Abstract
Introduction
Neurological manifestations and complications in coronavirus disease-2019 (COVID-19) patients are frequent. Prior studies suggested a possible association between neurological complications and fatal outcome, as well as the existence of potential modifiable risk factors associated to their occurrence. Therefore, more information is needed regarding the incidence and type of neurological complications, risk factors, and associated outcomes in COVID-19.
Methods
This is a pre-planned secondary analysis of the international multicenter observational study of the COVID-19 Critical Care Consortium (which collected data both retrospectively and prospectively from the beginning of COVID-19 pandemic) with the aim to describe neurological complications in critically ill COVID-19 patients and to assess the associated risk factors, and outcomes. Adult patients with confirmed COVID-19, admitted to Intensive Care Unit (ICU) will be considered for this analysis. Data collected in the COVID-19 Critical Care Consortium study includes patients' pre-admission characteristics, comorbidities, severity status, and type and severity of neurological complications. In-hospital mortality and neurological outcome were collected at discharge from ICU, and at 28-days.
Ethics and Dissemination
The COVID-19 Critical Care Consortium main study and its amendments have been approved by the Regional Ethics Committee of participating sites. No further approval is required for this secondary analysis.
Trial Registration Number
ACTRN12620000421932.
Keywords: COVID-19, neurological complications, disability, stroke, neurological outcome
Introduction
Coronavirus disease 2019 (COVID-19) presents with a wide spectrum of symptoms, from mild to severe, up to sequential organ failure and multiple-organ dysfunction (1). Reports of neurological manifestations associated with COVID-19 are increasing in the literature (2, 3). COVID-19 neurological signs can involve either the central nervous system (CNS), peripheral nervous system (PNS), or musculoskeletal system. Fatigue, myalgia, impaired sense of smell and taste, and headache are common neurological manifestations of COVID-19 (4, 5), whereas dizziness, confusion, delirium, agitation, stroke, hypoxic ischemic injury, seizures, encephalitis and coma among others have been reported neurological complications of hospitalized patients (4, 5). In some cases, neurological manifestations have been reported even without a primary respiratory involvement (4, 5). Several explanations have been proposed for the cause of neurological symptoms of COVID-19, but the underlying pathophysiology is not well defined. Putative mechanisms include viral neurotropism, a hyperinflammatory and hypercoagulable state, or pathological brain–lung crosstalk (6). Endothelial dysregulation (7–9) and pro-thrombotic state (10–12) have been widely suspected to be the possible main contributors of the increased risk of neurologic events. Indeed, COVID-19 patients are at high risk of hypoxia, hypotension, and microvascular abnormalities (13–15) which can promote neuroinflammation and excitotoxicity and increased permeability of the blood brain barrier (16). The risk is even more increased by the use of extracorporeal membrane oxygenation (ECMO) support that is a salvage option in COVID-19 critically ill patients with refractory hypoxemia (17). Prior studies suggested a possible association between neurological complications and mortality (18), but more information is required to delineate this association with respect to regional variation, as well as the risk factors associated to the occurrence of neurological complications (19). The aim of this study is to estimate the incidence of neurological complications in critically ill COVID-19 patients. Associations between neurological complications, patient-level variables and outcomes will also be assessed.
Methods and Analysis
Study Design
This is a pre-planned sub-analysis of a large international multicenter observational study of patients in participating intensive care units (ICUs) with COVID-19 of the COVID-19 Critical Care Consortium incorporating the ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease (ECMOCARD). The collaborative consists of investigators from the Asia-Pacific extracorporeal life support organization (APELSO) in collaboration with centers within the SPRINT-SARI and International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) Network. In Australia, this study is also supported by collaboration with the “National registry on the treatment and outcomes of patients requiring ECMO” (EXCEL Registry). A panel of 13 experts in neurocritical care was created in 2020 together with the main protocol of the COVID-19 Critical Care Consortium by the Steering committee of the consortium. The panel planned this subanalysis and the electronic case report form (eCRF) in February 2020 and followed it up through monthly meeting. The study will be conducted in compliance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) (20) (Supplementary Item 1). Trial registration number: ACTRN12620000421932.
Objectives
The primary objective is to identify and describe the type and incidence of neurological complications in COVID-19 patients before and after admission to ICU, for all ICU patients selected patient subgroups (sex, age, country, treatment, COVID-19 wave).
Secondary objectives include: To evaluate the effect of neurological complications on outcomes after COVID-19, i.e., mortality, duration of ICU and hospital stay, neurological outcome (modified Rankin scale, mRS) at discharge, incidence of delirium and cognitive outcome at discharge. To identify factors related to the occurrence of neurological complications (including neurological injury due to the antiviral therapy).
Specific Sub-analysis
Secondary sub-analyses will also include the investigation of (1) magnetic resonance images (MRI) or computed tomography (CT) features; (2) serum biomarkers [neuronal injury markers (S100B, neuron specific enolase, NSE), endothelial dysfunction markers, inflammatory markers].
Inclusion and Exclusion Criteria
The COVID-19 Critical Care Consortium included all COVID-19 patients (≥18 years) admitted to ICU for receiving critical care with confirmed or suspected COVID-19 respiratory disease. For this specific sub-analysis, further inclusion criteria will be available data on neurological complications/manifestations. Patients treated with mechanical ventilation or ECMO for other causes than COVID-19 will be excluded.
Study Procedures and Setting
The protocol of the main study has been previously published (21). Participants in the COVID-19 Critical Care Consortium Observational Study are recruited at multiple sites in over 52 countries from 1st January 2020 onwards.
