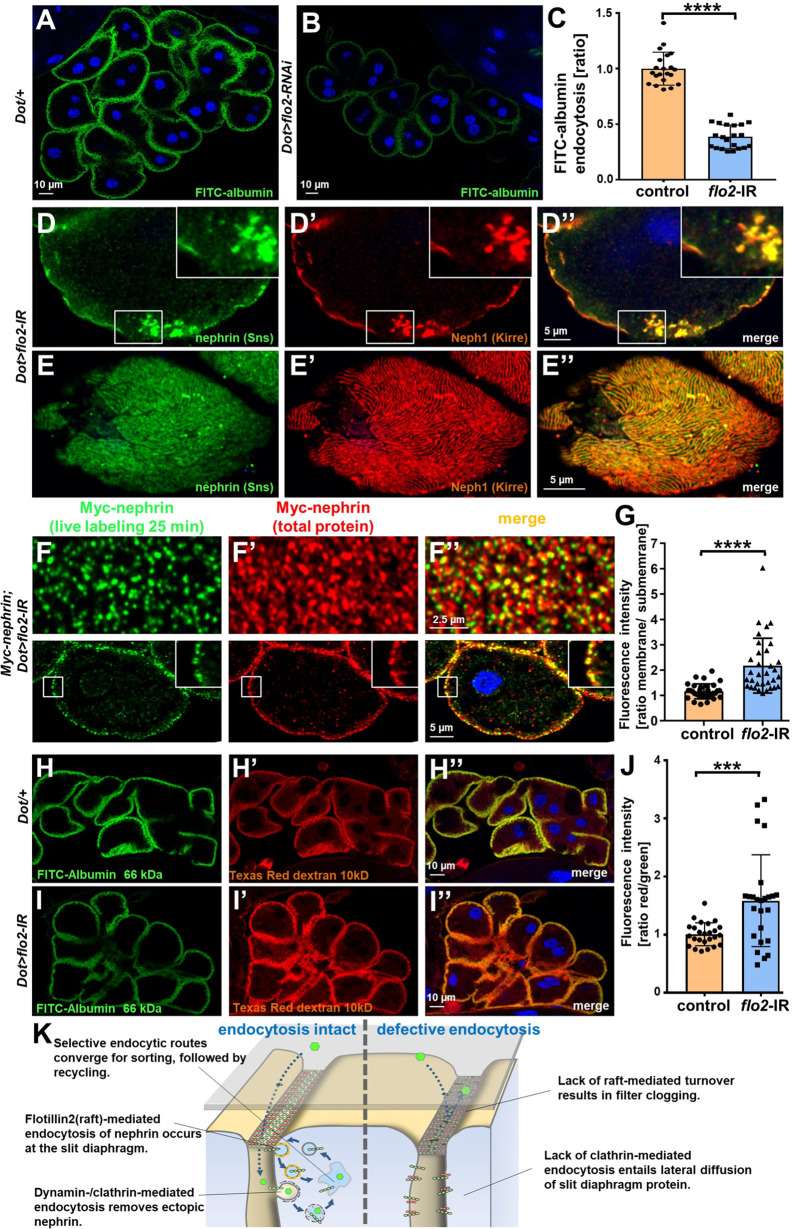
Figure 7. Flotillin2-mediated endocytosis is required for nephrin turnover in Drosophila nephrocytes.
(A–B) Confocal microscopy images of nephrocytes after uptake of FITC-albumin as read-out of nephrocyte function are shown. Control nephrocytes exhibit stronger uptake (A) than nephrocytes expressing flo2-RNAi (B). (C) Quantitation of results analogous to (A–B) in ratio to a control experiment performed in parallel (mean ± standard deviation, n=7 animals per genotype, p<0.0001 for flo2-RNAi). (D–E’’) Confocal images of nephrocytes expressing flo2-RNAi show localized breakdown of slit diaphragms in cross-sectional (D–D’’) and tangential planes (E–E’’). (F–F’’) Confocal microscopy images in tangential sections (upper row) and cross sections (lower row) of nephrocytes are shown after live antibody labeling with 2 hr of chasing. Animals express flo2-RNAi under control of Dorothy-GAL4. Nephrin turnover is strongly reduced compared to control (Figure 5—figure supplement 2A). The diffuse intracellular signal from live labeling was similar to control (Figure 5—figure supplement 2A). (G) Quantitation of results from (F) compared to control experiments. Results are expressed as ratio of the fluorescence intensity between surface and subcortical regions for individual cells (mean ± standard deviation, n=11 animals per genotype, p<0.0001 for flo2-RNAi). (H–I’’) Confocal microscopy images of nephrocytes after simultaneous uptake of FITC-albumin (66 kDa, green) and Texas-Red-Dextran (10 kDa) are shown. Control nephrocytes show significant uptake of both tracers (H–H’’). Silencing of flo2 causes a stronger decrease in the uptake of the larger tracer FITC-albumin compared to smaller Texas-Red-Dextran (I–I’’). (J) Quantitation of fluorescence intensity expressed as a ratio of Texas-Red-Dextran/FITC-albumin (small/large tracer) confirms a disproportionate reduction for flo2-RNAi (mean ± standard deviation, n=9 animals per genotype, p<0.001 for flo2-RNAi). (K) Schematic illustrating the proposed mechanistic role of endocytosis for maintenance of the filtration barrier. Left: Ectopic fly nephrin within the channels is removed by clathrin-dependent endocytosis that returns most of the protein to the surface through recycling pathways. The nephrin that is bound within the slit diaphragm complex is subject to turnover in a shorter circuit that is raft-mediated and feeds into recycling as well. Right: Upon disruption of endocytosis filtration is impaired by clogging of the filter due to lack of cleansing and the architecture of the slit diaphragms is disturbed by unhindered lateral diffusion of slit diaphragm protein.