Abstract
A random five-codon gene library was used to isolate minigenes whose expression causes cell growth arrest. Eight different deleterious minigenes were isolated, five of which had in-frame stop codons; the predicted expressed peptides ranged in size from two to five amino acids. Mutational analysis demonstrated that translation of the inhibitory minigenes is essential for growth arrest. Pulse-labeling experiments showed that expression of at least some of the selected minigenes results in inhibition of cellular protein synthesis. Expression of the deleterious minigenes in cells deficient in peptidyl-tRNA hydrolase causes accumulation of families of peptidyl-tRNAs corresponding to the last minigene codon; the inhibitory action of minigene expression could be suppressed by overexpression of the tRNA corresponding to the last sense codon in the minigene. Experimental data are compatible with the model that the deleterious effect of minigene expression is mediated by depletion of corresponding pools of free tRNAs.
Short open reading frames (ORFs) are abundant in genomes of all the studied organisms. Some mini-ORFs, especially those found in transcribed RNAs (mRNA, rRNA, or others), may be potentially translated into short, functionally active peptides. For example, it has been shown recently that translation of a pentapeptide minigene present in 23S rRNA of Escherichia coli renders cells resistant to the ribosome-targeted antibiotic, erythromycin; this resistance was apparently caused by specific interaction of the translated short peptide with the ribosome (34, 36).
Several documented examples suggest that expression of specific short peptides may be detrimental for the cell. In these cases, again, the physiological effect of expression of short peptides is mediated by protein synthesis machinery. It has been shown that translation of naturally occurring two-codon minigenes (bar) in bacteriophage lambda is deleterious in cells with a low level of peptidyl-tRNA hydrolase (PTH) activity (10, 13). It has been proposed that lambda minigene translation in pth mutant cells results in accumulation of peptidyl-tRNA corresponding to the last minigene codon; the resulting tRNA starvation abolishes translation of the cellular genes (14, 15, 31).
Understanding the peculiarities of translation of short ORFs may provide important insights into mechanisms of protein synthesis and gene regulation. In order to identify ORFs whose expression may be deleterious to the cell and, in particular, inhibit translation of cellular proteins, we used a random five-codon minigene expression library. Library screening revealed that expression of certain short ORFs may dramatically inhibit cell growth due to interference with protein synthesis. Results of analysis of several selected library clones are compatible with the model that the deleterious effect of minigene expression is mediated by accumulation of specific peptidyl-tRNAs and depletion of corresponding pools of free tRNAs.
MATERIALS AND METHODS
Strains and materials.
Inhibitory peptide minigenes were selected and propagated in E. coli JM109. In some experiments, E. coli P90C (CSH142) [F− ara Δ(gpt-lac)5] (29) or its derivative P90C pth(rap), which contains a mutant allele for peptidyl-tRNA hydrolase, was used. The rap derivative was constructed by P1 cotransduction of pth(rap) and zch::Tn10 (12). Strains were propagated in Luria-Bertani (LB) medium containing antibiotics when necessary. dl-cycloserine was from SIGMA, restriction enzymes were from Promega or MBI Fermentas, and the chemicals were from Fisher Scientific. [35S]methionine (specific activity, 28 Ci/mmol), [3H]uridine (specific activity, 3.4 Ci/mmol), [3H]lysine (specific activity, 82 Ci/mmol) [3H]isoleucine (specific activity, 113 Ci/mmol), [3H]-glutamic acid (specific activity, 250 Ci/mmol), and [14C]serine (specific activity, 162 mCi/mmol) were from Amersham.
Selection of the deleterious minigenes.
Construction of the pPOT1AE vector and preparation of the random minigene library were described previously (34, 35). For selection of plasmids carrying deleterious minigenes, E. coli cells (strain JM109) were transformed with the random pentapeptide library plasmids and grown in 2.5 ml of LB medium for 1 h (at this and all subsequent steps, liquid cultures were grown at 37° with constant shaking at 250 rpm). Ampicillin was added to a final concentration of 100 μg/ml, and transformed cells were grown in liquid culture for 7 h at 37° and stored overnight at +4°C. The next day cells were pelleted, resuspended in 2.5 ml of fresh medium containing 100 μg of ampicillin/ml, and grown for 1 h. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a 2 mM final concentration, and incubation was continued for 1 h. This was followed by the addition of cycloserine to a final concentration of 12 mM, and the culture was grown for 3 more hours. At the end of incubation with cycloserine, cells were pelleted, resuspended in 15 ml of fresh LB medium containing 100 μg of ampicillin/ml, stored overnight at 4°C, and then grown several hours at 37°C until culture density reached an A650 of 0.4. Plasmid was isolated from a 3-ml culture and used to transform fresh E. coli cells, strain JM109. The selection cycle was repeated four more times, after which cells were plated onto agar plates containing 100 μg of ampicillin/ml; individual colonies were isolated and replica plated onto LB-agar plates containing 100 μg of ampicillin/ml with or without 2 mM IPTG. Plasmids were isolated from the clones that failed to grow in the presence of IPTG and transformed into fresh JM109 cells. Cotransference of IPTG sensitivity with the plasmid was tested by replica plating transformants on the plates with or without IPTG. Plasmids containing peptide minigenes conferring IPTG sensitivity were isolated and sequenced.