Data Collection
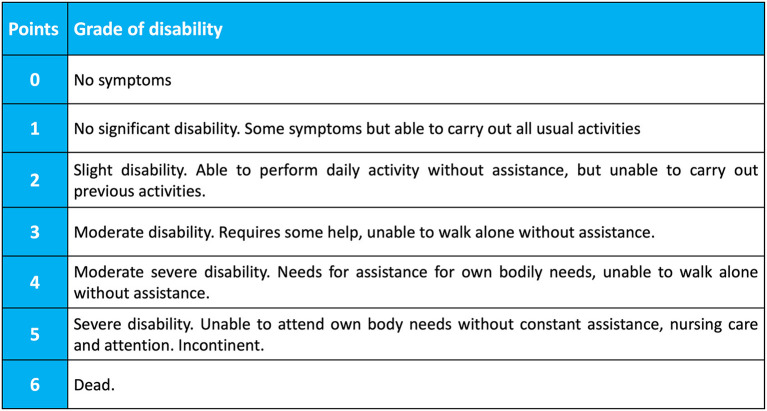
Data collection started from the commencement of COVID-19 pandemic and is planned to continue until completion of COVID-19 pandemic, as judged by the World Health Organization. According to the COVID-19 Critical Care Consortium Observational Study protocol (21) and neurological sub-study protocol, the following data will be collected: general patient characteristics, age, gender, body mass index (BMI), country, previous chronic comorbidities, scores of severity; premorbid scores [modified Rankin scale (0–6 points), Figure 1; new neurological complications, laboratory findings, imaging, and management of neurological complications (Supplementary Item 2); patient outcome (mortality at discharge, at 28-days, withdrawal of life-saving therapy and reason; mRS at ICU discharge, mRS at 28 days after discharge). Main eCRF of the COVID-19 critical care consortium study and neuro sub-study are provided in the Supplementary Items 3, 4.
Figure 1.
Modified Rankin Scale (mRS). The Modified Rankin Score (mRS) is a 6-point disability scale with possible scores ranging from 0 to 6 (from 0 = no symptoms to 6 = dead). A score of 0–3 indicate mild to moderate disability and a score of 4–5 indicate severe disability. From Wade (22).
Data Management
Data are stored in the central online eCRF database managed by the Oxford University in anonymized form, in order to preserve confidentiality of information in medical records. The Username and password will be assigned by the Oxford University during the registration process for individual Research Coordinators or Site Investigators. All electronic data transfer between study site and database will be username and password protected. The Participant List of the Neurology sub-study is maintained locally and is not to be transferred to any other location. confidentiality of the participant will be maintained unless disclosure is required by law.
Data entry and management will be coordinated by ISARIC and ECMOCARD steering committee, including programming and data management support. ANZIC-RC and ISARIC will act as custodian of the data. The University of Queensland (Australia) will receive data from the data custodians via data sharing agreements. The management committee of the trial will take responsibility for the content and integrity of any data.
Definition of Neurological Complications
Definition of neurological complications (23–32) is listed in Table 1.
Table 1.
Definition of neurological complications/manifestations.
| Neurological complication | Definition |
|---|---|
| Central nervous system | |
| Ischemic stroke (23) | Neurological deficit due to an acute focal injury in the central nervous system caused by vascular involvement such as occlusion and cerebral infarction. |
| Intracranial hemorrhage (23, 24) | Bleeding that occurs inside the skull. Hemorrhagic stroke: neurological deficit due to an acute focal injury in the central nervous system caused by vascular involvement with intracerebral or subarachnoid hemorrhage. Subdural hematoma: collection of blood under the dura mater. |
| Encephalitis/meningitis (25) | Severe inflammatory disorder of the brain or meninges or parenchyma. |
| Transverse myelitis and other spinal cord pathologies (26) | Inflammatory disorder with acute or subacute motor-sensory and autonomic spinal cord dysfunction. |
| Epilepsy, seizures, and generalized convulsive status epilepticus (27, 28) | Epilepsy is a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures, and by neurobiological, cognitive, psychological, and social consequences. Seizure is a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain. Generalized convulsive status epilepticus is defined in adults and children older than 5 years as ≥5 min of (1) continuous seizure or (2) two or more discrete seizures between which there is incomplete recovery of consciousness. |
| Delirium (29) | Acute change in consciousness and attention caused by an organic condition. |
| Peripheral nervous system | |
| Guillain-Barré Syndrome (30) | Inflammatory immune-mediated polyradiculoneuropathy with acute onset that manifests with tingling, progressive weakness, autonomic disfunction and pain. |
| Critical illness myopathy/neuropathy (31) | Neuromuscular weakness in the intensive care setting. |
| Hypogeusia/hyposmia (32) | Quantitative disorders characterized by reduction of taste or smell. |
| Others | |
| Hypoxic-ischemic brain injury (33) | Reduction in blood supply, oxygen supply or utilization that determines a decreased oxygen delivery to the brain and post cardiac arrest hypoxic ischemic brain injury (reduction in blood supply, oxygen supply or utilization that determines a decreased oxygen delivery to the brain due to cardiac arrest). |
Statistical Analysis Plan
Planned analyses will comprise of descriptive summaries and regression-based methods for estimating associations between patient-level variables, neurological complications, and outcomes. Descriptive statistics for summarizing the study cohort will be presented as medians with interquartile ranges and frequencies with percentages for continuous and categorical variables, respectively. As an observational study, missing data are expected; a data completeness summary will accompany descriptive summaries for all variables considered. The incidence of neurological complications will be calculated as the number of events per 1,000 ICU days and as the number of events divided by the total number of ICU admissions. Incidence will be estimated per complication using logistic and Poisson regression; Poisson models will include patient days as an offset to account for varying ICU exposure. Baseline models will be adjusted for patient-level variables (e.g., sex, age, country) and calendar time to account for the timing of different COVID-19 waves. Additional covariates will be informed by univariate analysis and penalized regression techniques to address the secondary objective related to incidence.
Analysis of associations between neurological complications and clinical outcomes will be examined using generalized linear mixed models for binary outcomes and parametric survival models for time-to-event outcomes. Evidence of potential associations, including patient demographics and clinical signs assessed during ICU admission, will initially be assessed using univariate analysis. Results of univariate analysis will be used to inform variable selection for multivariable analysis.
Multivariable models for all study objectives will be adjusted for known confounders as fixed or random effects, including study center, country, and calendar time. Model results will be presented as odds ratios (binary outcomes), relative risks (count outcomes) or hazard ratios (time-to-event outcomes) with 95% confidence intervals and p-values from hypothesis tests as appropriate.
Study Status
The protocol version is 1.2.8 of the COVID-19 Critical Care Consortium Observational Study available at https://www.elso.org/COVID19/ECMOCARD.aspx. Data collection started from the commencement of COVID-19 pandemic and is planned to continue until completion of COVID-19 pandemic, as judged by the World Health Organization, as reported in the protocol.
Discussion
This neurological sub-analysis of the COVID-19 Critical Care Consortium Observational Study is designed with the aim to obtain a detailed overview on neurological complications in a large international multicenter cohort of critically ill COVID-19 patients admitted to ICU, to determine incidence and risk factors of neurological complications, and the association of neurological complications with outcome. This study will provide real-time global data without geographic restrictions.
