Summary
Background
Introduction of the Omicron variant caused a steep rise in SARS-CoV-2 infections despite high vaccination coverage in the Danish population. We used blood donor serosurveillance to estimate the percentage of recently infected residents in the similarly aged background population with no known comorbidity.
Methods
To detect SARS-CoV-2 antibodies induced due to recent infection, and not vaccination, we assessed anti-nucleocapsid (anti-N) immunoglobulin G (IgG) in blood donor samples. Individual level data on SARS-CoV-2 RT-PCR results and vaccination status were available. Anti-N IgG was measured fortnightly from January 18 to April 3, 2022. Samples from November 2021 were analysed to assess seroprevalence before introduction of the Omicron variant in Denmark.
Findings
A total of 43 088 donations from 35 309 Danish blood donors aged 17–72 years were screened. In November 2021, 1·2% (103/8 701) of donors had detectable anti-N IgG antibodies. Adjusting for test sensitivity (estimates ranging from 74%–81%) and November seroprevalence, we estimate that 66% (95% confidence intervals (CI): 63%–70%) of the healthy, similarly aged Danish population had been infected between November 1, 2021, and March 15, 2022. One third of infections were not captured by SARS-CoV-2 RT-PCR testing. The infection fatality rate (IFR) was 6·2 (CI: 5·1–7·5) per 100 000 infections.
Interpretation
Screening for anti-N IgG and linkage to national registers allowed us to detect recent infections and accurately assess assay sensitivity in vaccinated or previously infected individuals during the Omicron outbreak. The IFR was lower than during previous waves.
Funding
The Danish Ministry of Health.
Keywords: SARS-CoV-2, Seroprevalence, Infection fatality rate
Research in context.
Evidence before this study
We searched PubMed for published research articles using the following search string and a combination of the included search terms: ((SARS-CoV-2) OR (COVID-19)) AND (seroprevalence) AND (Omicron). No language or date restrictions were imposed. At the initiation of the study period, we found no literature describing seroprevalence during the Omicron wave that began in November 2021 in Denmark.
Seroprevalence screening of blood donors has previously been employed to estimate the extent of SARS-CoV-2 infections in the population and the infection fatality rate. Previous studies of SARS-CoV-2 antibodies in Danish blood donors found that approximately 29%–64% of infections were captured by PCR tests during the preceding SARS-CoV-2 waves. However, the studies were performed prior to introduction of the SARS-CoV-2 Omicron variant and primarily among unvaccinated individuals.
Added value of this study
Through linkage of national SARS-CoV-2 surveillance data and anti-Nucleocapsid IgG measurements of Danish blood donors we were able to validate the sensitivity of the assay and to follow the evolving seroprevalence throughout the Omicron wave. We estimate that 66% (95% confidence interval (CI): 63%–70%) of the healthy, similarly aged Danish population had been recently infected and that one third of the infections were not detected in the national surveillance system. Infection fatality rate was low with a 30-day mortality of 6·2 (CI: 5·1–7·5) per 100 000 infections.
Implications of all the available evidence
The current initiative shows that seroprevalence study designs can be adapted and used to produce up-to-date knowledge of recent outbreaks, crucial both in the surveillance of SARS-CoV-2 and other future/emerging infections. We propose that blood donor serosurveillance is considered in future emerging infectious disease preparedness and surveillance plans.
Alt-text: Unlabelled box
Introduction
The SARS-CoV-2 variant Omicron (B.1.1.529) was first detected in Denmark November 28, 2021, and had become the dominant variant by mid-December 2021.1 Studies have suggested that the Omicron variant is able to better evade immunity in vaccinated or previously infected individuals as compared to the previously dominant Delta variant.2,3 In line with this, rapid transmission of the Omicron variant was observed during January and February 2022 when most restrictions were lifted in Denmark, a European country with high testing capacity, high vaccination coverage, and limited natural immunity acquired through SARS-CoV-2 infection.4 The actual number of SARS-CoV-2 infections in Denmark is likely substantially higher than observed based on RT-PCR test results due to asymptomatic infections and periodically strained test capacity.
The symptoms of infection with the Omicron variant are often milder than for the Delta variant with lower risk of severe disease and hospitalization.5,6 The frequency of mild and completely asymptomatic infections with the Omicron variant is unknown. In previous serosurveys of SARS-CoV-2 antibodies in Danish blood donors, 29% (CI: 24%–47%) of infections were captured by PCR tests during the first wave of the epidemic in the spring of 2020, while 64% (CI: 59%–69%) of infections were captured with the greater test capacity during the second wave in the winter of 2020/2021.7 Similar findings were reported from another Danish serosurvey.8
Anti-Nucleocapsid (anti-N) antibodies are produced after natural infection and not after vaccination with Spike based vaccines, which are the only vaccines that have been utilized in Denmark to date. It is known from previous studies that the concentration of anti-N IgG antibodies decreases over time,9,10 and it has been suggested that the sensitivity and/or specificity of anti-N IgG assays may change depending on vaccination status.11 Owing to the low SARS-CoV-2 incidence during summer and autumn of 2021 in Denmark and in light of the waning of anti-N IgG induced from earlier outbreaks in 2020 and early 2021, we expected that few donors would have a detectable anti-N antibody response in November 2021. National health register data with complete data on SARS-CoV-2 RT-PCR results and vaccination status, linked to our donor anti-N IgG surveillance data, allowed for the assessment of assay performance during the Omicron wave, where most recent infections were vaccine breakthrough and/or re-infections.
