Abstract
Approximately one in every 700 babies in the United States is born with Down syndrome, or 0.14%. Children with Down syndrome have cognitive impairment and congenital malformations necessitating frequent occurrences of general anesthesia and surgery. The thoughtful perioperative care of children with Down syndrome is relevant and acutely complex for the pediatric anesthesiologist. Behavior, sedation, hypotonia, upper airway obstruction, venous access, and bradycardia are omnipresent concerns apart from the surgical pathology. Down syndrome is also associated with autonomic nervous system dysfunction, a comorbidity that is overlooked in discussions of perioperative care and is described thus far in adults. Autonomic nervous system function or dysfunction may explain the phenotypical features of the perioperative challenges listed above. For this reason, understanding the development and measurement of autonomic nervous system function is important for the pediatric anesthesiologist. Definition and quantification of sympathetic and parasympathetic function will be reviewed.
Keywords: autonomic nervous system, down syndrome, perioperative period, trisomy 21
1 |. INTRODUCTION
Down syndrome is the most common chromosomal abnormality among live-born infants. Through full, partial, or mosaic nondisjunction and resultant trisomy of chromosome 21, Down syndrome is associated with cognitive impairment and congenital malformations. Down syndrome affects most organ systems including cardiovascular, respiratory, gastrointestinal, nervous, musculoskeletal, immune, hematologic, endocrine, ophthalmic, and auditory. Children with Down syndrome frequently present with microcephaly, macroglossia, endocardial cushion defects, duodenal atresia, atlantoaxial instability, subglottic stenosis, and an elevated risk of leukemia. Because of these sequalae, children with Down syndrome undergo higher rates of surgery and general anesthesia than their typically developing peers. Children with Down syndrome present unique considerations and challenges to perioperative safety.
Perioperative care of the child with Down syndrome is classically discussed in terms of airway and organ system comorbidities. A less often discussed comorbidity is autonomic dysfunction. The reach and application of autonomic dysfunction in pediatric patients with Down syndrome undergoing general anesthesia have yet to be described. Typically, anesthesiologists measure perioperative autonomic function via surrogate measures such as resting heart rate, blood pressure, and thermoregulation. The anesthesiologist manipulates each parameter to maintain homeostasis and minimize surgical stress on the child. However, the availability of non-invasive, continuous, ambulatory autonomic nervous system monitors enables routine quantification of aspects of the sympathetic and parasympathetic nervous system such as pre-ejection period and respiratory sinus arrhythmia. Such monitors may have a role in the perioperative setting especially for children with Down syndrome as these children are known to have autonomic variability as described in this review.
The aim of this educational review is to provide pediatric anesthesiologists with a brief overview of the development and measurement of autonomic nervous system function as well as its variation in children with Down syndrome.
1.1 |. A brief overview of the autonomic nervous system
The role of the autonomic nervous system is to maintain physiologic homeostasis. It provides adaptive responses to acute physiologic perturbations. The autonomic nervous system regulates involuntary physiologic functions such as heart rate, blood pressure, respiration, digestion, and sexual arousal. The sympathetic and parasympathetic nervous systems largely oppose each other. The sympathetic nervous system innervates the heart via cardiac nerves off the sympathetic chains. The medulla oblongata, though part of the parasympathetic nervous system, tonically sends excitatory signals to spinal sympathetic preganglionic neurons. Neurons in the rostral ventrolateral medulla mediate sympathetic and parasympathetic tone in the heart and blood vessels. The nucleus ambiguus provides preganglionic cardiac parasympathetic neurons.
1.2 |. Development of the autonomic nervous system
In typically developing individuals, the autonomic nervous system matures and myelinates throughout fetal life and infancy. This process may be altered in children with Down syndrome, as autonomic nervous system differences have been noted in very young infants.1 Vagal tone increases dramatically at 37–38 weeks. After birth, there is linear increase in parasympathetic influence on resting heart rate with increasing chronological age followed by a plateau phase in childhood. By age 8 years, children have resting heart rates, and by extension parasympathetic activity, comparable to adults. There is another rise in parasympathetic activity in adolescence and teenage years.
