | 49.6 | 0.86 |
| Yes | 50.7 | |
| Maintained ideal anger-out* | ||
| No | 50.3 | 0.53 |
| Yes | 47.3 | |
| Maintained low levels of daily discrimination* | ||
| No | 49.9 | 0.07 |
| Yes | 43.0 |
BP = blood pressure
*These factors were available at multiple study visits. For each study visit where these variables were available, we categorized participants as having ideal or non-ideal levels of each of these factors. We then categorized participants as maintaining ideal levels of each factor if participants were in the ideal category at all visits in which they were collected. S1 Table in S1 File lists these study variable definitions, visits at which they were collected, collection methods, and their classification for ideal level status.
Stress scale tertile cut points: low (0–31), moderate (32–80), high (81–482)
Adjusted associations of participant characteristics with persistent BP control
After adjustment for sex, participants ≥65 years of age were less likely to have persistent BP control compared with participants <65 years of age (Table 3 –Left Panel). After age and sex adjustment, participants were more likely to have persistent BP control if they maintained a family income ≥ $25,000 a year, had a high school education, maintained adherence to antihypertensive medication, had health insurance at baseline, and visited a health professional in the year prior to each study visit. In a model with all demographic, clinical, behavioral, access to healthcare and psychosocial factors, participants ≥65 years of age were less likely to have persistent BP control, while women, participants who maintained an income ≥ $25,000 a year, and who reported visiting a health professional in the year before each study visit were more likely to have persistent BP control (Table 3 –Right Panel). S4 Table in S1 File presents the RRs for persistent BP control associated with participant characteristics among JHS participants with controlled BP at Visit 1.
Table 3. Adjusted risk ratios for persistent blood pressure control among participants taking antihypertensive medication (n = 1,604).
| Characteristic | Risk ratio (95% CI) Model 1 | p-value | Risk ratio (95% CI) Model 2 | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Age: ≥65 years compared to <65 years | 0.73 (0.64–0.82) | <0.001 | 0.77 (0.67–0.88) | <0.001 |
| Sex: women vs men | 1.08 (0.97–1.21) | 0.155 | 1.13 (1.01–1.28) | 0.035 |
| Maintained income ≥$25,000 per year*: yes vs no | 1.28 (1.14–1.43) | <0.001 | 1.19 (1.04–1.35) | 0.012 |
| High school education: yes vs. no | 1.20 (1.03–1.41) | 0.020 | 1.06 (0.90–1.26) | 0.494 |
| Marital status: married vs not married | 1.08 (0.97–1.19) | 0.160 | 1.01 (0.91–1.12) | 0.867 |
| Clinical factors | ||||
| Number of antihypertensive medication classes | ||||
| 1 | Ref | Ref | ||
| 2 | 1.12 (0.99–1.26) | 0.073 | 1.13 (1.00–1.27) | 0.053 |
| 3 | 1.05 (0.90–1.22) | 0.533 | 1.05 (0.91–1.22) | 0.490 |
| ≥4 | 0.96 (0.78–1.18) | 0.708 | 0.97 (0.79–1.19) | 0.797 |
| Maintained ideal body mass index*: yes vs no | 0.94 (0.73–1.22) | 0.663 | 0.95 (0.74–1.24) | 0.727 |
| Maintained ideal glycemic control*: yes vs no | 0.96 (0.82–1.14) | 0.658 | 0.94 (0.79–1.11) | 0.456 |
| Behavioral factors | ||||
| Maintained adherence to antihypertensive medication: yes vs. no* | 1.11 (1.01–1.23) | 0.037 | 1.09 (0.99–1.20) | 0.094 |
