Abstract
Cardiac adverse effects of the COVID-19 vaccine are very rare, myocarditis and pericarditis are the most common amid them, and constrictive pericarditis (CP) is reported to be restricted to a few cases following mRNA COVID-19 vaccines. We report a case of a 72-year-old male patient who developed symptoms of right-sided heart failure, which started after 8 days of receiving the third dose of inactivated virus COVID-19 vaccine and his diagnostic tests comprising transthoracic echocardiography, chest CT scan, cardiac magnetic resonance were in favor of CP. Ultimately, invasive cardiac catheterization confirmed the diagnosis of CP. Due to the lack of satisfactory response to corticosteroid therapy, pericardiectomy was performed, which gave rise to symptom relief progressively and substantially. Considering the temporal course of the patient's symptoms and exclusion of other possible etiologies based on the patient's medical history and diagnostic evaluation, immunization with the COVID-19 vaccine was recognized as a culprit for developing CP. Despite being a scarce phenomenon, the COVID-19 vaccine could have a tendency to provoke pericardial inflammation in so far as causing CP. Hence, physicians should have a high index of suspicion in these circumstances and accelerate the diagnostic investigation.
Keywords: BBIBP-CorV (Vero Cells), Inactivated vaccine, COVID-19, SARS-CoV-2, Constrictive pericarditis, Adverse effect
Introduction
Vaccination against SARS-CoV-2 infection has appeared as a vital weapon to overcome the global burden of the COVID-19 pandemic [1,2]. Although efficacy and safety of approved COVID-19 vaccines are evident and their adverse effects are predominantly negligible and ephemeral, rare but potentially fatal complications of these immunizations warrant attention [3,4]. Given the cardiac adverse events of the vaccine, myocarditis and pericarditis were the most common complications, and there were only a few case reports which described patients affected by constrictive pericarditis (CP) after receiving the COVID-19 vaccine of mRNA type [4,5]. Herein we present a case of CP following administration of the third dose of an inactivated virus COVID-19 vaccine for the first time.
Case presentation
A 72-year-old man presented to the pulmonary outpatient clinic complaining of exertional dyspnea. He developed subacute progressive dyspnea on exertion for 1 month, which started after 8 days of his third dose of inactivated SARS-CoV-2 vaccine administration. He also reported unusual fatigue, decreased appetite, and abdominal swelling as concurrent symptoms, but he did not cite chest pain, orthopnea, or abdominal pain. The patient did not encounter any significant adverse effects with the first and second doses of the COVID-19 vaccine, which were of the same type as the third dose. His medical history consisted of ischemic heart disease with left anterior descending (LAD) artery and right coronary artery (RCA) angioplasty, ischemic stroke 8 months ago, diabetes mellitus, and hypertension, but he did not have any history of previous cardiac surgery, tuberculosis infection, mediastinal radiotherapy, and connective tissue diseases or malignancies. He also did not report being affected by COVID-19 illness since the onset of the pandemic. Upon physical examination, tachypnea (respiratory rate: 28 breaths per minute) was evident; otherwise, vital signs were stable. Jugular venous distention at 30-degree with Kussmaul's sign was apparent in the neck examination. There were normal heart sounds but diminished lung sound at bases bilaterally in chest examination. Abdominal distension with shifting dullness was noted, which affirmed the presence of ascites, and on extremities, bilateral 2+ pitting edema of lower limbs was noticed. The only paraclinical investigation that was done until the presentation was an abdominal ultrasound showing severe ascites and mild splenomegaly 6 days before this visit.