In contrast, the sympathetic division of the autonomic nervous system develops steadily throughout the fetal period and early infancy. This linear increase is attributed to myelination of sympathetic neurons. Then, sympathetic activity shows a linear decrease from 0.5 to 20 years. Developmental trajectories described for sympathetic and parasympathetic nervous system activity are for typically developing children. Children with Down syndrome have variable autonomic function at a young age, suggesting developmental aberrations. In the sections that follow, autonomic variation in children with Down syndrome is compared to the autonomic function of typically developing children and the anesthetic relevance of these variations are explained to the extent possible. Figure 1 depicts the autonomic innervation of the mature heart.
FIGURE 1.
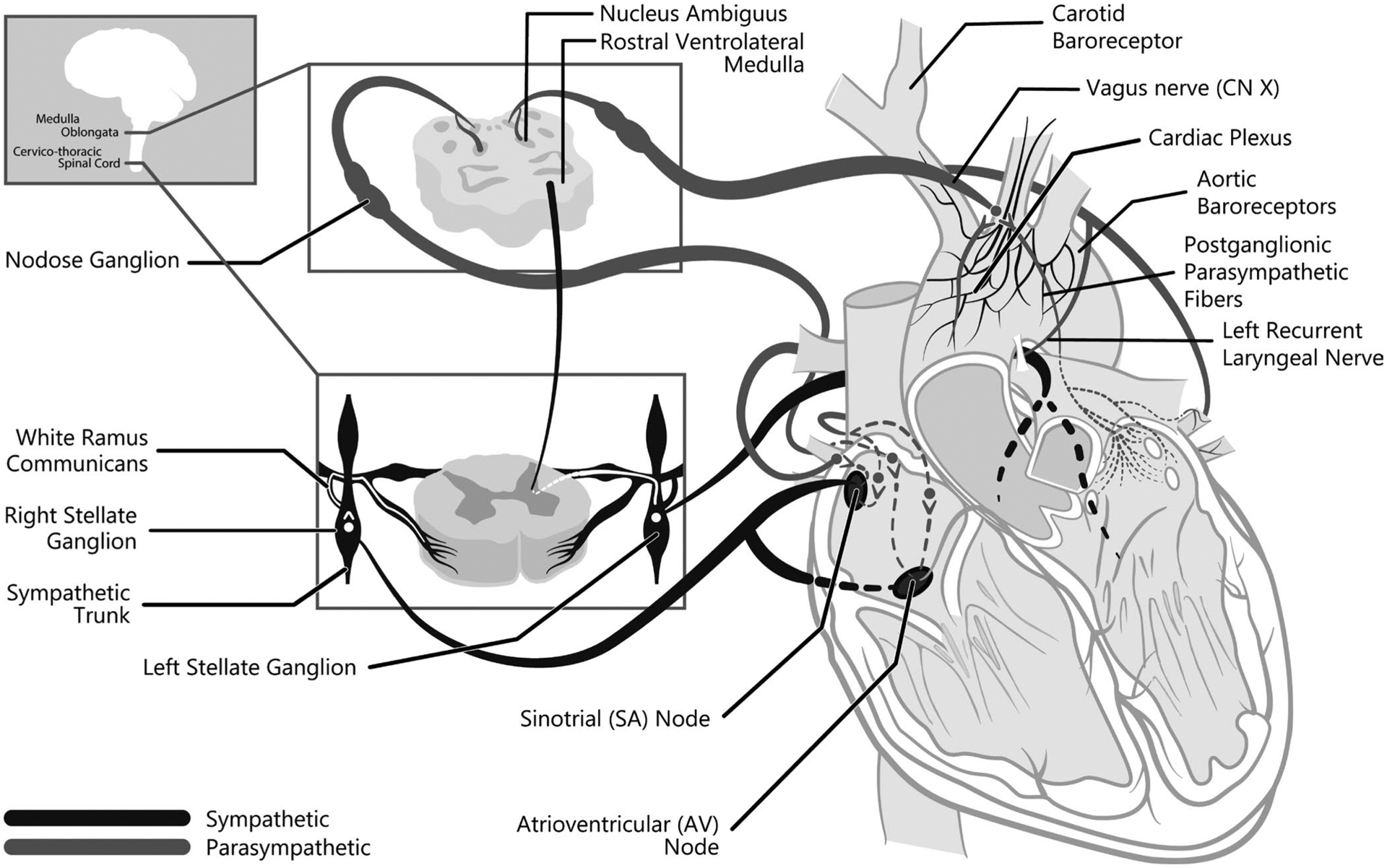
The course of the nerves providing autonomic innervation to the heart. Craniosacral, parasympathetic outflow originates in the medulla oblongata. The left and right vagus nerves comprise the parasympathetic input to the heart. Preganglionic parasympathetic nerves synapse near the heart and innervate the sinoatrial node, atria and atrioventricular node and great vessels. Cardiac sympathetic nerves arise from the thoracolumbar spinal cord with some cervical cord contributions. They exit the spinal cord and course to the sympathetic chain where they may travel a few cord levels prior to synapsing. Fibers exiting the sympathetic chain course to the sinoatrial node, atrioventricular node, and ventricles
2 |. HEART RATE CONTROL AND VARIATIONS IN THE CHILD WITH DOWN SYNDROME
Normal heart rate is impacted by numerous autonomic, developmental, environmental, and structural factors. Beat-to-beat changes of heart rate are affected by respiration, vasomotor tone, body temperature, and central input. Sympathetic influence on the heart causes increased chronotropy of the sinoatrial node, increased dromotropy at the atrioventricular node, and increased contractility. In contrast, parasympathetic influence causes deceleration and vagal arrest at the sinoatrial node as well as decreased atrial contraction. For these reasons, episodic heart rate measurements are considered over a wide range. Note that normal heart rate increases for the first month of life indicating the end of the rapid rise of parasympathetic input relative to the continual increase of sympathetic input on the sinoatrial node. Table 1 reflects interindividual HR differences based on age; however, intra-individual heart rates fluctuate with activity and healthy children may have bradycardia during rest or sleep.
TABLE 1.