| Current cigarette smoking: yes vs. no | 0.97 (0.81–1.16) | 0.752 | 1.02 (0.85–1.23) | 0.824 |
| Maintained non-drinker status*: yes vs. no | 0.93 (0.84–1.03) | 0.184 | 0.97 (0.87–1.08) | 0.554 |
| Maintained ideal physical activity*: yes vs. no | 0.99 (0.82–1.20) | 0.929 | 0.94 (0.78–1.14) | 0.537 |
| Access to health care | ||||
| Health insurance: insured vs. uninsured | 1.24 (1.03–1.49) | 0.025 | 1.12 (0.92–1.36) | 0.256 |
| Reported visiting a healthcare professional in the past year at each study visit*: yes vs no | 1.23 (1.09–1.37) | <0.001 | 1.19 (1.06–1.34) | 0.003 |
| Maintained no difficulty in obtaining health services*: yes vs no | 1.08 (0.98–1.20) | 0.112 | 1.00 (0.90–1.10) | 0.933 |
| Psychosocial factors | ||||
| Stress | ||||
| Low | Ref | Ref | ||
| Moderate | 0.93 (0.80–1.08) | 0.336 | 0.95 (0.81–1.10) | 0.481 |
| High | 0.95 (0.81–1.12) | 0.557 | 1.01 (0.86–1.20) | 0.858 |
| Depressive symptoms vs with no symptoms | 0.86 (0.73–1.01) | 0.063 | 0.91 (0.76–1.08) | 0.266 |
| Maintained ideal anger-in*: yes vs no | 1.04 (0.89–1.21) | 0.646 | 1.01 (0.86–1.18) | 0.945 |
| Maintained ideal anger-out*: yes vs no | 0.99 (0.84–1.17) | 0.941 | 1.01 (0.85–1.19) | 0.915 |
| Maintained low levels of daily discrimination*: yes vs no | 0.94 (0.80–1.11) | 0.448 | 0.95 (0.80–1.12) | 0.522 |
CI = confidence interval
Model 1 adjusted for age and sex.
Model 2 adjusted for all variables listed in the table.
*These factors were available at multiple study visits. For each study visit where these variables were available, we categorized participants as having ideal or non-ideal levels of each of these factors. We then categorized participants as maintaining ideal levels of each factor if participants were in the ideal category at all visits in which they were collected. S1 Table in S1 File lists these study variable definitions, visits at which they were collected, collection methods, and their classification for ideal level status.
Stress scale tertile cut points: low (0–31), moderate (32–80), high (81–482)
Persistent BP control and risk for incident CVD
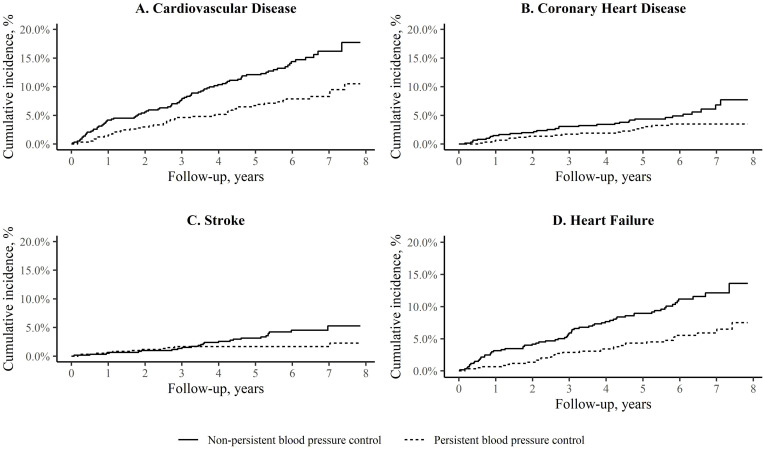
Among 1,151 participants without CVD at Visit 3, there were 127 incident CVD events over a median follow-up time of 6.0 years (25th-75th percentile: 5.2–6.9). The incidence rate for CVD was 13.7 (95% CI: 9.8–17.7) and 25.6 (95% CI: 20.1–31.2) per 1,000 person-years among participants with and without persistent BP control (Table 4). The cumulative incidence for CVD, CHD, stroke, and heart failure by persistent BP control status are presented in the Fig 1. After multivariable adjustment for age, sex, diabetes, current smoking, SBP, total cholesterol and HDL cholesterol, the HR for CVD comparing participants with versus without persistent BP control was 0.71 (95% CI: 0.46–1.10) (Table 4). The multivariable adjusted HRs for CHD, stroke, and heart failure were 0.68 (95% CI: 0.34–1.34), 0.65 (95% CI: 0.27–1.52), and 0.55 (95% CI: 0.33–0.90), respectively. Compared to those with controlled BP at Visit 3 but without persistent BP control, the adjusted HR for CVD, CHD, stroke, and heart failure for participants with persistent BP control was 0.70 (95% CI: 0.44–1.12), 0.77 (95% CI: 0.36–1.66), 0.64 (95% CI: 0.26–1.59), and 0.53 (95% CI: 0.32–0.90), respectively (S5 Table in S1 File).
