Patients treated recently for a B-lymphoid malignancy are shown to have particularly high risks of severe COVID-19 compared to multiple control cohorts of patients with cancer and COVID-19.
Abstract
Patients with B-lymphoid malignancies have been consistently identified as a population at high risk of severe COVID-19. Whether this is exclusively due to cancer-related deficits in humoral and cellular immunity, or whether risk of severe COVID-19 is increased by anticancer therapy, is uncertain. Using data derived from the COVID-19 and Cancer Consortium (CCC19), we show that patients treated for B-lymphoid malignancies have an increased risk of severe COVID-19 compared with control populations of patients with non–B-lymphoid malignancies. Among patients with B-lymphoid malignancies, those who received anticancer therapy within 12 months of COVID-19 diagnosis experienced increased COVID-19 severity compared with patients with non–recently treated B-lymphoid malignancies, after adjustment for cancer status and several other prognostic factors. Our findings suggest that patients recently treated for a B-lymphoid malignancy are at uniquely high risk for severe COVID-19.
Significance:
Our study suggests that recent therapy for a B-lymphoid malignancy is an independent risk factor for COVID-19 severity. These findings provide rationale to develop mitigation strategies targeted at the uniquely high-risk population of patients with recently treated B-lymphoid malignancies.
This article is highlighted in the In This Issue feature, p. 171
INTRODUCTION
The disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known as coronavirus disease 2019 (COVID-19), was classified as a pandemic by the World Health Organization (WHO) in February 2020. As of December 19, 2021, more than 273 million cases have been reported worldwide, with more than 5.3 million deaths (https://apps.who.int/iris/bitstream/handle/10665/338703/nCoV-weekly-sitrep12Jan21-eng.pdf). The effects of the pandemic have been especially profound on high-risk patients, including patients with cancer. Of patients with cancer, those with hematologic malignancies are at particularly high risk of severe viral infections due to disease-related myelosuppression, deficits in humoral and cellular immunity, as well as the immunosuppressive effects of therapy (1–4). Multiple studies have reported a relatively high risk of severe COVID-19 and mortality for patients with hematologic malignancies, although the precise risks reported are highly variable and depend on factors related to the patient's other comorbidities, specific type of cancer and cancer therapy, and external factors such as local epidemiology and resources available in health care systems (5–10). Patients with B-lymphoid malignancies in particular have been documented to have inferior outcomes, likely due to the humoral and cellular immune dysfunction associated with these cancers (11–14).
The impact of drug therapies for B-lymphoid malignancies on COVID-19 outcomes is not clear. Early in the pandemic, some therapies that are widely used to treat lymphoid malignancies, particularly Bruton's tyrosine kinase (BTK) inhibitors, were hypothesized to have efficacy as anti–COVID-19 therapies, but clinical trials of these agents did not demonstrate a clear benefit (9, 15). The association between cancer treatment and risk of severe COVID-19 has varied across studies. Some studies have demonstrated an increased risk of severe COVID-19, whereas other studies have demonstrated no association after adjustment for other pertinent risk factors such as age, sex, and relevant comorbidities (16, 17). Although dedicated studies of patients with B-lymphoid malignancies have shown poor outcomes for this population as a whole, these studies have not demonstrated an increase in COVID-19–associated mortality or severity in patients who have recently received anticancer drug therapy after adjustment for other important risk factors, such as disease control (11, 13). However, these studies were potentially inadequately powered to demonstrate an effect.
The COVID-19 and Cancer Consortium (CCC19) is an international consortium that collects data on patients with current or prior history of cancer who have developed presumed or laboratory-confirmed COVID-19. One of the largest databases of its kind, the CCC19 registry, enables granular analysis of the independent association of clinical risk factors with outcomes in this unique patient population, including controlled analyses not possible in smaller databases or those generated solely from electronic health records. The CCC19 framework facilitates relatively robust analysis of the independent association of B-lymphoid malignancy diagnoses and B-lymphoid malignancy therapies with COVID-19–specific outcomes. Using data derived from the CCC19 registry, we sought to investigate the impact of anticancer therapy on COVID-19 outcomes in patients with B-lymphoid malignancies. We hypothesized that B-lymphoid malignancies themselves as well as therapies for B-lymphoid malignancies would be independently associated with increased COVID-19 severity.
RESULTS
Patient Characteristics
A total of 10,380 records from the CCC19 registry were evaluated. After exclusion criteria were applied, a total of 8,759 records were included in the primary analysis (Supplementary Fig. S1). Most (n = 7,509, 86%) of the cohort had follow-up information after diagnosis of COVID-19 of 30 days or greater, and were symptomatic at presentation (n = 7477, 85%). The median age of the included cohort was 65 years (interquartile range: 55–74), and the majority (n = 4596, 53%) were female. Approximately half of the cohort was non-Hispanic White, while non-Hispanic Black patients represented 18% and Hispanic patients represented 16%. Similar proportions of patients were diagnosed with cancer within 1 year prior to COVID-19 (n = 2,397, 26%), within 5 years prior to COVID-19 (n = 3,258, 37%), and greater than or equal to 5 years prior to COVID-19 (n = 2,786, 32%).
The most common cancer diagnoses among the control groups of patients without B-lymphoid malignancies (n = 7,764) were breast adenocarcinoma (n = 1,622), prostate adenocarcinoma (n = 1,009), and non–small cell lung cancer (n = 519). Eleven percent (n = 995) of the entire cohort had a B-lymphoid neoplasm. Approximately half of these (n = 499) had low-grade non-Hodgkin lymphoma [chronic lymphocytic leukemia (CLL) included]. The most common B-lymphoid malignancy diagnosis was CLL (n = 255). Of the patients with B-lymphoid malignancies, approximately half had received systemic therapy within one year of COVID-19 diagnosis (n = 516, 52%), and half had not (n = 479, 48%). These were similar proportions to the patients without B-lymphoid malignancies [n = 3,653 (46%) treated within a year, 4,111 (54%) not treated within a year]. Additional patient characteristics are shown in Table 1 and Supplementary Table S1.
Table 1.