In the latest 2 years, knowledge has increased regarding extra-pulmonary complications of COVID-19 and their effect on outcome. Severe COVID-19 disease potentially involves multiple organs, including pulmonary, coagulation, cardiac, neurological, renal, hepatic, and gastrointestinal manifestations (34). Many neurological manifestations have been described recently in small observational studies, but additional evidence is needed from large multicentric cohorts. For this reason, in the present study we aim to depict the incidence, risk factors, and impact on outcome of neurological complications in critically ill COVID-19 patients from a large observational multicentric cohort. Data regarding pre-admission neurological manifestations, in-hospital neurological complications as well as ICU-and-hospital length of stay, neurological outcome (mRS), and mortality are available in the eCRF. This sub-analysis of the COVID-19 Critical Care Consortium Observational Study was pre-planned during the first/second wave of the pandemic (late 2020), thus increasing the data quality and minimizing the chance of spurious results and limiting the potential of exploratory learning. The number of patients included in the main study is continuously growing since the beginning of pandemic, allowing to obtain a large sample size, which can provide important information on the current incidence and characteristics of neurological manifestations in COVID-19 patients, evaluating potential associations between predictors and development of neurological complications, and assessing outcomes at discharge from ICU and from hospital and 28-days patients' outcomes. The included patients will be from different countries and centers, including low incoming countries. The patients will be also included during different waves and years of the pandemic, before and after the advent of vaccination campaigns, and with different variants of COVID-19 (i.e., omicron, delta, etc.). This will provide interesting insights on the differences in epidemiology, management strategies, geographical, and economical characteristics of COVID-19 adult patients who manifest neurological complications admitted to ICU. This global research context will provide the lens through which the study as well as its methodological approaches, findings, conclusions, and recommendations can be viewed.
Incidence and Types of Neurological Manifestations and Complications of COVID-19
The importance of investigating neurological manifestations in COVID-19, assessing their risk factors, and association with outcome is justified by the increasing identification in the available literature of many studies which reported high morbidity and mortality and poor neurological outcome in COVID-19 patients who manifest neurological complications, with the need for identifying and investigating such alterations in a bigger cohort of COVID-19 critically ill patients. Indeed, regarding each of the identified neurological manifestations of COVID-19, the data are fragmentary and come from different small cohorts. Myalgia, dysgeusia, and taste dysfunction were frequently reported (33% of cases), altered mental status in 32%, headache 29%, encephalopathy 26%, alteration of consciousness 13%, stroke 12%, dizziness 10%, vision impairment 6%, intracerebral hemorrhage, 5%, seizure 4%, encephalitis 2%, and GBS 1% (35). Intracranial hemorrhage was identified in 477 patients with a prevalence of 0.85% and a mortality of 52% suggesting a very poor prognosis despite rare incidence (36). The prevalence of intracranial hemorrhage, ischemic stroke, and hypoxic ischemic brain injury was higher in patients with COVID-19 who underwent ECMO support (5.9%) with a mortality of 92% (17). Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis have been reported in 46 patients with COVID-19 only, of whom 32% died (37).
Risk Factors for Neurological Manifestations and Complications of COVID-19
Regarding risk factors and association of neurological manifestations with outcome, a systematic review revealed that patients who suffer from a severe COVID-19 have more CNS involvement, neurological symptoms, and association with stroke. More severe patients had higher D-dimer and C-reactive protein levels than non-severe patients and presented multiple organ involvement (38). Myalgia, acute cerebrovascular disease, elevated creatin kinase, and lactate dehydrogenase were associated with more severe disease (3), while delirium on admission is a good predictor of mortality outcome in COVID-19 (39). In a cohort of 1,072 patients, age, headache at presentation, preexisting neurologic disease, invasive mechanical ventilation, and neutrophil/lymphocyte ratio ≥ 9 were independent predictors of new neurologic complications (40). In another study, the CT lung disease severity score was predictive of acute abnormalities on neuroimaging in patients with COVID-19 with neurologic manifestations (41). In a retrospective analysis, previous neurological history did not impact mortality, whereas new neurological manifestations were predictors of death (42). In a large cohort of 3,055 COVID-19 patients, preexisting neurological disorders were associated with higher risk of developing new neurological manifestations (2).
Outcome of COVID-19 Patients With Neurological Manifestations and Complications
Patients affected by COVID-19 with neurological manifestations were noted to have an impaired quality of life in 49% of cases, with a residual disability at 6-months in 52%, impaired cognition in 69%, and persistence of anxiety and depression in 32% (43). Neurological outcome in 135 patients with COVID-19 at 3-months follow-up was impaired (44), and a significant patient number still suffer from neurological sequelae 1 year after SARS-CoV-2 infection (45). A large multicentric study investigating delirium in 4,530 COVID-19 patients revealed that acute brain dysfunction was highly prevalent and prolonged in critically ill patients with COVID-19, with benzodiazepines and lack of family visitation identified to be risk factors for its development (46). After 6 months, in a cohort of 236,379 patients with COVID-19, neurological and psychiatric manifestations had an estimated incidence of 33.62 and 12.84%, respectively (47). Clinical outcome was evaluated in a cohort of 267 patients, concluding that patients with cerebrovascular disease had the worst prognosis (48).
Potential Pitfalls and Unintended Effects of This Study
Taken together, a large number of case reports and case series, despite coming mainly from small cohorts and local studies raise interest around the need for clarification about type and incidence of COVID-19 neurological manifestations, risk factors, and association with outcome on large scale, thus encouraging to better plan for possible management and therapeutics for neurological complications in critically ill COVID-19 patients. A limitation of current available data in the literature is that most of the data come from small cohorts, that could be addressed by using the larger COVID-19 Critical Care Consortium. Our study is unique in a way that we can address both limitations by studying the questions with international cohort with granular neurological variables. According to the design of our study, no unintended effects are expected. However, some limitations should be addressed. Being an observational study, it can be exposed to bias and confounding. Additionally, it cannot be used to demonstrate causality.
Conclusions
In conclusion the present study will provide new information on a global scale regarding the incidence and type of neurological complications, risk factors, and associated outcomes in COVID-19 with clinical applications.
