Abstract
Background: Ischemic stroke is a leading cause of morbidity and mortality in neurological diseases. Numerous studies have evaluated the efficacy and safety of ischemic stroke therapies, but clinical data were largely inconsistent. Therefore, it is necessary to summarize and analyze the published clinical research data in the field.
Objective: We aimed to perform an umbrella review to evaluate the efficacy and safety of ischemic stroke therapies.
Methods: We conducted a search for meta-analyses and systematic reviews on PubMed, the Cochrane Library, and the Web of Science to address this issue. We examined neurological function deficit and cognitive function scores, quality of life, and activities of daily living as efficacy endpoints and the incidence of adverse events as safety profiles.
Results: Forty-three eligible studies including 377 studies were included in the umbrella review. The results showed that thrombolytic therapy (tPA; alteplase, tenecteplase, and desmoteplase), mechanical thrombectomy (MTE), edaravone with tPA, stem cell-based therapies, stent retrievers, acupuncture with Western medicines, autologous bone marrow stromal cells, antiplatelet agents (aspirin, clopidogrel, and tirofiban), statins, and Western medicines with blood-activating and stasis-dispelling herbs (NaoShuanTong capsule, Ginkgo biloba, Tongqiao Huoxue Decoction, Xuesaitong injection) can improve the neurological deficits and activities of daily living, and the adverse effects were mild for the treatment of ischemic stroke. Moreover, ligustrazine, safflower yellow, statins, albumin, colchicine, MLC601, salvianolic acids, and DL-3-n-butylphthalide showed serious adverse events, intracranial hemorrhage, or mortality in ischemic stroke patients.
Conclusion: Our study demonstrated that tPA, edaravone and tPA, tPA and MTE, acupuncture and Western medicines, and blood-activating and stasis-dispelling herbs with Western medicines are the optimum neurological function and activities of daily living medication for patients with ischemic stroke.
Systematic Review Registration: https://inplasy.com/, identifier [INPLASY202250145].
Keywords: ischemic stroke, clinical trial, systematic review, umbrella review, neurological functional
Introduction
Ischemic stroke is a major cause of death and disability, so prevention and effective treatment of stroke are of utmost importance in China and the West. The World Health Organization has suggested that an incidence of stroke occurs once every 5 s worldwide, approximately one-third of strokes are fatal, and another third leave survivors with permanent disability (Donkor, 2018). Moreover, surviving stroke patients impose a heavy medical burden on families and communities (Go et al., 2014). However, little is known about the efficacy and safety of treatments of ischemic stroke in the hyper-acute (0–24 h) and acute phases (1–7 days) and recovery period (>7 days) post-stroke in humans (Marzolini et al., 2019). The key challenge in the treatment of stroke is to identify the most effective way to implement the efficacious interventions currently available.
Some evidence supports national guidelines recommending the use of recombinant tissue plasminogen activator (tPA) thrombolysis for the treatment of hyperacute ischemic stroke, which can significantly improve neurological deficits (Li et al., 2017; Zhou et al., 2020). In addition, the guidelines also recommend antithrombotic (including antiplatelet and anticoagulant therapy), neuroprotection, traditional Chinese medicine, statins, and control of high-risk factors for secondary prevention of ischemic stroke (Practice, 2021). Additionally, as a bradykinin B1 and B2 receptor agonist, HUK provides functional benefits (Patel and McMullen, 2017). Furthermore, other neuroprotective drugs are supported by comprehensive clinical reports that demonstrate their efficacy and safety in improving cognitive impairment or other major domains (Practice, 2021).
Attempts to many systematic reviews and meta-analyses have been conducted to analyze the different stroke treatments. These studies, however, did not provide comprehensive appraisals of stroke therapies, and some results are still conflicting (Wu et al., 2007). A review of the latest literature, having removed repeated studies and research involving complications, followed by a meta-analysis to derive at pooled prevalence, was needed. Therefore, the present study aimed to perform an umbrella review of the systematic reviews and meta-analyses of stroke therapies through a comprehensive and updated literature search and to reach a definitive conclusion by integrating all available meta-analyses to identify which of the commercially available treatments for ischemic stroke patients are efficacious and safe.
Materials and Methods
Our study was performed in accordance with the standard guidelines of Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) (Moher et al., 2009). The protocol for this review was prospectively registered at INPLASY PROTOCOL (INPLASY202250145).
Search Strategy and Quality Assessment
A systematic search of published peer-reviewed English language literature was conducted using PubMed, Web of Science, and the Cochrane Library until March 2022. The database search terms were as follows: (Ischemic stroke) and (systematic review or meta-analysis) and clinical trial. We included meta-analyses and systematic reviews that determined the efficacy and safety of treatments in patients with stroke. Inclusion criteria were: 1) written in English; 2) published systematic review or meta-analyses; 3) including any evaluation of clinical assessment scales for stroke; 4) published in peer-reviewed journals. Studies were excluded if 1) unpublished studies; 2) no necessary sample data; 3) patients were diagnosed with other strokes; 4) the study reported insufficient details and other outcomes; and 5) the study presented the risk of bias/study limitations.
The AMSTAR2 tool was used to evaluate systematic reviews and meta-analyses (Shea et al., 2007; De Santis et al., 2021). The methodological quality of the studies was determined by the percentage of AMSTAR2 score. The percentage of AMSTAR2 score was classified into 0–33%, 34–66%, and 67%–100% indicating low quality, medium quality, and high quality, respectively.
We searched for related articles using keywords and filtering titles, and two investigators screened the literature independently. Articles were downloaded and the abstracts screened using inclusion criteria, deleting any irrelevant or repetitive articles. Thereafter, we manually searched the reference lists of the chosen studies for any other relevant studies not found in our initial search. Finally, a full-text search was performed to extract and then analyze the data from articles.
Data Extraction
According to the following criteria, three investigators (Yongbiao Li, Ruyi Cui, and Fangcheng Fan.) independently selected those trials that met the inclusion criteria. The main characteristics of the selected study were extracted in a table including the year of publication, study design, number of studies, and regimens for the treatment. We included results evaluating the efficacy of drugs in patients with at least one of the clinical assessment scales: 1) the incidence of intracranial hemorrhage (sICH); 2) the primary outcomes included: global neurological deficit scores such as the National Institutes of Health Stroke Scale (NIHSS) score ≤1 and the Neurological Function Deficit Scores (NFDS); 3) all-cause mortality; 4) dependence assessed by Barthel Index (BI) scores ≥95; 5) modified Rankin Scale score of 0–1 or return to baseline (mRS); 6) clinical effect, defined according to the nationally approved criteria, is divided into essentially recovered, significant improvement, improvement, no change, deterioration, and death (the first three categories are judged to be effective); 7) the secondary outcomes included the following: cognitive function scoring; related hemorheology and lipid metabolism outcomes; quality of life; and 8) incidence of adverse events (AE). The selection of assessments was extracted on study size, sample size, mean difference (Fixed, 95% CI) or odds ratio (Fixed, 95% CI), and heterogeneity (I2). A percentage of 0–25% was classified as mild, 26–50%, as moderate, and 51–75%, as significant between-study heterogeneity. If I2 > 50%, a random-effects model was used for the analysis, or the data were analyzed on the fixed-effects model (Wang et al., 2016).
Statistical Analysis
The sample size and mean difference were used to calculate the four clinical assessment scales. NIHSS/mRS/BI scores were used to evaluate neurological status, and behavioral symptoms in patients were calculated by NFDS. We focused on the clinical effect is divided into essentially recovered, significant improvement, no change, deterioration; cognitive function scoring; quality of life as activities of daily living. All data analyses were performed by GraphPad Prism 5.0 software. The results were expressed as OR ± SD (standard deviation). The adverse events have assessed the incidence of adverse events, and the OR was calculated. Therefore, mean difference or odds ratio with 95% CI and p values were used to assess the efficacy and safety of the study medications.
Results
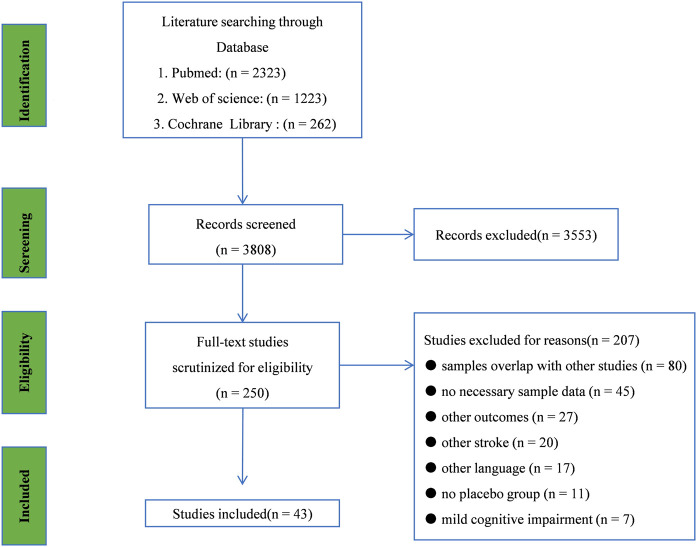
Literature search and study selection through the initial search, we retrieved a total of 3,808 records from PubMed, Web of Science, and Cochrane Library. After examining the titles and abstracts, 250 studies were selected for further full-text scrutiny. In all, 207 studies were excluded due to the following reasons: samples overlap with other studies (n = 80), no necessary sample data (n = 45), other outcomes (n = 27), other stroke (n = 20), other language (n = 17), no placebo group (n = 11), mild cognitive impairment (n = 7), (Figure 1). Thus, 43 studies were included in the umbrella review: Pan et al., 2020; Pan et al., 2020); Liu et al., 2021); (Liu et al., 2011); Blann et al., 2015); (Blann et al., 2015); Emberson et al., 2014) (Emberson et al., 2014); Peng et al., 2014); (Peng et al., 2014); Zhang et al., 2019); Shang et al., 2019); Puñal-Riobóo et al., 2015; Yuan et al., 2008; (Fu et al., 2013), Fan et al., 2014), Lin et al., 2014); Xu et al., 2015); Cao and Li, (2015); Marmagkiolis et al., 2015); Zheng et al., 2017), Li et al., 2017), Zhang et al., 2017), Chong et al., 2020); (Zhao et al., 2021), Li et al. (2020); (Li et al., 2020), Gao et al., 2021); Huang et al., 2020); (Huang and Xiao, 2021), Liu et al., 2022), Feng et al., 2021), Lee et al. (2010); Zhou et al., 2022); Hu et al., 2021); Hong and Lee, 2015, Liu et al., 2021); (Liu et al., 2021), Xin et al., 2020); Katsanos et al., 2020), Wang et al., 2021), Liu et al., 2019; (Liu et al., 2019), Xu et al., 2019); (Zhang et al., 2019), (Huang et al., 2020), Yang et al., 2015), Yang et al., 2015), (Ni et al., 2020); (Thelengana et al., 2019), Shi et al., 2014) and (Siddiqui et al., 2013), (Liu et al., 2016), Kaesmacher et al., 2019); (Kaesmacher et al., 2019), Li et al., 2014). The main characteristics, bias analysis, and the quality scores of the included studies are shown in Table 1 and Supplementary material.