Baseline patient characteristics
| Characteristics | Nonrecently treated control | B-lymphoid malignancies off therapy | Recently treated control | Recently treated B-lymphoid malignancies |
|---|---|---|---|---|
| (n = 4111) | (n = 479) | (n = 3653) | (n = 516) | |
| Cancer status | ||||
| Remission/NED | 71% (2901) | 56% (266) | 23% (837) | 27% (137) |
| Active, stable or responding | 14% (555) | 29% (137) | 47% (1706) | 45% (232) |
| Active, progressing | 8% (311) | 6% (31) | 21% (777) | 16% (81) |
| Unknown | 8% (342) | 9% (44) | 9% (326) | 13% (66) |
| Missing | 0% (<5) | 0% (<5) | 0% (7) | 0% (<5) |
| Age [years, median (IQR)] | 67 (57–77) | 63 (52.5–74) | 63 (53–72) | 62 (47–73) |
| Sex | ||||
| Female | 50% (2073) | 43% (204) | 58% (2108) | 41% (211) |
| Male | 49% (2034) | 57% (275) | 42% (1544) | 59% (304) |
| ECOG performance status | ||||
| Zero | 33% (1360) | 41% (194) | 34% (1225) | 30% (157) |
| One | 17% (717) | 20% (97) | 35% (1265) | 38% (196) |
| Two or greater | 12% (513) | 10% (48) | 16% (576) | 15% (75) |
| Unknown | 37% (1517) | 29% (140) | 16% (576) | 17% (87) |
| Missing | <1% (<5) | <1% (<5) | <1% (11) | <1% (<5) |
| Smoking status | ||||
| Never | 52% (2119) | 58% (277) | 53% (1953) | 59% (305) |
| Current or former | 46% (1889) | 38% (183) | 43% (1569) | 37% (190) |
| Missing | 3% (103) | 4% (19) | 4% (131) | 4% (21) |
| Modified Charlson Comorbidity index (median, IQR) | 2 (1–3) | 2 (1–3) | 1 (1–2) | 1 (1–2) |
| Timing of COVID-19 diagnosis | ||||
| Jan–Apr 2020 | 25% (1039) | 26% (125) | 23% (829) | 27% (137) |
| May–Aug 2020 | 40% (1653) | 43% (205) | 41% (1505) | 37% (191) |
| Sep–Dec 2020 | 20% (832) | 19% (90) | 20% (746) | 20% (102) |
| Jan–Apr 2021 | 14% (555) | 12% (58) | 15% (534) | 15% (79) |
| May–Aug 2021 | 1% (23) | <1% (<5) | 1% (23) | 1% (<5) |
| Missing | <1% (9) | <1% (<5) | <1% (16) | <1% (<5) |
| Race | ||||
| Non-Hispanic White | 56% (2302) | 58% (276) | 49% (1801) | 50% (259) |
| Hispanic | 13% (514) | 15% (71) | 19% (703) | 24% (122) |
| Non-Hispanic Black | 19% (787) | 14% (66) | 18% (669) | 14% (70) |
| Other | 11% (449) | 12% (59) | 11% (401) | 12% (61) |
| Missing | 1% (59) | 1% (7) | 2% (79) | 1% (<5) |
| Obesity | ||||
| Not obese | 60% (2460) | 66% (317) | 63% (2294) | 62% (322) |
| Obese | 40% (1636) | 34% (161) | 36% (1329) | 37% (191) |
| Missing | <1% (15) | <1% (<5) | 1% (30) | <1% (<5) |
| Region | ||||
| U.S. Northeast | 31% (1278) | 39% (187) | 41% (1482) | 42% (219) |
| U.S. Midwest | 32% (1333) | 25% (118) | 20% (746) | 21% (106) |
| U.S. South | 15% (597) | 15% (70) | 17% (607) | 18% (95) |
| U.S. West | 19% (763) | 17% (82) | 17% (625) | 15% (78) |
| Other | 3% (140) | 5% (22) | 5% (193) | 3% (18) |
| Vaccination status | ||||
| Any vaccine before COVID-19 | 1% (50) | 1% (6) | 2% (57) | 3% (15) |
| No vaccine before COVID-19 | 30% (1213) | 27% (129) | 29% (1065) | 30% (155) |
| Unknown | 3% (111) | 3% (15) | 3% (102) | 2% (100) |
| Missing | 67% (2737) | 69% (329) | 66% (2429) | 65% (336) |
NOTE: These are intended to avoid potential identification of patients based on relatively rare permutations of variables. To this end, data are only presented for categories in which displaying these outcome data is feasible without violation of this standard, to protect patient privacy.
Abbreviations: ECOG, Eastern Cooperative Oncology Group. Counts less than 5 are not presented pursuant to CCC19 consortium publication standards; NED, no evidence of disease.
Patient Outcomes
Of the 8,759 patients in the cohort overall, the majority (n = 4,840, 55%) required hospitalization during their COVID-19 illness; patients with B-lymphoid malignancy had a higher hospitalization rate (n = 659, 67%). Mortality in the cohort overall was 12%, with mortality of 13% in patients with B-lymphoid malignancies. Across malignancies, outcomes were worst for patients with chronic lymphocytic leukemia (CLL), 72% of whom required hospitalization and 19% of whom died. Mortality was 21% for patients diagnosed with COVID-19 during the first four months of 2020, declining to 10% by the middle four months and 8% by the last four months of 2020. Mortality was particularly high in patients with malignancies that were either active and progressing (26%) or who had an ECOG performance status of 2 or greater (31%). Additional descriptive outcomes are demonstrated in Supplementary Table S2.
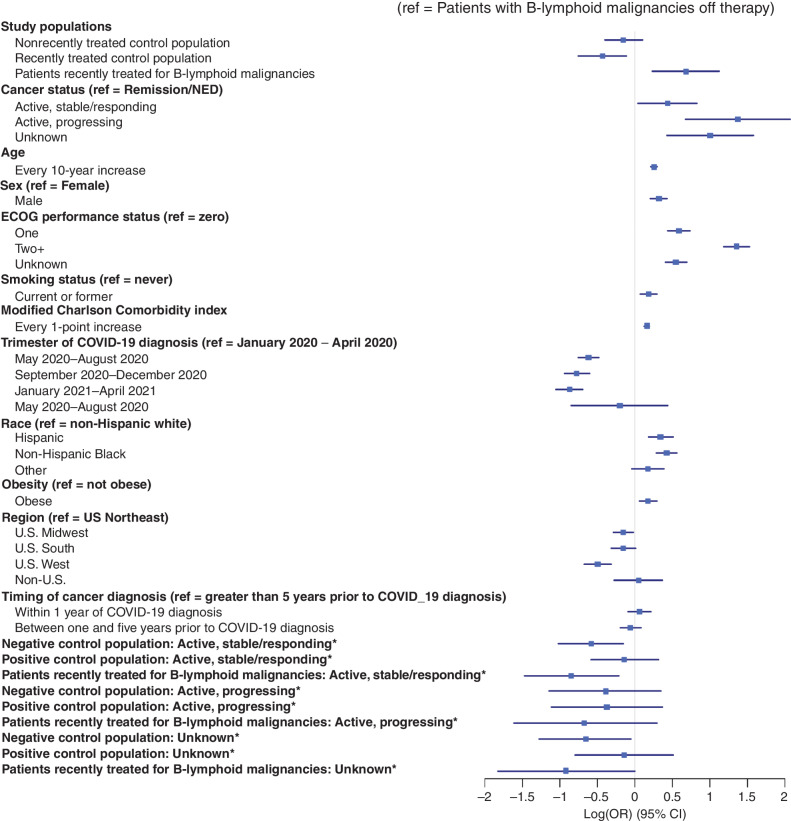
The cohort was divided into four mutually exclusive groups: (i) patients with solid tumors or non–B-lymphoid hematologic malignancies not treated for cancer within one year of COVID-19 diagnosis (“nonrecently treated control population”); (ii) patients with B-lymphoid malignancies not treated for cancer within one year of COVID-19 diagnosis (“patients with nonrecently treated B-lymphoid malignancies”); (iii) patients with solid tumors or non–B-lymphoid hematologic malignancies treated for cancer within one year of COVID-19 diagnosis (“recently treated control population”), and (iv) patients with B-lymphoid malignancies treated within a year of diagnosis (“patients recently treated for B-lymphoid malignancies”). In the primary analysis, compared with the population of patients with nonrecently treated B-lymphoid malignancies, COVID-19 severity was similar in the nonrecently treated control population (AOR, 0.86; 95% CI, 0.67–1.22), and the recently treated control population had reduced COVID-19 severity (AOR, 0.65; 95% CI, 0.47–0.89). Central to this analysis, patients recently treated for B-lymphoid malignancies had increased COVID-19 severity compared with patients with nonrecently treated B-lymphoid malignancies (AOR, 1.98; 95% CI, 1.27–3.08) as well as to both the nonrecently treated and recently treated control populations. Beyond these primary findings, multivariable analysis for the entire cohort revealed increasing COVID-19 severity with increasing age, male sex, progressing malignancy, obesity, Hispanic ethnicity and non-Hispanic Black race, and diagnosis of cancer within one year prior to COVID-19 diagnosis. ECOG performance status of 2 or greater and increasing modified Klabunde comorbidity index were also associated with increased COVID-19 severity. Calendar time had a statistically significant association with COVID-19 severity after adjustment, with greatest mortality during the initial phase of the pandemic in the first four months of 2020 (Fig. 1; Supplementary Table S3).