The aim of this study was to estimate the proportion of the adult healthy population in Denmark who had been infected with SARS-CoV-2 during the extensive surge of Omicron infections in the winter and spring of 2022 in Denmark, to assess the degree of underdiagnosis through the national SARS-CoV-2 RT-PCR test system, and to estimate the Infection Fatality Rate (IFR) among 17–72-year-old Danes without comorbidities.
Material and methods
Data sources
We collected blood samples from blood donors from each of the five administrative regions in Denmark and tested for anti-N IgG antibodies (see below). Anti-N IgG seroprevalence data was combined with information on the participating blood donors and the background population was obtained from the Danish COVID-19 surveillance and included: (1) SARS-CoV-2 RT-PCR test results measured in oropharyngeal swaps from residents in Denmark based on the Danish Microbiological Database;12 (2) information on underlying diseases based on diagnosis codes from the Danish National Patient Registry;13 (3) information on vital status and region of residence from the Danish Civil Registration System; (4) registrations of death from the Danish Register of Causes of Death; and (5) COVID-19 vaccination data from the Danish Vaccination Register. Population counts from the first quarter of 2022 were obtained from Statistics Denmark. All data sets were restricted to people aged 17–72 years. No sample size calculation was performed prior to initiation of the study. We followed the STROBE reporting checklist.
Study populations
-
1)
Seroprevalence baseline: Anti-N IgG measurements for blood donors who donated blood between October 26 and November 31, 2021. These donations were included to establish an anti-N IgG seroprevalence baseline before introduction of the SARS-CoV-2 Omicron variant in Denmark. Only anti-N IgG antibody measurements and basic characteristics were available for this cohort.
-
2)
Omicron seroprevalence: Anti-N IgG measurements for blood donors who donated blood in the study period (January 18, 2022, to April 3, 2022). Both Anti-N IgG antibody measurements and surveillance data were available for these serial cross-sectional studies of blood donors. Blood donations were collected and tested on alternate weeks in this study period.
-
3)
Background population: Surveillance data for all residents in Denmark aged 17–72 years on January 1, 2022. SARS-CoV-2 RT-PCR test results and death registered within 30 or 60 days of a positive RT-PCR test for SARS-CoV-2 were identified for this cohort. We excluded 7 953 positive SARS-CoV-2 PCR tests (0·4% of positive RT-PCR tests) due to missing information about administrative region.
Serological testing of blood donors in Denmark
Every week 5–6 000 blood donors aged 17–72 years voluntarily donate blood in Denmark. Blood donors constitute approximately 5% of the Danish population in the above-mentioned age stratum.
In the current study, we used the SARS-CoV-2 IgG assay (Abbott Diagnostics, Abbott Park, Illinois, United States) to identify infection-induced immunity. The Abbott SARS-CoV-2 anti-N IgG assay is based on the chemiluminescent microparticle immunoassay (CMIA) technique and was performed on automated Architect or Alinity systems. The anti-N IgG antibody result was interpreted as positive if the signal to cut-off (S/CO) ratio was above 1·4 as recommended by the manufacturer. The anti-N IgG antibody measurements were performed over six rounds with sampling on odd-numbered calendar weeks during the study period. We additionally tested donations from the first two weeks of November 2021, before Omicron was introduced into Denmark, thus representing a baseline for tracking subsequent increased seroprevalence consequent to Omicron infections. The specificity of the assay was previously assessed in a national validation in the laboratories also analysing the current samples (Abbott Alinity: 99·3% (98·3–99·7); Abbott Architect: 99·5% (98·5–99·8)).14
Statistical analyses
The assay sensitivity was assessed as the probability of anti-N IgG positive test results among blood donors with a recent PCR-confirmed SARS-CoV-2 infection. A recent SARS-CoV-2 infection was defined as a positive SARS-CoV-2 RT-PCR result on or after November 1, 2021, and until two weeks before the median test date of the given test week. This period, hereafter referred to as the PCR reference period, was chosen as donors were asked to self-defer for two weeks after they tested positive for SARS-CoV-2. If multiple positive tests had been recorded during the PCR reference period, the first was chosen. Infections were considered re-infections if the blood donor had a PCR-confirmed infection before November 1, 2021, and a minimum of 60 days prior to the re-infection.
The anti-N IgG antibody seroprevalence among blood donors by test week was calculated in a Poisson model. The estimated cumulative incidence of SARS-CoV-2-infected individuals in the background population was then calculated as the difference between the anti-N IgG seroprevalence in November and the seroprevalence for the given test week divided by the observed test sensitivity. The November seroprevalence was subtracted to both adjust for the assay specificity and to remove signals from earlier infections. Stratified versions of the test week-specific estimates were generated, stratified by sex, age (age strata: 17–35, 36–50, 51–60, and 61–72 years), administrative region, and whether the donor had previously been infected. In addition, the estimated cumulative incidence of SARS-CoV-2-infected individuals was weighted to ensure that the age, sex, and regional distribution of the donors each week and within each strata matched that of the general population. Each test week was considered independently and recurring donors could thus be included more than once. Confidence intervals for this combined measure were derived via the delta method.