Pulse-labeling of RNA and proteins.
Cells harboring the pMR plasmid, carrying the MR peptide minigene in the pPOT1AE vector, or the control, the empty pPOT1AE vector, were grown in morpholinepropanesulfonic acid (MOPS) medium (30). When optical density reached an A600 = 0.08, IPTG was added to a final concentration of 2 mM. Two 80-μl aliquots were taken at defined time points from each culture for pulse labeling of RNA and proteins. For protein labeling, 2 μl of [35S]methionine (17 μmol, 28 Ci/mmol) was added to the cell culture, and the mixture was incubated 8 min at 37°C. After the addition of 80 μl of 20% trichloroacetic acid, samples were incubated for 25 min at 100°C and filtered through G4 glass fiber filters (Fisher).
For RNA labeling, 5 μl of [3H]uridine (27 μmol, 3.4 Ci/mmol) was added to an 80-μl culture, and the mixture was incubated 4 min at 37°C. After the addition of 80 μl of 20% trichloracetic acid, the sample was kept on ice for 30 min and then filtered through G4 glass fiber filters. Filters were dried and counted.
Mutagenesis of MR minigene.
The MR peptide minigene was randomly mutagenized by using PCR-mediated mutagenesis. The PCR primers CCW and CC (GCCATCGGAAGCTGTGG and CTCCGCTATCGCTACGTG, respectively), complementary to pPOT1AE sequences upstream from the Ptac promoter and downstream from the Ttrp terminator (Fig. 1), were used to PCR amplify the minigene in the presence of Mn2+ (3). The mutagenized PCR products were cut with restriction enzymes AflII and EcoRI and cloned back into the pPOT1AE vector cut with the same enzymes. The recombinant plasmids were transformed into E. coli JM109 cells, and clones able to grow on agar plates containing 100 μg of ampicillin/ml and 2 mM IPTG were selected. The phenotypes were confirmed by retransforming the plasmids isolated from individual colonies into fresh cells.
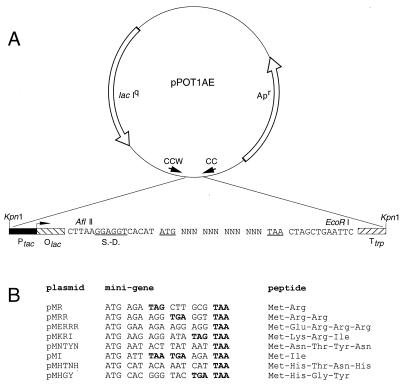
FIG. 1.
Inhibitory minigenes selected from the random minigene library. (A) Physical map of the pPOT1AE vector with the five-codon minigene library. tac promoter (Ptac), lac operator (Olac), and trp terminator (Ttrp) are shown as well as AflII and EcoRI sites used for the library construction (35). The Shine-Dalgarno region (S.-D.) and initiator and terminator codons of the library minigene sense strands are underlined. Location and direction of sequencing primers, CCW and CC, are indicated. (B) Inhibitory minigenes selected from the library and the encoded peptides. The plasmids in the selected clones were named according to the single-letter amino acid sequence of the peptides encoded in the minigenes. The in-frame stop codons are shown in boldface.
Insertion of four extra nucleotides into the EcoRI or AflII sites flanking the peptide minigene was achieved by cutting the plasmid at corresponding sites, filling up protruding ends, and religation. To insert longer DNA sequences into the EcoRI or AflII sites, an excess of a 10-bp NheI linker was present during the religation step.
Recloning MR peptide minigenes into the pACYC184 vector.
The MR minigene with the Ptac promoter and Ttrp terminator was PCR amplified from the pPOT1AE construct with a pair of primers, GGAAGCTTTTGACAATTAATC and CTAAGCTTCCAAAAAAAAGCCCG, which contained HindIII sites in their sequence. The PCR product was cut with the HindIII restriction enzyme and cloned into the HindIII site of the pACYC184 vector, producing the pACMR plasmid.
Genomic library screening.
The genomic library was constructed in the modified pTrc99A vector (Pharmacia), in which the lacIq gene was disrupted by cutting the vector at the unique BstEII site located in the lacIq gene, filling protruding DNA ends, and religating the vector. E. coli genomic DNA was partially hydrolyzed with Sau3A and ligated into the BamHI site of the modified pTrc99A vector. The ligation mixture was transformed into ultracompetent XL-1 Blue cells (Stratagene), producing a library of approximately 30,000 clones. The plasmid library was amplified and introduced into E. coli JM109 cells containing the pACMR plasmid.