FIGURE 1.
Searching and screening process: literature search and study selection Through the initial search, we retrieved a total of 3,808 records from PubMed, Web of Science, and Cochrane Library. After examining the titles and abstracts, 250 studies were selected for further full-text scrutiny. In all, 207 studies were excluded due to the following reasons: sample overlap with other studies (n = 80), no necessary sample data (n = 45), other outcomes (n = 27), other stroke (n = 20), other language (n = 17), no placebo group (n = 11), and mild cognitive impairment (n = 7).
TABLE 1.
Description and AMSTAR2 scores of included studies.
| Study | Condition | Studies included | Study duration (median, range) | Daily dose (median, range) | Outcome | AMSTAR2 score | Study quality |
|---|---|---|---|---|---|---|---|
| Ni et al. (2013) | Ligustrazine versus placebo | 3 | 14w (2w–48w) | 240 mg/day | 1. Effect and 2. sICH | 5/11 | low |
| Xin et al. (2020) | Heparin versus Placebo | 9 | 12w | <40 mg/day | 1. mRS, 2. NIHSS, 3. sICH, 4. DOS, and 5. AE | 7/11 | middle |
| Shang et al. (2019) | MTE versus placebo | 7 | 12w | NA | 1. mRS and 2. sICH | 8/11 | high |
| Kaesmacher et al. (2019) | tPA plus MTE versus placebo | 12 | 12w | NA | 1. mRS and 2. sICH | 9/11 | high |
| Li et al. (2014) | Acupuncture plus XM versus placebo | 17 | 12W | NA | 1. Effect | 8/11 | high |
| Liu et al. (2021) | Nimodipine versus placebo | 8 | 18w (12w–24w) | NA | 1. Effect, and 2. NFDs | 10/11 | high |
| Blann et al. (2015) | Aspirin plus clopidogrel versus placebo | 24 | 12w | 60 mg/day | 1. Effect and2. sICH | 9/11 | high |
| Emberson et al. (2014) | tPA versus placebo | 12 | 3 h (0–6 h) | <0.85 mg/kg/day | 1. Effect, 2. sICH, and 3. NIHSS | 10/11 | high |
| Peng et al. (2014) | XNJ versus placebo | 13 | 4w | 45 ml (30–60 ml/day) | 1. Effect, 2. NFDs, and 3. AE | 6/11 | middle |
| Zhang et al. (2019) | NST versus placebo | 13 | 12w | 50 mg/day | 1. Effect, 2. NFDs, 3 .BI, and 4. mRS | 10/11 | high |
| Yuan et al. (2008) | Chuanxiong versus Placebo | 3 | 24w (1w–48w) | 120 mg (80–160 mg/day) | 1. NFDs and 2. AE | 10/11 | high |
| Fu et al. (2013) | XXMT versus placebo | 8 | 12w (4w–24w) | NA | 1. NIHSS, 2. mRS, and 3. Effect | 5/11 | low |
| Fan et al. (2014) | Safflower yellow versus placebo | 7 | 2w | 50 mg/day | 1. Effect, 2. NFDs, and 3. AE | 5/11 | low |
| Lu et al. (2014) | Rhubarb versus placebo | 12 | 2w (1w–4w) | NA | 1. Effect, 2. NFDs, 3. BI, 4. NIHSS, and 5. AE | 6/11 | middle |
| Xu et al. (2015) | WD versus placebo | 13 | 2w (2w–4w) | NA | 1. Effect, 2. sICH, and 3. NFDS | 4/11 | low |
| Cao and Li. (2015) | MSCs versus placebo | 5 | 3w (1w–6w) | 5 × 107-2.6 × 108 cell | 1. NIHSS, 2. mRS, 3. BI, and 4. AE | 6/11 | middle |
| Marmagkiolis et al. (2015) | stent retrievers versus placebo | 5 | 12w | NA | 1. mRS, 2. sICH, and 3. AE | 8/11 | high |
| Zheng et al. (2017) | Puerarin versus placebo | 16 | 1w (1w–2w) | 300 mg (100–500 mg/day) | 1. Effect and 2. NFDs | 6/11 | middle |
| Li et al. (2017) | Alpha1 versus placebo | 6 | 6 h (3–9 h) | 90 mg/kg/day | 1. Effect, 2. sICH, and 3. AE | 8/11 | high |
| Zhang et al. (2017) | Cerebrolysin versus placebo | 7 | 12w (1w–12w) | 50 ml/day | 1. mRS, 2. BI, and 3. AE | 9/11 | high |
| Chong et al. (2020) | Ginkgo biloba versus placebo | 12 | 12w (1w–12w) | 100 mg (40–160 mg)/day | 1. NIHSS, 2. NFDs, 3. sICH, and 4. AE | 9/11 | high |
| Li et al. (2020) | Stem cell-based versus placebo | 9 | 12w (1w–12w) | 5 × 106-2.97 × 109 cell | 1. NIHSS, 2. mRS, 3. BI, and 4. AE | 9/11 | high |
| Zhou et al. (2020) | tirofiban versus placebo | 6 | 18w (12w–24w) | (0.1-0.4 ug/kg/day) | 1. Effect, 2. sICH, and 3. AE | 5/11 | low |
| Gao et al. (2021) | BHD versus placebo | 11 | 16w (8w–24w) | NA | 1. Effect, 2. NIHSS, and 3. AE | 9/11 | high |
| Huang and Xiao. (2021) | Albumin versus placebo | 4 | 15w (2w–48w) | 1.3 mg (0.6–2 mg/kg/day) | 1. Effect | 9/11 | high |
| Liu et al. (2022) | DZSM versus placebo | 28 | 7w (1w–13w) | NA | 1. mRS, 2. NFDs, 3. BI, and 4. NIHSS | 10/11 | high |
| Feng et al. (2021) | XST plus XM versus placebo | 12 | 2w (2w–4w) | NA | 1.Effect and 2. NIHSS | 5/11 | middle |
| Lee et al. (2010) | Intra-A versus placebo | 5 | 12w | NA | 1. mRS, 2. BI, and 3. NIHSS | 5/11 | middle |
| Zhou et al. (2022) | TQHX plus XM versus placebo | 12 | 4w | NA | 1. Effect and 2. NFDs | 9/11 | high |
| Hu et al. (2021) | Edaravone plus rt-PA versus placebo | 17 | 2w (1w–4w) | 60 mg/day | 1. sICH and 2. NIHSS | 5/11 | middle |
| Hong and Lee. (2015) | Statins versus placebo | 18 | 6w (1w–12w) | 8 mg/kg/day | 1. Effect and 2. NFDs | 9/11 | high |
| Liu et al. (2021) | ZL versus placebo | 7 | 2w | 1.4 mg (1.2–1.6 g/day) | 1. mRS, 2. BI, and 3. NIHSS | 7/11 | middle |
| Xin et al. (2020) | salvianolic acids versus placebo | 12 | 2w (1w–4w) | 200 mg (100–300 mg/day) | 1. Effect, 2. NIHSS, 3. mRS, and 4. BI | 4/11 | low |
| Katsanos et al. (2020) | Colchicine versus placebo | 4 | 74w (4w–144w) | 0.5 mg/day | 1. AE | 3/11 | low |
| Liu et al. (2019) | ANP versus placebo | 18 | 2w | 3 g/day | 1. Effect, 2. NIHSS, and 3. NFDs | 9/11 | high |
| Xu et al. (2015) | NBP versus placebo | 12 | 6w (1w–12w) | 100 mg/day | 1. BI, 2. NIHSS, and 3. AE | 9/11 | high |
| Wang et al. (2021) | Pntsp versus placebo | 20 | 6w (2w–10w) | 470 mg (140–800 mg/day) | 1. NIHSS, 2. mRS, 3. BI, and 4. AE | 10/11 | high |
| Huang et al. (2020) | HUK versus placebo | 16 | 3 h (0–6 h) | 0.15 PNA | 1. NIHSS, 2. NFDs, and 3. AE | 7/11 | middle |
| Yang et al. (2015) | Mailuoning versus Placebo | 21 | 12w | 204 mg (8–400 mg/day) | 1. Effect, 2. NFDs, 3. BI, 4. NIHSS, and 5. AE | 9/11 | high |
| Ni et al. (2020) | Cinepazide maleate versus placebo | 4 | 7w (2w-12w) | 320 mg/day | 1. mRS, 2. BI, and 3. AE | 7/11 | middle |
| Thelengana et al. (2019) | TNK versus placebo | 4 | 3 h (0–6 h) | 0.15 mg (0.1–0.2 mg/kg/day) | 1. Effect, 2. NFDs, 3. BI, 4. NIHSS, and 5. AE | 9/12 | high |
| Shi et al. (2014) | Cilostazol versus placebo | 6 | 30w (1w-60w) | 690 mg (80–1300 mg/day) | 1. sICH and 2. AE | 10/11 | high |
| Siddiqui et al. (2013) | MLC601 versus placebo | 2 | 13w (2w-24w) | 405 mg (10–800 mg/day) | 1. NFDs and 2. BI | 5/11 | low |
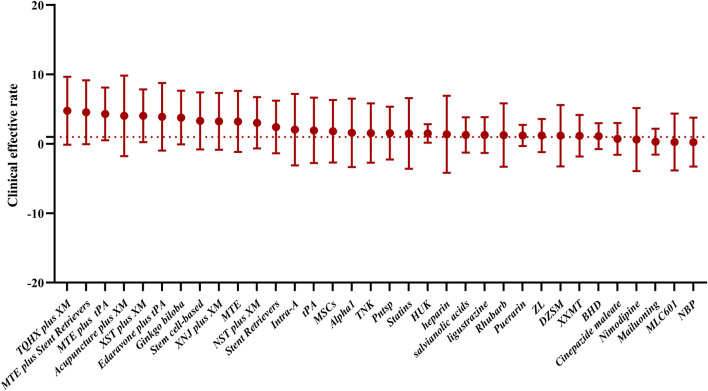
As shown in Table 1, a total of 377 clinical trials were included, with 43 drug therapies in the treatment groups. All studies were randomized controlled clinical trials, and the treatment duration ranged from 1 to 72 weeks. In total, 24 meta-analyses included were of high quality according to AMSTAR2 score, 12 meta-analyses included were of middle quality according to AMSTAR2 score, and seven meta-analyses included were of low quality according to AMSTAR2 score. The total clinical efficacy was used to evaluate the effect of drug therapy on ischemic stroke (Figure 2).