Depicts normal, awake heart rate medians for age. Bradycardic heart rates are those below the 10th percentile in the aggregate population. Adapted from Fleming and colleagues3
| Age | Median heart rate (beats/min) | Bradycardia (beats/min) |
|---|---|---|
| 0–3 months | 143 | <123 |
| 3–6 months | 140 | <120 |
| 6–9 months | 134 | <114 |
| 9–12 months | 128 | <109 |
| 12–18 months | 123 | <103 |
| 18 months to 2 years | 116 | <98 |
| 2–3 years | 110 | <92 |
| 3–4 years | 104 | <86 |
| 4–6 years | 98 | <81 |
| 6–8 years | 91 | <74 |
Autonomic heart rate control in individuals with Down syndrome is characterized by sympathetic failure during tasks such as treadmill exercise. In children with Down syndrome, bradycardia, with associated blood pressure reduction, has been reported to occur during inhalation induction of anesthesia with sevoflurane. Autonomic mechanisms, such as parasympathetic excess or sympathetic failure, have been postulated as the likely cause of this bradycardia.
3 |. BLOOD PRESSURE CONTROL ALTERATIONS IN CHILDREN WITH DOWN SYNDROME
Normal blood pressure in children is age-dependent, controversial and hypotension is often defined as in Table 2. The autonomic nervous system contributes to blood pressure regulation via carotid and aortic baroreceptors. Mechanoreceptors in the aorta and carotid arteries detect stretch (increases in arterial pressure) and increase their firing rate. Aortic baroreceptors synapse on vagus nerve fibers and travel to the nucleus tractus solitarius.
TABLE 2.
Hypotension in children
| Age | Systolic blood pressure (mmHg) |
|---|---|
| 0–28 days | <60 |
| 1–12 months | <70 |
| 1–10 years | <70 + 2 × age in years |
| Age > or = 10 years | <90 mmHg |
Santoro and colleagues reported that the blood pressure profiles of children with Down syndrome were approximately 6 percentiles lower than that of age-and weight-matched non-Down syndrome peers.2 However, these blood pressures were typically still within normal limits (approximately the 38th percentile). Notably, no child in this study had a blood pressure that met World Health Organization criteria for prehypertension, hypertension and only 10% of children had blood pressures greater than the 70th percentile.2 Explanations for blood pressure differences in children with Down syndrome include comorbidities such as hypothyroidism, cardiac anomalies, endovascular and lipid metabolism differences, Moyamoya syndrome, physical deconditioning, and sympathetic dysautonomia. However, the authors pointed out that sympathetic control of blood pressure is relevant during exercise and does not predominate when the child is at rest, such as during a medical visit where blood pressure is measured.
