Introduction
Technology used in tandem with active learning can pave the way for improving the quality of teaching throughout science disciplines. The rapid advancement of technology has brought forth discussions on best practices in utilizing technology in education. Teachers, with support from administrators, must manage technology-rich classrooms while maintaining student engagement (Sandlots 1997). Enhanced educational experiences using technology have been implemented in science classrooms; these include the use of computer technologies (Dori, 1997) and clickers (MacArthur, 2008). Several organizations have developed online interactive educational capabilities, such as the University of Colorado’s PhET models (Moore, 2014) and nanoHUB’s 400 science simulation tools and 4500 resources which reach 1.4 million users (Madhavan, 2013). These technological advancements have led to the development of innovative pedagogical designs in science curricula. These technology-based pedagogical advances have been instrumental in improving the achievement gap in underrepresented student populations.
Active learning in science curricula has been an area of focus exemplified by the establishment of common core assessments, as well as by the cultivation of Next Generation Science Standards (NGSS). The utilization of active learning techniques such as problem-based learning (PBL), process-oriented guided inquiry learning (POGIL) and peer-led team learning (PLTL) has been shown to increase student engagement in science classes (Eberlein, 2008). In order for students to achieve the expectations of NGSS, novel use of technology in the classroom can foster an environment of these student-centered active learning techniques.
Technology-dependent pedagogical designs have been explored. For example, a flipped classroom, which utilizes online resources such as video lectures that students access outside of the classroom enables faculty to focus on in-class problem solving. Jonathan Leo and Kelly Puzio found that flipped classroom pedagogy had a positive effect on student achievement in a 9th grade biology classroom, leading students to deeper understandings of principles in biology (Leo & Puzio, 2016). However, although students in the classroom described by Leo and Puzio preferred out-of-class use of online media such as videos and lectures to learn basic scientific principles, these flipped classroom approaches did not lead these learners to the insight and deeper understanding that in-class active learning activities can provide. The flipped classroom strategy is valuable primarily because it sets the stage for educators to enrich students’ in-class time through active problem solving and activities that promote deeper scientific understanding and insight.
In a separate study, J.H. Rivera describes the use of virtual and simulated labs in a blended classroom, and proposes the potential for using technology concurrently with traditional lecture approaches to provide an optimal learning environment for science majors at colleges and universities (Rivera, 2016).
Utilizing advanced technologies in conjunction with active learning pedagogical approaches has been a successful model in helping close achievement gaps in science education. There exists a disparity of participation and success between genders and specific ethnic groups in STEM education fields (Else-Quest, 2013). It is imperative that we as a society devise strategies that reduce these achievement gaps in order to optimize success in the sciences for all students, from all backgrounds. Fostering an environment of inclusion that taps the minds of students left behind in underfunded and ignored urban K-12 schools will provide the impetus for greater science discoveries. Inequities in the availability of technologies to these students have been a major barrier to the success of these students (Brown, 2000). Integrating advanced technologies (Mayer-Smith, 2000) with active-learning activities can promote an inclusive experience and provides disproportionate benefit to underprepared students, thereby, helping to reduce the achievement gap between the disadvantaged and non-disadvantaged student (Haak, 2011). Obtaining increased diversity in science education is essential and achievable (Wilson, 2014), but a major barrier to this goal is insufficient technologies available in the urban classroom (Shin, 2003). Advances in computer simulations and in remotely accessible instrumentation can help shatter this barrier.
Access to high-end technologies such as microscopes has been shown effective for increasing a student’s understanding of scientific theories (Penn, 2007). For example, the use of a scanning electron microscope (SEM) can stimulate an interest in science, motivating kinesthetic learners to seek more traditional in-class knowledge (Furlan, 2009). Increasingly, affordable hands-on activities and technology that can make a facile transition to the classroom are becoming available. In an important current trend, remote access laboratories (Lowe, 2013) and simulations (Sauter, 2013) that lead to increased student engagement are being deployed. One example involves the utilization of remotely accessible microscopy, which has been shown to complement a histology laboratory (Munoz, 2014), promoting active and independent student work. Designing remote learning environments in which students can control advanced technologies, such as an SEM, can make science seem more “real” (Childers, 2015). This is the ultimate goal of the RAIN network: To bring hands-on, authentic scientific opportunities to the science classroom to stimulate students’ interest in science and cultivate student success.