FIGURE 2.
Total clinical efficacy was used to evaluate the effect of drug therapy on ischemic stroke. In this study, the possible order of efficacy of the drugs was TQHX plus XM, MTE plus stent retrievers, MTE plus tPA, acupuncture plus XM, XST plus XM, edaravone plus tPA, Ginkgo biloba, stem cell-based therapy, XNJ plus XM, MTE, NST plus XM, stent retrievers, intra-A, tPA, MSCs, Alpha1, TNK, Pntsp, statins, HUK, heparin, salvianolic acids, ligustrazine, rhubarb, puerarin, ZL, DZSM, XXMT, BHD, cinepazide maleate, nimodipine, Mailuoning, MLC601, and NBP.
Clinical Effect
Clinical effective rate was observed in 18 studies. Detailed characteristics of included studies are listed in Table 2. The clinical effect of ligustrazine (OR: 1.28, 95% CI: 1.10–1.50), nimodipine (OR: 0.62, 95% CI: 0.50–0.78), aspirin plus clopidogrel (OR: 1.82, 95% CI: 1.08–2.57), tissue plasminogen (tPA) (RR: 1.95, 95% CI: 1.10–2.56), Wen Dan Decoction (WD) (OR: 1.60, 95% CI: 1.43–1.79), Xingnaojing capsule and Western medicines (XNJ) (OR: 3.25, 95% CI: 2.30–4.59), NaoShuanTong capsule plus Western medicines (NST plus XM) (OR: 3.04, 95% CI: 1.76–5.26), Xiaoxuming decoction (XXMT) (OR: 1.17, 95% CI: 1.09–1.26), Rhubarb (OR: 1.27, 95% CI: 1.18–1.37), stem cell-based (OR: 3.31, 95% CI: 2.54–4.31), puerarin (RR: 1.22, 95% CI: 1.17–1.28), Buyang Huanwu decoction (BHD) (OR: 1.12, 95% CI: 0.99–1.27), statins (OR: 1.5, 95% CI: 1.29–1.75), salvianolic acids (OR: 1.29, 95% CI: 1.25–1.33), Panax notoginseng saponin (Pntsp) (RR: 1.55, 95% CI: 1.37–2.55), Xuesaitong injection plus western medicines (XST plus XM) (OR: 4.04, 95% CI: 2.86–5.73), Tongqiao Huoxue Decoction plus Western medicines (TQHX plus XM) (OR: 5.43, 95% CI: 3.77–7.82), Ginkgo biloba (RR: 3.79, 95% CI: 2.49–5.78), edaravone plus rt-PA (OR: 3.90, 95% CI: 3.02–5.02) Zhilong Huoxue Tongyu capsule (ZL) (RR: 1.2, 95% CI: 1.12–2.29), desmoteplase (alpha1) (OR: 1.59, 95% CI: 1.08–2.35), acupuncture plus XM (OR: 4.04, 95% CI: 2.93–5.57), and DZSM (Dengzhan Shengmai capsule) (OR: 1.18, 95% CI: 1.12 to 1.24) was significantly better compared with placebo. Moreover, ANP, ZL, and edaravone combined with western medicines significantly improve the total clinical effective rate compared to placebo.
TABLE 2.
Results of pairwise meta-analyses for the clinical effect.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Ligustrazine | Placebo | 3 | 321 | 322 | 1.28 | [1.10, 1.50] | NA | 0.05 |
| Acupuncture | Placebo | 14 | 643 | 536 | 4.04 | [2.93, 5.57] | 0 | 0.00001 |
| tPA | Placebo | 4 | 814 | 804 | 1.95 | [1.10, 2.56] | NA | 0.002 |
| Nimodipine | Placebo | 8 | 677 | 806 | 0.62 | [0.50, 0.78] | NA | 0.0001 |
| Aspirin plus clopidogrel | Placebo | 12 | 100 | 100 | 1.82 | [1.08, 2.57] | NA | 0.001 |
| XNJ | Placebo | 13 | 431 | 408 | 3.25 | [2.30, 4.59] | 0 | 0.00001 |
| NST | Placebo | 13 | 246 | 243 | 3.04 | [1.76, 5.26] | 0 | 0.00001 |
| Stem cell-based therapy | Placebo | 20 | 950 | 844 | 3.31 | [2.54, 4.31] | 0 | 0.0001 |
| Edaravone plus rt-PA | Placebo | 15 | 591 | 591 | 3.90 | [3.02, 5.02] | 0 | 0.0001 |
| XXMT | Placebo | 8 | 242 | 289 | 1.17 | [1.09, 1.26] | 0 | 0.0001 |
| Rhubarb | Placebo | 12 | 350 | 438 | 1.27 | [1.18, 1.37] | 18 | 0.00001 |
| WD | Placebo | 13 | 3,773 | 3,341 | 1.60 | [1.43, 1.79] | 46 | 0.0001 |
| Puerarin | Placebo | 16 | 1,427 | 1,540 | 1.22 | [1.17, 1.28] | 47 | 0.00001 |
| Alpha1 | Placebo | 6 | 217 | 222 | 1.59 | [1.08, 2.35] | 0 | 0.019 |
| BHD | Placebo | 11 | 350 | 334 | 1.12 | [0.99, 1.27] | 69 | 0.002 |
| XST plus XM | Placebo | 12 | 879 | 890 | 4.04 | [2.86, 5.73] | NA | 0.001 |
| Ginkgo biloba | Placebo | 9 | 417 | 416 | 3.79 | [2.49, 5.78] | NA | 0.0001 |
| TQHX plus XM | Placebo | 12 | 733 | 755 | 5.43 | [3.77, 7.82] | NA | 0.0001 |
| ZL | Placebo | 7 | 293 | 278 | 1.2 | [1.12, 2.29] | 0 | 0.0001 |
| HUK | Placebo | 9 | 338 | 338 | 1.30 | [1.21, 1.41] | 0 | 0.00001 |
| Statins | Placebo | 18 | 3,013 | 2,988 | 1.5 | [1.29, 1.75] | 0 | 0.01 |
| Salvianolic acids | Placebo | 12 | 1884 | 1893 | 1.29 | [1.25, 1.33] | 14 | 0.00001 |
| Pntsp | Placebo | 20 | 48 | 48 | 1.55 | [1.37, 2.55] | 0 | 0.0001 |
| DZSM | Placebo | 5 | 341 | 340 | 1.18 | [1.12, 1.24] | 85.7 | 0.0001 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity, NST, NaoShuanTong capsule; XNJ, Xingnaojing capsule; XXMT, Xiaoxuming decoction; Pntsp, Panax notoginseng Saponin; XST, plus XM: Xuesaitong injection plus Western medicines; TQHX, Tongqiao Huoxue decoction; ZL, Zhilong Huoxue Tongyu capsule; BHD, Buyang Huanwu decoction; Alpaga1: Desmoteplase; WD, Wen Dan Decoction. Western medicines (XM) (tPA, antiplatelet agents, statins, and edaravone).
NIHSS Score
The effects of the medications on clinical change were assessed by National Institutes of Health Stroke Scale (Table 3). Eight studies (20.0%) showed that XXMT (MD: −1.86, 95% CI: −3.25–−0.48), safflower yellow (MD: −3.42, 95% CI: −5.38–−2.98), MSCs (MD: −1.85, 95% CI: −2.77–−0.93), ZL (MD: −2.6, 95% CI: −3.41–−1.79), salvianolic acids (MD: −1.44, 95% CI: −1.97–−0.91), heparin (OR: 1.95, 95% CI: 0.74–5.11), XST (MD: −3.17, 95% CI: −4.14 to −2.20), intra-arterial fibrinolysis (Intra-A) (OR: 2.24, 95% CI: 1.27–3.95), edaravone plus rt-PA (MD: 3.95, 95% CI: 2.92–4.99), and human urinary kallidinogenase (HUK) (MD: −1.65, 95% CI, −2.12−−1.71) were significantly different compared with placebo. In contrast, DL-3-n-butylphthalide (NBP) (OR: 0.73, 95% CI: −0.14 to 1.59, p = 0.1), BHD (MD: 1.66, 95% CI: −1.08 to 4.40, p = 0.1), and DZSM (MD: 0.57, 95% CI: 0.44.0.73, p = 0.11) showed no change or a deterioration.
TABLE 3.