4 |. RESPIRATION AND CONSIDERATIONS IN DOWN SYNDROME
Respiratory control has many determinants. While there are defined age-based normal respiratory rates for children,3 it is unclear to what degree these values apply to children with Down syndrome. Control of breathing in children with Down syndrome may be impacted by dysautonomia, multilevel airway collapse, sleep-disordered breathing, loop gain, pseudohypoxia, oral motor coordination, gastroesophageal reflux, and lower respiratory considerations. Sleep-disordered breathing is common in children with Down syndrome due to anatomic features such as small nares, large tongue, laryngomalacia, and hypotonia leading to multilevel airway collapse. Central sleep apnea due to brainstem dysfunction may also be present in children with Down syndrome. Children with Down syndrome are known to have high loop gain (a measure of instability of ventilatory control) if sleep-disordered breathing is present.4 High loop gain can have a myriad of complex effects on autonomic function.
Altered respiratory control may present at a very young age in children with Down syndrome. A study of infants in the neonatal intensive care unit found greater degrees and frequencies of hypoxia in infants with Down syndrome than those without.1 Beyond autonomic control, this study attributes more frequent hypoxia to breathing while eating and oral motor coordination. Interestingly, cells from individuals with Down syndrome are in a “pseudohypoxic state,” where cellular metabolites resemble those associated with hypoxia even when their supply of oxygen is not disrupted.
Lower respiratory considerations also exist for children with Down syndrome. Bronchiolar smooth muscle normally relaxes in response to sympathetic stimulation and contracts in response to parasympathetic stimulation. In adults with Down syndrome who have known sympathetic dysfunction, bronchodilation in the operating room (endogenous or iatrogenic) may be submaximal. Alveolar simplification is well described and may predispose to endothelial changes that ultimately contribute to respiratory infections and pulmonary hypertension.
The interplay among these determinants of respiratory and autonomic function could be relevant perioperatively. Quantification of autonomic function in this population may inform anesthetic considerations.
5 |. TEMPERATURE REGULATION AND CHALLENGES IN CHILDREN WITH DOWN SYNDROME
The hypothalamus primarily regulates body temperature. The autonomic regulation of vascular tone impacts temperature regulation. Sympathetic nervous stimulation causes vascular smooth muscle contraction at the skin and splanchnic vessels but relaxation of skeletal muscle vessels. These sympathetic effects are mechanisms by which animals preferentially retain cardiac output in central vessels at the expense of peripheral, cutaneous temperature. In contrast, the parasympathetic nervous system has no effect on vascular smooth muscle.
Infants and adults with Down syndrome are more prone to hypothermia and have lower resting and post-exercise body temperatures than those without Down syndrome. Surma and colleagues explain that temperature instability may be due to hypothalamic dysfunction, subclinical hypothyroidism, and dysregulated vascular tone, but do not directly implicate the autonomic nervous system.5 However, the impact of autonomic function on vasomotor tone, and therefore temperature, has been suggested in reports of children with Down syndrome and cutis marmorata, post-anesthetic livedo reticularis and culture-negative fever following infant cardiac surgery.5–7 Both skin conditions arise from altered cutaneous vasomotor control. Temperature control in children with Down syndrome has yet to be described in detail but could be an important anesthetic consideration given temperature variability in children with Down syndrome and the importance of temperature for homeostasis. Comorbidities that may be affected by inadequate temperature regulation include an increased in pulmonary vascular resistance; this is more likely to be clinically relevant in a child with Down syndrome who is already predisposed to elevations in pulmonary arterial pressure.