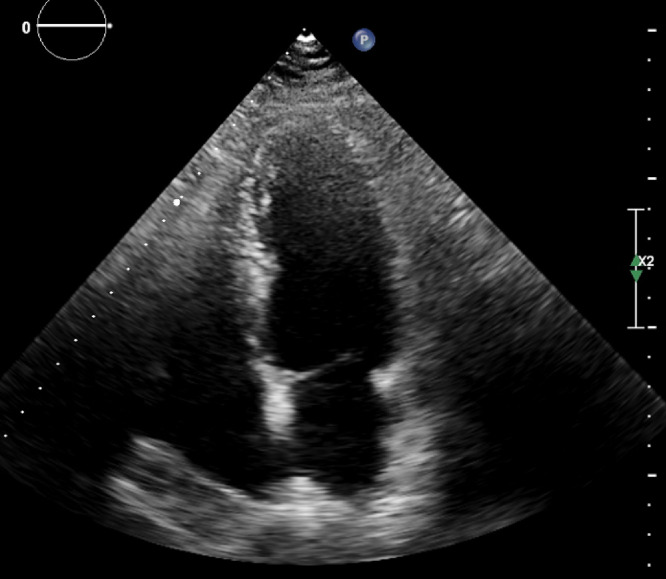
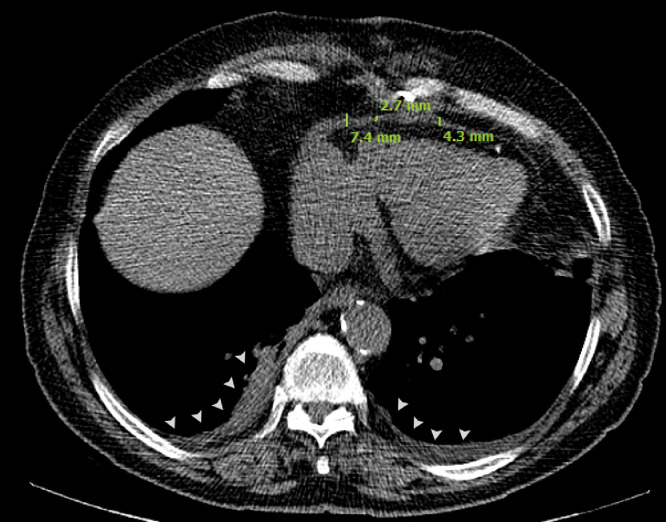
Given the aforementioned history and physical examinations, he was considered to be evaluated for his heart failure symptoms in an inpatient setting; therefore, he was admitted to our hospital on the same day. Transthoracic echocardiography was performed, which revealed the following results: normal left ventricular ejection fraction (EF: 55%) but thickened pericardium (5 mm) with septal bounce (Video 1, Fig. 1), significant inspiratory variation (59%) in mitral valve flow, annulus reversus, annulus paradoxus, inferior vena cava (IVC) plethora and expiratory diastolic flow reversal in hepatic vein flow, findings which all were compatible with the diagnosis of CP. High-resolution computed tomography of the chest indicated mild-to-moderate pleural effusion in both hemithoraces (Fig. 2). Ascitic fluid aspiration was done, and its analysis revealed high serum ascites albumin gradient with high ascitic protein (serum albumin 3.9 g/dL and ascitic albumin 2.8 g/dL (serum ascites albumin gradient: 1.1), total protein: 4.1 g/dL) which was in favor of CP as ascites source.
Fig. 1.
Transthoracic echocardiography of the patient in four chamber view suspicious of pericardial thickening.
Fig. 2.
Mediastinal window of thoracic HRCT of the patient revealing bilateral pleural effusion (arrow heads) and evidence of mild to moderate pericardial thickening (pericardial width >2 mm) in inferior aspect of heart. HRCT, high-resolution computed tomography.
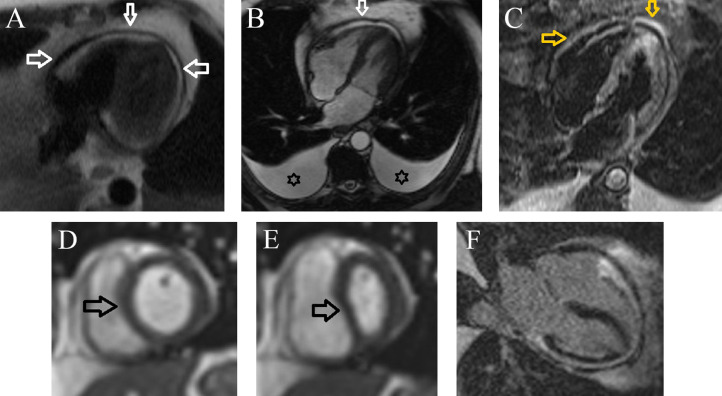
Additionally, the patient underwent cardiac magnetic resonance (CMR) 2 days after admission, which showed diffuse thickening of the pericardium (maximum 7 millimeters at inferior aspect, parietal pericardial thickness at the diaphragmatic level: 7 mm, around the apex: 4 mm), mild pericardial inflammation but no evidence of myocardial and pericardial enhancement or pericardial effusion. In addition, dilated IVC, septal bounce (apparent abnormal ventricular septal motion toward the left ventricle [LV] in early diastole), and respiratory phase ventricular interdependence were detected (Fig. 3). All these CMR findings were suggestive of CP. According to the presence of pericardial inflammation and edema, intravenous hydrocortisone was initiated with a dose of 50 milligrams 3 times a day besides supportive therapy. Laboratory data, including complete blood count, inflammatory markers (erythrocyte sedimentation rate and C-reactive protein), and liver function tests, were unremarkable. COVID-19 polymerase chain reaction and also viral markers panel were negative.