Methodologies of RAIN
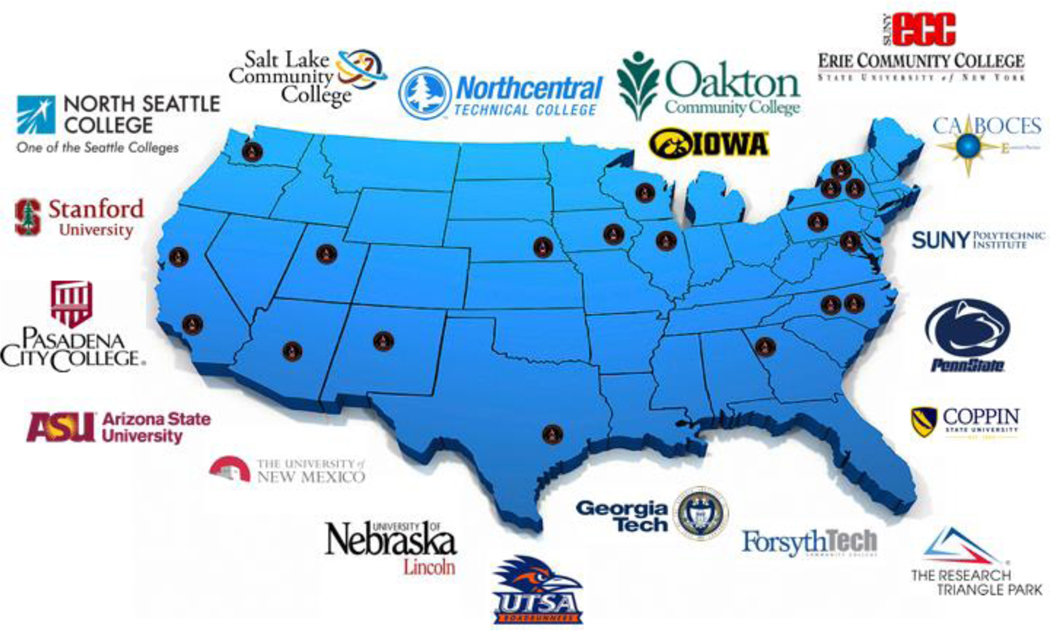
RAIN is a network of nineteen universities and community colleges (Figure 1) seeking to bring free access to advanced technologies in educational settings. These settings range from K-12, undergraduate science courses and technical degree programs. Anyone can access RAIN at nano4me.org/remote access.
Figure 1:
RAIN campuses across the United States
Each RAIN site has access to various high-end technologies used in advanced science laboratories. These instruments are expensive and without RAIN they would not be available at most high schools, community colleges or undergraduate labs within four-year universities. All learners, regardless of school funding, will benefit from the effortless use of the free facilities. The following is a description of several of the institutions participating in and making instruments available through the RAIN Network.
Penn State Nanotechnology Applications and Career Knowledge Network
A collaborative effort led by the Pennsylvania State University Nanotechnology Applications and Career Knowledge Network (NACK) was the impetus for the formation of RAIN. NACK is an Advanced Technology Education (ATE) Center that promotes increasing the nanotechnology workforce by sharing resources, and providing nano-based course materials and educational workshops. The NACK National Support Center for Nanotechnology Workforce Development has a mission to provide assistance to existing or developing micro and nanotechnology workforce education programs at community or technical colleges. NACK also advocates for universities to form partnerships with the community and technical colleges within the NACK Network. Within RAIN, the Penn State facility boasts the most remotely accessible instruments and the highest usage rate of instruments. The following instruments are available for remote use at Penn State.
Scanning Electron Microscope (SEM)
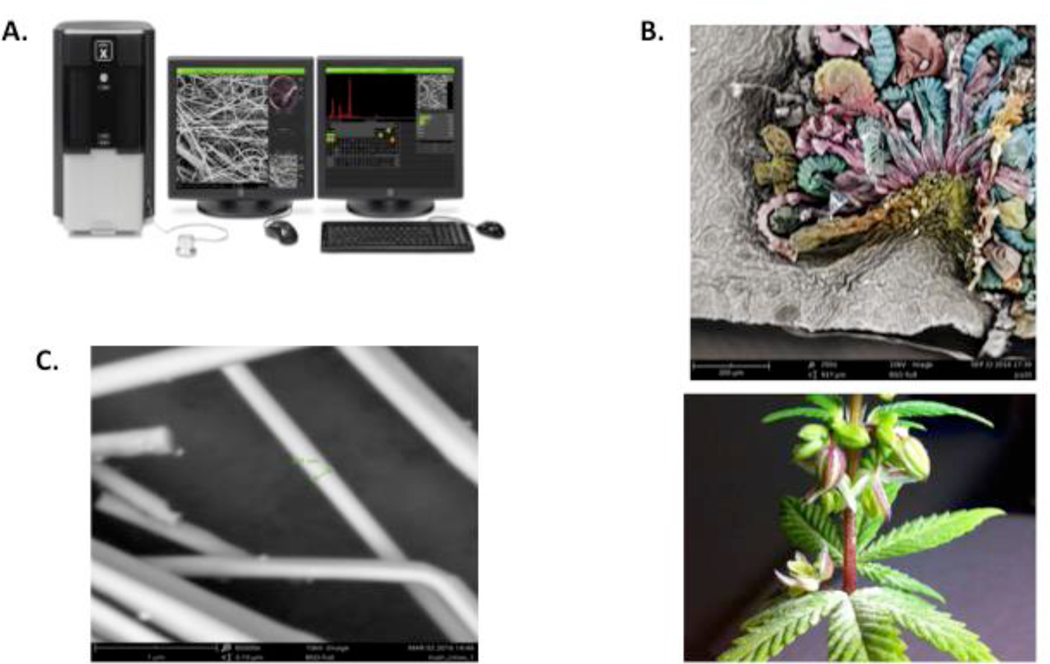
In 1937 Manfred von Ardenne used a focused electron beam to scan a rectangular pattern known as a raster. This was the advent of the scanning electron microscope (Figure 2A). In an SEM, an electron beam is scanned over a sample, causing electron interactions with atoms from the sample. Through several different types of interactions, various signals are obtained that create an image of the scanned sample, such as the hydrothermal worm (Figure 2B) taken by Philippe Crassous using a Quant SEM for the 2010 FEI Owner Image Contest at 525x magnification.