Ethics Statement
The study will be conducted in compliance with the current version of the COVID-19 Critical Care Consortium and Neurologic sub-study protocol. Protocol version and subsequent amendment will be submitted and approved by the Local Ethics Committee in compliance to national standards. Sites wishing to participate will be required to provide the COVID-19 Critical Care Consortium Research Coordinator with an Institutional Review Board (IRB) approval certificate. The regulations of the COVID-19 Critical Care Consortium state that this study will not require individual patient consent as an observational study. Data of this study is already recorded as part of routine clinical care, therefore justifying participant enrolment using a waiver of consent. However, for any location that deems individual consent necessary, informed consent will be managed in accordance with the local regulations of each involved IRB. In particular, in patients who meet the inclusion/exclusion criteria, informed consent will be obtained directly from the patient, either before the study or retrospectively in case the patient is unconscious at the time of enrolment. If the patient is unable to provide a consent form upon admission, informed consent will be obtained by his/her next of kin.
Author Contributions
DB drafted the manuscript and planned the methodology and the outcomes. S-MC and CR revised the manuscript and supervised the methodology and outcomes. DB, LP, MGr, SH, JF, GW, DBP, RA, LD, EG, MA, VW, AN, SD, GS, BP, DK, EM, AA-F, S-SS, MP, MGi, GF, PP, AP, NW, GL, JS, CR, and S-MC helped in the revision and methodology and approved the final version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Bill & Melinda Gates Foundation, Grant number INV-034765; The University of Queensland; The Wesley Medical Research; The Prince Charles Hospital Foundation; The Health Research Board of Ireland. GL was a recipient of the BITRECS fellowship; the BITRECS project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 754550 and from the La Caixa Foundation (ID 100010434), under the agreement LCF/PR/GN18/50310006. JS was funded by the Advance Queensland fellowship program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- APELSO
Asia-Pacific extracorporeal life support organization
- BMI
body mass index
- CNS
central nervous system
- COVID-19
Coronavirus disease 2019
- CT
computed tomography
- ECMO
extracorporeal membrane oxygenation
- ECMOCARD
ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease
- eCRF
electronic case report form
- ICU
intensive care unit
- IRB
institutional review board
- ISARIC
International Severe Acute Respiratory and emerging Infection Consortium
- MRI
magnetic resonance images
- mRS
modified Rankin scale
- NSE
neuron specific enolase
- PNS
peripheral nervous system.
Appendix
| Prefix/First Name/Last Name | Site Name |
|---|---|
| Eugeni Roure Marta Roure |
The University of Queensland, Australia |
| Fatima Nasrallah | The Queensland Brain Institute, The University of Queensland, St. Lucia, QLD, Australia |
| Katie McMahon | School of Clinical Sciences and Centre for Biomedical Technologies, Queensland University of Technology, Brisbane, Queensland, Australia |
| Judith Bellapart | Royal Brisbane and Women's Hospital, Herston, Queensland, Australia. |
| Fabio Silvio Taccone | Erasmus Hospital, Free University of Brussels, Evere, Belgium |
| Tala Al-Dabbous Huda Alfoudri Mohammed Shamsah |
Al Adan Hospital |
| Subbarao Elapavaluru Ashley Berg Christina Horn |
Allegheny General Hospital |
| Yunis Mayasi | Avera McKennan Hospital & University Health Centre |
| Stephan Schroll | Barmherzige Bruder Regansburg |
| Dan Meyer Jorge Velazco Ludmyla Ploskanych Wanda Fikes Rohini Bagewadi Marvin Dao Haley White Alondra Berrios Laviena Ashley Ehlers Maysoon Shalabi-McGuire Trent Witt |
Baylor Scott & White Health |
| Lorenzo Grazioli Luca Lorini |
Bergamo Hospital |
| E. Wilson Grandin Jose Nunez Tiago Reyes |
Beth Israel Deaconess Medical Centre |
| Diarmuid O'Briain Stephanie Hunter |
Box Hill Hospital |
| Mahesh Ramanan Julia Affleck |
Caboolture Hospital |
| Hemanth Hurkadli Veerendra Sumeet Rai Josie Russell-Brown Mary Nourse |
Canberra Hospital |
| Mark Joseph Brook Mitchell Martha Tenzer |
Carilion Clinic |
| Ryuzo Abe | Chiba University Graduate School of Medicine |
| Hwa Jin Cho In Seok Jeong |
Chonnam National University Hospital |
| Nadeem Rahman Vivek Kakar |
Cleveland Clinic- Abu Dhabi |
| Nicolas Brozzi | Cleveland Clinic - Florida |
| Omar Mehkri Sudhir Krishnan Abhijit Duggal Stuart Houltham |
Cleveland Clinic - Ohio |
| Jerónimo Graf | Clinica Alemana De Santiago |
| Roderigo Diaz Roderigo Orrego Camila Delgado Joyce González Maria Soledad Sanchez Michael Piagnerelli Josefa Valenzuela Sarrazin |
Clinica Las Condez |
| A/Prof. Gustavo Zabert Lucio Espinosa Paulo Delgado Victoria Delgado |
Clinica Pasteur National- University of Comahue |
| Diego Fernando Bautista Rincón Angela Maria Marulanda Yanten Melissa Bustamante Duque |
Clinica Valle de Lilli |
| Daniel Brodie | Medical ICU, Columbia College of Physicians and Surgeons, New-York-Presbyterian Hospital, NY, NY, USA |
| Alyaa Elhazmi Abdullah Al-Hudaib |
Dr Sulaiman Alhabib Medical Group – Research Center, Riyadh, Saudi Arabia |
| Maria Callahan | Emory University Healthcare System |
| M. Azhari Taufik Elizabeth Yasmin Wardoyo Margaretha Gunawan Nurindah S Trisnaningrum Vera Irawany Muhammad Rayhan |
Fatmawati Hospital |
| Mauro Panigada Antonio Pesenti Alberto Zanella Giacomo Grasselli Sebastiano Colombo Chiara Martinet Gaetano Florio |
Fondazione IRCCS Policlinico of Milan (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico) |