Figure 1.
Factors associated with COVID-19 severity. Forest plot visualization of the independent association of the covariates in the primary analysis with COVID-19 severity. Associations are represented as the log of the odds ratio of COVID-19 severity. Interaction terms are indicated with an asterisk.
There was a statistically significant prespecified interaction between the study populations of interest and cancer status. Compared to patients in the nonrecently treated control population in remission, patients with nonrecently treated B-lymphoid malignancies experienced increased COVID-19 severity if the malignancy was active but stable or responding (AOR, 1.80; 95% CI, 1.16–2.80); the interaction effect for patients with active and progressing B-lymphoid malignancies off therapy was not statistically significant (AOR, 1.48; 95% CI, 0.70–3.10). Cancer status did not appear to further modify the statistically significant association of recently treated B-lymphoid malignancies with increased COVID-19 severity (Fig. 1; Table 2).
Table 2.
Results of regression analysis for primary outcome: COVID-19 severity (N = 8759)
| Characteristics | Multivariable AOR (95% CI) |
|---|---|
| Study populations (ref = Patients with nonrecently treated B-lymphoid malignancies) | |
| Nonrecently treated control population | 1.16 (0.90–1.49) |
| Recently treated control population | 0.75 (0.61–0.93) |
| Patients recently treated for B-lymphoid malignancies | 2.30 (1.58–3.36) |
| Cancer status (ref = remission/NED) | |
| Active, stable/responding | 0.86 (0.70–1.06) |
| Active, progressing | 2.67 (2.05–3.48) |
| Unknown | 1.41 (1.10–1.81) |
| Age (every 10-year increase) | 1.29 (1.24–1.35) |
| Sex (ref = Female) | |
| Male | 1.38 (1.23–1.54) |
| ECOG Performance Status (ref = zero) | |
| One Two or greater Unknown | 1.80 (1.56–2.08) 3.89 (3.27–4.63) 1.73 (1.50–2.00) |
| Smoking status (ref = never) | |
| Current or former | 1.20 (1.07–1.34) |
| Modified Charlson Comorbidity Index | 1.18 (1.13–1.22) |
| Trimester of COVID-19 diagnosis (ref = January 2020–April 2020) | |
| May 2020–Aug 2020 | 0.54 (0.47–0.62) |
| Sep 2020–Dec 2020 | 0.46 (0.39–0.55) |
| Jan 2021–Apr 2021 | 0.42 (0.35–0.50) |
| May 2021–Jun 2021 | 0.82 (0.43–1.56) |
| Race (ref = non-Hispanic white) | |
| Hispanic | 1.41 (1.20–1.67) |
| Non-Hispanic Black | 1.53 (1.33–1.76) |
| Other | 1.19 (0.96–1.47) |
| Obesity (ref = not obese) | |
| Obese | 1.19 (1.06–1.34) |
| Region (ref = US Northeast) | |
| U.S. Midwest | 0.86 (0.75–0.98) |
| U.S. South | 0.86 (0.73–1.01) |
| U.S. West | 0.61 (0.51–0.73) |
| Non-U.S. | 1.05 (0.76–1.44) |
| Timing of cancer diagnosis (ref = Greater than 5 years prior to COVID-19 diagnosis) | |
| Within 1 year of COVID-19 diagnosis | 1.06 (0.91–1.24) |
| Between one and five years prior to COVID-19 diagnosis | 0.94 (0.82–1.09) |
| Interaction terms (ref = patients with nonrecently treated B-lymphoid malignancies in remission/NED) | |
| Negative control population x active and stable/responding cancer | 1.80 (1.16–2.80) |
| Positive control population x active and stable/responding cancer | 1.57 (1.16–2.12) |
| Patients recently treated for B-lymphoid malignancies x active and stable/responding cancer | 0.78 (0.46–1.31) |
| Negative control population x active and progressing cancer | 1.48 (0.70–3.10) |
| Positive control population x active and progressing cancer | 1.02 (0.71–1.46) |
| Patients recently treated for B-lymphoid malignancies x active and progressing cancer | 0.76 (0.38–1.54) |
| Negative control population x unknown cancer status | 1.94 (1.05–3.58) |
| Positive control populationx unknown cancer status | 1.69 (1.13–2.51) |
| Patients recently treated for B-lymphoid malignancies x unknown cancer status | 0.77 (0.36–1.64) |
NOTE: An OR > 1 indicates increased risk of high COVID-19 severity; Odds Ratio < 1 indicates decreased risk of high COVID-19 severity.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NED, no evidence of disease.
Analyses of individual secondary outcomes revealed increased rates of hospitalization both in patients recently treated for B-lymphoid malignancies (AOR, 2.84; 95% CI, 1.67–4.85) and patients with nonrecently treated B-lymphoid malignancies (AOR, 1.36; 95% CI, 1.00–1.85) compared with the nonrecently treated control population. ICU utilization was increased in patients recently treated for B-lymphoid malignancies (AOR, 2.12; 95% CI, 1.07–4.22), but not in patients with nonrecently treated B-lymphoid malignancies (AOR, 1.12; 95% CI, 0.78–1.61). There was no independent association with either category of patients with B-lymphoid malignancies and increased 30-day mortality or rates of mechanical ventilation (Supplementary Tables S4–S6).
Sensitivity analyses limited to patients with complete 30-day follow-up information and symptomatic COVID-19 did not reveal significant differences (Supplementary Tables S7 and S8). Of note, after adjustment for convalescent plasma receipt, patients with treated B-lymphoid malignancies no longer had increased COVID-19 severity compared with patients with nonrecently treated B-lymphoid malignancies (AOR, 1.34; 95% CI, 0.85–2.11; Supplementary Table S9).