6 |. CHILDREN WITH DOWN SYNDROME REACT DIFFERENTLY TO PERIOPERATIVE SEDATIVES AND ANALGESICS
In children with Down syndrome, altered sensation can have perioperative implications for general anesthesia, sedation, and pain management. Children with Down syndrome react differently than their typically developing peers to induction of anesthesia with sevoflurane. Bai and colleagues found that children with Down syndrome had a significantly higher prevalence and degree of bradycardia and greater decreases in heart rate from baseline during and following inhalation induction of anesthesia with sevoflurane as compared to a similarly matched sample. Down syndrome status and lower American Society of Anesthesiologists physical status were found to be significant risk factors for bradycardia.8
Children with Down syndrome react differently than their typically developing peers in response to midazolam and dexmedetomidine. As dexmedetomidine is a central alpha-2 agonist, it directly reduces sympathetic outflow. Following cardiac surgery, children with Down syndrome had a higher risk of adverse events such as bradycardia and hypotension following dexmedetomidine administration in the intensive care unit.9 The authors define hypotension similarly for those with and without Down syndrome. As described above, this may result in a greater proportion of children with Down syndrome meeting criteria for hypotension. Parasympathetic data for children in this study were not collected.
Autonomic relevance to mechanisms of pain transduction have not been described in children with Down syndrome but are implied in sympathetically mediated or generated pain. In contrast, pain perception and coping are different in children with and without Down syndrome. Instead of an autonomic mechanism, developmental status (i.e., ability to communicate pain) is likely culpable. Valkenburg and colleagues reported less sensitivity to cold and warmth of children with Down syndrome compared to their siblings when measured as reaction time to exposure to cold or warmth and inability to quantify and localize pain.10 Measurements of parasympathetic tone before and after administrations of opioid administration were not available. In a similar patient population, the Newborn Infant Parasympathetic Evaluation (NIPE™) measure of parasympathetic function was increased following administration of opioid.11 These studies list examples of clinical scenarios in which receipt of usual medical care in children with Down syndrome produces aberrant or conflicting clinical outcomes. Phenotypic differences in reactions to common anesthetic medications in children with Down syndrome warrant further study. Autonomic nervous system monitoring may provide insight into when clinical courses of children with Down syndrome diverge from typically developing children.
7 |. RESEARCH PERSPECTIVES
Autonomic measurement is slightly more complex and elegant than the measurement of routine vital signs. Heart rate variability has been used as a surrogate measure of autonomic function. Pre-ejection period and respiratory sinus arrhythmia are quantifiable measures of sympathetic and parasympathetic function, respectively, and are used in research settings. Technical considerations and availability prevent widespread use of these monitors perioperatively. Autonomic dysfunction in adults with Down syndrome is well described. However, in children, the age of onset and characteristics of sympathetic failure are unknown. Thus, perioperative monitoring of autonomic function may be of utility. Details of autonomic measurement follow.
7.1 |. Utility of heart rate variability in the operating room
While heart rate variability is fundamental to classic autonomic data analysis, it is less familiar to pediatric anesthesiologists. Heart rate variability measures the relative balance between the sympathetic and parasympathetic input into heart rate control. Quantitative assessment of heart rate variability reflects the interaction of the cardiac and respiratory systems to produce heart rate. Heart rate variability describes variations in both instantaneous heart rates, that is, R-R interval on the electrocardiogram. Examples of heart rate variability use in pediatrics include monitoring of premature infants in whom the autonomic nervous system is susceptible to dysmaturity (poor balance between the sympathetic and parasympathetic nervous systems) and intraoperative detection of nociception in children up to two years of age.11 To the authors’ knowledge, heart rate variability monitoring has yet to be employed systematically in the perioperative care of children with Down syndrome.
The standard measurement of autonomic function for clinical research and patient care is heart rate variability monitoring and is described by Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.12 Heart rate variability measurements can be derived from electrocardiogram (ECG) data. Heart rate variability data are analyzed in the time domain and in the frequency domain. Time-domain analysis of heart rate variability is useful to quantify activity of the parasympathetic nervous system on the sinoatrial node.12 Time-domain analysis comprises the standard deviation of all R-R intervals on the electrocardiogram. It is expressed as root mean square of successive differences.
Frequency domain analysis of heart rate variability comprises frequency-specific input from the sympathetic and parasympathetic nervous systems and is computed via power spectrum analysis, first described by Sayers B et al. For a detailed description of the methodology, refer to Akselrod et al.13 Power spectral analysis is an algorithm comprising the time derivatives of the series of R-R intervals (tachogram) from the electrocardiogram signal. Sections in the frequency band from 0.03 to 0.15 Hz are defined as low frequency and represent alterations in heart rate due to peripheral vasomotor tone and thermoregulation. Low-frequency heart rate variability is impacted by both sympathetic and parasympathetic nervous systems. Inversely, high-frequency heart rate variability in the range from 0.15 to 0.4 Hz is synchronous with respiration corresponding to a respiratory rate of 9 to 24 breaths per minute. High-frequency heart rate variability is modulated mostly by the parasympathetic nervous system. The ratio of low-to high-frequency heart rate variability power indicates sympathetic and parasympathetic balance.14 This is not clinically available in most operating rooms, and heart rate inspection is inadequate for estimation of low-frequency and high-frequency heart rate variability.