Figure 2:
(A) Scanning Electron Microscope (B) SEM image of Hydrothermal worm (C) SEM image of the wings of a blue morpho butterfly
A variety of images can be obtained, including dry or wet biological samples, as well as conductive, or nonconductive samples (presuming these latter samples are coated with an electrically conducting material). Sample preparation and imaging processes are uncomplicated and the imaging process via remote access is well-suited for any educational setting due to the ease of use inherent in SEM instrument design. Samples are imaged in a vacuum at room temperature (cryogenic attachments do exist that allow for imaging at low temperatures) and can be imaged at a resolution of 1 nanometer. The majority of RAIN SEM instruments can visualize specimens at the 100 nanometer range.
The Zeiss 55 Ultra SEM at Penn State uses a confined electron beam to image samples. Surface morphology can be attained using secondary electron detectors. The samples are bombarded with electrons and the detector collects the emitted electrons as an outcome of the bombardment under high vacuum conditions. The Zeiss 55 Ultra can achieve a resolution between 1–3 nanometers.
Figure 2C shows the wings of a blue morpho butterfly. This species has distinctive blue color on their wings due to the color that is formed with the aid of light scattering. Light gets diffracted by the nano-scale apertures on the wings, such that some reflected wavelengths are filtered out. This phenomenon produces structural blue coloration of the butterfly wing. The nano apertures generally have a width ranging from 400 nm up to 600 nm.
Energy Dispersive Spectroscopy (EDS)
Energy-dispersive X-ray spectroscopy (Figure 3A) is an instrument used for elemental analysis. As is true of human fingerprints, each element exhibits a unique pattern in its X-ray emission spectrum. When an electron beam is focused on a sample, the high-energy beam excites the atom’s electrons from ground state to higher, discrete energy levels, resulting in a cascading effect wherein electrons throughout the atom will jump from electron shell to electron shell. This movement of electrons leads to emission of X-ray signals from the sample due to energy differences that occur from electron movement.
Figure 3:
(A) EDS spectrum and elemental analysis of the mineral titanite (B) SEM image of titanite (C) Energy-dispersive X-ray spectrometer (D) SEM image and EDS analysis of silver nanowires
Emitted X-rays are analyzed with a detector and provide information regarding the shape of the features on the sample. X-ray spectroscopy can differentiate the chemical composition of a sample from X-ray peaks on the spectrum that are distinctively associated with particular elements. The SEM image (Figure 3B) and X-ray emission spectrum (Figure 3C) of a titanite mineral shows an elemental composition of oxygen, silicon, titanium and calcium with percent abundances of 61.7, 13.9, 12.8 and 11.6 percents, all with certainties greater than 98%.
An Oxford Instruments X-act EDS is incorporated to the Zeiss Ultra 55. X-ray emission can be obtained via electron bombardment on samples. For example, imaging and elemental analysis of silver (Ag) nanowires (Figure 3D) show varying diameters ranging between hundreds of nanometers down to several tens of nanometers. The chemical composition (shown in the inset) consists of a combination of oxygen (57%), silicon (25%), sodium (6.6%), copper (3.8%), calcium (2.2%), magnesium (2%) and silver (1.3%) along with small traces of other elements. This composition corresponds with the synthesis of silver nanowires, which were fabricated on top of sputter-coated copper coatings on a glass substrate made from silicon, oxygen, sodium, calcium and magnesium.
Atomic Force Microscope
Atomic Force Microscopy (AFM) is an imaging technique that utilizes the atomic forces between a sample and a cantilevered probe tip to scan the surface of a sample, utilizing the reflection of a laser and a photodetector to track the surface for imaging and measurement. Penn State’s Nanosurf AFM (Figure 4A) can image and measure at a resolution smaller than a nanometer, which is 1000 times better than the optical diffraction limit. The AFM can be used to establish the size of nanoparticles (Figure 4B) where the heights of ultra-short carbon nanotubes were determined to be between 1.1 and 2.1 nm (Figure 4D) using the scanning mode of the AFM. Scanning mode AFM is a method in which the probe is scanned directly over a sample in order to image and measure the surface topography of infinitesimal materials. Tapping mode, an alternative scanning technique, involves setting the probe to oscillate continuously at the surface of the sample, tapping the sample in order to obtain an image. Tapping mode is advantageous due to decreasing interactions with the sample, eliminating sample degradation as well as preventing movement of the sample as the sample is being scanned.
Figure 4:
(A) Nanosurf Atomic Force Microscope (B) AFM image of ultra-short carbon nanotubes (C) AFM scan of a DVD (D) Height analysis of ultra-short carbon nanotubes
Figure 4C depicts a scan of a DVD sample obtained from a Bruker Innova AFM. The holes correspond to the bits that are used to store information. Scratches, mainly caused by handling the DVD with tweezers, are observed in the image. Some of the bits have clearly been deformed by these scratches. The software-generated color of the AFM image depicts the relative heights of the bits. These “nanoholes” representing the bits have an observable depth of roughly 100 nm.