Outcomes for Patients Stratified by Therapeutic Regimen
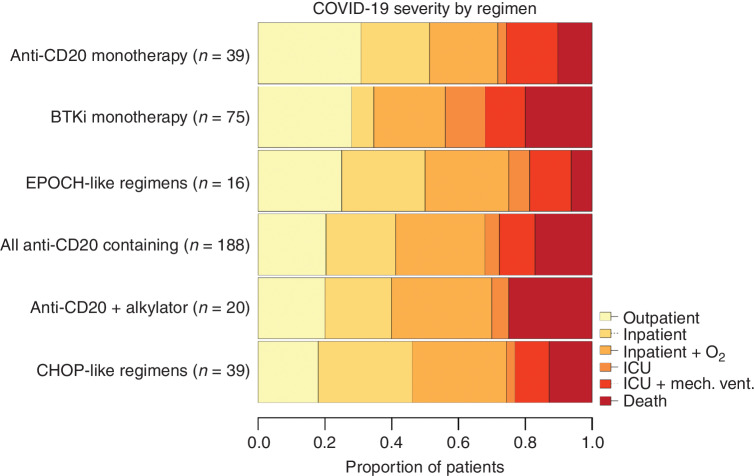
For the 516 patients recently treated for B-lymphoid malignancies, a specific regimen received was extractable in 436 (84%). COVID-19 severity was generally high in this group of patients. The outcomes for patients with the most widely used regimens are shown in Fig. 2. COVID-19 severity was particularly high in patients who recently received BTK inhibitor monotherapy and in patients receiving combinations of anti-CD20 mAbs and alkylators, with each group demonstrating 20% mortality at 30 days. More data on the most recent regimens received in these patients are provided in Supplementary Table S10.
Figure 2.
Descriptive outcomes for patients receiving various regimens. Bar plot demonstrating rates of the ordinal outcome for patients recently treated for B-lymphoid malignancies stratified by the type of regimen received. No statistical comparisons between these groups were undertaken due to small numbers of patients in some of these categories, and these data are presented for the purposes of description only. More data on regimens received by the patients in this study are presented in the supplement.
Outcomes for Patients Developing COVID-19 after COVID-19 Vaccinations
In total, 128 patients in this cohort had an infection following full or partial COVID-19 vaccination. A relatively small number of breakthrough infections after COVID-19 vaccination were recorded in patients recently treated for B-lymphoid malignancies (n = 15) and in patients with nonrecently treated B-lymphoid malignancies (n = 6). Of the patients recently treated for B-lymphoid malignancies who developed COVID-19 after COVID-19 vaccination, 12 (80%) required hospitalization.
DISCUSSION
COVID-19 poses unique risks to patients with B-lymphoid malignancies. These patients are recognized to be at high risk of morbidity and mortality in the context of respiratory viruses. Potential etiologies include impaired host immunity from effects of the malignancies and treatments, as well as a high burden of noncancer comorbidities, and low rates of seroconversion to relevant vaccinations (18–21). Indeed, seroconversion after COVID-19 vaccination has been found to be impaired in this patient population, perhaps more so in the setting of several lymphocytotoxic therapies, including anti-CD20 mAbs and BTK inhibitors (18–22). As such, COVID-19 remains a particularly salient concern even with widely available vaccinations, and deeper understanding of factors associated with COVID-19 severity in this population remains of paramount importance to inform prevention and management strategies.
Prior studies showed high rates of COVID-19 severity in patients with B-lymphoid malignancies, but no increase in COVID-19 severity or mortality associated with treatment of B-lymphoid malignancies. However, these studies were smaller than the current study and did not compare outcomes with untreated control populations (11, 14, 23, 24). This is the largest study of patients specifically with B-lymphoid malignancies and COVID-19 to date, with a cohort of 995 of such patients. Using multiple internal control populations, we demonstrated that compared with both treated and untreated control populations of patients with other malignancies, patients recently treated for B-lymphoid malignancies experienced increased COVID-19 severity after adjustment. Patients recently treated for B-lymphoid malignancies also had increased rates of hospitalization and ICU utilization compared with the control populations. These findings were stable to retesting the hypothesis under several preplanned sensitivity analyses in distinct study subpopulations.
Although this is a retrospective study, there are clinical implications. While the association of therapy for B-lymphoid malignancies with increased COVID-19 severity is troublesome, we note that the association of progressive malignancy with COVID-19 severity is also independently associated with increased COVID-19 severity. This is concordant with prior publications from the CCC19 and other groups (17, 25, 26). The etiology of this relationship is not clear from the data; it may be that patients with progressive malignancy are disproportionately deferring aggressive care in the setting of severe COVID-19, or that improved control over the primary malignancy results in reduced disease-related immunosuppression. In any case, these data do not support deferring highly effective anticancer therapies. A study of the effects of deferring chemotherapy in the setting of COVID-19 is not within the purview of CCC19. Our findings do suggest that increased potential for severe COVID-19 should enter into informed consent discussions of treatments for B-lymphoid malignancies. Clinicians may consider temporarily delaying therapy in situations where community transmission is high, or to allow for patients to be fully vaccinated. The increased risk of severe COVID-19 should factor into risk–benefit discussions about using long-term anti-CD20 maintenance therapies in scenarios where that approach is not associated with a proven overall survival benefit. Both individual and population-wide prevention strategies will remain of paramount importance to protect this vulnerable population.
Although during the later part of the study period COVID-19 vaccinations became widely available, the number of infections that occurred following vaccination was too small to analyze the independent association of vaccination with COVID-19 severity in patients with B-lymphoid malignancies, and any effect from boosters is not yet observed within the timeframe. Many patients recently treated for B-lymphoid malignancies will have impaired responses and immunity following COVID-19 vaccination, and these results likely remain salient to those patients. However, it is not possible to say with the clinical data available to the CCC19 registry whether those patients with B-cell malignancy who develop breakthrough infection are also those with the impaired response; prior studies have shown that even patients with an impaired antibody response can manifest T cell–mediated immunity; focused prospective studies are better equipped to answer this ongoing question (27). Although responses to primary vaccination series in this patient population have been shown to be suboptimal, there are data suggesting that boosters significantly increase vaccine efficacy in patients with B-lymphoid malignancies (21). These data highlight the importance of optimal prevention of COVID-19 in this population, including widespread deployment of boosters. Many health care systems are having to ration critical COVID-19 therapeutics, such as sotrovimab, tixagevimab/cilgavimab, and oral antivirals, given critical shortages. Our data suggest that patients recently treated for B-lymphoid malignancies should be prioritized to receive these therapies. More study of additional strategies, such as mAb or oral antiviral prophylaxis for high-risk exposures, are needed to reduce the morbidity of COVID-19 in this population.
The study period (March 2020–June 2021) ended prior to the high prevalence of the delta and omicron SARS-CoV-2 variants. At the time of publication of this manuscript, the omicron variant is most prevalent in the United States (https://covid.cdc.gov/covid-data-tracker/#variant-proportions). Although this variant may result in milder disease than the wild type coronavirus, the degree to which that finding is due to intrinsic properties of the omicron variant or prior immunity is not clear (28). As patients treated for B-lymphoid malignancies have impaired humoral immunity and have been shown to have impaired response to vaccination, COVID-19 due to the omicron variant may not be milder in this vulnerable population. It is also possible that future variants will exhibit greater immune escape potential and virulence than omicron. For these reasons, these results remain relevant in the current state of the COVID-19 pandemic.