We calculated the fraction of undetected SARS-CoV-2 infections as the ratio of cumulative SARS-CoV-2 RT-PCR confirmed infections among the background population in the PCR reference period divided by the estimated cumulative incidence of SARS-CoV-2-infected individuals. We also calculated the ratio of the development in the SARS-CoV-2 RT-PCR confirmed and the estimated infections between the test weeks. Confidence intervals for these ratios were derived by assuming each week's ratio was normally distributed and independent of the other weeks’ results and applying the delta method.
Furthermore, we estimated the IFR as the number of deaths within 30 or 60 days after a SARS-CoV-2 RT-PCR positive test result during the PCR reference period divided by the estimated number of SARS-CoV-2 infections in the population. The number of SARS-CoV-2 infections was calculated in two different ways: (1) as the number of confirmed SARS-CoV-2 infections during the PCR reference period (“the case fatality rate (CFR)”); and (2) the difference between our first and final estimates of SARS-CoV-2-infected individuals (IFR), which corresponds to the difference in estimated SARS-CoV-2 infections between January 5, 2022 and March 15, 2022.
The IFR estimations were weighted to match the general population aged 17–72 years excluding individuals with the comorbidities listed in Supplementary Table 1. Comorbidity was defined broadly covering both severe diseases (e.g., cancers) and benign diseases (e.g., hypertension). Comorbidity information was only available for residents in Denmark with at least one RT-PCR test. A total of 422 (1·4%) of blood donors and 160 603 (3·9%) of the background population were excluded from CFR and IFR analyses due to missing information about comorbidity status.
Results are reported as numbers and percentages with 95% confidence intervals. Statistical analyses were performed in R version 4.1.1 (R Foundation for Statistical Computing).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all results of the analyses and had the final responsibility for the decision to submit for publication.
Ethics
The study was approved as a surveillance study in all five Danish administrative regions and the appropriate institutional forms obtained. The participating blood donors were informed about the study and received the result of their SARS-CoV-2 antibody test. SARS-CoV-2 antibody test results were transferred from the blood centers to Statens Serum Institut through an encrypted e-mail connection. Data was linked to health register data through the unique personal identification number assigned to all residents in Denmark. Access to identifiable data was limited to data managers employed by Statens Serum Institut and authors CE, ADL, and LE. Furthermore, we used administrative register data and, according to Danish law, ethics approval is exempt for such research. The Danish Data Protection Agency, which is a dedicated ethics and legal oversight body, thus waives ethical review and approval for the use of administrative register data when no individual contact of participants is necessary and only aggregate results are included as disseminated findings. The study is therefore fully compliant with all legal and ethical requirements.
Results
Cohort characteristics
A total of 43 090 blood samples from 38 588 unique blood donors were tested for anti-N IgG antibodies. Among the samples, 8 701 were collected to determine baseline seroprevalence and 34 389 were collected during the study period. Thus, only a single donation sample per donor was tested from the November 2021 baseline period, while an average of 1·15 donations per donor were tested during the 2022 Omicron outbreak study period.
Table 1 shows basic characteristics of blood donors tested for anti-N IgG antibodies in calendar week 3–13, 2022 compared to the background population. The distribution according to gender and age was similar between the donors and the background Danish population. The Capital Region blood donor population comprised a lower percentage of the total blood donor population than what was found for the general population aged 17–72 years. More blood donors had been vaccinated compared to the general population (97·5% versus 89·4% vaccinated). Before November 1, 2021, approximately 7% of both blood donors and individuals in the background population had tested PCR positive for SARS-CoV-2 RNA at least once. From November 1, 2021, to January 1, 2022, the blood donors were more frequently PCR tested for SARS-CoV-2 RNA and more donors tested positive compared to the background population. The fraction of positive PCR tests (i.e., the numbers of positive PCR tests divided by the numbers of PCR tests performed) was lower for donors (8·2%) than for the background population (12·3%). Detailed distribution of PCR positive tests among blood donors and the background population stratified for gender, age, and region can be found in the Supplementary Table 2.
Table 1.
Characteristics of the blood donors and the background population aged 17–72 years.