In the operating room, heart rate monitoring is of course available; however, neither the time between successive R peaks of the electrocardiogram nor the time series that a group of these peaks would create, that is, the tachogram, is readily available to the anesthesiologist. Qualitative inspection of the anesthetic record for variations in heart rate is a practical way to consider autonomic function; however, it is not granular enough to change clinical management. Tachogram data are available in processed form (akin to the relationship between an electroencephalogram and a Bispectral Index™) in NIPE™, a proprietary scale derived from electrocardiogram signals.11 Alternatively, heart rate variability can now be recorded with photoplethysmography in the form of wrist and finger-worn devices. This has led to a plethora of heart rate variability monitors available to the public. These monitors are marketed to assess athletic training and to measure stress. However, their routine use in clinical care has been hindered by challenges including concern for device accuracy, lack of regulatory approval, or robust clinical trials to support their use.
7.2 |. Advantages to the addition of impedance cardiography to routine electrocardiography
The measurement of thoracic bioimpedance plus electrocardiogram is called impedance cardiography, providing autonomic function monitoring that measures sympathetic and parasympathetic function separately. The sympathetic function is quantified by the pre-ejection period and the parasympathetic by respiratory sinus arrhythmia. Sympathetic and parasympathetic nervous systems largely oppose each other but are not mutually exclusive. Therefore, separate measurement of each provides an advantage over heart rate variability in which only relative contributions of sympathetic and parasympathetic function are measured. The Vrije University Ambulatory Monitoring System (VU-AMS) is one such autonomic nervous system monitor (combined electrocardiography and impedance cardiography) that has been validated for use in children. The VU-AMS has recently been used to define normal parameters for SNS and PNS function for awake children ages 6 months to 20 years.15 These normative values may be used for comparison in children with Down syndrome or during administration of anesthetic agents. Ambulatory, continuous, non-invasive autonomic nervous system monitors have been used in the fields of psychology and cardiology extensively, but their use in pediatric anesthesiology is undocumented. Broader potential applications of the combination of impedance and electro-cardiography include monitoring of regional anesthetic function, the stress response to anesthesia and hypovolemia due to prolonged fasting.
7.3 |. Pre-ejection period and relevance in Down syndrome
Pre-ejection period is a measure of sympathetic influences on heart rate. Pre-ejection period is the time (measured in milliseconds) between electrical and mechanical events of the heart and represents the duration of isovolumic contraction. Isovolumic contraction occurs between the Q wave onset of the electrocardiogram and the opening of the aortic valve, or B point on impedance cardiogram. It is modulated by stimulation of beta-1 adrenoceptors. If the heart contracts sooner than usual after ventricular depolarization, then sympathetic tone is high, and pre-ejection period is short. A long pre-ejection period value indicates low sympathetic activity. Presently, pre-ejection period is derived noninvasively from the electrocardiogram Q wave onset to the rapid decline in thoracic bioimpedance (as measured by the impedance cardiogram), that is, onset of aortic ejection. Impedance cardiogram measurement is relatively unobtrusive and wearable in an ambulatory setting. The differential diagnosis of increased (or decreased) pre-ejection period is broad but may indicate a relevant physiologic change. Thyrotoxicosis is associated with short pre-ejection period. Children with Down syndrome are predisposed to thyroid dysfunction, and this may be reflected in subclinical disease. Also, in children with Down syndrome who become bradycardic on inhalation induction of anesthesia with sevoflurane, pre-ejection period would be expected to lengthen if sympathetic failure was the cause.
7.4 |. Respiratory sinus arrhythmia and relevance in Down Syndrome
Respiratory sinus arrhythmia, as the name implies, depicts an interaction between cardiovascular and respiratory systems. During inhalation, intrathoracic pressure becomes negative, pulling air into the lungs and venous blood into the heart. Respiratory neurons in the medulla oblongata inhibit preganglionic cardiac parasympathetic neurons resulting in a slightly faster heart rate during inspiration.