In this study, the point estimate for mortality in patients with B-lymphoid malignancies and COVID-19 (13%) is somewhat lower than that in other reported studies, which have demonstrated mortalities for patients with hematologic malignancies in general and COVID-19 between 28 and 37% (7, 8, 29). The patients included in many of these studies were diagnosed relatively early in the pandemic, whereas the majority of the patients in this study were diagnosed after May 2020. As was shown in this and other studies, the outcomes for patients with cancer and COVID-19 have been improving throughout the pandemic (17, 30). During the early periods, many health care systems were under significant strain due to surging cases, in many cases requiring rationing of care. In addition, the therapeutic paradigm for COVID-19 itself has changed since the onset of the pandemic in ways which are beneficial to patients with hematologic malignancies, in that the widespread off-study use of ineffective or even harmful COVID-19 therapies (31, 32) has been replaced by COVID-19 therapies with proven benefit (33–35). In the case of mAbs, it may be the case that these therapies abrogate some of the unique risk that COVID-19 poses to patients with B-lymphoid malignancies given the relative inability of these patients to produce neutralizing antibodies to COVID-19 (36).
Our finding that patients with nonrecently treated B-lymphoid malignancies did not experience increased COVID-19 severity compared with the nonrecently treated control population initially seems at odds with the wider literature. However, analysis of interaction effects suggested that the association of a B-lymphoid malignancy with COVID-19 severity was modified by cancer status. Although patients with B-lymphoid malignancies did not experience increased COVID-19 severity than patients in the nonrecently treated control population in general, patients with nonrecently treated B-lymphoid malignancies with active and stable or responding malignancies experienced increased COVID-19 severity. This effect was not statistically significant for patients with active and progressive B-cell malignancies off therapy, although the number of such patients may have been too small to observe an independent effect (n = 31). The observation that patients with nonrecently treated B-lymphoid malignancies in this study did not experience increased COVID-19 severity compared to control populations may have been driven by the fact that most of these patients (n = 266, 56%) were in remission, and therefore had minimal or no disease-related immunosuppression.
Of note, a small number of patients recently treated for B-lymphoid malignancies (n = 29, 6%) had been treated with transplantation or cellular therapy within a year of developing COVID-19; this group had a mortality of 20%. Although the study is too small to study that population in a dedicated capacity, it may be that recent stem cell transplantation or cellular therapy have unique modifying effects on the risk of severe COVID-19; the numbers of patients receiving these interventions in this cohort were too small to analyze this independent association. Such analyses should be a focus of future work as this and other disease-specific registries continue to expand (37).
This study has important limitations. This is a registry analysis, and although there may be some clinical implications of the findings, these data are primarily useful for hypothesis-generation. Furthermore, as a registry analysis, there is inherent selection bias in that patients with very mild disease may not come to the attention of sites reporting to the registry. As such, it is likely that the study population has greater COVID-19 severity than the general population of patients with cancer and COVID-19. With that said, this study includes a greater proportion of nonhospitalized patients than other registry studies and may have improved generalizability as a result. To maximize sample size, the study populations are heterogeneous, including with respect to most recent therapeutic exposures, which limits the generalizability of the findings. There are significant differences between the timing of COVID-19 diagnosis in this study and the epidemiology of the pandemic in the general population, with relative overrepresentation of diagnoses earlier in the pandemic. This may be due to high vigilance against COVID-19 in this patient population resulting in improved prevention over time but may also reflect reporting bias. As the ordinal outcome of COVID-19 severity includes metrics such as hospitalization and ICU admission, it is possible that patients with B-lymphoid malignancies are preferentially admitted to relatively higher level of care due to their malignancy as opposed to intrinsic features of COVID-19. As increased mechanical ventilation was observed in both patients recently treated for B-lymphoid malignancies and patients with nonrecently treated B-lymphoid malignancies this does not likely explain all of the increased COVID-19 severity observed in this population but may explain some of the findings. Conversely, a subset of patients with B-lymphoid malignancies receive their treatment as an inpatient (e.g., DA-EPOCH-R and R-HyperCVAD) and may have been diagnosed through routine screening protocols, and it is possible that some patients would have been recorded as hospitalized for COVID-19. As most patients who test positive for COVID-19 in this setting are discharged to quarantine and do not receive the planned chemotherapy, and a sensitivity analysis excluding asymptomatic patients showed similar results to the primary analysis, we do not think this possibility impacted our findings. Although this is the largest study of patients with B-lymphoid malignancies to date, and sufficiently large to observe an association of therapy in general on COVID-19 severity, the sample size was insufficiently large to investigate the independent effects of specific therapies, such as rituximab and ibrutinib, on COVID-19 severity.
In conclusion, this analysis suggests that the general population of patients with B-lymphoid malignancies is at relatively increased risk of severe COVID-19, and that this risk is further increased in the setting of recent anticancer therapy. These findings provide important context for informed consent discussions for patients with B-lymphoid malignancies considering anticancer therapy, but do not support deferring highly effective anticancer therapy in the vast majority of cases. Individual patient scenarios need to be considered in the context of community infectivity, hospital resources, patient and tumor characteristics, and disease response and prognosis. This remains a population of high need for improved primary prevention and therapeutic strategies.
METHODS
Study Design and Population
The origin, methodology for patient accrual, and data structure used by the CCC19 registry are described elsewhere (38–40). The CCC19 data are collected and managed using REDCap software hosted at Vanderbilt University Medical Center (Nashville, TN; refs. 41, 42). REDCap is developed and supported by the Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from NCATS/NIH). This study was partly supported by grants from the NCI (grant number P30CA068485 to Vanderbilt University Medical Center). Reports were accessed for patients diagnosed with COVID-19 from March 17, 2020 through June 13, 2021. Included patients were adults with cancer diagnoses (including cancers in remission) greater than 18 years of age with laboratory-confirmed diagnosis of SARS-CoV-2. Laboratory confirmation can include PCR, antigen testing/ELISA, antibodies to SARS-CoV-2, or any other test that would be consistent with a current or prior SARS-CoV-2 infection. Patients with precursor hematologic neoplasms [e.g., monoclonal gammopathy of uncertain significance (MGUS), monoclonal B-lymphoid lymphocytosis (MBL)] were excluded. The cohort captures cancer diagnosis by a terminology derived from the NCI Thesaurus. Records with incomplete outcome information such that the primary outcome could not be determined, quality scores greater than 4 (described below) indicative in general of high missingness of data or excessive unknowns, and missing data on the timing of cancer therapy receipt were also excluded from the analysis.
The CCC-19 uses a quality scoring system to determine the suitability of records for inclusion in analyses. A score greater than 5 was considered insufficient for inclusion in the analysis presented. Scores are tabulated as follows:
Minor problems (+1 point per problem):
ADT missing/unknown (prostate cancers only)
Biomarkers missing/unknown (breast cancers only)
ICU admission missing/unknown
Hospitalization missing/unknown
Mechanical ventilation missing/unknown
O2 ever needed missing/unknown
Days to death missing/unknown
Cancer status unknown
ECOG PS unknown
Missing cancer drug names for patients on systemic anticancer treatment
Missing or unknown categorical lab values if labs were drawn.