| Blood Donors |
Background population | ||
|---|---|---|---|
| Characteristics | November | In 2022 Study Period | |
| Number of participants (N) | 8 701 | 29 887 | 4 098 183 |
| Number of samples tested (N) | 8 701 | 34 389 | - |
| N (%) | N (%) | N (%) | |
| Vaccination statusa | |||
| Not vaccinated | - | 756 (2·5) | 436 728 (10·6) |
| 1st dose | - | 154 (0·5) | 70 916 (1·7) |
| 2nd dose | - | 9 558 (32·0) | 1 487 471 (36·2) |
| 3rd dose | - | 19 413 (65·0) | 2 111 485 (51·4) |
| Comorbidity status | |||
| Known comorbidity | - | 2 000 (6·7) | 640 022 (15·6) |
| No known comorbidity | - | 27 465 (91·9) | 3 297 558 (80·4) |
| Information not available | - | 422 (1·4) | 160 603 (3·9) |
| Gender | |||
| Women | 3 889 (44·7) | 14 471 (48·4) | 2 038 166 (49·7) |
| Men | 4 812 (55·3) | 15 416 (51·6) | 2 060 017 (50·3) |
| Ageb | |||
| 17–35 years | 2 767 (31·8) | 10 536 (35·3) | 1 437 005 (35·1) |
| 36–50 years | 2 719 (31·2) | 9 315 (31·2) | 1 080 460 (26·4) |
| 51–60 years | 2 094 (24·1) | 6 688 (22·4) | 799 448 (19·5) |
| 61–72 years | 1 121 (12·9) | 3 348 (11·2) | 781 270 (19·1) |
| Administrative region | |||
| Capital Region of Denmark | 1 795 (20·6) | 7 691 (25·7) | 1 330 114 (32·5) |
| Region Zealand | 1 546 (17·8) | 4 161 (13·9) | 576 633 (14·1) |
| Region of Southern Denmark | 2 686 (30·9) | 6 713 (22·5) | 845 114 (20·6) |
| Central Denmark Region | 1 532 (17·6) | 7 505 (25·1) | 936 228 (22·8) |
| North Denmark Region | 1 142 (13·1) | 3 817 (12·8) | 410 094 (10·0) |
| Number of SARS-CoV-2 RT-PCR tests from Nov 1, 2021 to January 1, 2022 | |||
| 0 | - | 2 991 (10·0) | 692 619 (16·9) |
| 1–2 | - | 7 872 (26·3) | 1 330 855 (32·5) |
| 3–5 | - | 8 812 (29·5) | 1 102 572 (26·9) |
| 6–9 | - | 5 764 (19·3) | 570 014 (13·9) |
| 10–14 | - | 2 747 (9·2) | 244 331 (6·0) |
| 15+ | - | 1 701 (5·7) | 157 792 (3·9) |
| At least one positive SARS-CoV-2 RT-PCR test before Nov 1, 2021 | - | 2 130 (7·1) | 300 858 (7·3) |
| At least one positive SARS-CoV-2 RT-PCR test between Nov 1, 2021 and January 1, 2022 | - | 2 933 (9·8) | 326 630 (8·0) |
Vaccination status was ascertained January 1, 2022 for the blood donors and January 4, 2022 for the general population.
The age of blood donors was determined at the date of donation and the age of the background population was determined at January 1, 2022.
Assay sensitivity following recent infection
In November 2021, the anti-N IgG antibody seroprevalence was 1·2% (103/8 701, CI: 1·0%–1·5%), which represents the proportion of blood donors with detectable anti-N IgG despite waning of SARS-CoV-2 IgG antibodies in individuals infected during previous waves. The sensitivity of the antibody assay to detect antibodies among recently infected donors defined by RT-PCR-confirmed infections during the Omicron wave was estimated for each of the test weeks and was consistently found to be 74%–81% (Table 2). The assay sensitivity was higher for blood donors with a RT-PCR-confirmed infection before November 1, 2021, and a minimum of 60 days from the re-infection (sensitivity estimates ranging from 93%, CI: 76%–100%, to 98%, CI: 82%–100%) than for donors with no known previous infection (73%, CI: 70%–76%, to 80%, CI: 76%–85%, Supplementary Table 3).
Table 2.
Sensitivity to detect anti-N IgG antibodies after recent SARS-CoV-2 RT-PCR positive test.
| Test week | Last date of PCR reference period | Number of donors tested for anti-N IgG antibodies | Observed seroprevalence among SARS-CoV-2 RT-PCR positives |
|---|---|---|---|
| N | % | ||
| Week 3 | January 4, 2022 | 4 722 | 77 (70–84) |
| Week 5 | January 18, 2022 | 5 847 | 77 (72–82) |
| Week 7 | February1, 2022 | 5 310 | 81 (77–85) |
| Week 9 | February 15, 2022 | 5 771 | 81 (78–84) |
| Week 11 | March 1, 2022 | 6 132 | 79 (76–82) |
| Week 13 | March 15, 2022 | 6 605 | 74 (71–77) |
Anti-nucleocapsid IgG antibody response after recent infection
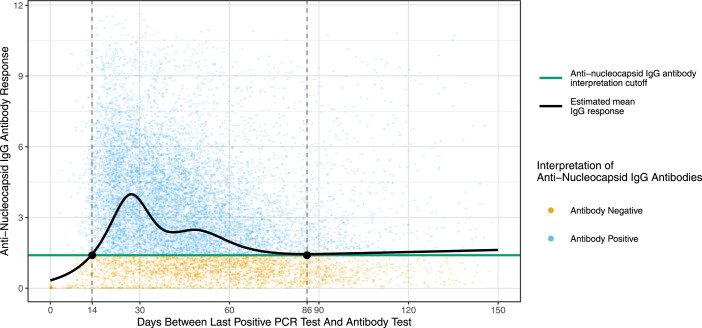
Figure 1 shows the time since last positive PCR test and the level of the anti-N IgG antibody response displayed as S/CO values. Fourteen days after a positive PCR test the mean anti-N IgG response increased to the assay threshold and remained above the threshold until day 86.