| Massimo Antonelli Simone Carelli Domenico L. Grieco |
Fondazione Policlinico Universitario Agostino Gemelli IRCCS |
| Motohiro Asaki | Fujieda Municipal General Hospital |
| Kota Hoshino | Fukuoka University |
| Leonardo Salazar Mary Alejandra Mendoza Monsalve |
Fundación Cardiovascular de Colombia |
| John Laffey Bairbre McNicholas David Cosgrave |
Galway University Hospitals |
| Joseph McCaffrey Allison Bone |
Geelong Hospital |
| Yusuff Hakeem | Glenfield Hospital |
| James Winearls Mandy Tallott |
Gold Coast University Hospital |
| David Thomson Christel Arnold-Day Jerome Cupido Zainap Fanie Malcom Miller Lisa Seymore Dawid van Straaten |
Groote Schuur Hospital |
| Ali Ait Hssain Jeffrey Aliudin Al-Reem Alqahtani Khoulod Mohamed Ahmed Mohamed Darwin Tan Joy Villanueva Ahmed Zaqout |
Hamad General Hospital - Weill Cornell Medical College in Qatar |
| Ethan Kurtzman Arben Ademi |
|
| Ana Dobrita Khadija El Aoudi Juliet Segura |
Hartford HealthCare |
| Gezy Giwangkancana | Hasan Sadikin Hospital (Adult) |
| Shinichiro Ohshimo | Hiroshima University |
| Javier Osatnik | Hospital Alemán |
| Anne Joosten | Hospital Civil Marie Curie |
| Antoni Torres Minlan Yang Ana Motos |
Hospital Clinic, Barcelona |
| Carlos Luna | Hospital de Clínicas |
| Francisco Arancibia | Hospital del Tórax |
| Virginie Williams Alexandre Noel |
Hospital du Sacre Coeur (Universite de Montreal) |
| Nestor Luque | Hospital Emergencia Ate Vitarte |
| Marina Fantini | Hospital Mater Dei |
| Ruth Noemi Jorge García Enrique Chicote Alvarez |
Hospital Nuestra Señora de Gracia |
| Anna Greti | Hospital Puerta de Hierro |
| Adrian Ceccato | Hospital Universitari Sagrat Cor |
| Angel Sanchez | Hospital Universitario Sant Joan d'Alacant |
| Ana Loza Vazquez | Hospital Universitario Virgen de Valme |
| Ferran Roche-Campo Diego Franch-Llasat |
Hospital Verge de la Cinta de Tortosa |
| Divina Tuazon | Houston Methodist Hospital |
| Marcelo Amato Luciana Cassimiro Flavio Pola Francis Ribeiro Guilherme Fonseca |
INCOR (Universidade de São Paulo) |
| Heidi Dalton Mehul Desai Erik Osborn Hala Deeb |
INOVA Fairfax Hospital |
| Antonio Arcadipane Gennaro Martucci Giovanna Panarello Chiara Vitiello Claudia Bianco Giovanna Occhipinti Matteo Rossetti Raffaele Cuffaro |
ISMETT |
| Sung-Min Cho Glenn Whitman |
Johns Hopkins |
| Hiroaki Shimizu Naoki Moriyama |
Kakogawa Acute Care Medical Center |
| Jae-Burm Kim | Keimyung University Dong San Hospital |
| Nobuya Kitamura | Kimitsu Chuo Hospital |
| Johannes Gebauer | Klinikum Passau |
| Toshiki Yokoyama | Kouritu Tousei Hospital |
| Abdulrahman Al-Fares Sarah Buabbas Esam Alamad Fatma Alawadhi |
|
| Kalthoum Alawadi | Al-Amiri and Jaber Al-Ahmed Hospitals, Kuwait Extracorporeal Life Support Program |
| Hiro Tanaka | Kyoto Medical Centre |
| Satoru Hashimoto Masaki Yamazaki |
Kyoto Prefectural University of Medicine |
| Tak-Hyuck Oh | Kyung Pook National University Chilgok Hospital |
| Mark Epler Cathleen Forney |
|
| Louise Kruse Jared Feister Joelle Williamson Katherine Grobengieser |
Lancaster General Health |
| Eric Gnall Sasha Golden Mara Caroline Timothy Shapiro Colleen Karaj Lisa Thome Lynn Sher Mark Vanderland Mary Welch Sherry McDermott |
Lankenau Institute of Medical Research (Main Line Health) |
| Matthew Brain Sarah Mineall |
Launceston General Hospital |
| Dai Kimura | Le Bonheur Children's Hospital |
| Luca Brazzi Gabriele Sales Giorgia Montrucchio |
Le Molinette Hospital (Ospedale Molinette Torino) |
| Tawnya Ogston | Legacy Emanuel Medical Center |
| Dave Nagpal Karlee Fischer |
London Health Sciences Centre |
| Roberto Lorusso | Maastricht University Medical Centre |
| Rajavardhan Rangappa Sujin Rai Argin Appu |
Manipal Hospital Whitefield |
| Mariano Esperatti Nora Angélica Fuentes Maria Eugenia Gonzalez |
Hospital Privado de Comunidad. Mar del Plata. Escuela Superior de Medicina. Universidad Nacional de Mar del Plata |
| Diarmuid O'Briain | Maroondah Hospital |
| Edmund G. Carton | Mater Misericordiae University Hospital |
| Ayan Sen Amanda Palacios Deborah Rainey |
Mayo Clinic College of Medicine |
| Gordan Samoukoviv Josie Campisi |
McGill University Health Centre |
| Lucia Durham Emily Neumann Cassandra Seefeldt Octavio Falcucci Amanda Emmrich Jennifer Guy Carling Johns Kelly Potzner Catherine Zimmermann Angelia Espinal |
Medical College of Wisconsin (Froedtert Hospital) |
| Nina Buchtele Michael Schwameis Andrea Korhnfehl Roman Brock Thomas Staudinger |
Medical University of Vienna |
| Stephanie-Susanne Stecher Michaela Barnikel Sófia Antón Alexandra Pawlikowski |
Medical Department II, LMU Hospital Munich |
| Akram Zaaqoq Lan Anh Galloway Caitlin Merley |
MedStar Washington Hospital Centre |
| Alistair Nichol | Monash University |
| Marc Csete Luisa Quesada Isabela Saba |
Mount Sinai Medical Centre |
| Daisuke Kasugai Hiroaki Hiraiwa |
|
| Taku Tanaka | Nagoya University Hospital |
| Eva Marwali Yoel Purnama Santi Rahayu Dewayanti Ardiyan Dafsah Arifa Juzar Debby Siagian |
National Cardiovascular Center Harapan Kita, Jakarta, Indonesia |
| Yih-Sharng Chen | National Taiwan University Hospital |
| Mark Ogino | Nemours Alfred I duPont Hospital for Children |
| Indrek Ratsep Andra-Maris Post Piret Sillaots Anneli Krund Merili-Helen Lehiste Tanel Lepik |
North Estonia Medical Centre |
| Frank Manetta Effe Mihelis Iam Claire Sarmiento Mangala Narasimhan Michael Varrone |
Northwell Health |
| Mamoru Komats | Obihiro-Kosei General Hospital |
| Julia Garcia-Diaz Catherine Harmon |
Ochsner Clinic Foundation |
| S. Veena Satyapriya Amar Bhatt Nahush A. Mokadam Alberto Uribe Alicia Gonzalez Haixia Shi Johnny McKeown Joshua Pasek Juan Fiorda Marco Echeverria |
Ohio State University Medical Centre |