UV-vis Spectrophotometer
The Cary 300 UV-vis spectrophotometer (Figure 5A) is used to investigate the optical response of synthesized solutions. It scans from 200 to 800 nanometer wavelengths, collecting the transmission and absorption spectrum of solutions placed in a quartz cuvette. Gold (Au) nanoparticle size can be determined using a UV-vis spectrometer. Absorption of the Au nanoparticles with roughly 30 nm diameter shows a peak at approximately 520 nm (Figure 5B) due to localized surface plasmon resonance (LSPR). The addition of the sodium chloride (NaCl) into the Au solution is expected to ionize the salt and reduce the effect of LSPR and thereby the total absorption of the solution. The procedure for Au nanoparticle synthesis and characterization using remote access to the UV-vis spectrometer are located on the RAIN website.
Figure 5:
(A) Varian Cary 300 Ultra-Violet/Visible Spectrometer (B) Wavelength analysis of colloidal gold solution using UV-VIS
Profilometer
The Veeco Dektak 6M profilometer (Figure 6A) consists of a probe that gathers one dimensional scans along the surface of the sample together with an integrated microscope. This characterization tool is used to investigate patterns created on surfaces such as occur after the completion of lithographic manufacturing steps used in developing silicon-based microchips. Patterned structures (Figure 6B) can show the heights of etched silicon prior to adhesion of electro-mechanical devices, such as resistors and transistors to the silicon surface. The profilometer’s probe is aligned such that it takes a scan along the dashed arrow shown in the inset. During this mechanical scan, the probe encounters a narrow aluminum feature, leading to its height measurement of 100 nm and width of ~200 μm. One can also investigate the surface roughness along the scanning direction. In the example shown, a tiny contaminant causes a sharp peak right after the narrow hill.
Figure 6:
(A) Veeco Dektak 6M Profilometer (B) Height analysis completed at the end of a patterned lithography experiment
Seattle’s Hub for Industry-driven Nanotechnology Education
Seattle’s Hub for Industry-driven Nanotechnology Education (SHINE) was established as a National Science Foundation (NSF) ATE Regional Center at North Seattle College (NSC) in 2012. NSC is a member of the Seattle Colleges District, the second largest district in the state serving nearly 50,000 students each year. SHINE also serves as the destination for interested students from across the Pacific Northwest (PNW) region to enter into and pursue a formal undergraduate nanotechnology education program, providing an educational pathway that exists nowhere else in the PNW. SHINE’s two-year college program at NSC continues to expand, preparing graduates for immediate technician-level employment or for transfer to a 4-year institution to pursue an advanced degree. SHINE serves as a model and a resource for emerging nanotechnology programs in the region.
SHINE developed a state-of-the-art user facility at NSC—the Nano Lab—as a core resource in its work with students, educators and industry. Since joining the RAIN Network, SHINE’s Nano Lab Facility has hosted 30 remote access sessions, bringing nanotechnology to over 500 students and teachers across the nation who otherwise would not have access to specialized equipment. SHINE has remote access capabilities to an Aspex Explorer Scanning Electron Microscope with Energy Dispersive X-ray Spectroscopy, Bruker DektakXT Surface Contact Profilometer, NanoSurf EasyScan 2 Atomic Force Microscope and an Olympus Fluoview FV10i confocal microscope (Figure 7A).
Figure 7:
(A) Olympus Fluoview FV10i Confocal Microscope (B) Tahr ovary epithelium cells (C) Mutagenic cells (D) Lily pollen
Confocal Microscope
Confocal microscopy utilizes point illumination and a pinhole to eliminate out of focus signals when imaging a sample. It is a noninvasive technique that does not cause degradation to the sample. Sample fluorescence is required to generate 2D (Figure 7B and 7C) and 3D images, so specimens for this technique must autofluoresce or be treated with fluorescent dyes that bind to an area of interest in the specimen. The confocal microscope is often used for biological applications, but it has also been implemented in nano-crystal imaging. The Olympus Fluoview instrument consists of a solid-state laser diode with excitation wavelengths of 450 nm, 535 nm, 570 nm and 620 nm. It comes equipped with a 10x and 60x oil-immersion objective lenses, photomultiplier tube fluorescent photon detectors and a fully enclosed vibration isolated bench-mount system. Figure 7D is a 3D image of lily pollen obtained by the SHINE Nano Lab; the image shows the 3D rendering of the sample that is inherent in confocal microscopy imaging.
Erie Community College
Erie Community College (ECC)completed construction of a state of the art Class 10,000 (ISO 7) cleanroom (Figure 8A). The cleanroom consists of a gowning area, airlock, clean storage area and a cleanroom totaling about 1,600 square feet. There are 12 HD cameras mounted on the ceiling. The cameras are set up to transmit lab demonstrations anywhere in the world. There are several network connections that enable remote sessions using a JEOL JSM-6010LA Scanning Electron Microscope (SEM) with EDS attachment.