Fig. 3.
(Panel-A) Axial view Haste sequence shows circumferential thickening of the pericardium (white arrow). (Panel-B) Four-chamber view Cine SSFP sequence shows thickened and low signal pericardium (white arrow), bilateral large pleural effusion (Asterix). (Panel-C) Four-chamber Short Tau Inversion Recovery (STIR) sequence reveals hypersignal pericardium (orange arrow) suggestive of edema. (Panel-D and E) Short-axis view Cine Real-time navigator sequence demonstrates interventricular dependence (septal bounce) during deep inspiration (black arrow). (Panel-F) Four-chamber view Late Gadolinium Enhancement (LGE) sequence reveals thickened pericardium without enhancement.
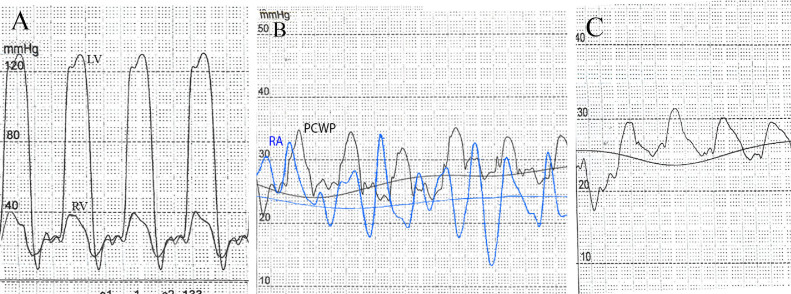
Moreover, other diagnostic workups for investigating the underlying etiologies were inconclusive. On the seventh day of admission, the patient underwent coronary angiography and right and left heart catheterization. Pulmonary capillary wedge pressure, right atrium, left atrium (LA), right ventricle (RV), and LV pressure during inspiration and expiration were measured. Equalization of four chambers' end-diastolic pressures was noted, and a dip and plateau pattern (or square root sign) was observed (Fig. 4). According to these findings, the diagnosis of CP was confirmed.
Fig. 4.
Four-chamber catheterization of the patient demonstrating equalization of pressure within atriums and ventricles. (Panel-A) Systolic, diastolic, and end-diastolic pressures were 130/9/25 mm Hg in LV, and 42/14/25 mm Hg in RV, respectively; revealing similar pressure curves in ventricles in diastole, discernible with “dip-and-plateau” or “square root” pattern. (Panel-B) Elevation and equalization of RA pressure and pulmonary capillary wedge pressure (PCWP) with means of 25 mm Hg and 29 mm Hg, respectively. The RA curve depicts the “M” or “W” outline. (Panel-C) Pulmonary artery pressure measurement indicates mild pulmonary artery hypertension (an average of 27 mm Hg).
In the course of the hospital stay, the patient did not respond significantly to the corticosteroid and supportive therapy. Accordingly, he became a candidate for pericardiectomy on day 32 of admission. During operation, following midsternotomy, a very thick pericardium with severe adhesions around the myocardium was noticed. Through midline, the pericardium was opened, and severe adhesions were released. There were also severe adhesions around left anterior descending and IVC which resulted in a minor laceration of IVC and RV. Cardiopulmonary bypass was established, and the whole myocardium, SVC, and IVC were released from adhesions of myocardium lacerations, and IVC and RV got repaired. After 3 days of surgery, pericardial biopsy demonstrated sclerotic and thick pericardium, and it showed negative results for vasculitis, neoplastic involvement, and granuloma. Following surgery, the patient's symptoms abated markedly, and eventually, after 5 days of his surgery on the 37 day of admission, he was discharged in generally stable condition. Upon his follow-up in the next 2 months, he was doing well without recurrence of his symptoms.