Table 4. Incidence rates and adjusted hazard ratios for cardiovascular events among participants with versus without persistent blood pressure control.
| Hazard ratio (95% confidence interval) | p-value | Hazard ratio (95% confidence interval) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Events | Incidence rate* | Model 1 | Model 2 | ||||
| Cardiovascular disease | |||||||
| Non-persistent BP Control | 81 | 25.6 (20.1–31.2) | 1 (Ref) | 1 (Ref) | |||
| Persistent BP Control | 46 | 13.7 (9.8–17.7) | 0.64 (0.44–0.92) | 0.015 | 0.71 (0.46–1.10) | 0.125 | |
| Coronary heart disease | |||||||
| Non-persistent BP Control | 32 | 9.3 (6.1–12.6) | 1 (Ref) | 1 (Ref) | |||
| Persistent BP Control | 19 | 5.4 (2.9–7.8) | 0.65 (0.36–1.14) | 0.134 | 0.68 (0.34–1.34) | 0.263 | |
| Stroke | |||||||
| Non-persistent BP Control | 25 | 7.1 (4.3–9.9) | 1 (Ref) | 1 (Ref) | |||
| Persistent BP Control | 11 | 3.0 (1.2–4.8) | 0.52 (0.25–1.05) | 0.069 | 0.65 (0.27–1.52) | 0.319 | |
| Heart failure | |||||||
| Non-persistent BP Control | 64 | 19.0 (14.3–23.6) | 1 (Ref) | 1 (Ref) | |||
| Persistent BP Control | 33 | 9.2 (6.1–12.4) | 0.57 (0.37–0.87) | 0.010 | 0.55 (0.33–0.90) | 0.019 | |
BP = blood pressure.
*Incidence rate per 1,000 person-years (95% confidence intervals).
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, diabetes, current smoking, systolic blood pressure, total cholesterol, and high-density lipoprotein.
Fig 1. Cumulative incidence of cardiovascular disease events among adults taking antihypertensive medication.
Persistent blood pressure (BP) control was defined as having controlled BP (systolic BP <140 mm Hg and diastolic BP <90 mm Hg) across three Jackson Heart Study visits (Visit 1 from 2000–2004, Visit 2 from 2005–2008, and Visit 3 from 2009–2013). Cardiovascular disease was a composite of coronary heart disease, stroke, and heart failure.
Discussion
Less than half of JHS participants taking antihypertensive medication at baseline maintained persistent BP control over a median of 8 years of follow-up. Several participant characteristics were associated with a higher likelihood of persistent BP control including younger age, maintaining family income ≥$25,000 a year, and visiting a health professional in the year prior to each study visit. Persistent BP control was associated with a lower risk for heart failure.
Overall, 48.9% of participants had persistent BP control in the current study. Secondary analyses of BP lowering trials have reported a high proportion of adults do not have persistent BP control [10, 15–17]. A prior study using data from ALLHAT reported that overall, only 20.0% of participants had controlled BP at eight study visits, conducted over a 22 month period [10]. Analyses of data from the Valsartan Antihypertensive Long-term use Evaluation trial, ALLHAT, the International Verapamil SR-Trandolapril Study, and the Coronary Disease Trial Investigating Outcome with Nifedipine report the proportion of participants with controlled BP <140/90 mm Hg at ≥75% of study visits was 33.9%, 36.4%, 36.8%, and 51.5%, respectively [10, 15–17]. Randomized trials often enroll high risk participants who may have BP that is hard to control. However, together with data from the current community-based study, these data highlight the substantial treatment gap in care for adults taking antihypertensive medication. A number of evidence-based approaches are available to increase BP control. The US Surgeon General’s Call to Action to Control Hypertension recommends that clinicians connect patients with community resources to assist in controlling BP, address low rates of antihypertensive medication adherence by utilizing electronic prescribing and 90-day refills, and also counsel patients on how to use home BP monitors and transmit BP readings to a clinical care team [18]. These factors may aid in achieving persistent BP control.