Figure 8:
(A) Cleanroom at Erie Community College (B) 5 μm x 5 Pm scan of Blu-ray disc by AFM (C) Micro-Electro-Mechanical Systems (MEMS) chip optical image
Recently Erie Community College acquired an AFM which will be utilized in ECC characterization classes and also will be made available for remote access. Figure 8B illustrates an image of a Blu-ray disc obtained from the AFM. A unique instrument at ECC is a Zeiss Axio Imager D1.M Optical Microscope. Students can characterize multiple samples efficiently; the micro-electro-mechanical systems (MEMS) chip shown in Figure 8C provides one example.
In future, the ECC site plans to obtain a JEOL IT-600 SEM with preinstalled options to accept the Nabity E-bean lithography system and a Perkin Elmer UV-Vis 750 Spectrophotometer that will analyze both solid and liquid samples. ECC also has a micro plotter available that can print patterns with conductive inks at a resolution as low as 10um.
Salt Lake Community College
Salt Lake Community College (SLCC) has a microscopy laboratory as a core resource for students and faculty in science and engineering programs. Prior to and since joining the RAIN network the SLCC microscopy lab has provided remote demonstrations for classrooms on and off campus for K-12 schools, colleges and universities. SLCC has a diverse set of microscopy tools for investigating the micro and nanoscopic worlds. Electron microscope work can be performed with the Hitachi TM 3000 tabletop scanning electron microscope for imaging and attached Bruker XFlash 430-H EDS for elemental analysis. Recently SLCC acquired a Delong Instrument LVEM5 transmission electron microscope (TEM), which provides high resolution imaging in transmission, scanning transmission (STEM), and diffraction modes.
The TEM configures images by transmitting electrons through ultra-thin samples where they interact and are then collected. Bright field images are generated by collecting the transmitted electrons whose intensity will vary due to the thickness of the sample. Diffraction patterns are collected from electrons that are scattered due to diffraction from the planes or regular structure of the sample. Figure 9A displays a diffraction pattern generated by layers of graphene. Note the hexagonal symmetry of the spots in the figure. Samples can also be imaged in a scanning transmission electron mode similar to traditional SEM equipment.
Figure 9:
(A) Diffraction pattern of graphene (B) AFM scan of PVP crosshatch nanogrids (C, D) Cancer cells
SLCC has a trio of surface probe microscopes. The Agilent 5400 scanning probe microscope can operate in both scanning tunneling (STM) and atomic force (AFM) modes. In addition to dual modes of operation this instrument has liquid cell capabilities. Complementing the Agilent instrument is a pair of AFMs from Nanosurf, an easyScan 2 AFM and the fully portable NaioAFM. Figure 9B is an AFM scan of polyvinylpyrrolidone (PVP) crosshatch nanogrids made using a standard nano-lab which can be easily performed in any science classroom.
In addition to microscope equipment, SLCC has a variety of specimen preparation equipment. The Buehler isomet low speed diamond saw and Buehler mounting and polishing equipment can be used to prepare metallurgical and ceramic samples. A Denton Desk V sputter coating machine is used to coat nonconductive samples with copper, gold or silver for electron microscope inspection. Nanoparticles, nanowires or nanotubes can be separated with ultrasonic and centrifuging equipment. Biological samples can be prepared using a Leica EM CPD300 critical point dryer. Figure 9C and 9D show cancer cells grown in the SLCC biotechnology program which were fixated and dried in the critical point dryer so they can be safely imaged in the vacuum environment of the scanning electron microscope. As shown in the image, the cells were actually dividing when the samples were prepared for imaging.
Pasadena City College
RAIN has become an integral aspect of the chemistry department at Pasadena City College (PCC). A remotely accessible Phenom ProX SEM with EDS attachment from Nanoscience Instruments (Figure 10A) has been made available for outreach to local K-12 schools and provides research experiences for students at PCC. A high percentage of PCC students from underrepresented backgrounds are enrolled in the science pathway program. Access to these high end instruments leads to increased passion and engagement in the sciences in both STEM majors and students who are as yet undecided about their academic track.
Figure 10:
(A) Phenom ProX SEM/EDS instrument at PCC (B) Top: Fern sporangia image from SEM Bottom: Fern plant (C) SEM image of nickel nanowires, with the diameter of one indicated to be 201 nm
Students from the PCC community can directly use the SEM instrument and obtain nanoscale images, such as the fern sporangia (Figure 10B). Bridging the PCC community with the wider educational community of Los Angeles, PCC is working with K-12 schools to develop remotely accessible labs to integrate into existing science curricula. The K-12 outreach is a combined effort of PCC faculty, students and industry professionals. The labs are available on the NACK RAIN site. These labs involve using steel wool to measure percentage of oxygen in the air, determining an unknown mineral sample using chemical and physical characteristics, and studying phytotoxicity of nanomaterials using mung beans. These labs, as well as several other nano-based experiments available at the NACK RAIN site, can be performed at a low cost and the procedures include imbedded remote access activities for learners.
Arizona State University/NCI-SW
The Nanotechnology Collaborative Infrastructure Southwest (NCI-SW) is based at Arizona State University (ASU), and is one node of the National Nanotechnology Coordinated Infrastructure (NNCI) funded by the NSF. The NNCI is a collection of 16 schools and affiliated partners that have agreed to make their laboratories and in-house expertise available to academic and industrial users from across the nation, to strengthen the national infrastructure supporting nanotechnology discovery and innovation.