Figure 1.
Anti-nucleocapsid IgG antibody response after recent infection. The level of the anti-N IgG antibody response is displayed as signal to cut-off (S/CO) ratio with an interpretation cut-off of 1·4 S/CO as recommended by the manufacturer (green line). The dotted vertical lines at 14 and 86 days indicate the end of the self-deferral period for blood donors after first positive SARS-CoV-2 test result and when the estimated mean IgG response was equal to the interpretation cut-off, respectively.
Seroprevalence due to recent infections during the Omicron wave
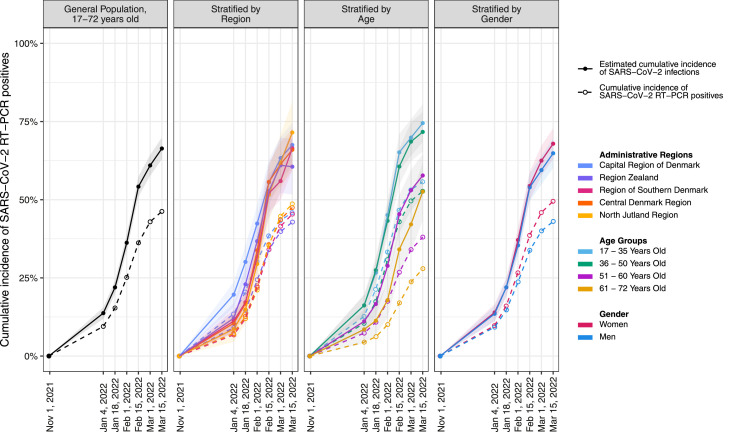
The cumulative incidence of confirmed and estimated SARS-CoV-2 infected individuals is shown in Figure 2 stratified by region, age, and gender. The unadjusted anti-N IgG seroprevalence increased from 1·2% (CI: 1·0%–1·4%) in November 2021 to 12% (CI: 11%–13%) in week 3 (January 18–23, 2022, Supplementary Table 4). During February and March 2022, the anti-N seroprevalence rose with increments of up to 15% points between the test weeks before the increase waned. Based on the anti-N IgG seroprevalence, with adjustment for assay sensitivity with regard to PCR-confirmed infections during the Omicron wave, we estimate that 66% (CI: 63%–70%) of the healthy adult Danish population were infected with SARS-CoV-2 between November 1, 2021, and March 15, 2022 (Supplementary Table 5). The ratio between estimated cumulative SARS-CoV-2 infections and RT-PCR confirmed infections was 1·45 (CI:1·08–1·82) for the study period. The ratio between development of estimated cumulative SARS-CoV-2 incidence and cumulative incidence of RT-PCR positives was gradually increasing from week 3 to week 9, indicating that the estimated number of infected individuals increased more than the number of PCR-confirmed infections during this period (Supplementary Table 6).
Figure 2.
Cumulative percentage of SARS-CoV-2 RT-PCR positive test results and the estimated cumulative SARS-CoV-2 infections during the study period. November 1, 2021 was set to zero percent. Due to the 14 days delay between the PCR reference period and the median date of the test week, the calculated cumulative proportion of infected individuals for each test week is indicated as the percentage infected on the last date of the corresponding PCR reference period.
Infection fatality rate (IFR)
The IFR stratified by gender, age, and region can be seen in Table 3. For the adjusted IFR the 30-day mortality was 6·2 (CI: 5·1–7·5) per 100 000 infected and the 60-day mortality was 10·2 (CI: 8·8–11·9) per 100 000 infected. The IFR was higher for men and increased with age.
Table 3.
Case fatality rate and infection fatality rate for the background population, aged 17–72 years, with no known comorbidities calculated from the RT-PCR confirmed and estimated SARS-CoV-2 infections.