Transformed cells were selected on agar plates containing 100 μg of ampicillin/ml (required for maintenance of the library plasmids), 25 μg of chloramphenicol/ml (required for maintenance of pACMR), and 2 mM IPTG. Plasmids were isolated from individual clones and library plasmids were segregated from pACMR by transforming fresh cells and selecting Ampr-Cams transformants. A clone with a library plasmid containing a 2.8-kb insert of genomic DNA that allowed cell growth in spite of expression of the MR peptide minigene from pACMR was chosen for further analysis.
The insert was reduced to 400 bp without the loss of resistance to the MR peptide minigene. Sequencing of the 400-bp insert revealed the presence of a tRNA4Arg gene expressed from its own promoter. To verify that overexpression of tRNA4Arg renders cells tolerant to expression of the MR minigene, the tRNA4Arg gene with its promoter was PCR amplified from E. coli genomic DNA with primers GGGAATTCACTTGTTCAGCAAC and CTAAGCTTGAGGATATAAAGAAGGC. The PCR product was cut with HindIII and EcoRI and inserted into the pUC19 vector cut with the same enzymes. The resulting plasmid was introduced into E. coli cells containing pACMR. Transformants were selected on agar plates containing 100 μg of ampicillin/ml and 25 μg of chloramphenicol/ml, and their ability to grow in the presence of 2 mM IPTG was tested by replica plating.
Rescue of P90C pth(rap) cells expressing inhibitory minigenes by tRNA overexpression.
To study the effect of tRNA overexpression on the growth of cells expressing inhibitory minigenes, P90C pth(rap) cells were cotransformed with the minigene containing pPOTAE1 constructs and compatible plasmids overexpressing either tRNAArg (pDC952) or tRNAIle (pDPT489) (6). Cells were grown in liquid cultures, and cell growth was monitored spectrophotometrically after induction of minigene expression by IPTG.
Estimation of amounts of peptidyl-tRNAs in the cell.
Cultures of the P90C pth(rap) strain transformed with pPOT1AE constructs carrying inhibitory peptide minigenes were grown at 37°C to an A600 = 0.4, at which point cultures were split between two flasks and IPTG was added to one flask to a final concentration of 1 mM. Cells were grown for 30 min at 37°C with constant shaking and were then harvested. The amounts of individual peptidyl-tRNAs in the cell were determined by the amino acyl-accepting activity in the cellular tRNA pool unmasked by treatment with PTH as described previously (14, 23, 24).
RESULTS
Selection of inhibitory peptide minigenes.
Construction of the five codon minigene library was described previously (Fig. 1A) (34, 35). Due to the random occurrence of in-frame stop codons, the library contains ORFs coding not only for pentapeptides but for shorter peptides as well. Minigenes are expressed under the control of an IPTG-inducible Ptac promoter.
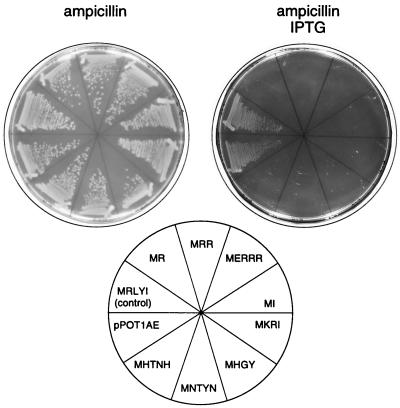
Negative selection with cycloserine was used to isolate minigenes whose expression inhibits cell growth. Cycloserine is a bactericidal antibiotic which kills fast-growing cells; therefore, cells expressing deleterious minigenes would preferentially survive in the presence of the drug. E. coli cells were transformed with the total plasmid library, and minigene expression was induced by IPTG which was followed by addition of cycloserine after 1 h. Incubation with cycloserine resulted in killing about 99% of bacteria in the culture, as determined by colony-forming ability. To avoid selection of chromosomal mutations, total plasmid was isolated from cells that survived cycloserine treatment and transformed into fresh cells. After four rounds of negative selection, individual clones were replica plated on plates with and without IPTG and eight clones unable to grow on IPTG-containing plates were isolated (Fig. 2). The inability of cells to grow in the presence of IPTG was cotransferrable with the plasmid; furthermore, when minigenes were moved from the originally selected plasmids into a fresh pPOTAE1 vector, newly constructed plasmids also caused cell growth arrest in the presence of IPTG. We concluded that eight selected clones contained plasmid-borne minigenes whose expression abolished cell growth.
FIG. 2.
Effect of IPTG-induced minigene expression on colony formation. pPOT1AE empty vector or an unselected, randomly picked clone encoding the peptide MRLYI were used as controls.
Sequencing of minigenes from isolated plasmids revealed that five of eight minigenes contained in-frame stop codons within the randomized minigene sequence (Fig. 1A and B). Since the frequency of occurrence of in-frame stop codons in unselected library clones is significantly lower (35), it appears that deleterious ORFs are preferentially found among smaller minigenes.
Comparison of minigene nucleotide sequences or amino acid sequences of the encoded peptides did not reveal any clear sequence consensus which would unveil a possible mechanism of inhibition of cell growth. Although certain prevalence of positively charged amino acids can be noted, its significance remains unclear.