Results of pairwise meta-analyses for the NIHSS score.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Heparin | Placebo | 9 | 260 | 317 | 1.95 | [0.74, 5.11] | 80 | 0.03 |
| XXMT | Placebo | 8 | 91 | 95 | −1.86 | [−3.25, −0.48] | 10 | 0.008 |
| Safflower yellow | Placebo | 7 | 368 | 394 | −3.42 | [−5.38, −2.98] | 82 | 0.004 |
| MSCs | Placebo | 5 | 52 | 57 | −1.85 | [−2.77, −0.93] | 24 | 0.0001 |
| BHD | Placebo | 11 | 96 | 96 | 1.66 | [−1.08, 4.40] | 64 | 0.1 |
| XST | Placebo | 12 | 879 | 890 | −3.17 | [−4.14, −2.20] | NA | 0.001 |
| Intra-A | Placebo | 5 | 130 | 204 | 2.24 | [1.27, 3.95] | 0 | 0.005 |
| Edaravone plus rt-PA | Placebo | 17 | 860 | 859 | 3.95 | [2.92, 4.99] | 92 | 0.0001 |
| ZL | Placebo | 7 | 115 | 330 | −2.6 | [−3.41, −1.79] | 50 | 0.0001 |
| Salvianolic acids | Placebo | 12 | 435 | 462 | −1.44 | [−1.97, −0.91] | 57 | 0.001 |
| NBP | Placebo | 12 | 108 | 108 | 0.73 | [−0.14, 1.59] | 89 | 0.1 |
| HUK | Placebo | 16 | 667 | 659 | –1.65 | [−2.12, −1.71] | 84 | 0.00001 |
| DZSM | Placebo | 5 | 341 | 340 | 0.57 | [0.44, 0.73] | 44.2 | 0.11 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity; rt-PA, alteplase; MSCs, autologous bone marrow stromal cells; XXMT, Xiaoxuming decoction; XST, Xuesaitong injection; NBP, DL-3-n-butylphthalide; BHD, Buyang Huanwu decoction; Intra-A, intra-arterial Fibrinolysis; HUK, human urinary kallidinogenase.
Rankin Scale (mRS) Score
From our search, the effects of the medications on clinical change were assessed by Rankin Score (mRS) (Table 4). In total, 18 studies (42.5%) including tPA (OR: 1.31, 95% CI: 1.07–3.59), tPA plus mechanical thrombectomy (MTE) (OR: 4.32, 95% CI: 2.16–7.46), MTE (OR: 3.23, 95% CI: 1.75–7.33), stent retrievers (OR: 2.43, 95% CI: 1.91–3.09), cerebrolysin (RR: −049, 95% CI: −1.21 to 0.24), ZL (MD: −0.57, 95% CI: −0.84 to −0.30), salvianolic acids (MD: −0.88, 95% CI: −1.11–−0.64), heparin (OR: 1.38, 95% CI: 0.61–3.56) and Rhubarb (OR: 3.11, 95% CI: 2.06–4.68), Intra-A (RR: 2.05, 95% CI: 1.33–3.14), DZSM (MD: −0.75, 95% CI: −1.02–−0.48), and cinepazide maleate (MD: 0.607, 95% CI: 0.46–0.801) showed better outcomes for mRS score than placebo. The other treatments “Safflower yellow (MD: −4.18, 95% CI: −5.38–−2.98, p = 0.1) and MSCs (RR: 1.81, 95% CI: 0.37–8.95, p = 0.47)” indicated no significant difference in effectiveness as compared to placebo.
TABLE 4.
Results of pairwise meta-analyses for the mRS score.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Heparin | Placebo | 12 | 2,145 | 550 | 1.38 | [0.61, 3.56] | 83 | 0.01 |
| Safflower yellow | Placebo | 13 | 368 | 394 | −4.18 | [−5.38,−2.98] | 52 | 0.1 |
| Rhubarb | Placebo | 13 | 350 | 438 | 3.11 | [2.06, 4.68] | 18 | < 0.05 |
| MSCs | Placebo | 7 | 86 | 86 | 1.81 | [0.37, 8.95] | 57 | 0.47 |
| tPA | Placebo | 4 | 814 | 804 | 1.31 | [1.07, 3.59] | NA | 0.01 |
| MTE | Placebo | 5 | 414 | 404 | 3.23 | [1.75, 7.33] | NA | 0.008 |
| MTE plus stent retrievers | Placebo | 5 | 142 | 143 | 4.56 | [2.63, 7.9] | 0 | 0.0001 |
| tPA plus MTE | Placebo | 17 | 2639 | 2640 | 4.32 | [2.16, 7.46] | 51 | 0.01 |
| Stent retrievers | Placebo | 5 | 653 | 634 | 2.43 | [1.91, 3.09] | 0 | 0.00001 |
| Cerebrolysin | Placebo | 5 | 971 | 808 | −0.49 | [−1.21, 0.24] | 73.6 | 0.052 |
| Intra-A | Placebo | 12 | 171 | 224 | 2.05 | [1.33, 3.14] | 0 | 0.001 |
| ZL | Placebo | 9 | 45 | 60 | −0.57 | [−0.84, −0.30] | 37 | 0.0001 |
| Salvianolic acids | Placebo | 7 | 210 | 242 | −0.88 | [−1.11, −0.64] | 0 | 0.001 |
| DZSM | Placebo | 28 | 341 | 340 | −0.75 | [−1.02, −0.48] | 85.9 | 0.0001 |
| Cinepazide maleate | Placebo | 4 | 236 | 234 | 0.607 | [0.46, 0.801] | NA | 0.0004 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity; MSCs, autologous bone marrow stromal cells; NST, NaoShuanTong capsule; tPA: tissue plasminogen XNJ, Xingnaojing capsule; MTE: mechanical thrombectomy, ZL, Zhilong Huoxue Tongyu capsule; Intra-A, intra-arterial fibrinolysis.
Barthel Index Score
The effects of the medications on clinical change were assessed by Barthel Index (BI) Score (Table 5). Ten studies (25%) showed that autologous bone marrow stromal cells (MSCs) (MD: 2.50, 95% CI: −4.69–9.68), TQHX plus XM (MD: 2.45, 95% CI: 1.16–3.73), ZL (MD: 9.75, 95% CI: 7.15–12.36), NST (MD: 8.15, 95% CI: 3.79–12.52), Intra-A (MD: 1.6, 95% CI: 1.01–2.51), DZSM (MD: 8.97, 95% CI: 5.88,12.05) and cinepazide maleate (MD: 0.719, 95% CI: 0.542, 0.956), and MLC601 (MD: 2.35, 95% CI: 1.31, 4.23) were significantly different compared with placebo. In contrast, NBP (MD: 1.65, 95% CI: 1.25–2.04), p = 0.08) showed no difference compared to placebo.
TABLE 5.
Results of pairwise meta-analyses for the BI score.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| NST | Placebo | 13 | 304 | 289 | 8.15 | [3.79, 12.52] | 75 | 0.0005 |
| MSCs | Placebo | 5 | 88 | 88 | 2.50 | [−4.69,9.68] | 74 | < 0.05 |
| Intra-A | Placebo | 5 | 139 | 204 | 1.6 | [1.01, 2.51] | 0 | 0.04 |
| TQHX plus XM | Placebo | 12 | 225 | 226 | 2.45 | [1.16, 3.73] | 89 | 0.0001 |
| ZL | Placebo | 7 | 115 | 130 | 9.75 | [7.15, 12.36] | 0 | 0.001 |
| NBP | Placebo | 12 | 165 | 160 | 1.65 | [1.25, 2.04] | 67 | 0.08 |
| DZSM | Placebo | 5 | 341 | 340 | 8.97 | [5.88, 12.05] | 85.9 | 0.0001 |
| Cinepazide maleate | Placebo | 4 | 236 | 236 | 0.719 | [0.542, 0.956] | 0 | 0.012 |
| MLC601 | Placebo | 2 | 237 | 436 | 2.35 | [1.31, 4.23] | 0 | 0.004 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity; MSCs, autologous bone marrow stromal cells; NST, NaoShuanTong capsule; TQHX, Tongqiao Huoxue Decoction; ZL, Zhilong Huoxue Tongyu capsule; NBP, DL-3-n-butylphthalide; BHD, Buyang Huanwu decoction; Intra-A, intra-arterial fibrinolysis.
Neurological Function Deficit Score
Table 6 presents the results of the comparisons of behavioral symptoms; a total of seven studies were assessed by NFD scores. Patients treated with XNJ (MD: −3.78, 95% CI: −4.75 to −2.81), NST (MD: 8.15, 95% CI: 10.11–49.10), Chuanxiong (MD: −3.11, 95% CI: −5.22–−1.00), Safflower yellow (MD: 3.11, 95% CI: 2.06–4.68), Rhubarb (MD: −3.36, 95% CI: −6.10–−0.62), Puerarin (MD: −3.69, 95% CI: −4.67–−2.71), Pntsp (MD: −3.36, 95% CI: −4.20–−2.53), HUK (MD, 1.30, 95% CI, 1.21 to 1.41), and Mailuoning (OR: 0.31, 95% CI: 0.23–0.42) showed better behavioral symptoms than those administered (p < 0.05). Moreover, Ginkgo biloba use was also associated with an improvement in activities of daily living and functional outcomes (MD: 9.52; 4.66 to 14.33, p < 0.001). Subgroup analysis suggests that the impact was larger when using an injectable formulation of Ginkgo biloba compared to the oral formulation. The other treatments indicated no significant difference in effectiveness as compared to placebo (p > 0.05) (Albumin (MD: 1.04, 95% CI: 0.85–1.27). TNK (MD: 1.56, 95% CI: 1.0–2.43), DZSM (MD: −2.81, 95% CI: 4.17–−1.44), and MLC601 (MD: 0.27, 95% CI: −0.02–0.55).
TABLE 6.