Moderate problems (+3 points per problem):
Cancer status missing
ECOG PS missing
Death status missing/unknown
Baseline COVID-19 severity missing/unknown
Should have 30-day follow-up but doesn't
Major problems (+5 points per problem)
High levels of missingness
High levels of unknowns
The study was exempt from institutional review board (IRB) review (VUMC IRB#200467) and approved by IRBs at participating sites according to local institutional policies. A list of participants indexed by institution is included in Supplementary Appendix S1. The CCC19 study is registered on ClinicalTrials.gov (NCT04354701) and is ongoing. The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Outcomes
The primary outcome was an ordinal scale of COVID-19 severity based on each patient's most severe reported disease status graded as (i) not meeting any of the other severity outcomes; (ii) hospitalized without supplemental oxygen; (iii) hospitalized with supplemental oxygen; (iv) admitted to an intensive care unit (ICU) without mechanical ventilation; (v) mechanical ventilation, and (vi) death from any cause within 30 days of COVID-19 diagnosis. A similar ordinal outcome has been used in prior publications from the CCC19 (17, 43) Secondary analyses with primary outcomes of 30-day all-cause mortality, ICU requirement, and mechanical ventilation requirement were conducted.
Subpopulations of Interest and Covariates
To test the hypothesis that B-lymphoid malignancies and associated anti-cancer drug therapies for lymphoid malignancies were independently associated with increased COVID-19 severity, we divided the cohort into four mutually exclusive groups: (i) patients with solid tumors or non-B-lymphoid hematologic malignancies not treated for cancer within one year of COVID-19 diagnosis (“nonrecently treated control population”); (ii) patients with B-lymphoid malignancies not treated for cancer within one year of COVID-19 diagnosis (“patients with nonrecently treated B-lymphoid malignancies”); (iii) patients with solid tumors or non-B-lymphoid hematologic malignancies treated for cancer within one year of COVID-19 diagnosis (“recently treated control population”), and (iv) patients with B-lymphoid malignancies treated within a year of diagnosis (“patients recently treated for B-lymphoid malignancies”). The one year timepoint was chosen because B-cell repletion following anti-CD20 mAbs has been shown to take up to 12 months (44–46).
Covariates of interest were identified a priori, and included the following: age; sex; smoking status (ever smokers, never smokers); obesity; race/ethnicity; Eastern Cooperative Oncology Group (ECOG) performance status; cancer status (remission/no evidence of disease, active/stable or responding to treatment, active/progressing, unknown); timing of COVID-19 diagnosis; timing of cancer diagnosis with respect to COVID-19 diagnosis; region (U.S. West, U.S. Midwest, U.S. South, U.S. Northeast, and non-US); time since cancer diagnosis with respect to COVID-19, and modified Klabunde comorbidity index, which was treated as a continuous variable (47). Race/ethnicity was categorized as Hispanic, non-Hispanic Black, non-Hispanic White, or other. ECOG performance status was categorized as 0, 1, or 2 or greater. Timing of COVID-19 diagnosis was categorized as follows: January–April 2020, May–August 2020, September–December 2020, January–April 2021. Time since cancer diagnosis with respect to COVID-19 diagnosis was categorized as within a year of COVID-19 diagnosis, within five years of COVID-19 diagnosis, and greater than five years prior to COVID-19 diagnosis. The modified Klabunde comorbidity index was derived from information collected in the CCC19 regarding patient comorbidities, as data regarding hemiplegia and peptic ulcer disease are not included in the CCC19, these elements of the Klabunde index were omitted.
We accounted for interaction effects to better understand the independent association of B-lymphoid malignancies, their therapies, and COVID-19 severity. In brief, interaction effects occur when the impact of a clinical characteristic on an outcome depends on the presence or absence of another characteristic. As such, we hypothesized that the association of B-lymphoid malignancy off therapy with COVID-19 severity would depend on whether that malignancy was active or inactive (48). To test this hypothesis, we added interaction terms between the four populations of interest (nonrecently treated control population, patients with nonrecently treated B-lymphoid malignancies, recently treated control population, patients recently treated for B-lymphoid malignancies) and cancer status (remission/no evidence of disease, active/stable or responding to treatment, active/progressing, unknown) to the primary analysis to investigate differential effects.
Statistical Analysis
The primary objective of the analysis was to compare rates of the primary outcome (COVID-19 severity) between the four populations of interest (nonrecently treated control population, patients with nonrecently treated B-lymphoid malignancies, recently treated control population, patients recently treated for B-lymphoid malignancies). A multivariable proportional odds logistic regression model was applied, adjusting for the above variables of interest. Before we conducted the logistic regression analysis, we performed multiple imputations (with 10 imputations) for missing values of variables through additive regression, bootstrapping, and predictive mean matching. Then, to reduce the impact of confounders among the four populations in the nonrandomized study, for each imputed dataset, we balanced the distributions of covariates between the four populations through inverse probability of treatment weighting (IPTW) by a multinomial logistic regression model. The reported AORs were the average results of the logistic regression model applied to the 10 weighted imputed dataset. We hypothesized that after adjustment, patients with nonrecently treated B-lymphoid malignancies would experience increased COVID-19 severity compared to both nonrecently treated, and recently treated control populations, and that patients recently treated for B-lymphoid malignancies would experience the greatest COVID-19 severity among all four groups. Regarding interaction terms, we hypothesized that for patients who have not recently received therapy, the presence of active cancer would be associated with increased COVID-19 severity, and cancers in remission would be associated with reduced COVID-19 severity. Similarly, multivariable binary logistic regression models along with multiple imputation and IPTW were applied in secondary analyses.
Sensitivity Analyses and Descriptive Analyses
A number of prespecified sensitivity analyses testing the stability of findings focused on the primary outcome in the group of interest were conducted. These were analyses restricted to patients with completed 30-day follow up, patients with documented symptomatic COVID-19, and adjustment for the receipt of convalescent plasma in the subset of patients requiring hospitalization which in a previous analysis of CCC19 data was shown to be associated with improved 30-day mortality for patients with hematologic malignancies and COVID-19 (49). The procedure of analysis was the same as mentioned above: multiple imputation for missing values of variables, IPTW for balance of covariate distributions, and multivariable proportional odds logistic regression analyses.
When available, free text descriptions of the most recent systemic anticancer regimen received were extracted and normalized using the HemOnc ontology (50). We described the differential impact of specific regimens on COVID-19 severity. Representative examples of how free text descriptions were abstracted into most recent systemic anticancer regimen are demonstrated in Supplementary Table S11.
We also recorded vaccination status as follows: (i) any dose of vaccine prior to COVID-19 diagnosis; (ii) no dose of vaccine prior to COVID-19; (iii) unknown. Of note, much of this study period occurred before COVID-19 vaccinations were available, and as the CCC19 did not start collecting data on vaccination until vaccinations were available, there is a high degree of missingness.
Counts less than 5 are not presented pursuant to CCC19 consortium publication standards. These are intended to avoid potential identification of patients based on relatively rare permutations of variables. To this end, data are only presented for categories in which displaying these outcome data is feasible without violation of this standard, to protect patient privacy. Analyses were performed in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), including the Hmisc, ipw, survey, and forestplot extension packages.
Data Availability
The data dictionary for the CCC19 is publicly available on Github (https://github.com/covidncancer/CCC19_dictionary). The data generated in this study are not publicly available due to the possibility that patients with a rare combination of variables could be reidentified, but are available upon reasonable request from the corresponding author. All aggregate deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than U.S. Census Divisions will be made publicly available for any purpose through the CCC19 website (https://www.ccc19.org) beginning 6 months and ending 72 months after publication of this article. These data will be displayed with an interactive graphical tool, allowing for visual analytics of the data. Individual deidentified patient data with site identifiers removed and geographic region of patient residence masked to a level no smaller than U.S. Census Divisions will be made available to researchers who provide a methodologically sound proposal, and whose proposed use of the data has been approved by an independent review committee identified for this purpose. External proposals can be submitted beginning 6 months and up to 72 months after publication of this article; the CCC19 is open to additional collaborators as well. All proposals should be directed to contact@ccc19.org; to gain access, data requestors will need to sign a data access agreement.