The NCI-SW joined RAIN in early 2016, with the objective of providing remote access as part of its education and outreach mission. A Phenom Pro SEM from Nanoscience Instruments (similar to the unit at PCC) is used to introduce nanotechnology to K-12 students in their classrooms and to members of the general public in the Phoenix metropolitan area, using examples such as nickel nanowires (Figure 10C). The NCI-SW also reaches out to rural communities across Arizona as well as the southwest – through the extensive network of community college programs managed by one of its partners, Science Foundation Arizona – and offers remote access to the SEM. The Maricopa County Community College District, another NCI-SW partner, has developed a two-year AAS degree program in nanotechnology at one of its campuses (Rio Salado College). The remote access capability will also be used in a portion of the laboratory sessions in the curriculum.
University of Texas at San Antonio (UTSA)-Kleberg Advanced Microscopy Center
The Kleberg Advanced Microscopy Center (KAMC) started in 2003 with the UTSA Physics and Astronomy Department, and was tasked with the study of nanomaterials, and polymeric and photonic structured materials. After incorporation of a JEOL-ARM200F in 2010, and X-ray Diffraction (XRD) and Focused Ion Beam (FIB) capabilities in the past two years, UTSA has one of the most complete electron microscopy centers in the country for the study of nanomaterials. Equipped with state-of- the-art instruments in electron microscopy and other advanced microscopy equipment, KAMC is focused on high-resolution imaging, electron diffraction, electron holography, electron tomography, cryo-TEM and Electron energy loss spectroscopy (EELS). In addition, some in situ electron microscopy measurements can be performed: mechanical, electrical and optical measurements. The center produces world-class research on nanotechnology, biology, chemistry, and condensed matter.
The center actively promotes collaboration with other institutions and providing researchers and students with access to instrumentation. KAMC is a user facility that operates on a philosophy of empowering researchers, including undergraduates, to use equipment themselves. KAMC participates in the Research Centers in Minority Institutions (RCMI) Program within the “Nanotechnology and Human Health Core” and provides students with hands-on experience of materials characterization techniques through courses, workshops and outreach activities. Microscopy training is provided to students in the Advanced Materials Technology program from Northwest Vista College, part of the Alamo College system.
KAMC’s idea of providing researchers and students with remote access to microscopes has been a constant goal since the arrival of their ARM200F TEM. KAMC achieved this goal by joining the RAIN network. Currently, the center participates with the HR-STEM Hitachi S5500 (Figure 11A), a field-emission gun SEM for high spatial resolution (0.4 nm), equipped with bright and dark field STEM detectors in addition to secondary- and backscattered-electron detectors. Spatially resolved chemical analysis, line scan and mapping is possible using the Bruker EDS system attached to the microscope. Figure 11B demonstrates elemental mapping capabilities of the STEM. The green dots in the image represent gold particles in the star shaped structure. A second image (Figure 11C) is a cluster of gold nanoparticles of different sizes and shapes.
Figure 11:
(A) Hitachi S5500 STEM microscope (B) Gold star-shaped particle and EDS maps (C) Group of gold nanoparticles
Other RAIN sites exist across the United States, including Forsyth Technical Community College in North Carolina. Forsyth College has a Nanotechnology Program that utilizes experiential learning to teach the fundamental concepts of nanotechnology in order to train their students for employment in industry and academia. Forsyth College provides access to an AFM for remote purposes and sites at Northcentral Technical College in Wisconsin and Oakton Community College in Illinois have remote access instruments available for use.
Discussion
Since RAIN’s inception in 2014, over 100 remote sessions have been requested by and provided to 36 K-12 education sites, 35 two-year institutions and 16 four-year colleges. Additionally, RAIN has hosted a webinar with over 50 educators from across different science disciplines and academic levels. In some instances, the remote access requests have been made specifically to target underrepresented groups of learners. In one case, for example, an enrichment program was developed for and delivered to underrepresented high school students at Suffolk Community College. In October 2016, RAIN also hosted “It’s RAINing Across America Open House” in support of National Nanotechnology Day. Nine RAIN sites participated in this event which provided teachers and students with opportunities to make remove use of a SEM, the most utilized instrument in the RAIN collection. It is common for students and teachers to image a variety of entities, including butterfly wings and gold nanoparticles (Figure 12).
Figure 12:
SEM images of (A) Butterfly wing (B) Gecko feet (C) Ant eye
RAIN currently has seven remote access laboratory guides, and the goal of adding more in the near future. Member institutions in the RAIN network are seeking to develop unique experiments that incorporate remote access. Educators can use these labs, which are NGSS compliant, in conjunction with their curricula. Students are given background information on each experiment, perform labs and prepare the samples for use in remote access sessions. Currently, RAIN has several nano-based labs in which students synthesize and characterize colloidal gold (McFarland, 2004) and silver nanoparticles (Sanders, 2014), perform electrodeposition of nickel nanowires (Bentley, 2005) and make dye-sensitized nano-crystalline solar cells (Gratzel, 2001). Several fundamental chemistry labs are also available based on studying the phytotoxicity of nanomaterials on mung beans (Ross, 2016), determining percent of oxygen on air using steel wool (Birk, 1981) and a lab involving inquiry based mineral identification(Xornam, 2006). RAIN also has a video resource library available for educators to share with their classes. The videos provide an introduction to remote access, background information and operation of the various remote access tools and instructions on how to remotely control the instruments. Videos are also available on SEM and AFM for students to familiarize themselves with the theory of these devices.