Participants ≥65 years of age were less likely than their younger counterparts to have persistent BP control. BP is more difficult to control among older adults due to factors including arterial stiffening and vascular injury [19–21]. However, prior studies report that a high proportion of older adults can achieve guideline-recommended BP levels, but that BP may be undertreated in this population due to concerns about side effects [6, 19, 22]. Randomized controlled trials have found benefit and little harm from intensive BP treatment among older adults [6, 23, 24]. Among participants ≥75 years in the Systolic Blood Pressure Intervention Trial [23], lowering BP to a target of 120 mm Hg (i.e., intensive) compared with 140 mm Hg (i.e., standard) reduced the risk for CVD by 34% (HR 0.66; 95% CI 0.51–0.85) [23]. There was no difference in number of severe adverse events, including injurious falls, between the intensive and standard treatment groups. Therefore, the benefit of treating BP to guideline recommended levels among older adults outweigh the potential harm.
Having an income ≥$25,000 per year was associated with increased likelihood of persistent BP control. A prior study reported that adults receiving care at ALLHAT study sites in the lowest income quintile (median income $21,800) had a 52% lower odds of BP control at year six of the trial (odds ratio 0.48; 95% CI 0.37–0.63) compared to those in the highest income quintile (median income $49,600) [25]. Policy makers should consider addressing low income as a public health priority given consistent evidence of the association between low income and increased BP [25, 26]. Providing opportunities for people to move from high- to low-poverty neighborhoods may be a potential way to address the effects of low income on BP control as relocation has been associated with a 5 mm Hg decrease in SBP [27, 28]. Healthcare system level interventions may improve BP control for adults with low income [29, 30]. Adapting an evidence-based hypertension treatment protocol used by Kaiser Permanente health system [29] to 12 safety-net clinics resulted in an increase in BP control rates from 60% to 66% among black adults [30]. Also, using existing infrastructure in predominantly black neighborhoods can improve BP control [31]. For instance, a pharmacist-led BP intervention administered to black men in barbershops lowered SBP by 21.6 mm Hg compared to a control group [31].
Attending a visit to a health professional in the year prior to each study visit was associated with a 21% increased likelihood of persistent BP control. The association between visits to a health professional in the past year and rates of BP control have been reported in prior studies [7, 32]. The Affordable Care Act expanded healthcare coverage to millions of adults in the US and resulted in an increase in antihypertensive medication use in states that expanded Medicaid [33, 34]. Additional state and federal health insurance expansions should be considered in the future in order to increase persistent BP control and prevent CVD.
Persistent BP control in the current study was associated with a lower risk for heart failure. A study using ALLHAT data reported an increased risk for CHD, stroke, and heart failure among participants with BP control at <50% compared with 100% of visits [10]. The risk of heart failure in the current study was lower for participants with persistent BP control compared to those with controlled BP at Visit 3 but without persistent BP control. While prior studies have reported a stronger association between cumulative exposure to high BP and CVD risk compared to BP measurements obtained at a single time point [4], the current study demonstrates that assessing long-term BP control may also be more informative for assessing CVD risk than measuring BP control at a single time point.