Supplementary Material
Acknowledgments
We thank all members of the CCC19 steering committee: Toni K. Choueiri, MD; Narjust Duma, MD; Dimitrios Farmakiotis, MD; Petros Grivas, MD PhD; Gilberto de Lima Lopes Jr., MD MBA; Corrie A. Painter PhD; Solange Peters, MD PhD; Brian I. Rini, MD; Dimpy Shah, MD PhD; Michael A. Thompson, MD PhD; and Jeremy L. Warner, MD MS, for their invaluable guidance of the CCC19 consortium. The authors have the following relevant funding to report: GL P30CA015704-45; MAB P30CA014236; CRF T32-CA236621 and P30-CA046592; RAM and DPS P30-CA054174 as well as grants from the American Cancer Society and Hope Foundation.
Footnotes
Note: Supplementary data for this article are available at Blood Cancer Discovery Online (https://bloodcancerdiscov.aacrjournals.org/).
Authors’ Disclosures
S.M. Rubinstein reports personal fees from Eusa Pharma, Janssen, Roche, Sanofi, and personal fees from Glaxo Smith Kline outside the submitted work. D. Bhutani reports grants from Sanofi Pharmaceuticals outside the submitted work. Y. Shyr reports grants from NIH/NCI during the conduct of the study; grants from NIH outside the submitted work. S. Mishra reports grants from NCI, American Association for Cancer Research, and grants from International Association for the Study of Lung Cancer during the conduct of the study; personal fees from National Geographic outside the submitted work. K.E. Stockerl-Goldstein reports grants and personal fees from Janssen and GSK; grants from Caelum Biosciences, Takeda, and grants from Ionis outside the submitted work. A. Beeghly-Fadiel reports grants from NIH P30 CA068485 (CCC19) during the conduct of the study; grants from NIH U24 MD010722 PMHDC Pilot Study, and grants from NIH U54 CA163072 MVTCP Pilot Study outside the submitted work. S.E. Assouline reports grants and personal fees from Roche Canada and Novartis; grants from Takeda; personal fees from Pfizer, Abbvie, and personal fees from Amgen outside the submitted work. Z. Bakouny reports grants from Genentech/imCORE; non-financial support from Bristol Myers Squibb, and personal fees from UpToDate outside the submitted work. B. Bashir reports other support from Amgen, Bicycle Therapeutics, Boehringer Ingelheim, Syros Pharmaceuticals, Tarveda Therapeutics, and other support from Ikena Oncology outside the submitted work. S. Berg reports personal fees and non-financial support from Exelexis; personal fees from BMS; personal fees and non-financial support from Eisai; personal fees from Seattle Genetics, and personal fees from Pfizer outside the submitted work. M.A. Bilen reports personal fees from Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, SeaGen, and Sanofi and grants from Merck, Xencor, Bayer, Bristol Myers Squibb, Genentech/Roche, SeaGen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer outside the submitted work. S. Gupta reports grants and personal fees from BMS; personal fees from EMD Sorono and Merck; grants and personal fees from Pfizer; personal fees from Janssen, Seattle Genetics, Natera, and other support from Moderna outside the submitted work. C. Hwang reports grants from Merck, Bayer, AstraZeneca; personal fees from Tempus, and personal fees from EMD Sorono outside the submitted work; and stock holdings in Johnson and Johnson. N.A. Johnson reports personal fees from Roche, Abbvie, Beigene, Merck, and personal fees from BMS outside the submitted work. M. Joshi reports grants from Astrazeneca, Pfizer, Eisai; and personal fees from Seagen outside the submitted work. C. Labaki reports grants from Roche - Genentech outside the submitted work. G.H. Lyman reports grants from Amgen; personal fees from Sandoz, and personal fees from Kallyope outside the submitted work. R.R. McKay reports Advisory board/consultant AstraZeneca, Aveo, Bayer, BMS, Calithera, Caris, Dendreon, Exelixis, Janssen, Myovant, Novartis, Pfizer, Sanofi, Tempus, Vividion. A.J. Olszewski reports other support from Genentech; other support from Precision Biosciences; personal fees and other support from Genmab; personal fees from TG Therapeutics; other support from Celldex Therapeutics; and non-financial support from Adaptive Biotechnologies outside the submitted work. R. Rosovski reports grants and personal fees from BMS and Janssen; personal fees from Dova, and personal fees from Inari outside the submitted work. A. Schmidt reports personal fees from Astellas outside the submitted work. A. Shastri reports personal fees from Janssen Pharmaceuticals; grants from Kymera Therapeutics; other support from GLG & Guidepoint; other support from Rigel Pharmaceuticals; and other support from Onclive outside the submitted work. P. Torka reports personal fees from Genentech, TG therapeutics, ADC therapeutics, and personal fees from Kura Oncology outside the submitted work. L. Zubiri reports grants from SEOM (Sociedad Española de Oncología Médica) during the conduct of the study; personal fees from MERCK outside the submitted work. J.L. Warner reports grants from NIH/NCI during the conduct of the study; grants from AACR; personal fees from Westat, Roche, Flatiron Health, Melax Tech; and other support from HemOnc.org LLC outside the submitted work. M.A. Thompson reports personal fees from Adaptive, Abbvie, Elsevier Clinical Path, Epizyme, Janssen, Sanofi, GRAIL/Illumina; non-financial support from Strata Oncology, and non-financial support and other support from Syapse outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
S.M. Rubinstein: Conceptualization, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. D. Bhutani: Conceptualization, formal analysis, validation, writing–original draft, writing–review and editing. R.C. Lynch: Conceptualization, resources, formal analysis, investigation, methodology. C. Hsu: Formal analysis, methodology. Y. Shyr: Formal analysis, methodology. S. Advani: Conceptualization, writing–original draft, writing–review and editing. R.A. Mesa: Conceptualization, writing–original draft, writing–review and editing. S. Mishra: Resources, project administration. D.P. Mundt: Conceptualization, writing–original draft, writing–review and editing. D.P. Shah: Methodology. R. Sica: Conceptualization, methodology, writing–review and editing. K.E. Stockerl-Goldstein: Conceptualization, writing–original draft, writing–review and editing. C. Stratton: Conceptualization, methodology. M. Weiss: Conceptualization, writing–original draft, writing–review and editing. A. Beeghly-Fadiel: Data curation. M. Accordino: Writing–review and editing. S.E. Assouline: Writing–review and editing. J. Awosika: Conceptualization, writing–original draft, writing–review and editing. Z. Bakouny: Conceptualization, writing–review and editing. B. Bashir: Writing–review and editing. S. Berg: Writing–review and editing. M.A. Bilen: Writing–original draft. C.A. Castellano: Writing–review and editing. J.C. Cogan: Data curation, writing–review and editing. D. KC: Data curation, writing–review and editing. C.R. Friese: Writing–review and editing. S. Gupta: Writing–review and editing. D. Hausrath: Data curation, writing–review and editing. C. Hwang: Data curation, writing–review and editing. N.A. Johnson: Writing–review and editing. M. Joshi: Writing–review and editing. A. Kasi: Writing–review and editing. E.J. Klein: Writing–review and editing. V.S. Koshkin: Writing–review and editing. N.M. Kuderer: Resources, writing–review and editing. D.H. Kwon: Writing–review and editing. C. Labaki: Writing–review and editing. T. Latif: Data curation, writing–review and editing. E. Lau: Writing–review and editing. X. Li: Data curation, writing–review and editing. G.H. Lyman: Conceptualization, writing–review and editing. R.R. McKay: Data curation, writing–review and editing. G. Nagaraj: Writing–review and editing. A. Nizam: Writing–review and editing. T.K. Nonato: Writing–review and editing. A.J. Olszewski: Writing–review and editing. H.V. Polimera: Writing–review and editing. A.J. Portuguese: Writing–review and editing. M.M. Puc: Writing–review and editing. P. Razavi: Writing–review and editing. R. Rosovski: Writing–review and editing. A. Schmidt: Conceptualization, writing–review and editing. S.A. Shah: Writing–review and editing. A. Shastri: Writing–review and editing. C. Su: Writing–review and editing. P. Torka: Writing–review and editing. T.M. Wise-Draper: Writing–review and editing. L. Zubiri: Writing–review and editing. J.L. Warner: Conceptualization, resources, data curation, software, formal analysis, visualization, methodology, writing–original draft, writing–review and editing. M.A. Thompson: Conceptualization, supervision, writing–original draft, writing–review and editing.