| Rita Moreno | Oklahoma Heart Institute |
| Bishoy Zakhary | Oregon Health and Science University Hospital (OHSU) |
| Marco Cavana Alberto Cucino |
Ospedale di Arco (Trento Hospital) |
| Giuseppe Foti Marco Giani Benedetta Fumagalli |
Ospedale San Gerardo |
| Davide Chiumello Valentina Castagna |
Ospedale San Paolo |
| Andrea Dell'Amore Paolo Navalesi |
Padua University Hospital (Policlinico of Padova) |
| Hoi-Ping Shum | Pamela Youde Nethersole Eastern Hospital |
| Alain Vuysteke | Papworth Hospitals NHS Foundation Trust |
| Asad Usman Andrew Acker Benjamin Smood Blake Mergler Federico Sertic Madhu Subramanian Alexandra Sperry Nicolas Rizer |
Penn Medicine (Hospital of the University of Pennsylvania) |
| Erlina Burhan Menaldi Rasmin Ernita Akmal Faya Sitompul Navy Lolong Bhat Naivedh |
Persahabatan General Hospital |
| Simon Erickson | Perth Children's Hospital |
| Peter Barrett David Dean |
|
| Julia Daugherty | Piedmont Atlanta Hospital |
| Antonio Loforte | Policlinico di S. Orsola, Università di Bologna |
| Irfan Khan Mohammed Abraar Quraishi Olivia DeSantis |
Presbyterian Hospital Services, Albuquerque |
| Dominic So Darshana Kandamby |
Princess Margaret Hospital |
| Jose M. Mandei Hans Natanael |
Prof Dr R. D. Kandou General Hospital - Paediatric |
| Eka YudhaLantang Anastasia Lantang |
Prof Dr R. D R. D. Kandou General Hospital - Adult |
| Surya Oto Wijaya | Dr Sulianti Saroso Hospital |
| Anna Jung | Providence Saint John's Health Centre |
| George Ng Wing Yiu Ng |
Queen Elizabeth Hospital, Hong Kong |
| Pauline Yeung Ng Shu Fang |
The University of Hong Kong |
| Alexis Tabah Megan Ratcliffe Maree Duroux |
Redcliffe Hospital |
| Shingo Adachi Shota Nakao |
Rinku General Medical Center (and Senshu Trauma and Critical Care Center) |
| Pablo Blanco Ana Prieto Jesús Sánchez |
Rio Hortega University Hospital |
| Meghan Nicholson | Rochester General Hospital |
| Warwick Butt Alyssa Serratore Carmel Delzoppo |
Royal Children's Hospital |
| Pierre Janin Elizabeth Yarad |
Royal North Shore Hospital |
| Richard Totaro Jennifer Coles |
Royal Prince Alfred Hospital |
| Bambang Pujo | RSUD Soetomo |
| Robert Balk Andy Vissing Esha Kapania James Hays Samuel Fox |
|
| Garrett Yantosh Pavel Mishin |
Rush University, Chicago |
| Saptadi Yuliarto Kohar Hari Santoso Susanthy Djajalaksana |
Saiful Anwar Malang Hospital (Brawijaya University) (Paediatrics) |
| Arie Zainul Fatoni | Saiful Anwar Malang Hospital (Brawijaya University) (Adult) |
| Masahiro Fukuda | Saiseikai Senri Hospital |
| Keibun Liu | Saiseikai Utsunomiya Hospital |
| Paolo Pelosi Denise Battaglini |
San Martino Hospital |
| Juan Fernando Masa Jiménez | San Pedro de Alcantara Hospital |
| Diego Bastos | Sao Camilo Cura D'ars |
| Sérgio Gaião | São João Hospital Centre, Porto |
| Desy Rusmawatiningtyas | Sardjito Hospital (Paediatrics) |
| Young-Jae Cho | Seoul National University Bundang Hospital |
| Su Hwan Lee | Severance Hospital |
| Tatsuya Kawasaki | Shizuoka Children's Hospital |
| Laveena Munshi | Sinai Health Systems (Mount Sinai Hospital) |
| Pranya Sakiyalak Prompak Nitayavardhana |
Siriraj Hospital |
| Tamara Seitz | Sozialmedizinisches Zentrum Süd – Kaiser-Franz-Josef-Spital |
| Rakesh Arora David Kent |
St Boniface Hospital (University of Mannitoba) |
| Daniel Marino | St Christopher's Hospital for Children |
| Swapnil Parwar Andrew Cheng Jennene Miller |
St George Hospital |
| Shigeki Fujitani Naoki Shimizu |
St Marianna Medical University Hospital |
| Jai Madhok Clark Owyang |
Stanford University Hospital |
| Hergen Buscher Claire Reynolds |
St Vincent's Hospital |
| Olavi Maasikas AleksanBeljantsev Vladislav Mihnovits |
Tartu University Hospital |
| Takako Akimoto Mariko Aizawa Kanako Horibe Ryota Onodera |
Teine Keijinkai Hospital |
| Carol Hodgson Aidan Burrell Meredith Young |
The Alfred Hospital |
| Timothy George | The Heart Hospital Baylor Plano, Plano |
| Kiran Shekar Niki McGuinness Lacey Irvine |
The Prince Charles Hospital |
| Brigid Flynn | The University of Kansas Medical Centre |
| Tomoyuki Endo | Tohoku Medical and Pharmaceutical University |
| Kazuhiro Sugiyama | Tokyo Metropolitan Bokutoh Hospital |
| Keiki Shimizu | Tokyo Metropolitan Medical Center |
| Eddy Fan Kathleen Exconde |
Toronto General Hospital |
| Shingo Ichiba | Tokyo Women's Medical University Hospital |
| Leslie Lussier | Tufts Medical Centre (and Floating Hospital for Children) |
| Gösta Lotz | Universitätsklinikum Frankfurt (University Hospital Frankfurt) (Uniklinik) |
| Maximilian Malfertheiner Lars Maier Esther Dreier |
Universitätsklinikum Regensburg (Klinik für Innere Medizin II) |
| Neurinda Permata Kusumastuti | University Airlangga Hospital (Paediatric) |
| Colin McCloskey Al-Awwab Dabaliz Tarek B Elshazly Josiah Smith |
University Hospital Cleveland Medical Centre (UH Cleveland Hospital) |
| Konstanty S. Szuldrzynski Piotr Bielański |
University Hospital in Krakow |
| Yusuff Hakeem | University Hospitals of Leicester NHS Trust (Glenfield Hospital) |
| Keith Wille | University of Alabama at Birmingham Hospital (UAB) |
| Srinivas Murthy | University of British Columbia |
| Ken Kuljit S. Parhar Kirsten M. Fiest Cassidy Codan Anmol Shahid |
University of Calgary (Peter Lougheed Centre, Foothills Medical Centre, South Health Campus and Rockyview General Hospital) |
| Mohamed Fayed Timothy Evans Rebekah Garcia Ashley Gutierrez Hiroaki Shimizu |
University of California, San Francisco-Fresno Clinical Research Centre |
| Tae Song Rebecca Rose |
University of Chicago |
| Suzanne Bennett Denise Richardson |
University of Cincinnati Medical Centre |
| Giles Peek | University of Florida |
| Lovkesh Arora Kristina Rappapport Kristina Rudolph Zita Sibenaller Lori Stout Alicia Walter |