Discussion
CP is a disorder of cardiac filling which arises from a diseased, inelastic pericardium that limits the expansion of cardiac chambers and should be regarded as one of the differential diagnoses when a patient presents with symptoms of heart failure, particularly when right-sided symptoms and signs are predominant and ejection fraction is preserved as was observed in our patient [6,7]. Given the etiologies of CP, tuberculosis worldwide, and idiopathic, prior cardiac surgery and chest radiation in North America and Europe are the most common causes, and vaccines had not been distinctly described as a cause of CP based on our literature review [7]; however, with the emergence of COVID-19 vaccines, CP has been reported in 6 cases to date [5,[8], [9], [10], [11]].
All the patients illustrated in these case reports developed CP following injection of either the first or second dose of mRNA type COVID-19 vaccine. This fact differentiates our case from these cases. Our patient was afflicted by CP after the injection of the inactivated virus COVID-19 vaccine booster dose. This observation can suggest that even though mRNA vaccine-induced CP is still dominant in case numbers, the pathophysiology of vaccine-induced CP is beyond the type of vaccines and doesn't have a clear correlation with vaccine dose [5,[8], [9], [10], [11]]. Furthermore, when the possible role of the vaccine in the induction of CP was discussed, the temporal relationship between vaccine shots and the onset of CP symptoms, besides excluding other common and possible causes of CP, was considered as a basis like what we contemplated about our case. Moreover, due to the scarcity of literature in this context and the low level of evidence in the nature of these studies, causality could not be firmly established [10,11].
Viani et al postulated that the immune reaction against the spike protein of SARS-CoV-2 might give rise to CP as an infrequent adverse reaction to the vaccine, and also another potential mechanism is molecular mimicry of viral spike between viral spike protein and an unknown cardiac protein. They explained this pathogenesis for the mRNA vaccine, but a similar scenario could elucidate the clinical picture of our patient [8].
In view of the management of the patients with vaccine-induced CP, different therapeutic strategies were employed based on the severity and duration of symptoms and also imaging findings. In the case described by Nakanishi et al [5], symptoms controlled by oral diuretics and pericardiectomy were deferred owing to mild symptoms. In Viani et al [8] case, colchicine and NSAID were prescribed according to the effusive type of the patient's CP, and then the patient was started on oral corticosteroids because of persistently high inflammatory markers, and Hysi et al [11] chose pericardiectomy as a treatment plan to manage their patient who his clinical manifestations were ascribed to subacute CP. We firstly opted for medical therapy as there was evidence of edema in CMR and considering possible "transient" constriction that may respond to anti-inflammatory therapy, but when a significant treatment response was not observed, a surgical approach was advocated [7,12].
Eventually, it merits emphasizing that, nevertheless, our patient encountered a serious adverse event of COVID-19 vaccination; given the rarity of these reactions, vaccination benefits still outweigh, and the vaccine is known as the best weapon to overcome the global burden of COVID-19 pandemic [13], [14], [15], [16].
Conclusion
In essence, although there is not sufficient evidence to corroborate that the COVID-19 vaccines are implicated in the development of CP but at least when we consider these few reported cases and our experience with this case, we could suggest that there is a propensity for COVID-19 vaccines to provoke notable and perennial inflammation in the pericardium to the extent that CP could develop in an extremely rare occasion. Consequently, accelerating the diagnostic workup when we confront a patient with signs and symptoms of heart failure following COVID-19 vaccination could avert the patient's condition decompensation.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Video-1 Legend: Four-chamber view of patient's echocardiography indicating ventricular septal bounce. (respiratory phase ventricular interdependence).
Patient consent statement
We did not disclose any identifiable information while writing this case report; however written consent was obtained from the patient.
Footnotes
Competing Interests: All authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.07.021.