| 30-Day mortality (per 100,000) |
60-Day mortality (per 100,000) |
|||
|---|---|---|---|---|
| CFR | IFR | CFR | IFR | |
| Total | 8.3 (6.9–10.1) | 6.2 (5.1–7.5) | 13.8 (11.9–16.1) | 10.2 (8.8–11.9) |
| Gender | ||||
| Women | 6.4 (4.7–8.7) | 4.8 (3.6–6.6) | 11.0 (8.8–13.9) | 8.4 (6.7–10.6) |
| Men | 10.5 (8.2–13.4) | 7.5 (5.9–9.7) | 16.9 (13.9–20.5) | 12.1 (10.0–14.8) |
| Age | ||||
| 17–35 years | 1.9 (1.0–3.5) | 1.6 (0.9–3.1) | 3.0 (1.8–4.9) | 2.6 (1.6–4.3) |
| 36–50 years | 4.5 (2.8–7.2) | 4.1 (2.6–6.6) | 6.3 (4.2–9.5) | 5.8 (3.9–8.7) |
| 51–60 years | 13.0 (8.8–19.3) | 7.6 (5.2–11.3) | 25.0 (18.8–33.2) | 14.6 (11.0–19.4) |
| 61–72 years | 40.7 (30.9–53.6) | 15.1 (11.5–19.9) | 66.2 (53.4–82.2) | 24.6 (19.8–30.5) |
| Administrative Region | ||||
| Capital Region of Denmark | 7.0 (4.7–10.3) | 5.7 (3.8–8.4) | 10.9 (8.0–14.9) | 8.8 (6.4–12.1) |
| Region Zealand | 12.9 (8.3–19.9) | 9.0 (5.8–14.0) | 22.5 (16.2–31.3) | 15.8 (11.4–22.0) |
| Region of Southern Denmark | 9.2 (6.2–13.8) | 6.6 (4.4–9.8) | 14.6 (10.7–20.1) | 10.4 (7.6–14.3) |
| Central Denmark Region | 5.1 (3.1–8.3) | 3.8 (2.3–6.2) | 8.6 (5.9–12.5) | 6.5 (4.4–9.4) |
| North Denmark Region | 9.3 (5.4–15.9) | 5.8 (3.3–9.9) | 18.5 (12.6–27.2) | 11.5 (7.8–16.9) |
Case fatality rate (CFR) was calculated based on the SARS-CoV-2 RT-PCR test results between January 5, 2022, and March 15, 2022. The infection fatality rate (IFR) was calculated based on the seroprevalence measured in donors. To account for the differences in gender, age, and regional distribution of donors when compared to the background population (17–72 years with no known comorbidities), we weighted the estimates.
Discussion
In this large national serosurveillance study, we used anti-N IgG seroprevalence among blood donors to estimate the proportion of healthy Danish residents, aged 17–72 years, who had been infected with SARS-CoV-2 during the Omicron wave. We estimate that 66% (CI: 63%–70%) of this population were infected by the Omicron variant from November 2021 through March 15, 2022. The estimated cumulated SARS-CoV-2 infection prevalence was calculated based on the anti-N IgG antibody seroprevalence among blood donors and weighted to adjust for differences in sex, age, and regional distribution between blood donors and the background population aged 17–72 years. We calculated that approximately 1/3 of the estimated total infections were not detected through the national SARS-CoV-2 RT-PCR test system and that 32% of the adult Danish population were infected in just four weeks. These findings support other studies demonstrating the high transmissibility and immune evasiveness of the Omicron variant.15
Blood donor populations were among the first to be screened in SARS-CoV-2 serosurveys during the beginning of the epidemic in Denmark, and the results have been used extensively in Denmark and in several other countries to follow the development of the pandemic.7,16,17 We previously monitored SARS-CoV-2 seroprevalence using assays assessing anti-Spike antibodies produced after vaccination or infection.7,17,18 In the present study, we tested donor samples for anti-N IgG, which is known to wane over time.9,10,19 Even though we mainly assessed vaccine breakthrough infections the assay sensitivity was high. We found that only 1·2% (CI: 1·0–1·4) of donations were anti-N IgG positive in November 2021. Use of this anti-N IgG assay allowed us to establish a baseline proportion of past infections with residual anti-N IgG reactivity to track subsequent increases in seroprevalence attributable to the rapidly spreading Omicron variant.
We were able to establish the sensitivity of the anti-N IgG assay by linking antibody test results with national SARS-CoV-2 RT-PCR data at the individual level. Additionally, we could infer the proportion of undetected infections in the general population, which allowed us to make projections of the current progression of the epidemic throughout the surge and decline of Omicron infection incidence. Lastly, the design allowed us to use the number of SARS-CoV-2 RT-PCR positives to calculate the IFR among 17–72-year-old individuals without comorbidity. The seroprevalence results were used to inform the authorities and were published in fortnightly news releases. Since the commencement of this study, other SARS-CoV-2 seroprevalence studies assessing infection-induced antibodies have been published.20,21 However, these studies do not link data to national health registers or calculate IFRs.
The Omicron variant has been associated with an increased risk of re-infection and vaccine breakthrough infection although no specific rates have yet been reported.22,23 Only limited data is available on anti-N seroconversion after vaccine breakthrough infection. The number of study participants in early studies is generally low and the probability of seroconversion after breakthrough infection with variants prior to the emergence of the Omicron variant ranges from 26% to 82%.24, 25, 26 The differences in seroconversion may be due to differences in time from infection to serological testing as well as differences in severity of infection among the populations included. It was suggested that asymptomatic infections may exhibit lower seroconversion rates and level of antibodies than symptomatic infections.26 Seroconversion rates after Omicron vaccine breakthrough infection were therefore expected to be lower than what was previously reported. However, our data shows that 74–81% of blood donors who tested positive for SARS-CoV-2 from November 1, 2021 and until 14 days before their donation seroconverted. Figure 1 shows a rapid decline in anti-N IgG antibody response, and the mean IgG level drops to the assay threshold after about three months. Optimization of the threshold may lead to higher assay sensitivity while maintaining a high specificity. Of note, new thresholds may have to be further adjusted for potential upcoming waves of infections, due to the impact of prior infections and vaccinations and time on anamnestic serological responses associated with reinfections and/or vaccine breakthrough infections, and differential induction of anti-N seroreactivity associated with future variants. The region-stratified sensitivity estimates likely reflect the spatio-temporal differences, with high transmission in the Capital Region of Denmark early in the Omicron wave. The sensitivity estimates were higher for donors who had had a RT-PCR-confirmed infection before November 1, 2021, in addition to a RT-PCR-confirmed infection during the Omicron wave. The dependency of the sensitivity on both time since infection and multiple infections might affect the use of anti-N IgG assays for serosurveillance during potential epidemics with future variants.