Results of pairwise meta-analyses for NFDs.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| XNJ | Placebo | 13 | 356 | 347 | −3.78 | [−4.75, −2.81] | 54 | 0.00001 |
| NST | Placebo | 13 | 100 | 100 | 8.15 | [10.11, 49.10] | 95 | 0.0005 |
| Chuanxiong | Placebo | 3 | 80 | 81 | −3.11 | [−5.22, −1.00] | 0 | 0.0039 |
| Safflower yellow | Placebo | 7 | 368 | 394 | 3.11 | [2.06, 4.68] | 0 | 0.00001 |
| Rhubarb | Placebo | 12 | 210 | 210 | −3.36 | [−6.10, −0.62] | 89 | 0.00001 |
| Puerarin | Placebo | 16 | 659 | 699 | −3.69 | [−4.67, −2.71] | 70 | 0.00001 |
| Albumin | Placebo | 4 | 804 | 807 | 1.04 | [0.85, 1.27] | 0 | 0.65 |
| Salvianolic acids | Placebo | 12 | 235 | 235 | −8.65 | [−11.10, −6.20] | 31 | 0.001 |
| Pntsp | Placebo | 20 | 1464 | 1435 | −3.36 | [−4.20, −2.53] | 74 | 0.0001 |
| Nimodipine | Placebo | 8 | 677 | 806 | 0.54 | [0.50, 0.78] | NA | 0.0001 |
| HUK | Placebo | 9 | 338 | 338 | 1.30 | [1.21, 1.41] | 0 | 0.00001 |
| DZSM | Placebo | 5 | 341 | 340 | −2.81 | [−4.17, −1.44] | 85.9 | 0.1 |
| Mailuoning | Placebo | 15 | 736 | 755 | 0.31 | [0.23, 0.42] | 0 | 0.001 |
| TNK | Placebo | 4 | 656 | 671 | 1.56 | [1.0, 2.43] | 0 | 0.05 |
| MLC601 | Placebo | 2 | 275 | 520 | 0.27 | [−0.02, 0.55] | 66 | 0.06 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity, NST, NaoShuanTong capsule; XNJ, Xingnaojing capsule; Pntsp, Panax notoginseng Saponin; TQHX, Tongqiao Huoxue decoction; TNK, tenecteplase.
Extracranial Hemorrhage (sICH)
The sICH events resulting from administration of other treatments were mild, and Safflower yellow (p = 0.93), stent retrievers (OR: 1.08, 95% CI: 0.64–2.30), Alpha1 (OR: 1.25, 95% CI: 0.97–1.62), Ginkgo biloba (OR: 0.82, 95% CI: 0.43–1.57), tirofiban (OR: 1.14, 95% CI: 0.72–1.82), heparin (OR: 0.71, 95% CI: 0.25–2.05), edaravone plus rt-PA (OR: 0.44, 95% CI: 0.29–0.66), MTE plus stent retrievers (OR: 0.59, 95% CI: 0.35–0.97), MTE (OR: 3.05, 95% CI: 0.44–21.23), MTE plus tPA (OR: 0.93, 95% CI: 0.72–1.19), TNK (OR: 1.07, 95% CI: 0.6–1.93), and cilostazol (OR: 0.29, 95% CI: 0.15–0.56) had no significant difference on sICH events between these groups and placebo groups (Table 7).
TABLE 7.
Results of pairwise meta-analyses for extracranial hemorrhage.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Heparin | Placebo | 9 | 288 | 330 | 0.71 | [0.25, 2.05] | 32 | 0.22 |
| Safflower yellow | Placebo | 7 | 368 | 394 | NA | NA | 0 | 0.93 |
| Stent retrievers | Placebo | 5 | 652 | 634 | 1.08 | [0.64, 2.30] | 0 | 0.63 |
| Ginkgo biloba | Placebo | 12 | 266 | 281 | 0.82 | [0.43, 1.57] | 0 | 0.443 |
| Tirofiban | Placebo | 6 | 216 | 213 | 1.14 | [0.72, 1.82] | 0 | 0.57 |
| Edaravone plus rt-PA | Placebo | 8 | 221 | 221 | 0.44 | [0.29, 0.66] | 0 | 0.93 |
| Alpha 1 | Placebo | 6 | 467 | 595 | 1.25 | [0.97, 1.62] | 9 | 0.09 |
| TNK | Placebo | 4 | 658 | 676 | 1.07 | [0.6, 1.93] | 0 | 0.81 |
| MTE plus stent retrievers | Placebo | 5 | 146 | 144 | 0.59 | [0.35,0.97] | 0 | 0.83 |
| MTE | Placebo | 5 | 141 | 140 | 3.05 | [0.44, 21.23] | 0 | 0.25 |
| tPA plus MTE | Placebo | 7 | 2639 | 2640 | 0.93 | [0.72, 1.19] | 29 | 0.13 |
| Cilostazol | Placebo | 6 | 1728 | 1731 | 0.29 | [0.15, 0.56] | 0 | 0.77 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity, TNK, tenecteplase.
Mortality
Fifteen studies reported all-cause mortality at the end of follow-up. Ligustrazine (OR: 1.67, 95% CI: 1.02–2.67), statins (OR: 0.85, 95% CI: 0.77–0.93) were significant different compared with placebo. In contrast, stent retrievers (OR: 0.81, 95% CI: 0.58–1.12), cerebrolysin (OR: 0.82, 95% CI: 0.55–1.22), Ginkgo biloba (OR: 1.21, 95% CI: 0.29–5.09), stem cell-based (MD: 0.6, 95% CI: 0.35–1.03), tirofiban (OR: 0.53, 95% CI: 0.13–2.07), albumin (OR: 1.1, 95% CI: 0.9–1.34), Alpha1 (OR: 1.05, 95% CI: 0.7–1.59), heparin (OR: 0.9, 95% CI: 0.74–1.09), Intra-A (OR: 0.83, 95% CI: 0.48–1.39), edaravone plus rt-PA (MD: 0.43, 95% CI: 0.13–1.42), tPA (OR: 1.04, 95% CI: 0.75–1.43), DZSM (MD: 0.54, 95% CI: 0.31–0.95), TNK (MD: 1.03, 95% CI: 0.69–1.52), and cilostazol (MD: 0.80, 95% CI: 0.42 to 1.53, p = 0.52) had no significant differences of mortality events between these groups and placebo groups (p > 0.05) (Table 8).
TABLE 8.
Results of pairwise meta-analyses for mortality.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Ligustrazine | Placebo | 3 | 321 | 322 | 1.67 | [1.02, 2.67] | 95 | < 0.05 |
| Heparin | Placebo | 9 | 2703 | 1145 | 0.9 | [0.74, 1.09] | 1 | 0.42 |
| tPA | Placebo | 4 | 814 | 804 | 1.04 | [0.75, 1.43] | NA | 0.83 |
| Stent retrievers | Placebo | 5 | 653 | 634 | 0.81 | [0.58, 1.12] | 29 | 0.19 |
| Alpha 1 | Placebo | 6 | 467 | 595 | 1.05 | [0.7, 1.59] | 0 | 0.8 |
| Cerebrolysin | Placebo | 7 | 971 | 808 | 0.82 | [0.55, 1.22] | 0 | 0.81 |
| Ginkgo biloba | Placebo | 12 | 213 | 228 | 1.21 | [0.29, 5.09] | 43 | 1.8 |
| Stem cell-based therapy | Placebo | 9 | 218 | 217 | 0.6 | [0.35, 1.03] | 4 | 0.4 |
| Tirofiban | Placebo | 6 | 218 | 223 | 0.53 | [0.13, 2.07] | 63 | 0.1 |
| Albumin | Placebo | 4 | 1928 | 1938 | 1.1 | [0.9, 1.34] | 0 | 0.51 |
| Intra-A | Placebo | 5 | 171 | 224 | 0.83 | [0.48, 1.39] | 0 | 0.46 |
| Edaravone plus rt-PA | Placebo | 4 | 474 | 472 | 0.43 | [0.13, 1.42] | 0 | 0.87 |
| Statins | Placebo | 18 | 3034 | 3021 | 0.85 | [0.77, 0.93] | 0 | 0.003 |
| DZSM | Placebo | 5 | 341 | 340 | 0.54 | [0.31, 0.95] | 85.9 | 0.23 |
| TNK | Placebo | 4 | 658 | 676 | 1.03 | [0.69, 1.52] | 0 | 0.9 |
| Cilostazol | Placebo | 6 | 1728 | 1731 | 0.80 | [0.42, 1.53] | 0 | 0.52 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity; Intra-A, intra-arterial fibrinolysis; rt-PA, alteplase; TNK, tenecteplase.
Adverse Events
Adverse events of the meta-analysis of participants with at least one adverse event indicated a beneficial effect in favor of placebo treatment compared with salvianolic acids (OR: 1.45, 95% CI: 1.11–1.91, p = 0.007), Pntsp (RR: 0.62, 95% CI: 0.39–0.97, p = 0.04), colchicine (OR: 0.31, 95% CI: 0.13–0.71, p = 0.006), and NBP (RR: 3.55, 95% CI, 1.19 –10.56; p < 0:05). The adverse events resulting from administration of other treatments were mild, and Chuanxiong (OR: 1.02, 95% CI: 0.35–2.96), MSCs (RR: 0.43, 95% CI: 0.18–1.05), Cerebrolysin (OR: 1.18, 95% CI: 0.86–1.64), Ginkgo biloba (OR: 1.48, 95% CI: 0.51–2.71), Stem cell-based (MD: 2.59, 95% CI: 0.11–5.93), TQHX (OR: 1.78, 95% CI: 0.51–6.2), HUK (RR: 0.01, 95% CI: 0.02–0.04), Mailuoning (OR: 1.39, 95% CI: 0.28–6.76), and cinepazide maleate had no significant differences in adverse events between these groups and placebo groups (p > 0.05) (Table 9). Among all of the trials, in the HUK groups, six cases of hypotension, four cases of fever, two cases of flushing, two cases of vomiting, one case of headache, one case of arrhythmia, and one case of pruritus were reported. In addition, no deaths and four serious adverse events were reported in the MLC601 group.
TABLE 9.