References
- 1. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004;351:1860–73. [DOI] [PubMed] [Google Scholar]
- 2. Ison MG. Influenza prevention and treatment in transplant recipients and immunocompromised hosts. Influenza Other Respir Viruses 2013;7:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison VA. Infections in patients with leukemia and lymphoma [Internet], inStosor V, Zembower TR (eds): Infectious complications in cancer patients. Cham: Springer International Publishing; 2014, pp. 319–49. Available from: 10.1007/978-3-319-04220-6_11. [DOI] [PubMed] [Google Scholar]
- 4. Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005;23:9219–26. [DOI] [PubMed] [Google Scholar]
- 5. Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancers. Leukemia 2020;34:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020;7:e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020;136:2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mato AR, Roeker LE, Lamanna N, Allan JN, Leslie L, Pagel JM, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood 2020;136:1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee LY, Cazier J-B, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamure S, Duléry R, Di Blasi R, Chauchet A, Laureana C, Deau-Fischer B, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine 2020;27:100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol 2021;8:e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stack M, Sacco K, Castagnoli R, Livinski AA, Notarangelo LD, Lionakis MS. BTK inhibitors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systematic review. Res Sq 2021;319342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brar G, Pinheiro LC, Shusterman M, Swed B, Reshetnyak E, Soroka O, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol 2020;38:3914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu C-Y, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol 2021;32:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P, et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2: a prospective study. Blood Adv 2021;5:4398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021;137:3165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Monin-Aldama L, Laing AG, Munoz-Ruiz M, McKenzie DR, del Molino del Barrio I, Alaguthurai T, et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv 2021. [Google Scholar]
- 21. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021;39:1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, et al. Disease-and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2021;2:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonuomo V, Ferrarini I, Dell'Eva M, Sbisà E, Krampera M, Visco C. COVID-19 (SARS-CoV-2 infection) in lymphoma patients: A review. World J Virol 2021;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riches JC. Impact of COVID-19 in patients with lymphoid malignancies. World J Virol 2021;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Booth S, Curley HM, Varnai C, Arnold R, Lee LYW, Campton NA, et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy [Internet]. Br J Haematol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elkrief A, Wu JT, Jani C, Enriquez KT, Glover M, Shah MR, et al. Learning through a pandemic: the current state of knowledge on COVID-19 and cancer [Internet]. Cancer Discov 2022;12:303–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv 2020;4:5966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mileham KF, Bruinooge SS, Aggarwal C, Patrick AL, Davis C, Mesenhowski DJ, et al. Changes over time in COVID-19 severity and mortality in patients undergoing cancer treatment in the United States: initial report from the ASCO registry. JCO Oncol Pract 2021;OP2100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singh AK, Singh A, Singh R, Misra A. Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr 2020;14:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenberg E, Dufort E, Udo T, Wilberschied L, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020;323:2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19. N Engl J Med 2020;383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puing AG, Ho S, Frankel P, Tegtmeier B, Martin A, Ross J, et al. Severe acute respiratory syndrome coronavirus 2-specific monoclonal antibody for the treatment of mild to moderate coronavirus disease 2019 in cancer patients: a single-center experience. J Infect Dis 2022;225:352–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Desai A, Mohammed TJ, Duma N, Garassino MC, Hicks LK, Kuderer NM, et al. COVID-19 and cancer: a review of the registry-based pandemic response. JAMA Oncol 2021;7:1882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubinstein SM, Steinharter JA, Warner J, Rini BI, Peters S, Choueiri TK. The COVID-19 and cancer consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell 2020;37:738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desai A, Warner J, Kuderer N, Thompson M, Painter C, Lyman G, et al. Crowdsourcing a crisis response for COVID-19 in oncology. Nat Cancer 2020;1:473–6. [DOI] [PubMed] [Google Scholar]
- 41. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rivera DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu C-Y, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov 2020;10:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol 2007;122:139–45. [DOI] [PubMed] [Google Scholar]
- 45. Kurokawa T, Hase M, Tokuman N, Yoshida T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol Oncol 2011;29:5–9. [DOI] [PubMed] [Google Scholar]
- 46. Shree T, Shankar V, Lohmeyer JJK, Czerwinski DK, Schroers-Martin JG, Rodriguez GM, et al. CD20-targeted therapy ablates de novo antibody response to vaccination but spares preestablished immunity. Blood Cancer Discov 2022;3:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- 48. Karaca-Mandic P, Norton EC, Dowd B. Interaction terms in nonlinear models. Health Serv Res 2012;47:255–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol 2021;7:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Warner JL, Dymshyts D, Reich CG, Gurley MJ, Hochheiser H, Moldwin ZH, et al. HemOnc: a new standard vocabulary for chemotherapy regimen representation in the OMOP common data model. J Biomed Inform 2019;96:103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data dictionary for the CCC19 is publicly available on Github (https://github.com/covidncancer/CCC19_dictionary). The data generated in this study are not publicly available due to the possibility that patients with a rare combination of variables could be reidentified, but are available upon reasonable request from the corresponding author. All aggregate deidentified patient data with site identifiers removed and geographical region of patient residence masked to a level no smaller than U.S. Census Divisions will be made publicly available for any purpose through the CCC19 website (https://www.ccc19.org) beginning 6 months and ending 72 months after publication of this article. These data will be displayed with an interactive graphical tool, allowing for visual analytics of the data. Individual deidentified patient data with site identifiers removed and geographic region of patient residence masked to a level no smaller than U.S. Census Divisions will be made available to researchers who provide a methodologically sound proposal, and whose proposed use of the data has been approved by an independent review committee identified for this purpose. External proposals can be submitted beginning 6 months and up to 72 months after publication of this article; the CCC19 is open to additional collaborators as well. All proposals should be directed to contact@ccc19.org; to gain access, data requestors will need to sign a data access agreement.