The Omicron variant has proved to be highly transmissible but much less severe compared to previous variants.5,27,28 However, due to the large number of undetected cases, no precise estimates of the IFR have been published based on seroprevalence studies. We have calculated the IFR among adult individuals without comorbidity during the current peak and found that the IFR was indeed low at 6·2 (CI: 5·1–7·5) per 100 000 infections. The 30-day mortality for the oldest age group (61–72 years) was 15·1 (CI: 11·5–19·9). The IFR was lower than the CFR especially among individuals aged 51–60 years and 61–72 years, which is likely due to a higher ratio of undetected SARS-CoV-2 infections among the older age strata. The IFR was considerable lower than the IFRs calculated during previous waves in Denmark, with 30-day mortalities of 74·1 (CI: 55·6–137) to 281 (CI: 158–1 686) during the first wave (spring 2020) prior to vaccination roll-out and 50·3 (CI: 40·4–64·5) to 156 (CI: 114–228) during the second wave (winter 2020/2021) for Danish residents aged 51–69 years without comorbidity.7 The current lower IFR estimates are likely owing to several circumstances such as the vaccination coverage, societal interventions, testing strategy, age composition of the infected individuals, and, importantly, the dominant SARS-CoV-2 variant at the time. SARS-CoV-2 RT-PCR testing was available for all residents free of charge during the study.
Strengths and limitations
The Danish blood donor biobanks have served as an easy applicable tool in monitoring the COVID-19 epidemic in Denmark.7,17,18,29 Blood donors comprise approximately 5% of the similarly aged Danish population.30 Furthermore, the geographical distribution of blood donors and the possibility to perform continuous screening of a stable population allows us to assess the spatio-temporal development in seroprevalence of SARS-CoV-2 antibodies. In this study, we assume that the seroprevalence among donors is similar to that of the healthy background population aged 17–72 years. The estimated cumulative SARS-CoV-2 infections, IFR, and undetected infections have been weighted to adjust for differences in sex, age, and regional distribution between blood donors and the background population and improve the generalizability. Blood donors were more often vaccinated and had a higher SARS-CoV-2 test frequency than the background population, probably reflecting that blood donors are dutiful and part of the active work force including a high proportion of healthcare professionals. The lower fraction of positive PCR tests among blood donors supports that the reason blood donors are more often tested positive owes to the more frequent testing compared to the background population. Blood donors are healthy, and their all-cause mortality is lower than that of the background population.31 This may limit the generalizability of the findings. Furthermore, having a positive RT-PCR test has an impact on subsequent probability of donation. This means that donors experiencing severe symptoms are expected to be underrepresented in the study, and seroprevalence in the blood donors could have been underestimated.
Conclusion
In November 2021, the Omicron variant was introduced in Denmark, it quickly spread and became the dominant variant by mid-December 2021. Due to the increasing number of cases, testing strategies for close contacts were scaled down in order not to exceed the RT-PCR test capacity, and antigen self-tests were recommended to a larger extent. Given these changes there was a need to supplement the established surveillance system with serosurveillance. We measured anti-N IgG antibodies among Danish blood donors allowing us to detect recent, mainly vaccine breakthrough, infections, and determine the assay sensitivity during the Omicron wave. Less than five months after the introduction of the Omicron variant we estimate that 66% (63–70%) of the Danish background population aged 17–72 years with no known comorbidities have been infected, with one third of the population infected during only four weeks. Fortunately, the IFR for this population was considerably lower than during previous waves.
The current initiative shows that seroprevalence designs can be modified to the use of IgG assays detecting recent infections. This is crucial in the surveillance of future epidemics of SARS-CoV-2 variants. We propose that blood donor serosurveillance is included in future emerging infectious disease preparedness plans.
Contributors
CE, JG, KAK drafted the manuscript. CE, SE, ES, MPB, CSJ, TGK, HU, LE, OBVP, SO designed the study. ADL, JKB, MS, LH performed the statistical analyses. CE, TGK, HU, LE, OBVP, SO interpreted the data. CE, SM, SWJ, DKH, MTB, JN, TB, CM, SGS, LHH, BA, KMD planned and analysed laboratory analyses. All authors were involved in critically revising the manuscript and approved the final version before submission.
Data sharing statement
Access to individual-level data is governed by the Danish Authorities. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. Researchers at Danish research institutions may obtain the relevant approval and data. International researchers may gain data access if governed by a Danish research institution as approval and data access are required.
Declaration of interests
The authors declare no conflict of interests.