Data were collected on the effectiveness of the remote access experience for teachers (Figure 13) and students (Figure 14). Of the 34 educators who responded to RAIN’s survey, 33 felt that remote sessions went extremely well or were a good experience. Only one participant felt the activity did not go well. All but one said they would recommend remote access to a colleague. This demonstrates the effectiveness of RAIN from teachers’ perspectives. The majority of teachers felt that their remote access session facilitated their students’ understanding of scientific applications, that remote access motivated their students, and/or that the experience fulfilled outcomes for the course in which the remote access was performed. Overall, teachers who have participated in RAIN have had a fulfilling experience and feel that the remote access session contributed positively to the classroom environment. The following is a collection of individual teacher’s responses to conducting and participating in remote sessions using virtual instrumentation provided through the RAIN network:
Figure 13:
Teacher responses to effectiveness of RAIN (34 responses)
Figure 14:
Student responses to effectiveness of RAIN (198 responses)
Instructor 1: “I think this is a great resource for students across the country who may not be having ready access to state of the art nanotechnology tools.”
Instructor 2: “My students benefited a lot from this special learning environment.”
Instructor 3: “The remote access was pretty awesome.”
From a teacher’s perspective, RAIN shows potential to be a valuable tool. Most importantly, data show that RAIN has had a positive influence on student’s passion and increased motivation for student success in the sciences. Out of 198 students surveyed from a variety of educational settings, 87% of students rated themselves as having been either very or somewhat satisfied by the experience. Only 3% of the students expressed dissatisfaction with experience. Out of the 198 students, 129 found the session fun and interesting, and expressed a desire for remote access experiences in future educational settings. A high percent of students also felt the remote session activity was a great supplement to their standard lab and course material, and that the virtual labs helped demonstrate the relationship among the mathematics and sciences and technology and engineering. What follows are several individual student responses to the remote session:
Student 1: “There is nothing that could improve or would make the remote lab better. It was an amazing experience and at the same time a great honor to be part of this remote lab!”
Student 2: “Yo this is some slick stuff! 10/10 would do again. This was awesome!”
Student 3: “This was an exciting, fun learning experience. It would be a great addition to the class and learning experience to incorporate more of these sessions during the remaining of the semester and semesters to come.”
Student 4: “I thought remote access was an amazing tool to have in my science career. This was exactly what I was missing when I did research in high school!”
Students’ responses suggest to us that increased availability to remote access labs can provide an important new resource for STEM classroom learning and warrants continued support for the RAIN program.
RAIN is a community seeking to bring novel technologies to all educational settings in an uncomplicated manner at no cost to the institution gaining access. We believe this technology will enhance students’ passion and motivation to seek an education in the sciences. Remote access labs combined with hands-on activities involving advanced technologies can spark learners’ interest. We suggest readers explore the prospect of using this resource in their own classroom settings by signing up for a remote access session at http://nano4me.org/remoteaccess.
Table 1:
RAIN sites and Instrumentation
| RAIN Site | Remote Access Instruments |
|---|---|
| Arizona State University | SEM |
| Erie Community College | SEM/EDS |
| Forsythe Tech Community College | AFM |
| Northcentral Technical College | SEM, AFM, Flex AFM |
| North Seattle College | Confocal Microscope, AFM, Profilometer, SEM/EDS |
| Oakton Community College | SEM/EDS, Flex AFM, Profilometer |
| Pasadena City College | SEM/EDS |
| Pennsylvania State University | FESEM/EDS, SPM/AFM, Profilometer, UV-vis |
| Salt Lake Community College | SEM, AFM/SPM |
| University of Texas at San Antonio | SEM/EDS |
Acknowledgements:
RAIN is a network supported by grants from the following: The National Science Foundation under Grant Number DUE 1204279. The Nanotechnology Collaborative Infrastructure Southwest is supported by the National Science Foundation under Grant No. 1542160. Any opinions, findings, and conclusions or recommendations expressed in this paper are those of the authors and do not necessarily reflect the views of the National Science Foundation. Esteban Bautista is supported by BUILD PODER, which is funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number RL5GM118975. The National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Works Cited
- Apedoe XS, Walker SE and Reeves TC, (2006). Integrating Inquiry-based Learning into Undergraduate Geology. Journal of Geoscience Education. 54(3), pp. 414–421. [Google Scholar]