Strengths of the current analysis include the use of a well-characterized, community-based cohort study with standardized BP measurements at multiple visits, and adjudicated CVD events. Data were available to investigate a large number of factors with persistent BP control. However, the current study has several potential limitations. Generalizability of results may be limited as the JHS only included African Americans from the Jackson, MS metropolitan area. Statistical power to detect an association between persistent BP control and CVD events was limited due to the low number of CVD events. Only six years of CVD event follow-up were available after Visit 3. Additionally, adults with CVD events prior to Visit 3 were excluded from the CVD events analysis, lowering the overall CVD risk of the sample. The number of CVD events in the sample limited our ability to examine the association of BP control at different time points with CVD outcomes (i.e., the association of BP control at Visit 1 only versus at Visit 1 and Visit 2 with CVD outcomes) in comparison to persistent BP control. We were able to utilize a time-varying approach for antihypertensive medication adherence. However, due to the complexity of prescribing patterns over time, we only adjusted for the number of antihypertensive medications at baseline. Finally, while we conducted multivariable adjusted analyses, the study design was observational. Therefore, we cannot rule out the potential of residual confounding.
In conclusion, less than half of the African-American participants in the current study taking antihypertensive medication had persistent BP control. Having persistent BP control has the potential to lower the risk of heart failure events. Efforts to facilitate persistent BP control including ensuring adults with hypertension have a usual source of care, utilizing more intensive antihypertensive therapy among older adults, and improving quality of care among adults with low income through guideline informed standardized care and community outreach should be a public health priority.
Supporting information
(DOCX)
Acknowledgments
The authors wish to thank the staffs and participants of the JHS.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Data Availability
Access to the Jackson Heart Study data can be requested at the following website https://biolincc.nhlbi.nih.gov/studies/jhs/. The authors did not have any special access privileges that others would not have.
Funding Statement
This study was supported by grants from the National Heart, Lung, and Blood Institute (grant number K24 HL125704) awarded to DS, and (grant number 1K01HL151974-01) awarded to GT. This study was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases(grant number 3R01DK108628-05S1) awarded to GT. The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (grant number HHSN268201800013I), as well as Tougaloo College (grant number HHSN268201800014I), the Mississippi State Department of Health (grant number HHSN268201800015I) and the University of Mississippi Medical Center (grant numbers HHSN268201800010I, HHSN268201800011I and HHSN268201800012I).
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. Dec 14 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Archives of internal medicine. Mar 8 1993;153(5):598–615. doi: 10.1001/archinte.153.5.598 [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. Jan 26 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 4.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. Feb 5 2014;311(5):490–497. doi: 10.1001/jama.2013.285122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Q, Dillon CF, Burt VL, Gillum RF. Association of hypertension treatment and control with all-cause and cardiovascular disease mortality among US adults with hypertension. Am J Hypertens. Jan 2010;23(1):38–45. doi: 10.1038/ajh.2009.191 [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. Oct 23 2018;138(17):e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Hardy ST, Fine LJ, et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. Sep 9 2020. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. Jan 2015;65(1):54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. Mar 1995;25(3):305–313. doi: 10.1161/01.hyp.25.3.305 [DOI] [PubMed] [Google Scholar]
- 10.Bowling CB, Davis BR, Luciano A, et al. Sustained blood pressure control and coronary heart disease, stroke, heart failure, and mortality: An observational analysis of ALLHAT. J Clin Hypertens (Greenwich). Apr 2019;21(4):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor HA Jr., Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. Autumn 2005;15(4 Suppl 6):S6–4-17. [PubMed] [Google Scholar]
- 12.Seals SR, Colantonio LD, Tingle JV, et al. Calibration of blood pressure measurements in the Jackson Heart Study. Blood Press Monit. Jun 2019;24(3):130–136. doi: 10.1097/MBP.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keku E, Rosamond W, Taylor HA, Jr., et al. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethn Dis. Autumn 2005;15(4 Suppl 6):S6-62–70. [PubMed] [Google Scholar]