Acknowledgements
The authors thank participating blood donors and all the staff involved in performing this study. A special thanks to the laboratory technicians from the Departments of Clinical Immunology at Aarhus University Hospital, Odense University Hospital, Zealand University Hospital, and Aalborg University Hospital for their exceptional work of testing of the samples.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100479.
Appendix. Supplementary materials
References
- 1.Statens Serum Institut . Statens Serum Institut; Denmark: 2022. Ugentlige Tendenser - Covid-19 og Andre Luftvejsinfektioner. [Google Scholar]
- 2.Hansen C, Schelde A, Moustsen-Helm I, et al. Vaccine effectiveness against infection and COVID-19-associated hospitalisation with the Omicron (B.1.1.529) variant after vaccination with the BNT162b2 or mRNA-1273 vaccine: A nationwide Danish cohort study. PREPRINT, Res Sq. 2022 doi: 10.21203/rs.3.rs-1486018/v1. [DOI] [Google Scholar]
- 3.Eggink D, Andeweg SP, Vennema H, et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espenhain L, Funk T, Overvad M, et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaspersen KA, Hindhede L, Boldsen JK, et al. Estimation of SARS-CoV-2 infection fatality rate by age and comorbidity status using antibody screening of blood donors during the COVID-19 epidemic in Denmark. J Infect Dis. 2022;225:219–228. doi: 10.1093/infdis/jiab566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espenhain L, Tribler S, Sværke Jørgensen C, Holm Hansen C, Wolff Sönksen U, Ethelberg S. Prevalence of SARS-CoV-2 antibodies in Denmark: nationwide, population-based seroepidemiological study. Eur J Epidemiol. 2021;36:715–725. doi: 10.1007/s10654-021-00796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73:e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chansaenroj J, Yorsaeng R, Posuwan N, et al. Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci Rep. 2021;11:23216. doi: 10.1038/s41598-021-02659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asamoah-Boaheng M, Goldfarb DM, Barakauskas V, et al. Evaluation of the performance of a multiplexed serological assay in the detection of SARS-CoV-2 infections in a predominantly vaccinated population. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01454-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voldstedlund M, Haarh M, Mølbak K. MiBa board of representatives. The Danish microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol. 2021;59:e02520–e02596. doi: 10.1128/JCM.02596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyngse FP, Mølbak K, Denwood M, et al. Effect of vaccination on household transmission of SARS-CoV-2 Delta variant of concern. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed S, Uzicanin S, Lewin A, et al. Current challenges of severe acute respiratory syndrome coronavirus 2 seroprevalence studies among blood donors: a scoping review. Vox Sang. 2021;117:476–487. doi: 10.1111/vox.13221. [DOI] [PubMed] [Google Scholar]
- 17.Erikstrup C, Hother CE, Pedersen OBV, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2021;72:249–253. doi: 10.1093/cid/ciaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedersen OB, Nissen J, Dinh KM, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection fatality rate among elderly danes: a cross-sectional study on retired blood donors. Clin Infect Dis. 2021;73:e2962–e2969. doi: 10.1093/cid/ciaa1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallais F, Gantner P, Bruel T, et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine. 2021;71 doi: 10.1016/j.ebiom.2021.103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke KEN, Jones JM, Deng Y, et al. Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:606–608. doi: 10.15585/mmwr.mm7117e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cable R, Coleman C, Glatt T, et al. Estimates of prevalence of anti-SARS-CoV-2 antibodies among blood donors in eight provinces of South Africa in November 2021. PREPRINT, Res Sq. 2022 doi: 10.21203/rs.3.rs-1687679/v1. [DOI] [Google Scholar]
- 22.Kuhlmann C, Mayer CK, Claassen M, et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. 2022;399:625–626. doi: 10.1016/S0140-6736(22)00090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376 doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen N, Brady M, Carrion Martin AI, et al. Serological markers of SARS-CoV-2 infection; anti-nucleocapsid antibody positivity may not be the ideal marker of natural infection in vaccinated individuals. J Infect. 2021;83:e9–e10. doi: 10.1016/j.jinf.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. New England J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitaker HJ, Gower C, Otter AD, et al. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off. PREPRINT, medRxiv. 2021 doi: 10.1101/2021.10.25.21264964. [DOI] [Google Scholar]
- 27.Fall A, Eldesouki RE, Sachithanandham J, et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: An investigation of hospital admissions and upper respiratory viral loads. EbioMedicine. 2022;79 doi: 10.1016/j.ebiom.2022.104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veneti L, Bøås H, Bråthen Kristoffersen A, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hønge BL, Hindhede L, Kaspersen KA, et al. Long-term detection of SARS-CoV-2 antibodies after infection and risk of re-infection. Int J Infect Dis. 2022 doi: 10.1016/j.ijid.2022.01.041. S1201-9712(22)00046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloddonorerne i Danmark, Årsberetning 2019-2020 (The Danish Blood Donor Association, annual report 2019-2020), https://bloddonor.dk/wp-content/uploads/2020/11/BiD-Aarsberetning-19_20-komprimeret.pdf. Accessed 1 August 2022.
- 31.Ullum H, Rostgaard K, Kamper-Jørgensen M, et al. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion. 2015;55:2479–2485. doi: 10.1111/trf.13205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.