Appendix. Supplementary materials
References
- 1.Moreira ED, Jr, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. C4591031 Clinical trial group. safety and efficacy of a third dose of BNT162b2 Covid-19 Vaccine. N Engl J Med. 2022;386(20):1910–1921. doi: 10.1056/NEJMoa2200674. Epub 2022 Mar 23. PMID: 35320659; PMCID: PMC9006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol Ther. 2022:e15461. doi: 10.1111/dth.15461. Epub ahead of print. PMID: 35316551; PMCID: PMC9111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fazlollahi A, Zahmatyar M, Noori M, Nejadghaderi SA, Sullman MJM, Shekarriz-Foumani R, et al. Cardiac complications following mRNA COVID-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2021:e2318. doi: 10.1002/rmv.2318. Epub ahead of print. PMID: 34921468. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi Y, Honda S, Yamano M, Kawasaki T, Yoshioka K. Constrictive pericarditis after SARS-CoV-2 vaccination: a case report. Int J Infect Dis. 2022;116:238–240. doi: 10.1016/j.ijid.2022.01.027. Epub 2022 Jan 19. PMID: 35063679; PMCID: PMC8767927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch TD, Oh JK. Constrictive pericarditis. Cardiol Clin. 2017;35(4):539–549. doi: 10.1016/j.ccl.2017.07.007. PMID: 29025545. [DOI] [PubMed] [Google Scholar]
- 7.Welch TD. Constrictive pericarditis: diagnosis, management and clinical outcomes. Heart. 2018;104(9):725–731. doi: 10.1136/heartjnl-2017-311683. Epub 2017 Nov 25. PMID: 29175978. [DOI] [PubMed] [Google Scholar]
- 8.Viani GM, Pedrotti P, Seregni R, Antonio B. Effusive-constrictive pericarditis after the second dose of BNT162b2 vaccine (Comirnaty): a case report. Eur Heart J Case Rep. 2022;6(2):ytac012. doi: 10.1093/ehjcr/ytac012. PMID: 35233481; PMCID: PMC8874812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misumi I, Ogata A, Fukuda K, Sato K, Nagano M, Usuku H, Tsujita K. Constrictive pericarditis following mRNA COVID-19 vaccination in a patient with systemic sclerosis. J Cardiol Cases. 2022 doi: 10.1016/j.jccase.2022.03.014. Epub ahead of print. PMID: 35401886; PMCID: PMC8977375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diana D, Cardillo I, Polizzi V, Musumeci F. 544 Two cases of constrictive pericarditis temporally associated with mRNA-1273 COVID-19 vaccination. Eur Heart J Suppl. 2021 doi: 10.1093/eurheartj/suab142.014. Volume 23, Issue Supplement_Gsuab142.014. [DOI] [Google Scholar]
- 11.Hysi I, Rebet Ol, Vaquette B, Fabre O. Subacute constrictive pericarditis after mRNA Covid-19 vaccination. Arch Clin Med Case Rep. 2022;6:189–192. [Google Scholar]
- 12.Chang SA, Oh JK. Constrictive pericarditis: a medical or surgical disease? J Cardiovasc Imaging. 2019;27(3):178–186. doi: 10.4250/jcvi.2019.27.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidari A, Hassanzadeh M, Naderkhani M, Gholizadeh Mesgarha M, Pour Mohammad A, Azadeh A, et al. Predictors of critical COVID-19 in an Iranian population: Age and disabilities play a special role. Med J Islam Repub Iran. 2021;35:94. doi: 10.47176/mjiri.35.94. PMID: 34956940; PMCID: PMC8683779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahadorizadeh L, Emamikhah M, Pour Mohammad A, Gholizadeh Mesgarha M. Simultaneous occurrence of cerebral venous sinus thrombosis and immune thrombocytopenic purpura in a patient with a history of COVID-19 infection. Neurol Ther. 2022;11(1):491–497. doi: 10.1007/s40120-021-00294-9. Epub 2021 Oct 29. PMID: 34714517; PMCID: PMC8554500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pour Mohammad A, Mashayekhi F, Seirafianpour F, Gholizadeh Mesgarha M, Goodarzi A. COVID-19 and COVID-19 vaccine-related dermatological reactions: an interesting case series with a narrative review of the potential critical and non-critical mucocutaneous adverse effects related to virus, therapy, and the vaccination. Clin Case Rep. 2022;10(4):e05775. doi: 10.1002/ccr3.5775. PMID: 35498347; PMCID: PMC9040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moradians V, Shateri Amiri B, Bahadorizadeh L, Gholizadeh Mesgarha M, Sadeghi S. Concurrent COVID-19 and pneumocystis carinii pneumonia in a patient subsequently found to have underlying hairy cell leukemia. Radiol Case Rep. 2022 doi: 10.1016/j.radcr.2022.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Video-1 Legend: Four-chamber view of patient's echocardiography indicating ventricular septal bounce. (respiratory phase ventricular interdependence).