In order to gain insight into the mechanism of cell growth inhibition, a series of experiments were performed. The pMR clone was chosen as a model for genetic and biochemical studies. This MR minigene was attractive because it was one of the smallest among the selected deleterious minigenes.
Translation of the peptide minigene is required for cell growth arrest.
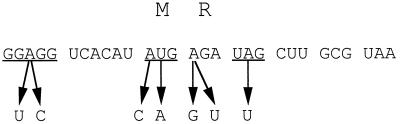
To find out which sequences of the MR minigene are important for the deleterious effect, mutations were introduced randomly in the peptide minigene and its flanking region by PCR mutagenesis, and clones that regained the ability to grow in the presence of IPTG were selected (Fig. 3). All the found mutations were located in the regions important for translation of the minigene. Two of the mutations reduced complementarity of the Shine-Dalgarno sequence to the 3′ end of 16S rRNA required for efficient initiation of translation (33); two other mutations eliminated the initiator AUG codon of the peptide ORF. One mutation converted the second codon of the minigene into a nonsense codon; the smallest possible minigene (AUG-UGA) was not inhibitory for the cell. Similarly, a missense mutation changing the second amino acid of the encoded Met-Arg dipeptide to Gly eliminated the inhibitory effect of minigene translation. Yet another mutation converted the UAG stop codon of the MR minigene into the Leu codon; however, in contrast to translation of the MR minigene, translation of the resulting MRLLA minigene was not inhibitory to cell growth. No mutations were found downstream from the minigene stop codon or in the spacer separating the Shine-Dalgarno region and initiator AUG codon. Thus, the results of mutational analysis were compatible with the idea that the size and the sequence of the minigene were essential for the inhibition of cell growth. Furthermore, it is minigene translation, rather than a mere presence of mRNA, that is required for cell growth inhibition.
FIG. 3.
Mutations in the MR minigenes that alleviate its inhibitory action. The encoded amino acids are shown above the corresponding minigene mRNA codons. The Shine-Dalgarno region and initiator and terminator codons of the MR minigene are underlined.
Expression of peptide minigene inhibits protein synthesis in the cell.
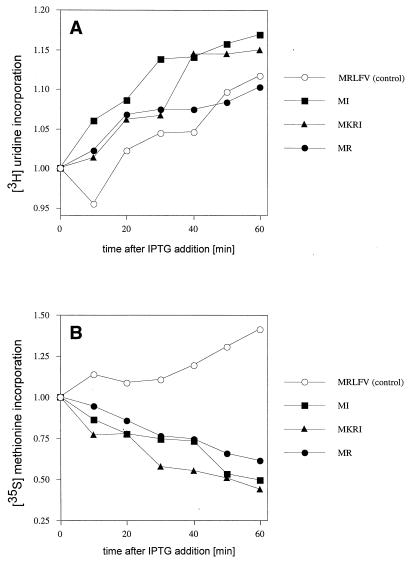
To identify which cellular process may be influenced by the minigene expression, the effect of expression of the MR minigene on the rate of RNA or protein synthesis was determined by in vivo pulse-labeling with [3H]uridine or [35S]methionine, respectively (Fig. 4). While RNA synthesis continued after induction of minigene expression (as can be judged by incorporation of uridine in trichloracetic acid-insoluble material), the rate of protein synthesis (methionine incorporation into the polypeptide fraction) was significantly reduced in cells expressing the MR minigene. Similar results were obtained for the other two studied minigenes, MKRI and MI.
FIG. 4.
Effect of minigene expression on incorporation rates of [3H]uridine (A) and [35S]methionine (B) after addition of IPTG. Data are shown for cells transformed with a control plasmid encoding pentapeptide MRLFV that does not inhibit cell growth (35) or with plasmids pMR, pMKRI, or pMI.
tRNA4Arg rescues cells expressing the MR minigene.
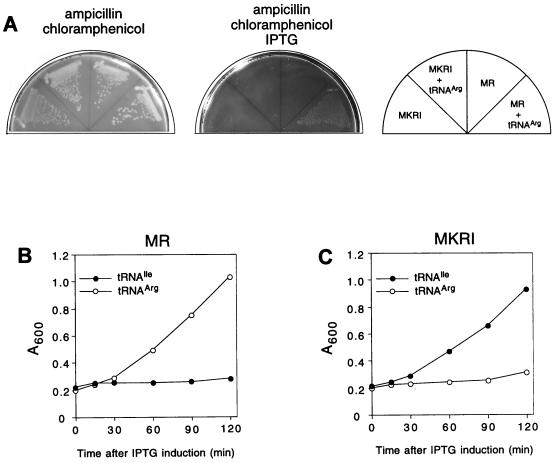
In order to understand the mechanism of protein synthesis inhibition resulting from minigene expression, we searched for a chromosomal gene whose overexpression could suppress the inhibitory action of the MR peptide. The MR minigene, with the Ptac promoter and Ttrp terminator (Fig. 1A), was transferred from the pPOT1AE into the pACYC184 vector to produce plasmid pACMR. Expression of the MR minigene even from a low-copy-number pACMR was sufficient to inhibit cell growth; cells carrying pACMR did not form colonies in the presence of IPTG. An E. coli genomic library was constructed in the modified pTrc99A vector (1) (see Materials and Methods). The library was introduced into cells carrying pACMR, and a clone which gained the ability to grow in the presence of IPTG was selected. The size of the cloned genomic DNA segment expressed in the selected clone was originally 2.8 kbp. However, it could be reduced to 400 bp without loss of its ability to suppress the inhibitory effect of the MR peptide minigene. Sequencing of the 400-bp insert showed the presence of the tRNA4Arg gene, suggesting that overproduction of this tRNA rendered cells tolerant to the minigene expression. To verify this conclusion, the tRNA4Arg gene with its own promoter was PCR amplified from the E. coli chromosome and inserted into pUC19 plasmid, and the resulting construct was introduced into cells carrying pACMR. Overproduction of tRNA4Arg in cells expressing the MR minigene restored their ability to form colonies on the agar plate. However, cells expressing the MKRI minigene were not rescued by tRNA4Arg (Fig. 5).