Results of pairwise meta-analyses for AE.
| Comparative medication | Reference medication | Number of studies | Pairwise meta-analyses | |||||
|---|---|---|---|---|---|---|---|---|
| Number of control | Number of patients | MD/OR/RR | 95% CI | I2 | P | |||
| Chuanxiong | Placebo | 3 | 50 | 49 | 1.02 | [0.35, 2.96] | NA | 0.09 |
| MSCs | Placebo | 5 | 64 | 44 | 0.43 | [0.18, 1.05] | 0 | 0.06 |
| Cerebrolysin | Placebo | 7 | 971 | 808 | 1.18 | [0.86, 1.64] | 23 | 0.27 |
| Ginkgo biloba | Placebo | 12 | 388 | 406 | 1.48 | [0.51, 2.71] | 54 | 0.07 |
| Stem cell-based therapy | Placebo | 9 | 136 | 139 | 2.59 | [0.11, 5.93] | 0 | 0.87 |
| TQHX plus XM | Placebo | 12 | 180 | 180 | 1.78 | [0.51, 6.2] | 0 | 0.36 |
| Salvianolic acids | Placebo | 12 | 1496 | 1498 | 1.45 | [1.11, 1.91] | 0 | 0.007 |
| Colchicine | Placebo | 4 | 2764 | 2788 | 0.31 | [0.13, 0.71] | 0 | 0.006 |
| NBP | Placebo | 4 | 108 | 108 | 3.55 | [1.19, 10.56] | 0 | < 0.05 |
| Pntsp | Placebo | 20 | 361 | 354 | 0.62 | [0.39, 0.97] | 0 | 0.04 |
| HUK | Placebo | 9 | 387 | 387 | 0.01 | [0.02, 0.04] | 0 | 0.50 |
| Mailuoning | Placebo | 2 | 64 | 65 | 1.39 | [0.28, 6.76] | 0 | 0.57 |
| cinepazide maleate | Placebo | NA | 648 | 643 | NA | NA | NA | 0.82 |
CI, confidence interval; MD, mean difference; OR, risk ratio; I2, heterogeneity; MSCs, Pntsp, Panax notoginseng Saponin; autologous bone marrow stromal cells; TQHX, Tongqiao Huoxue Decoction.
Discussion
Our umbrella review was conducted on the data derived from treatments for ischemic stroke patients, which was used to appraise the relative effectiveness and safety of therapies. We attempted to summarize data from published systematic reviews and meta-analyses to find if there are significant beneficial treatments for ischemic stroke patients. Our study showed that thrombolytic therapy (rt-PA, TNK, and alpha1), MTE, stem cell-based therapies, stent retrievers, acupuncture plus XM, MSCs, antiplatelet agents (aspirin, clopidogrel, and tirofiban), statins, and blood-activating and stasis-dispelling herbs can improve the neurological deficits and activities of daily living in patients with ischemic stroke. MTE plus Stent Retrievers or tPA, TQHX plus XM, XST plus XM, and NST plus XM show better clinical efficacy and safety. Ligustrazine, safflower yellow, statins, Pntsp, albumin, HUK, colchicine, MLC601, salvianolic acids, and NBP have no important impact on neurological deficits or activities of daily living. In addition, tPA, MTE, stem cell-based therapies, Stent Retrievers, Acupuncture, NST, Ginkgo biloba, TQHX, XST, and XNJ show no serious adverse events in ischemic stroke patients. Our results need to be interpreted with caution to determine the optimal treatment strategy for ischemic stroke patients.
The effects of tPA may be considerable for ischemic stroke which is incurable with current treatment paradigms, and other medications that may slow down the progression of ischemic stroke patients are worth exploring. Previous studies have showed that tPA or MTE has beneficial effects on hyperacute period ischemic stroke (Thelengana et al., 2019) (Liu et al., 2016), while one study demonstrated that tPA plus MTE performed best (Kaesmacher et al., 2019). Our results indicated that all tPA, MTE, MTE plus tPA, MTE plus Stent Retrievers, TQHX plus XM, XST plus XM, and NST plus XM were more effective for neurological function or activities of daily living compared with placebo. Researches have demonstrated that there was a higher effect of Stent Retrievers and MTE observed for acute ischemic stroke than that observed for the mild ischemic stroke patients (Punal-Rioboo et al., 2015). Similar to these studies, Stent Retrievers and MTE treatment showed statistically significant improvement in clinical effect compared to placebo in our study. Research studies have demonstrated that Human serum albumin has shown remarkable efficacy in rodent models of ischemic stroke (Huang and Xiao, 2021). Unfortunately, our study has demonstrated that showing no statistically significant difference between the albumin and control groups (p > 0.05). Considering pulmonary edema and other complications are more likely to occur in such patients after albumin infusion, the administration of albumin therapy for acute ischemic stroke should be carried out with utmost caution.
The behavioral symptoms of patients with ischemic stroke are often evaluated by NFDS/NIHSS/BI/mRS, which assesses the severity and frequency of neuropsychiatric symptoms. As a result, previous meta-analyses have reported that the efficacy of blood-activating and stasis-dispelling herbs may be related to the severity of ischemic stroke. In addition, tPA, MTE plus tPA, MTE plus Stent Retrievers, blood-activating and stasis-dispelling herbs plus XM was reported as only a modest but significant effect found on behavior in ischemic stroke patients (Peng et al., 2014; Punal-Rioboo et al., 2015; Kaesmacher et al., 2019; Shang et al., 2019; Zhang et al., 2019). In our study, Alpha1 was more effective for neurological improvement rate compared with placebo. Unfortunately, the lack of placebo controls in NFDS/NIHSS/BI/mRS score studies may limit their validity. Interestingly, MSCs are not significant in mRS score but significant in NIHSS/BI score. Moreover, nimodipine can significantly improve clinical outcomes compared with placebo, although it does not significantly reduce the incidence rate of recurrent hemorrhage and adverse reactions. In addition, tPA and MTE affected mRS scores and was recommended by the FDA. We considered treatment with ligustrazine, Safflower yellow albumin, MLC601, ANP, rhubarb, and NBP to not affect neurological deficits and activities of daily living because of the lack of statistical significance of results. Patients with ischemic stroke deteriorate progressively with varying degrees of severity of disease, which may affect the results obtained from pooling data. Moreover, measurement time after dosing can affect NFDS/NIHSS/BI/mRS scoring results and cause them to be biased.
Previous meta-analyses have demonstrated that patients treated with intra-arterial fibrinolysis provided a modest and better improvement in clinical effect change (Roaldsen et al., 2022). In addition, drug combination shows a statistically significant advantage compared to placebo the short-term and long-term analysis. Although the effect of single blood-activating and stasis-dispelling herbs (TQHX, NST, XST, etc.) use is not ideal (Erratum, 2017), they show a modest and better effect in combination with XM (Wu et al., 2007). Furthermore, ischemic stroke agents are likely to have an important effect on increasing neurological function or activities of daily living in mild to moderate ischemic stroke patients. In this study, the quality evaluated by AMSTAR2 scores of systematic reviews of ligustrazine, safflower yellow, cerebrolysin, BHD, salvianolic acids, and ZL was low, and these may not have an important impact on neurological function or activities of daily living. First, ischemic stroke is a sudden disease, our review mainly selected clinical studies to demonstrate short-term efficacy on neurological function. Although long-term clinical trials are ethically questionable, those that are high-quality are essential to uncover comparative differences between treatments of ischemic stroke. Second, we believe that further analyses are needed to clarify the factors associated with the increased placebo effect over time in global clinical trials. In the treatment of ischemic stroke, the safety of the treatments is critical since they should be taken on a long-term basis. The number of participants with at least one serious adverse event such as nausea, diarrhea, cardiovascular, gastrointestinal, and other disorders was extracted. Previous meta-analyses have demonstrated that acute and convalescent stroke patients treated with antiplatelet agents showed a modest improvement, although there is a risk of intracranial hemorrhage (Zhou et al., 2020). In this review, edoxaban was likely to provide more protection from stroke and sICH than placebo, aspirin alone, or aspirin plus clopidogrel in both clinical trials and unselected community populations. Moreover, statins were found to be effective for primary and secondary prevention of ischemic stroke in the study through the aggressive reduction of cholesterol. Some studies have found that using statins before an ischemic stroke can increase collateral circulation and improve prognosis. Despite an increased risk of bleeding conversion, thrombolytic use of statins resulted in overall improvement. Recent studies have also found statins to be associated with atrial fibrillation. In addition, the promotion of collateral circulation by neuroprotective drugs may be related to the induction of NO synthesis and angiogenesis in vascular endothelium (Hu et al., 2021). In addition, the incidence of withdrawals due to adverse events tended to be higher in the salvianolic acids albumin, MLC601, and NBP treatment than in placebo groups. Moreover, our study summarized that MTE, stem cell-based therapies, stent retrievers, acupuncture plus XM, NST, Ginkgo biloba, TQHX, XST, and XNJ show no serious adverse events in ischemic stroke patients.
In recent years, stem cell-based therapies (MSCs, stem cell-based) as a treatment to investigate ischemic stroke patients has been a potential therapy (Cao and Li, 2015; Li et al., 2020). A previous study has shown that Intra-A results in a better beneficial effect for cognition and activities of daily living (Lee et al., 2010). Similar to these studies, stem cell-based therapies may show effectiveness for neurological deficits and activities of daily living in this study. However, clinical trials of stem cell-based therapies for ischemic stroke are still in the early stage. Many factors such as cell types, cell numbers, delivery routes, time windows, and medical and rehabilitation therapies affect the efficacy of stem cells. Well-designed RCTs are necessary to explore the benefit of stem cell-based therapies as treatment in patients with ischemic stroke, and further research effects should be carefully explored.
In general, the treatment for patients with ischemic stroke is aimed at promoting independence, clear embolism, maintaining function, and treating symptoms. Previous meta-analyses and reviews have focused on the possible effectiveness and safety of stem cell-based therapies, stent retrievers, acupuncture, MSCs, antiplatelet agents, statins, and blood-activating and stasis-dispelling herbs (Li et al., 2014; Cao and Li, 2015; Punal-Rioboo et al., 2015; Shang et al., 2019; Zhang et al., 2019; Li et al., 2020; Zhao et al., 2021; Zhou et al., 2022), even though patients experience modest efficacy and many adverse events with the treatment. As a result, we need to identify an efficacious and safe treatment paradigm for ischemic stroke patients. Studies have shown that MTE plus tPA, MTE plus Stent Retrievers, TQHX plus XM, XST plus XM, NST plus XM, and acupuncture plus XM improved neurological deficits and activities of daily living, and the adverse effects were mild for the treatment of ischemic stroke (Li et al., 2014; Kaesmacher et al., 2019; Shang et al., 2019; Li et al., 2020; Shen et al., 2020). However, a larger sample size and long-term follow-up studies are needed to find the reliability of this medication. Due to tPA, MTE, tPA plus edaravone, blood-activating, and stasis-dispelling herbs plus XM efficacy in improving neurological deficits and activities of daily living, we believe that tPA or tPA plus other drugs can be employed as first-line treatment.