- 14.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. Jun 24 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Kjeldsen SE, Zappe DH, et al. Cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the VALUE trial. Eur Heart J. Mar 21 2016;37(12):955–964. doi: 10.1093/eurheartj/ehv633 [DOI] [PubMed] [Google Scholar]
- 16.Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the International Verapamil SR-Trandolapril Study. Hypertension. Aug 2007;50(2):299–305. doi: 10.1161/HYPERTENSIONAHA.107.090290 [DOI] [PubMed] [Google Scholar]
- 17.Meredith PA, Lloyd SM, Ford I, Elliott HL. Importance of sustained and "tight" blood pressure control in patients with high cardiovascular risk. Blood Press. 2016;25(2):74–82. doi: 10.3109/08037051.2015.1127528 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Control Hypertension. U.S. Department of Health and Human Services, Office of the Surgeon General. 2020. [Google Scholar]
- 19.Borzecki AM, Glickman ME, Kader B, Berlowitz DR. The effect of age on hypertension control and management. Am J Hypertens. May 2006;19(5):520–527. doi: 10.1016/j.amjhyper.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 20.Williamson JD. Blood Pressure Control in Older Adults: Toward a More Personalized Medicine Approach. J Am Geriatr Soc. Sep 18 2020. [DOI] [PubMed] [Google Scholar]
- 21.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. Mar 29 2019;124(7):1045–1060. doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 22.Currie G, Delles C. Blood pressure targets in the elderly. J Hypertens. Feb 2018;36(2):234–236. doi: 10.1097/HJH.0000000000001576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/ = 75 Years: A Randomized Clinical Trial. JAMA. Jun 28 2016;315(24):2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocco MV, Sink KM, Lovato LC, et al. Effects of Intensive Blood Pressure Treatment on Acute Kidney Injury Events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. Mar 2018;71(3):352–361. doi: 10.1053/j.ajkd.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahu A, Herrin J, Dhruva SS, et al. Disparities in Socioeconomic Context and Association With Blood Pressure Control and Cardiovascular Outcomes in ALLHAT. J Am Heart Assoc. Aug 6 2019;8(15):e012277. doi: 10.1161/JAHA.119.012277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anstey DE, Christian J, Shimbo D. Income Inequality and Hypertension Control. J Am Heart Assoc. Aug 6 2019;8(15):e013636. doi: 10.1161/JAHA.119.013636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chetty R, Hendren N, Katz LF. The Effects of Exposure to Better Neighborhoods on Children: New Evidence from the Moving to Opportunity Experiment. American Economic Review. 2016;106(4):855–902. doi: 10.1257/aer.20150572 [DOI] [PubMed] [Google Scholar]
- 28.Kershaw KN, Robinson WR, Gordon-Larsen P, et al. Association of Changes in Neighborhood-Level Racial Residential Segregation With Changes in Blood Pressure Among Black Adults: The CARDIA Study. JAMA Intern Med. Jul 01 2017;177(7):996–1002. doi: 10.1001/jamainternmed.2017.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim JJ, Handler J, Jacobsen SJ, Kanter MH. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. May 2014;30(5):544–552. doi: 10.1016/j.cjca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 30.Fontil V, Gupta R, Moise N, et al. Adapting and Evaluating a Health System Intervention From Kaiser Permanente to Improve Hypertension Management and Control in a Large Network of Safety-Net Clinics. Circ Cardiovasc Qual Outcomes. Jul 2018;11(7):e004386. doi: 10.1161/CIRCOUTCOMES.117.004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor RG, Lynch K, Li N, et al. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med. Apr 5 2018;378(14):1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinkler JM, Sugar CA, Escarce JJ, Ong MK, Mangione CM. Does Age Matter? Association Between Usual Source of Care and Hypertension Control in the US Population: Data From NHANES 2007–2012. Am J Hypertens. Aug 2016;29(8):934–940. doi: 10.1093/ajh/hpw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and Ethnic Disparities in Health Care Access and Utilization Under the Affordable Care Act. Med Care. Feb 2016;54(2):140–146. doi: 10.1097/MLR.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumarsono A, Lalani H, Segar MW, et al. Association of Medicaid Expansion with Rates of Utilization of Cardiovascular Therapies Among Medicaid Beneficiaries Between 2011 and 2018. Circ Cardiovasc Qual Outcomes. Nov 9 2020. doi: 10.1161/CIRCOUTCOMES.120.007492 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Access to the Jackson Heart Study data can be requested at the following website https://biolincc.nhlbi.nih.gov/studies/jhs/. The authors did not have any special access privileges that others would not have.