FIG. 5.
Effect of overexpression of tRNA cognate to the last sense codon of the minigene on cell growth. Growth of JM109 cells (wild-type PTH) transformed either with plasmids expressing MI or MKRI minigenes or double transformed with the same minigene plasmids plus a plasmid expressing the tRNA4Arg gene (A). Growth in liquid culture of P90C pth(rap) cells cotransformed with pMR (B) or pMKRI (C) plasmids and with the plasmids expressing either tRNA2Ile or tRNA4Arg.
tRNA4Arg decodes the last AGA codon of the MR peptide minigene. Therefore, we tested if overproduction of tRNA2Ile, cognate to the last codon of the MKRI ORF, could rescue cells expressing a MKRI minigene. As shown in Fig. 5C, expression of tRNA2Ile restored the growth of cells expressing the MKRI minigene but not that of cells expressing the MR minigene. Furthermore, overexpression of tRNALys, which corresponded to (AAG) codon of the MKRI ORF, or of tRNA4Arg did not improve the survival of cells expressing the MKRI peptide minigene (data not shown). Thus, it is likely that overexpression of the tRNA cognate to the last minigene codon suppresses the deleterious effect of minigene expression.
Cells expressing inhibitory minigenes accumulate peptidyl-tRNA corresponding to the last codon of the peptide ORF.
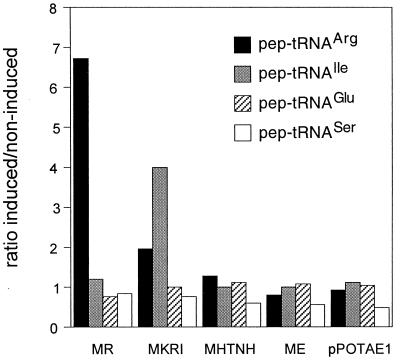
Previously, it was shown that expression of the bacteriophage lambda barI minigene, which is detrimental for PTH-deficient pth(rap) cells, is accompanied by accumulation of peptidyl-tRNA cognate to the last codon of barI (14, 15). Therefore, we studied the effect of expression of minigenes selected from the random library on the accumulation of peptidyl-tRNA in pth(rap) cells (Fig. 6). Expression of the MR peptide minigene resulted in a marked increase of the amount of peptidyl-tRNAArg, while expression of MKRI minigene caused preferential accumulation of peptidyl-tRNAIle. In contrast, expression of neither the inhibitory minigene MHTNH nor the harmless minigene ME led to accumulation of these peptidyl-tRNAs or of peptidyl-tRNAGlu (not shown). This result demonstrated that the inhibitory action of short peptide minigenes in pth(rap) cells correlates with accumulation of specific isoaccepting families of peptidyl-tRNAs.
FIG. 6.
Accumulation of families of isoaccepting peptidyl-tRNAs in cells transformed with inhibitory minigene MR, MKRI, or MHTNH, with noninhibitory minigene ME, or with the empty pPOT1AE vector. The amount of peptidyl-tRNA was determined as described in reference 14. The standard deviations of the measurements were around 20%.
DISCUSSION
From more than 50 clones randomly picked from the unselected pentapeptide minigene library none showed IPTG-dependent growth arrest (data not shown). Only eight inhibitory minigenes were isolated after four rounds of negative selection. Therefore, though the selection we used was nonexhaustive, we suspect that the number of inhibitory minigenes in the library of ca. 160,000 peptide sequences is rather small (35).
The mode of action of only one minigene, MR, was studied in detail. Therefore, strictly speaking, our conclusions regarding the mechanism of cell growth inhibition caused by minigene expression refer specifically to this minigene. However, several other selected minigenes, when studied in individual tests, showed properties similar to those of the MR minigene (see Fig. 4 to 6). Therefore, we suspect that the mode of inhibitory action of at least several selected minigenes resembles that of MR.