Limitations
The limitations to this study should be acknowledged. First, direct comparative evidence of treatments for ischemic stroke patients in our included studies was limited. Second, other factors may have led to the umbrella review inconsistencies, such as the duration and quality of studies. Furthermore, a considerable number of studies could not be included as they did not have the abovementioned data.
Conclusion
In conclusion, our study suggested that tPA, tPA plus MTE, acupuncture plus XM, tPA plus edaravone, and blood-activating and stasis-dispelling herbs plus XM are the optimum cognitive and activities of daily living medication for patients with ischemic stroke. In the future, the combination of well-tolerated agents and other significant beneficial treatments should be used for patients with ischemic stroke, which will contribute to the successful construction of a similar study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
QL and YC conceived and designed the review. YOL, RC, FF, YL, YA, HL, SL, YD, QY, ZQ, and WS looked up the literature. YOL wrote the manuscript. QL and YC revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82174085).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.924747/full#supplementary-material
References
- Blann A. D., Skjøth F., Rasmussen L. H., Larsen T. B., Lip G. Y. (2015). Edoxaban versus placebo, aspirin, or aspirin plus clopidogrel for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb. Haemost. 114 (2), 403–409. 10.1160/TH15-05-0383 PubMed Abstract | 10.1160/TH15-05-0383 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Cao W., Li P. (2015). Effectiveness and Safety of Autologous Bone Marrow Stromal Cells Transplantation After Ischemic Stroke: A Meta-Analysis. Med. Sci. Monit. 21, 2190–2195. 10.12659/MSM.895081 PubMed Abstract | 10.12659/MSM.895081 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong P. Z., Ng H. Y., Tai J. T., Lee S. W. H. (2020). Efficacy and Safety of Ginkgo biloba in Patients with Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Am. J. Chin. Med. 48 (3), 513–534. 10.1142/S0192415X20500263 PubMed Abstract | 10.1142/S0192415X20500263 | Google Scholar [DOI] [PubMed] [Google Scholar]
- De Santis K. K., Lorenz R. C., Lakeberg M., Matthias K. (2021). The application of AMSTAR2 in 32 overviews of systematic reviews of interventions for mental and behavioural disorders: A cross‐sectional study. Res. Syn. Meth. 10.1002/jrsm.1532 10.1002/jrsm.1532 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Donkor E. S. (2018). Stroke in the21stCentury: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 1–10. 10.1155/2018/3238165 PubMed Abstract | 10.1155/2018/3238165 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson J., Lees K. R., Lyden P., Blackwell L., Albers G., Bluhmki E., et al. (2014). Stroke Thrombolysis Trialists' Collaborative, GEffect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384 (9958), 1929–1935. 10.1016/S0140-6736(14)60584-5 PubMed Abstract | 10.1016/S0140-6736(14)60584-5 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erratum (2017). Erratum: Traditional chinese patent medicine for acute ischemic stroke: an overview of systematic reviews based on the GRADE approach: Erratum. Med. Baltim. 96 (32), e7810. 10.1097/MD.0000000000007810 PubMed Abstract | 10.1097/MD.0000000000007810 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S., Lin N., Shan G., Zuo P., Cui L. (2014). Safflower yellow for acute ischemic stroke: A systematic review of randomized controlled trials. Complement. Ther. Med. 22 (2), 354–361. 10.1016/j.ctim.2014.01.001 PubMed Abstract | 10.1016/j.ctim.2014.01.001 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Feng L., Wu X. J., Cao T., Wu B. (2021). The efficacy and safety of Xuesaitong injection combined with western medicines in the treatment of ischemic stroke: an updated systematic review and meta-analysis. Ann. Palliat. Med. 10 (9), 9523–9534. 10.21037/apm-21-1828 PubMed Abstract | 10.21037/apm-21-1828 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Fu D. L., Lu L., Zhu W., Li J. H., Li H. Q., Liu A. J., et al. (2013). Xiaoxuming decoction for acute ischemic stroke: a systematic review and meta-analysis. J. Ethnopharmacol. 148 (1), 1–13. 10.1016/j.jep.2013.04.002 PubMed Abstract | 10.1016/j.jep.2013.04.002 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Gao L., Xiao Z., Jia C., Wang W. (2021). Effect of Buyang Huanwu decoction for the rehabilitation of ischemic stroke patients: a meta-analysis of randomized controlled trials. Health Qual. Life Outcomes 19 (1), 79. 10.1186/s12955-021-01728-6 PubMed Abstract | 10.1186/s12955-021-01728-6 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Blaha M. J. (2014). Stroke Statistics, SExecutive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129 (3), 399–410. 10.1161/01.cir.0000442015.53336.12 PubMed Abstract | 10.1161/01.cir.0000442015.53336.12 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hong K. S., Lee J. S. (2015). Statins in Acute Ischemic Stroke: A Systematic Review. J. Stroke 17 (3), 282–301. 10.5853/jos.2015.17.3.282 PubMed Abstract | 10.5853/jos.2015.17.3.282 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Guo Y., Lin Y., Tang Y., Tang Q., Wang X., et al. (2021). Safety and efficacy of edaravone combined with alteplase for patients with acute ischemic stroke: A systematic review and meta-analysis. Pharmazie 76 (2), 109–113. 10.1691/ph.2021.0949 PubMed Abstract | 10.1691/ph.2021.0949 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Huang Y., Wang B., Zhang Y., Wang P., Zhang X. (2020). Efficacy and safety of human urinary kallidinogenase for acute ischemic stroke: a meta-analysis. J. Int. Med. Res. 48 (9), 300060520943452. 10.1177/0300060520943452 PubMed Abstract | 10.1177/0300060520943452 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Xiao Z. (2021). Albumin therapy for acute ischemic stroke: a meta-analysis. Neurol. Sci. 42 (7), 2713–2719. 10.1007/s10072-021-05244-9 PubMed Abstract | 10.1007/s10072-021-05244-9 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kaesmacher J., Mordasini P., Arnold M., López-Cancio E., Cerdá N., Boeckh-Behrens T., et al. (2019). Direct mechanical thrombectomy in tPA-ineligible and -eligible patients versus the bridging approach: a meta-analysis. J. Neurointerv Surg. 11 (1), 20–27. 10.1136/neurintsurg-2018-013834 PubMed Abstract | 10.1136/neurintsurg-2018-013834 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos A. H., Palaiodimou L., Price C., Giannopoulos S., Lemmens R., Kosmidou M., et al. (2020). Colchicine for stroke prevention in patients with coronary artery disease: a systematic review and meta-analysis. Eur. J. Neurol. 27 (6), 1035–1038. 10.1111/ene.14198 PubMed Abstract | 10.1111/ene.14198 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lee M., Hong K. S., Saver J. L. (2010). Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke 41 (5), 932–937. 10.1161/STROKEAHA.109.574335 PubMed Abstract | 10.1161/STROKEAHA.109.574335 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Li L., Zhang H., Meng S. Q., Qian H. Z. (2014). An updated meta-analysis of the efficacy and safety of acupuncture treatment for cerebral infarction. PLoS One 9 (12), e114057. 10.1371/journal.pone.0114057 PubMed Abstract | 10.1371/journal.pone.0114057 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ling L., Li C., Ma Q. (2017). Efficacy and safety of desmoteplase in acute ischemic stroke patients: A systematic review and meta-analysis. Med. Baltim. 96 (18), e6667. 10.1097/MD.0000000000006667 PubMed Abstract | 10.1097/MD.0000000000006667 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Dong X., Tian M., Liu C., Wang K., Li L., et al. (2020). Stem cell-based therapies for ischemic stroke: a systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 11 (1), 252. 10.1186/s13287-020-01762-z PubMed Abstract | 10.1186/s13287-020-01762-z | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa R. C., Jovanovic B. D., Alberts M. J. (2002). Analysis of the safety and efficacy of intra-arterial thrombolytic therapy in ischemic stroke. Stroke 33 (12), 2866–2871. 10.1161/01.str.0000038987.62325.14 PubMed Abstract | 10.1161/01.str.0000038987.62325.14 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu G. J., Luo J., Zhang L. P., Wang Z. J., Xu L. L., He G. H., et al. (2011). Meta-analysis of the effectiveness and safety of prophylactic use of nimodipine in patients with an aneurysmal subarachnoid haemorrhage. CNS Neurol. Disord. Drug Targets 10 (7), 834–844. 10.2174/187152711798072383 PubMed Abstract | 10.2174/187152711798072383 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu H., Yan Y., Pang P., Mao J., Hu X., Li D., et al. (2019). Angong Niuhuang Pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 237, 307–313. 10.1016/j.jep.2019.03.043 PubMed Abstract | 10.1016/j.jep.2019.03.043 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu M., Pu Y., Gu J., He Q., Liu Y., Zeng Y., et al. (2021). Evaluation of Zhilong Huoxue Tongyu capsule in the treatment of acute cerebral infarction: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 86, 153566. 10.1016/j.phymed.2021.153566 PubMed Abstract | 10.1016/j.phymed.2021.153566 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu X., Li Y., Bai N., Yu C., Xiao Y., Li C., et al. (2022). Updated evidence of Dengzhan Shengmai capsule against ischemic stroke: A systematic review and meta-analysis. J. Ethnopharmacol. 283, 114675. 10.1016/j.jep.2021.114675 PubMed Abstract | 10.1016/j.jep.2021.114675 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang L., Hong P. (2016). Efficacy and Safety of Mechanical Thrombectomy in Treating Acute Ischemic Stroke: A Meta Analysis. J. Invest Surg. 29 (2), 106–111. 10.3109/08941939.2015.1067738 PubMed Abstract | 10.3109/08941939.2015.1067738 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lu L., Li H. Q., Fu D. L., Zheng G. Q., Fan J. P. (2014). Rhubarb root and rhizome-based Chinese herbal prescriptions for acute ischemic stroke: a systematic review and meta-analysis. Complement. Ther. Med. 22 (6), 1060–1070. 