Respiratory sinus arrhythmia is used to quantify parasympathetic innervation of the heart—high respiratory sinus arrhythmia reflects high parasympathetic activity. Respiratory sinus arrhythmia is the heart rate variability during a respiratory cycle, where heart rate increases during inhalation and decreases during exhalation. Normally, the heartbeats several (approximately six) times during one respiratory cycle. Heart rate varies within that time frame. During the next few heart beats (exhalation), the heart is full, and heart rate slows as stroke volume, and therefore cardiac output, easily maintains oxygen delivery. Respiratory sinus arrhythmia is computed as the difference between expiratory and inspiratory R-R intervals. Tidal volume and R-R interval further impact these phasic heart rate changes. A high resting respiratory sinus arrhythmia indicates high vagal tone, while lower resting respiratory sinus arrhythmia indicates low vagal tone. On an evolutionary scale, respiratory sinus arrhythmia may modulate an animal’s energy exchange by synchronizing cardiovascular and respiratory processes in response to metabolic and environmental factors. Respiratory sinus arrhythmia is measured clinically by using a deep breathing test. More work is needed to understand the interpretation of respiratory sinus arrhythmia in the context of spontaneous ventilation and upper airway obstruction commonly seen in children with Down syndrome. Following in induction of anesthesia, pressure support ventilation may be instituted and the implications of this and respiratory sinus arrhythmia are not well described. Additionally, consideration could be given to the use of parasympathetic nervous system activity for the detection of pain in children with Down syndrome who are less able to describe and localize pain.10 A study of autonomic function in children with Down syndrome concluded that parasympathetic activity, as measured by high-frequency power spectrum analysis, is significantly reduced. The children in this study also had an increased low-frequency to high-frequency ratio indicating sympathetic activity that outweighs parasympathetic activity. The apneic episodes in the study were associated with hypoxia and the authors conclude that this may lead to increased risk of adverse cardiovascular outcomes.16 Chronic intermittent hypoxemia, in children with obstructive sleep apnea, disturbs the balance of sympathetic and parasympathetic function. Specifically, parasympathetic nervous system function is reduced to a greater degree than can be explained by sleep-disordered breathing in children with Down syndrome.16 The reason for this is not described; however, increased loop gain or upper airway obstruction (autonomic nervous system effects notwithstanding) may be contributing factors. Parasympathetic nervous system activity measurement using respiratory sinus arrhythmia in children with sleep-disordered breathing may yield unclear data. Therefore, it would be premature to recommend heart rate variability monitoring for the detection or tracking of sleep-disordered breathing in children with Down syndrome.
Inhalation induction of anesthesia involves a momentary relative overdose of sevoflurane. This overpressure speeds anesthetic induction and is associated with abrupt withdrawal of parasympathetic activity as measured by heart rate variability at the time of loss of eyelid reflex.17
8 |. CONCLUSION
Individuals with Down syndrome have intrinsic characteristics and comorbidities that affect their autonomic function. Differences in the development of the autonomic nervous system in children with Down syndrome versus typically developing children may explain different rates of bradycardia on induction, sedative effect of midazolam, dexmedetomidine, and pain behaviors. These effects may be age dependent, and it is unclear at what age these differences become clinically relevant. Consequently, autonomic function, measurement, and manipulation are relevant to the pediatric anesthesiologist. Autonomic nervous system monitoring may have a role in the perioperative environment. Routine measurement of autonomic function before, during, and after anesthesia is not directly mentioned by the American Society of Anesthesiologists but requires further study to determine its place (if any) in the perioperative care of children with Down syndrome.
9 |. REFLECTIVE QUESTIONS
What autonomic nervous system developmental differences can be expected in a 26-week newborn versus a full-term newborn?
What types of autonomic dysfunction can be expected in individuals with Down syndrome?
What impact does congenital heart disease status have on autonomic function?
What behavioral comorbidities in children with Down syndrome may have autonomic underpinnings?
ACKNOWLEDGMENTS
The authors thank Maria Ashton MS, RPH, and MBA for providing writing assistance, editing, and proofreading. The authors also thank Julia Bendon, MS for rendering Figure 1.