The following findings provide insights into mechanism of inhibitory action of the MR minigene: (i) expression of the minigene results in inhibition of protein synthesis, (ii) the inhibitory effect of minigene translation can be compensated by overexpression of the tRNA corresponding to the last minigene codon, and (iii) PTH-deficient cells expressing inhibitory minigenes accumulate peptidyl-tRNA corresponding to the last codon of the minigene. The observed characteristics of the MR minigene selected from the random library closely resemble those found for naturally occurring inhibitory minigenes of bacteriophage lambda (14, 15). The lambda minigenes (barI and barII [wild type] and the mutant, barA702) are extremely small (AUG AUA UAA, AUG AUA UGA, and AUG AAA UAA, respectively), coding for dipeptides Met-Ile (barI and barII) and Met-Lys (barA702) (14, 31). Translation of bacteriophage lambda minigenes results in inhibition of protein synthesis in PTH-deficient cells which correlates with accumulation of peptidyl-tRNA cognate to the last codon of the minigene (32). Furthermore, similar to minigenes selected from the random library, the deleterious effect of barI or barA702 on translation can be compensated by excess of tRNA cognate to the last minigene codon (14). It has been proposed that bar inhibitory action is mediated by accumulation of peptidyl-tRNA and resulting depletion of the uncharged tRNA pool in the cell (15). By analogy, we suggest that, at least in pth(rap) cells, a similar mode of cell growth inhibition applies to the MR, and maybe other minigenes, selected from the random library, namely, that minigene expression results in an accumulation of peptidyl-tRNA corresponding to the last codon of the minigene and resulting starvation for corresponding tRNA.
Peptidyl-tRNA accumulation is toxic to the cell because it depletes the pool of uncharged tRNA required for protein biosynthesis (2, 20, 25). In normal cells, free peptidyl-tRNAs are usually present in a low amount due to occasional drop-off from the translating ribosome (4, 7, 23). An essential enzyme, PTH, is responsible for peptidyl-tRNA recycling (23); PTH depletion results in cell death, most probably because of starvation for tRNALys sequestered in the form of peptidyl-tRNA (17). Several factors have been identified that may affect the rate of peptidyl-tRNA drop-off, including effect of antibiotics (26–28), codon-anticodon mispairing (4, 11), and presence of consecutive rare codons at the beginning of the genes (9). Involvement of ribosomal termination (16) and initiation factors (19) in peptidyl-tRNA dissociation has been demonstrated. However, the details of peptidyl-tRNA drop-off remain largely unknown.
The effect of lambda bar minigenes is most readily revealed in PTH-deficient cells. Similarly, all the minigenes selected from the library were also deleterious for pth(rap) cells (Fig. 5 and data not shown). At the same time, library minigenes were selected in (and accordingly, efficiently inhibited the growth of) the cells even with the normal level of PTH activity (Fig. 2). It remains unknown whether peptidyl-tRNA can accumulate to a significant level in pth+ cells, where theoretically it should be efficiently recycled. However, the fact that the growth of pth+ cells expressing MR minigenes was restored by overexpression of tRNA4Arg cognate to the last minigene codon is compatible with this possibility.
The last sense codon of the MR minigene is decoded by a scarce tRNA isoacceptor (8, 18, 37). The same is true for several other selected minigenes (MRR, MERRR, and MKRI). The trapping of even a small amount of a rare tRNA in the form of peptidyl-tRNA should rapidly exhaust its pool and would interfere with translation of cellular genes containing corresponding codons. Therefore, the presence of rare codons can potentially contribute to the deleterious effect of the selected minigenes on cellular protein synthesis. On the other hand, other selected minigenes (for example, MI) do not abide to this rule and are decoded by major tRNA isoacceptors. Thus, the importance of rare codons in deleterious minigenes remains unclear. Another factor that theoretically can contribute to the drop-off rate of peptidyl-tRNA is the size of the nascent peptide. It has been proposed that the translation complex is less stable during early rounds of translation (5), and the in vitro efficiency of tRNA drop-off decreases with the increasing length of the polypeptide (19). Based on the in vitro data, Karimi et al. (19) hypothesized that starting with the length of 4 to 6 amino acid residues, the growing polypeptide enters the nascent peptide tunnel which would preclude peptidyl-tRNA drop-off. In accordance with these observations, both the selected MR minigene and the lambda bar minigenes are extremely small. Furthermore, five of eight selected minigenes encoded peptides shorter than 5 amino acids long (Fig. 1), which is again compatible with peptidyl-tRNA drop-off as the mechanism of cell growth inhibition by selected minigenes.
An interesting question is whether an inhibitory effect of the selected and natural minigenes depends on the nature of the encoded peptide. Several experimental observations indicate that interaction of the nascent peptide with the ribosome may affect properties of the translation complex (21, 22, 36). Interestingly, barI and barII of lambda phage and the selected minigene MI encode the same peptide (Met-Ile), whereas the sequences of corresponding minigenes, including regulatory regions and an Ile codon are different, suggesting that the inhibitory action of minigene may be mediated by the peptide. It is conceivable that peptidyl-tRNAs with specific nascent peptides may be retained less efficiently on the ribosome.