10.1016/j.ctim.2014.10.002 PubMed Abstract | 10.1016/j.ctim.2014.10.002 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Marmagkiolis K., Hakeem A., Cilingiroglu M., Gundogdu B., Iliescu C., Tsitlakidou D., et al. (2015). Safety and Efficacy of Stent Retrievers for the Management of Acute Ischemic Stroke: Comprehensive Review and Meta-Analysis. JACC Cardiovasc Interv. 8 (13), 1758–1765. 10.1016/j.jcin.2015.07.021 PubMed Abstract | 10.1016/j.jcin.2015.07.021 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Marzolini S., Robertson A. D., Oh P., Goodman J. M., Corbett D., Du X., et al. (2019). Aerobic Training and Mobilization Early Post-stroke: Cautions and Considerations. Front. Neurol. 10, 1187. 10.3389/fneur.2019.01187 PubMed Abstract | 10.3389/fneur.2019.01187 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339 (7), b2535. 10.1371/journal.pmed.100009710.1136/bmj.b2535 PubMed Abstract | 10.1371/journal.pmed.100009710.1136/bmj.b2535 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Chen H., Chen G., Ji Y., Yi F., Zhang Z., et al. (2020). Efficacy and safety of cinepazide maleate injection in patients with acute ischemic stroke: a multicenter, randomized, double-blind, placebo-controlled trial. BMC Neurol. 20 (1), 282. 10.1186/s12883-020-01844-8 PubMed Abstract | 10.1186/s12883-020-01844-8 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Ni X., Liu S., Guo X. (2013). Medium- and long-term efficacy of ligustrazine plus conventional medication on ischemic stroke: a systematic review and meta-analysis. J. Tradit. Chin. Med. 33 (6), 715–720. 10.1016/s0254-6272(14)60002-9 PubMed Abstract | 10.1016/s0254-6272(14)60002-9 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Pan X., Li J., Xu L., Deng S., Wang Z. (2020). Safety of Prophylactic Heparin in the Prevention of Venous Thromboembolism After Spontaneous Intracerebral Hemorrhage: A Meta-analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 81 (3), 253–260. 10.1055/s-0039-3400497 PubMed Abstract | 10.1055/s-0039-3400497 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Patel R. A. G., McMullen P. W. (2017). Neuroprotection in the Treatment of Acute Ischemic Stroke. Prog. Cardiovasc Dis. 59 (6), 542–548. 10.1016/j.pcad.2017.04.005 PubMed Abstract | 10.1016/j.pcad.2017.04.005 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Peng W., Yang J., Wang Y., Wang W., Xu J., Wang L., et al. (2014). Systematic review and meta-analysis of randomized controlled trials of xingnaojing treatment for stroke. Evidence-Based Complementary Altern. Med. 2014, 1–9. 10.1155/2014/210851 PubMed Abstract | 10.1155/2014/210851 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice C. M. (2021). Guideline for primary care of ischemic stroke (2021). Chin. J. General Pract. 20 (9). 10.3760/cma.j.cn114798-20210804-00590 10.3760/cma.j.cn114798-20210804-00590 | Google Scholar [DOI] [Google Scholar]
- Puñal-Riobóo J., Atienza G., Blanco M. (2015). Safety and Efficacy of Mechanical Thrombectomy Using Stent Retrievers in the Endovascular Treatment of Acute Ischaemic Stroke: A Systematic Review. Interv. Neurol. 3 (3-4), 149–164. 10.1159/000430474 PubMed Abstract | 10.1159/000430474 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roaldsen M. B., Jusufovic M., Lindekleiv H. (2022). Cochrane Review on Endovascular Thrombectomy and Intra-Arterial Interventions for Acute Ischemic Stroke. Stroke 53 (5), e193–e194. 10.1161/STROKEAHA.121.036285 10.1161/STROKEAHA.121.036285 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X. J., Shi Z. H., He C. F., Zhang S., Bai Y. J., Guo Y. T., et al. (2019). Efficacy and safety of endovascular thrombectomy in mild ischemic stroke: results from a retrospective study and meta-analysis of previous trials. BMC Neurol. 19 (1), 150. 10.1186/s12883-019-1372-9 PubMed Abstract | 10.1186/s12883-019-1372-9 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea B. J., Grimshaw J. M., Wells G. A., Boers M., Andersson N., Hamel C., et al. (2007). Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 7, 10. 10.1186/1471-2288-7-10 PubMed Abstract | 10.1186/1471-2288-7-10 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T., Zhou J., Zhao Y. (2020). Efficacy and safety of different doses of tenecteplase for the treatment of acute ischemic stroke: A protocol for a systematic review and network meta-analysis. Med. Baltim. 99 (49), e23379. 10.1097/MD.0000000000023379 PubMed Abstract | 10.1097/MD.0000000000023379 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Pu J., Xu L., Malaguit J., Zhang J., Chen S. (2014). The efficacy and safety of cilostazol for the secondary prevention of ischemic stroke in acute and chronic phases in Asian population--an updated meta-analysis. BMC Neurol. 14, 251. 10.1186/s12883-014-0251-7 PubMed Abstract | 10.1186/s12883-014-0251-7 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui F. J., Venketasubramanian N., Chan E. S., Chen C. (2013). Efficacy and safety of MLC601 (NeuroAiD®), a traditional Chinese medicine, in poststroke recovery: a systematic review. Cerebrovasc. Dis. 35 (Suppl. 1), 8–17. 10.1159/000346231 PubMed Abstract | 10.1159/000346231 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Thelengana A., Radhakrishnan D. M., Prasad M., Kumar A., Prasad K. (2019). Tenecteplase versus alteplase in acute ischemic stroke: systematic review and meta-analysis. Acta Neurol. Belg 119 (3), 359–367. 10.1007/s13760-018-0933-9 PubMed Abstract | 10.1007/s13760-018-0933-9 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Wang L. D., Xu Z. M., Liang X., Qiu W. R., Liu S. J., Dai L. L., et al. (2021). Systematic Review and Meta-Analysis on Randomized Controlled Trials on Efficacy and Safety of Panax Notoginseng Saponins in Treatment of Acute Ischemic Stroke. Evid. Based Complement. Altern. Med. 2021, 4694076. 10.1155/2021/4694076 10.1155/2021/4694076 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wei X., Yang J., Suo J., Chen J., Liu X., et al. (2016). Chronic exposure to aluminum and risk of Alzheimer's disease: A meta-analysis. Neurosci. Lett. 610, 200–206. 10.1016/j.neulet.2015.11.014 PubMed Abstract | 10.1016/j.neulet.2015.11.014 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Wu B., Liu M., Liu H., Li W., Tan S., Zhang S., et al. (2007). Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 38 (6), 1973–1979. 10.1161/STROKEAHA.106.473165 PubMed Abstract | 10.1161/STROKEAHA.106.473165 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Xin M., Hao Y., Huang G., Wang X., Liang Z., Miao J., et al. (2020). The efficacy and safety of salvianolic acids on acute cerebral infarction treatment: A protocol for systematic review and meta analysis. Med. Baltim. 99 (23), e20059. 10.1097/MD.0000000000020059 PubMed Abstract | 10.1097/MD.0000000000020059 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. H., Huang Y. M., Ling W., Li Y., Wang M., Chen X. Y., et al. (2015). Wen Dan Decoction for hemorrhagic stroke and ischemic stroke. Complement. Ther. Med. 23 (2), 298–308. 10.1016/j.ctim.2015.01.00110.1155/2021/4265219 PubMed Abstract | 10.1016/j.ctim.2015.01.00110.1155/2021/4265219 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Xu Z. Q., Zhou Y., Shao B. Z., Zhang J. J., Liu C. (2019). A Systematic Review of Neuroprotective Efficacy and Safety of DL-3-N-Butylphthalide in Ischemic Stroke. Am. J. Chin. Med. 47 (3), 507–525. 10.1142/S0192415X19500265 PubMed Abstract | 10.1142/S0192415X19500265 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yang W., Shi Z., Yang H. Q., Teng J., Zhao J., Xiang G. (2015). Mailuoning for acute ischaemic stroke. Cochrane Database Syst. Rev. 1, CD007028. 10.1002/14651858.CD007028.pub3 PubMed Abstract | 10.1002/14651858.CD007028.pub3 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Zeng X., Luo Y., Li Z., Wu T. (2008). Chuanxiong-type preparations for acute ischemic stroke. Cochrane Database Syst. Rev. 4, CD005569. 10.1002/14651858.CD005569.pub2 PubMed Abstract | 10.1002/14651858.CD005569.pub2 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhang D., Dong Y., Li Y., Chen J., Wang J., Hou L. (2017). Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2017, 4191670. 10.1155/2017/4191670 PubMed Abstract | 10.1155/2017/4191670 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xing Y., Chang J., Wang L., An N., Tian C., et al. (2019). Efficacy and Safety of NaoShuanTong Capsule in the Treatment of Ischemic Stroke: A Meta-Analysis. Front. Pharmacol. 10, 1133. 10.3389/fphar.2019.01133 PubMed Abstract | 10.3389/fphar.2019.01133 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Zheng H., Du Y., Zhang R., Chen P., Ren R., et al. (2021). The Clinical Efficacy of Ginkgo biloba Leaf Preparation on Ischemic Stroke: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2021, 4265219. 10.1155/2021/4265219 10.1155/2021/4265219 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q. H., Li X. L., Mei Z. G., Xiong L., Mei Q. X., Wang J. F., et al. (2017). Efficacy and safety of puerarin injection in curing acute ischemic stroke: A meta-analysis of randomized controlled trials. Med. Baltim. 96 (1), e5803. 10.1097/MD.0000000000005803 PubMed Abstract | 10.1097/MD.0000000000005803 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Gao Y., Ma Q. L. (2020). Safety and efficacy of tirofiban in acute ischemic stroke patients Not receiving endovascular treatment: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 24 (3), 1492–1503. 10.26355/eurrev_202002_20208 PubMed Abstract | 10.26355/eurrev_202002_20208 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Zhou X., Shao T., Xie X., Ding M., Jiang X., Su P., et al. (2022). Tongqiao Huoxue Decoction for the treatment of acute ischemic stroke: A Systematic Review and meta-analysis. J. Ethnopharmacol. 283, 114693. 10.1016/j.jep.2021.114693 PubMed Abstract | 10.1016/j.jep.2021.114693 | Google Scholar [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.