University of Iowa |
| Daniel Herr Nazli Vedadi |
University of Maryland - Baltimore |
| Robert Bartlett | University of Michigan Medical Center |
| Antonio Pesenti | University of Milan |
| Shaun Thompson Julie Hoffman Xiaonan Ying |
University of Nebraska Medical Centre |
| Ryan Kennedy | University of Oklahoma Health Sciences Centre (OU) |
| Muhammed Elhadi | Faculty of Medicine, University of Tripoli |
| Matthew Griffee Anna Ciullo Yuri Kida |
University of Utah Hospital |
| Ricard Ferrer Roca JordI Riera Sofia Contreras Cynthia Alegre |
Vall d'Hebron University Hospital, Barcelona |
| Christy Kay Irene Fischer Elizabeth Renner |
Washington University in St. Louis/ Barnes Jewish Hospital |
| Hayato Taniguci | Yokohama City University Medical Center |
| John Fraser Gianluigi Li Bassi Jacky Suen |
|
| Adrian Barnett Nicole White Kristen Gibbons Simon Forsyth Amanda Corley India Pearse Samuel Hinton Gabriella Abbate Halah Hassan Silver Heinsar Varun A Karnik Katrina Ki Hollier F. O'Neill Nchafatso Obonyo Leticia Pretti Pimenta Janice D. Reid Kei Sato Kiran Shekar Aapeli Vuorinen Karin S. Wildi Emily S. Wilson Stephanie Yerkovich |
COVID-19 Critical Care Consortium |
| James Lee Daniel Plotkin Barbara Wanjiru Citarella Laura Merson |
ISARIC, Centre for Tropical Medicine and Global Health, University of Oxford, Oxford, UK |
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.930217/full#supplementary-material
References
- 1.Wong CKH, Wong JYH, Tang EHM, Au CH, Wai AKC. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep. (2020) 10:19765. 10.1038/s41598-020-74988-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou SH-Y, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. (2021) 4:e2112131. 10.1001/jamanetworkopen.2021.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yassin A, Nawaiseh M, Shaban A, Alsherbini K, El-Salem K, Soudah O, et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Neurol. (2021) 21:138. 10.1186/s12883-021-02161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huth SF, Cho S-M, Robba C, Highton D, Battaglini D, Bellapart J, et al. Neurological manifestations of coronavirus disease 2019: a comprehensive review and meta-analysis of the first 6 months of pandemic reporting. Front Neurol. (2021) 12:664599. 10.3389/fneur.2021.664599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19. Neurology. (2021) 97:e2269–81. 10.1212/WNL.0000000000012930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglini D, Brunetti I, Anania P, Fiaschi P, Zona G, Ball L, et al. Neurological manifestations of severe SARS-CoV-2 infection: potential mechanisms and implications of individualized mechanical ventilation settings. Front Neurol. (2020) 11:845. 10.3389/fneur.2020.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sashindranath M, Nandurkar HH. Endothelial dysfunction in the brain. Stroke. (2021) 52:1895–904. 10.1161/STROKEAHA.120.032711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savarraj J, Park ES, Colpo GD, Hinds SN, Morales D, Ahnstedt H, et al. Brain injury, endothelial injury and inflammatory markers are elevated and express sex-specific alterations after COVID-19. J Neuroinflammation. (2021) 18:277. 10.1186/s12974-021-02323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. (2022) 17:307–20. 10.1016/j.stemcr.2021.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas Z, Chaudhary A. COVID-19 associated coagulopathy resulting in cerebral venous thrombosis and pulmonary embolism. Cureus. (2021) 13:e19602. 10.7759/cureus.19602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manolis AS, Manolis TA, Manolis AA, Papatheou D, Melita H. COVID-19 infection: viral macro- and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management. J Cardiovasc Pharmacol Ther. (2021) 26:12–24. 10.1177/1074248420958973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robba C, Battaglini D, Ball L, Valbusa A, Porto I, Della Bona R, et al. Coagulative disorders in critically ill COVID-19 patients with acute distress respiratory syndrome: a critical review. J Clin Med. (2021) 10:140. 10.3390/jcm10010140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldrop G, Safavynia SA, Barra ME, Agarwal S, Berlin DA, Boehme AK, et al. Prolonged unconsciousness is common in COVID-19 and associated with hypoxemia. Ann Neurol. (2022) 91:740–55. 10.1002/ana.26342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutkai I, Mayer MG, Hellmers LM, Ning B, Huang Z, Monjure CJ, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. (2022) 13:1745. 10.1038/s41467-022-29440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambade V, Ambade S. SARS-CoV-2 infecting endothelial cells, biochemical alterations, autopsy findings and outcomes in COVID-19, suggest role of hypoxia-inducible factor-1. J Med Biochem. (2022) 41:14–20. 10.5937/jomb0-30659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Tan R, Mo Y, Zhang J. The blood-brain barrier in health, neurological diseases, and COVID-19. Fundam Res. (2022). 10.1016/j.fmre.2022.03.003. [Epub ahead of print]. [DOI] [Google Scholar]
- 17.Kannapadi N V., Jami M, Premraj L, Etchill EW, Giuliano K, Bush EL, et al. Neurological complications in COVID-19 patients with ECMO support: a systematic review and meta-analysis. Hear Lung Circ. (2022) 31:292–8. 10.1016/j.hlc.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frontera JA, Yang D, Lewis A, Patel P, Medicherla C, Arena V, et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. (2021) 426:117486. 10.1016/j.jns.2021.117486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidescu EI, Odajiu I, Tulbǎ D, Sandu CD, Bunea T, Sandu G, et al. Prognostic factors in COVID-19 patients with new neurological manifestations: a retrospective cohort study in a romanian neurology department. Front Aging Neurosci. (2021) 13:645611. 10.3389/fnagi.2021.645611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Li Bassi G, Suen J, Barnett AG, Corley A, Millar J, Fanning J, et al. Design and rationale of the COVID-19 critical care consortium international, multicentre, observational study. BMJ Open. (2020) 10:e041417. 10.1136/bmjopen-2020-041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade DT. Measurement in Neurological Rehabilitation. New York, NY: Oxford University Press; (1992). [PubMed] [Google Scholar]