Though short ORFs are abundant in all the sequenced genomes, the biological significance of minigenes has been essentially ignored. This is partly explained by difficulties in the detection of short peptides translated from small ORFs and resulting uncertainty of which small ORFs are actually expressed in the cell. The results of our experiments with the minigene library show that expression of some minigenes may have a profound effect on cell physiology (references 35 and 36 and this paper). Thus, we believe that studies of natural short ORFs will reveal functionally active cellular minigenes.
ACKNOWLEDGMENTS
We thank R. W. Simons for providing the P90C strain, R. F. Méndez-Canseco for construction of the pth(rap) derivative of P90C, B. J. Del Tito for donating the tRNA-expressing plasmids, and J. Menninger for fruitful discussions.
This work was supported by NSF grant MCB 9420768, the UIC Innovation Development Fund (to A.S.M.), and by grants from the Howard Hughes Medical Institute and the Consejo Nacional de Ciencia y Technología (CONACyT, México) (to G.G.). J.V.H. was a recipient of a loan-fellowship from CONACyT.
REFERENCES
- 1.Amann E, Ochs B, Abel K J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Atherly A G, Menninger J R. Mutant E. coli strain with temperature sensitive peptidyl-transfer RNA hydrolase. Nat New Biol. 1972;240:245–246. doi: 10.1038/newbio240245a0. [DOI] [PubMed] [Google Scholar]
- 3.Cadwell R C, Joyce G F. Randomization of genes by PCR mutagenesis. PCR Methods Applic. 1992;2:28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A B, Menninger J R. Tests of the ribosomal editing hypothesis: amino acid starvation differentially enhances the dissociation of peptidyl-tRNA from the ribosome. J Mol Biol. 1979;134:621–637. doi: 10.1016/0022-2836(79)90370-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen G-F T, Inouye M. Suppression of the negative effect of minor arginine codons on gene expression; preferential usage of minor codons within the first 25 codons of the Escherichia coli genes. Nucleic Acids Res. 1990;18:1465–1473. doi: 10.1093/nar/18.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Tito B J, Jr, Ward J M, Hodgson J, Gershater C J, Edwards H, Wysocki L A, Watson F A, Sathe G, Kane J F. Effects of a minor isoleucyl tRNA on heterologous protein translation in Escherichia coli. J Bacteriol. 1995;177:7086–7091. doi: 10.1128/jb.177.24.7086-7091.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Kurland C G. Ribosome mutants with altered accuracy translate with reduced processivity. J Mol Biol. 1995;248:551–561. doi: 10.1006/jmbi.1995.0242. [DOI] [PubMed] [Google Scholar]
- 8.Dong H J, Nilsson L, Kurland C G. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol. 1996;260:649–663. doi: 10.1006/jmbi.1996.0428. [DOI] [PubMed] [Google Scholar]
- 9.Gao W W, Tyagi S, Kramer F R, Goldman E. Messenger RNA release from ribosomes during 5′-translational blockage by consecutive low-usage arginine but not leucine codons in Escherichia coli. Mol Microbiol. 1997;25:707–716. doi: 10.1046/j.1365-2958.1997.5081871.x. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Villegas M R, De La Vega F M, Galindo J M, Segura M, Buckingham R H, Guarneros G. Peptidyl-tRNA hydrolase is involved in inhibition of host protein synthesis. EMBO J. 1991;10:3549–3555. doi: 10.1002/j.1460-2075.1991.tb04919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gast F U, Peters F, Pingoud A. The role of translocation in ribosomal accuracy. Translocation rates for cognate and noncognate aminoacyl- and peptidyl-tRNAs on Escherichia coli ribosomes. J Biol Chem. 1987;262:11920–11926. [PubMed] [Google Scholar]
- 12.Guarneros G, Machado G, Guzman P, Garay E. Genetic and physical location of the Escherichia coli rap locus, which is essential for growth of bacteriophage lambda. J Bacteriol. 1987;169:5188–5192. doi: 10.1128/jb.169.11.5188-5192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman P, Rivera-Chavira B E, Court D L, Gottesman M E, Guarneros G. Transcription of a bacteriophage DNA site blocks growth of Escherichia coli. J Bacteriol. 1990;172:1030–1034. doi: 10.1128/jb.172.2.1030-1034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Sanchez J, Gerardo Valadez J, Vega Herrera J, Ontiveros C, Guarneros G. Lambda bar minigene-mediated inhibition of protein synthesis involves accumulation of peptidyl-tRNA and starvation for tRNA. EMBO J. 1998;17:3758–3765. doi: 10.1093/emboj/17.13.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández J, Ontiveros C, Valadez J G, Buckingham R H, Guarneros G. Regulation of protein synthesis by minigene expression. Biochimie. 1997;79:527–531. doi: 10.1016/s0300-9084(97)82746-0. [DOI] [PubMed] [Google Scholar]