- Bentley AK, Farhoud M. and Ellis AB, (2005). Template Synthesis and magnetic Manipulation of Nickel Nanowires, J. Chem. Educ, 82(5), 765. [Google Scholar]
- Birk JP, McGrath L. and Gunter SK, (1981). A General Chemistry Experiment for the Determination of the Oxygen Content of Air, J. Chem. Educ, 58(10), 804–805. [Google Scholar]
- Brown Monica R., (2000). Access, Instruction and Barriers Technology Issues Facing Students at Risk, Remedial and Special Education, 21(3), 182–192. [Google Scholar]
- Childers G. & Jones MG (2015). Students as Virtual Scientists: An exploration of students’ and teachers’ perceived realness of a remote electron microscopy investigation. International Journal of Science Education, 37(15), 2433–2452. [Google Scholar]
- Dori YJ & Barnea N. (1997). In-service chemistry teachers’ training: the impact of introducing computer technology on teachers’ attitudes and classroom implementation. International Journal of Science Education, 19(5), 577–592. [Google Scholar]
- Eberlein T, Kampmeier J, Minderhout V, Moog RS, Platt T, Varma-Nelson P. & White HB, (2008). Pedagogies of engagement in science: A comparison of PBL, POGIL and PLTL. Biochem. Mol. Biol. Educ, 36(4), 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Mineo CC & Higgins A, (2013). Math and Science Attitudes and Achievement at the Intersection of Gender and Ethnicity, Psychology of Women Quarterly, 37(3), 293–309. [Google Scholar]
- Furlan PY, (2009). Engaging Students in Early Exploration of Nanoscience Topics Using Hands-On Activities and Scanning Tunneling Microscopy, J. Chem. Educ, 86(6), 705–711. [Google Scholar]
- Gratzel M. (2001). review article Photoelectrochemical cells, Nature, 414, 338–344. [DOI] [PubMed] [Google Scholar]
- Haak DC, HilleRisLambers J, Pitre E. & Freeman S. (2011). Increased Structure and Active Learning Reduce the Achievement Gap in Introductory Biology. Science, 332(6034), 1213–1216. [DOI] [PubMed] [Google Scholar]
- Leo J. & Puzio K. (2016). Flipped Instruction in a High School Science Classroom. Journal of Science Education and Technology, 25(5), 775–781. [Google Scholar]
- Lowe D, Newcombe P. & Stumpers B. (2013). Evaluation of the Use of Remote Laboratories for Secondary School Science Education. Research in Science Education, 43(3), 1197–1219. [Google Scholar]
- MacArthur JR & Jones LL (2008). A review of literature reports of clickers applicable to college chemistry classrooms. Chem. Educ. Res. Pract, 9, 187–195. [Google Scholar]
- Madhavan K, Zentner L, Farnsworth V, Shivarajapura S, Zentner M. Denny N. & Klimeck G. (2013). nanoHUB.org: cloud-based services for nanoscale modeling, simulation and education. Nanotechnology Reviews, 2(1), 107–117. [Google Scholar]
- Mayer-Smith J, Pedretti E. & Woodrow J. (2000). Closing of the gender gap in technology enriched science education: a case study. Computers and Education, 35(1), 51–63. [Google Scholar]
- McFarland AD, Haynes CL, Mirkin CA, Van Duyne RP and Godwin HA, (2004). Color My nanoworld, J. Chem. Educ, 81(4), 544A. [Google Scholar]
- Moore EB, Chamberlain JM, Parson R. & Perkins KK (2014). PhET Interative Simulations: Transformativ Tools for Teaching Chemistry. J. Chem. Educ, 91(8), 1191–1197. [Google Scholar]
- Muñoz A L, López JL Use of virtual microscopy to promote histology learning. Microscopy: Advances in Scientific Research and Education 2014: 1210–1213. [Google Scholar]
- Penn RL & Johnson P, (2007). Building a Successful Middle School Outreach Effort: Microscopy Camp, J. Chem. Educ, 84(6), 955–960. [Google Scholar]
- Rivera JH (2016). Science-based laboratory comprehension: an examination of effective practices within traditional, online and blended learning environments. Open Learning: the Journal of Open, Distance and eLearning, 31(3), 209–218. [Google Scholar]
- Ross SS, Owen MJ, Pedersen BP, Liu G. and Miller WJW, (2016). Using Mung Beans as a Simple, Informative Means to Evaluate the Phytotoxicity of Engineered Nanomaterials and Introduce Concept of Nanophytotoxicity to Undergraduate Students, J. Chem. Educ, 93(8), 1428–1433. [Google Scholar]
- Sanders WC, Ainsworth PD, Archer DM, Armajo ML, Emerson CE, Calara JV, Dixon ML Lindsey ST, Moore HJ and Swenson JD, (2014). Characterization of Micro- and Nanoscale Silver Wires Synthesized Using a Single-Replacement Reaction between Sputtered Copper Metal and Dilute Silver Nitrate Solutions, J. Chem. Educ, 91(5), 705–710. [Google Scholar]
- Sandholtz JH, Ringstaff C. & Dwyer DC (1997). Teaching with Technology: Creating Student-Centered Classrooms, Teachers College Press, Teachers College, Columbia University. [Google Scholar]
- Sauter M, Uttal DH, Rapp DN, Downing M. & Jona K. (2013). Getting real: the authenticity of remote labs and simulations for science learning. Distance Education, 34(1), 37–47. [Google Scholar]
- Shin Y, (2003). Virtual Experiment Environments Design for Science Education, Proceedings of the 2003 International Conference on Cyberworlds, 388–395. [Google Scholar]
- Wilson ZS, McGuire SY, Limbach PA, Doyle MP, Marzilli LG, & Warner IM, (2014). An Inquiry into Successful Approaches in Chemistry, J. Chem. Educ, 91, 1860–1866. [Google Scholar]