- 23.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, (Buddy), Culebras A, et al. An updated definition of stroke for the 21st century. Stroke. (2013) 44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone JL, Rifai MHS, Sugar O, Lang RGR, Oldershaw JB, Moody RA. Subdural hematomas. Surg Neurol. (1983) 19:216–31. 10.1016/S0090-3019(83)80005-6 [DOI] [PubMed] [Google Scholar]
- 25.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez Y, Rojas M, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Monsalve DM, et al. Guillain–Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. (2018) 15:547–62. 10.1038/cmi.2017.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher RS, Boas W van E, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia. (2005) 46:470–2. 10.1111/j.0013-9580.2005.66104.x [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. (1999) 40:120–2. 10.1111/j.1528-1157.1999.tb02000.x [DOI] [PubMed] [Google Scholar]
- 29.Setters B, Solberg LM. Delirium. Prim Care. (2017) 44:541–59. 10.1016/j.pop.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 30.Walling AD, Dickson G. Guillain-Barré syndrome. Am Fam Physician. (2013) 87:191–7. [PubMed] [Google Scholar]
- 31.Gutmann L, Gutmann L. Critical illness neuropathy and myopathy. Arch Neurol. (1999) 56:527. 10.1001/archneur.56.5.527 [DOI] [PubMed] [Google Scholar]
- 32.Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J Med Virol. (2020) 92:1793–4. 10.1002/jmv.25903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. (2017) 21:90. 10.1186/s13054-017-1670-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robba C, Battaglini D, Pelosi P, Rocco RMP. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. (2020) 14:865–8. 10.1080/17476348.2020.1778470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y, Bai X, Zhu T, Huang J, Zhang H. What can the neurological manifestations of COVID-19 tell us: a meta-analysis. J Transl Med. (2021) 19:363. 10.1186/s12967-021-03039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidbauer ML, Ferse C, Salih F, Klingner C, Musleh R, Kunst S, et al. COVID-19 and intracranial hemorrhage: a multicenter case series, systematic review and pooled analysis. J Clin Med. (2022) 11:605. 10.3390/jcm11030605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19. Neurol Neuroimmunol Neuroinflammation. (2021) 8:e1080. 10.1212/NXI.0000000000001080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severo Bem Junior L, do Rego Aquino PL, Nunes Rabelo N, do Rego Aquino MA, Veiga Silva AC, Ferreira Valenca Mota R de C, et al. SARS-CoV-2 and nervous system - neurological manifestations in patients with COVID-19: a systematic review. J Neurol Res. (2020) 10:113–21. 10.14740/jnr602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hariyanto TI, Putri C, Hananto JE, Arisa J, Fransisca V Situmeang R, Kurniawan A. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. J Psychiatr Res. (2021) 142:361–8. 10.1016/j.jpsychires.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flores-Silva FD, García-Grimshaw M, Valdés-Ferrer SI, Vigueras-Hernández AP, Domínguez-Moreno R, Tristán-Samaniego DP, et al. Neurologic manifestations in hospitalized patients with COVID-19 in Mexico City. PLoS ONE. (2021) 16:e0247433. 10.1371/journal.pone.0247433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahammedi A, Ramos A, Bargalló N, Gaskill M, Kapur S, Saba L, et al. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. Am J Neuroradiol. (2021) 42:1008–16. 10.3174/ajnr.A7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salahuddin H, Afreen E, Sheikh IS, Lateef S, Dawod G, Daboul J, et al. Neurological predictors of clinical outcomes in hospitalized patients with COVID-19. Front Neurol. (2020) 11:585944. 10.3389/fneur.2020.585944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaumont H, Meppiel E, Roze E, Tressières B, de Broucker T, Lannuzel A. Long-term outcomes after NeuroCOVID: a 6-month follow-up study on 60 patients. Rev Neurol. (2022) 178:137–43. 10.1016/j.neurol.2021.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rass V, Beer R, Schiefecker AJ, Kofler M, Lindner A, Mahlknecht P, et al. Neurological outcome and quality of life 3 months after COVID-19: a prospective observational cohort study. Eur J Neurol. (2021) 28:3348–59. 10.1111/ene.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rass V, Beer R, Schiefecker AJ, Lindner A, Kofler M, Ianosi BA, et al. Neurological outcomes 1 year after COVID-19 diagnosis: a prospective longitudinal cohort study. Eur J Neurol. (2022) 29:1685–96. 10.1111/ene.15307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. (2021) 9:239–50. 10.1016/S2213-2600(20)30552-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. (2021) 8:416–27. 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross Russell AL, Hardwick M, Jeyanantham A, White LM, Deb S, Burnside G, et al. Spectrum, risk factors and outcomes of neurological and psychiatric complications of COVID-19: a UK-wide cross-sectional surveillance study. Brain Commun. (2021) 3:fcab168. 10.2139/ssrn.3767901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.