- 16.Heurgué-Hamard V, Karimi R, Mora L, MacDougall J, Leboeuf C, Grentzmann G, Ehrenberg M, Buckingham R H. Ribosome release factor RF4 and termination factor RF3 are involved in dissociation of peptidyl-tRNA from the ribosome. EMBO J. 1998;17:808–816. doi: 10.1093/emboj/17.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heurgué-Hamard V, Mora L, Guarneros G, Buckingham R H. The growth defect in Escherichia coli deficient in peptidyl-tRNA hydrolase is due to starvation for Lys-tRNALys. EMBO J. 1996;15:2826–2833. [PMC free article] [PubMed] [Google Scholar]
- 18.Inokuchi H, Yamao F. Structure and expression of prokaryotic tRNA genes. In: Söll D, RajBhandary U, editors. tRNA: structure, biosynthesis and function. Washington, D.C: ASM Press; 1995. pp. 17–30. [Google Scholar]
- 19.Karimi R, Pavlov M Y, Heurgué-Hamard V, Buckingham R H, Ehrenberg M. Initiation factors IF1 and IF2 synergistically remove peptidyl-tRNAs with short polypeptides from the P-site of translating Escherichia coli ribosomes. J Mol Biol. 1998;281:241–252. doi: 10.1006/jmbi.1998.1953. [DOI] [PubMed] [Google Scholar]
- 20.Kurland C G, Ehrenberg M. Constraints on the accuracy of messenger RNA movement. Q Rev Biophys. 1985;18:423–450. doi: 10.1017/s0033583500005370. [DOI] [PubMed] [Google Scholar]
- 21.Lovett P S, Rogers E J. Ribosome regulation by the nascent peptide. Microbiol Rev. 1996;60:366–385. doi: 10.1128/mr.60.2.366-385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayford M, Weisblum B. ermC leader peptide. Amino acid sequence critical for induction by translational attenuation. J Mol Biol. 1989;206:69–79. doi: 10.1016/0022-2836(89)90524-x. [DOI] [PubMed] [Google Scholar]
- 23.Menninger J R. Peptidyl transfer RNA dissociates during protein synthesis from ribosomes of Escherichia coli. J Biol Chem. 1976;251:3392–3398. [PubMed] [Google Scholar]
- 24.Menninger J R. The accumulation as peptidyl-transfer RNA of isoaccepting transfer RNA families in Escherichia coli with temperature-sensitive peptidyl-transfer RNA hydrolase. J Biol Chem. 1978;253:6808–6813. [PubMed] [Google Scholar]
- 25.Menninger J R. Accumulation of peptidyl tRNA is lethal to Escherichia coli. J Bacteriol. 1979;137:694–696. doi: 10.1128/jb.137.1.694-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menninger J R, Coleman R A. Lincosamide antibiotics stimulate dissociation of peptidyl-tRNA from ribosomes. Antimicrob Agents Chemother. 1993;37:2027–2029. doi: 10.1128/aac.37.9.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menninger J R, Coleman R A, Tsai L-N. Erythromycin, lincosamides, peptidyl-tRNA dissociation, and ribosome editing. Mol Gen Genet. 1994;243:225–233. doi: 10.1007/BF00280320. [DOI] [PubMed] [Google Scholar]
- 28.Menninger J R, Otto D P. Erythromycin, carbomycin, and spiramycin inhibit protein synthesis by stimulating the dissociation of peptidyl-tRNA from ribosomes. Antimicrob Agents Chemother. 1982;21:810–818. doi: 10.1128/aac.21.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J H. A short course in bacterial genetics. Laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 30.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ontiveros C, Valadez J G, Hernández J, Guarneros G. Inhibition of Escherichia coli protein synthesis by abortive translation of phage lambda minigenes. J Mol Biol. 1997;269:167–175. doi: 10.1006/jmbi.1997.1017. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Morga D, Guarneros G. A short DNA sequence from lambda phage inhibits protein synthesis in Escherichia coli rap. J Mol Biol. 1990;216:243–250. doi: 10.1016/s0022-2836(05)80316-x. [DOI] [PubMed] [Google Scholar]
- 33.Shine J, Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 34.Tenson T, DeBlasio A, Mankin A. A functional peptide encoded in the Escherichia coli 23S rRNA. Proc Natl Acad Sci USA. 1996;93:5641–5646. doi: 10.1073/pnas.93.11.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenson T, Xiong L, Kloss P, Mankin A S. Erythromycin resistance peptides selected from random peptide libraries. J Biol Chem. 1997;272:17425–17430. doi: 10.1074/jbc.272.28.17425. [DOI] [PubMed] [Google Scholar]
- 36.Tripathi S, Kloss P S, Mankin A S. Ketolide resistance conferred by short peptides. J Biol Chem. 1998;273:20073–20077. doi: 10.1074/jbc.273.32.20073. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S P, Zubay G, Goldman E. Low-usage codons in Escherichia coli, yeast, fruit fly and primates. Gene. 1991;105:61–72. doi: 10.1016/0378-1119(91)90514-c. [DOI] [PubMed] [Google Scholar]