Funding information
Effort for this manuscript was funded by The National Heart, Lung and Blood Institute, Office of the Director as part of award number 1R21HL162572-01 for the project entitled, “Finding the contribution of the autonomic nervous system during perioperative events in children with Down Syndrome”. Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R21HL162572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
No conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated.
REFERENCES
- 1.Krahn KN, Nagraj VP, McCulloch MA, Zimmet AM, Fairchild KD. Hypoxemia in infants with trisomy 21 in the neonatal intensive care unit. J Perinatol. 2021;41:1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro JD, Lee S, Mlynash M, Mayne EW, Rafii MS, Skotko BG. Diminished blood pressure profiles in children with down syndrome. Hypertension. 2020;75:819–825. [DOI] [PubMed] [Google Scholar]
- 3.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011; 377: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siriwardhana LS, Nixon GM, Davey MJ, et al. Children with down syndrome and sleep disordered breathing display impairments in ventilatory control. Sleep Med. 2021; 77: 161–169. [DOI] [PubMed] [Google Scholar]
- 5.Surma V, Nagraj VP, Fairchild KD, Vergales J. Temperature instability in infants with trisomy 21 in the neonatal intensive care unit. J Perinatol. 2020;40:1167–1170. [DOI] [PubMed] [Google Scholar]
- 6.de Moraes PH, Sueli Pinheiro Modolob N, Humberto de Paiva D. Livedo reticularis by hypothermia during anesthesia for dental treatment in Down’s syndrome patient. Rev Bras Anestesiol. 2019;69:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergales J, Seckeler MD, Chew J, Gangemi J. Prevalence of culture-negative fever in infants with down syndrome undergoing cardiac surgery. World J Pediatr Congenit Heart Surg. 2019;10:599–603. [DOI] [PubMed] [Google Scholar]
- 8.Bai W, Voepel-Lewis T, Malviya S. Hemodynamic changes in children with down syndrome during and following inhalation induction of anesthesia with sevoflurane. J Clin Anesth. 2010;22:592–597. [DOI] [PubMed] [Google Scholar]
- 9.Ueno K, Ninomiya Y, Shiokawa N, Hazeki D, Eguchi T, Kawano Y. Dexmedetomidine is associated with an increased incidence of bradycardia in patients with trisomy 21 after surgery for congenital heart disease. Pediatr Cardiol. 2016;37:1228–1234. [DOI] [PubMed] [Google Scholar]
- 10.Valkenburg AJ, Tibboel D, van Dijk M. Pain sensitivity of children with down syndrome and their siblings: quantitative sensory testing versus parental reports. Dev Med Child Neurol. 2015;57:1049–1055. [DOI] [PubMed] [Google Scholar]
- 11.Weber F, Roeleveld HG, Geerts NJE, Warmenhoven AT, Schröder R, de Leeuw TG. The heart rate variability-derived newborn infant parasympathetic evaluation (NIPE) index in pediatric surgical patients from 0 to 2 years under sevoflurane anesthesia-a prospective observational pilot study. Pediatr Anesth. 2019;29:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik M, Bigger TJ, Camm AJ, et al. Heart rate variability standards of measurement, physiological interpretation and clinical use. Eur Heart J. 1996;93:354–381. [PubMed] [Google Scholar]
- 13.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power Spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. [DOI] [PubMed] [Google Scholar]
- 14.Iellamo F, Galante A, Legramante JM, et al. Altered autonomic cardiac regulation in individuals with down syndrome. Am J Physiol Heart Circ Physiol. 2005; 289: H2387–H2391. [DOI] [PubMed] [Google Scholar]
- 15.Harteveld LM, Nederend I, Ten Harkel ADJ, et al. Maturation of the cardiac autonomic nervous system activity in children and adolescents. J Am Heart Assoc. 2021;10:e017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne RSC. Consequences of paediatric sleep disordered breathing: contributions from Australian and New Zealand investigators. Sleep Med. 2021;77:147–160. [DOI] [PubMed] [Google Scholar]
- 17.Constant I, Dubois MC, Piat V, Moutard ML, McCue M, Murat I. Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology. 1999;91:1604–1615. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman ME, Chameides L, Schexnayder SM, et al. Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010; 126: e1361–e1399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable—no new data generated.


