Abstract
BRAF plus MEK inhibitor combinations are currently FDA-approved for melanoma, non–small cell lung cancer, and anaplastic thyroid cancer. The lack of clinical benefit with BRAF inhibition in BRAF V600–mutated colorectal cancer has prevented its tissue-agnostic drug development. We reviewed the AACR GENIE database for the prevalence of BRAF V600 mutations across tumor types. We reviewed the literature for case reports of clinical responses, outcomes in patients with BRAF V600 mutation—positive nonmelanoma malignancies who received BRAF inhibitor therapy, and data from published adult and pediatric trials. BRAF V600 mutations are prevalent across multiple nonmelanoma malignancies (>40 different tumor types), lead to oncogene addiction, and are clinically actionable in a broad range of adult and pediatric nonmelanoma rare malignancies. Continued tissue-agnostic drug development is warranted beyond the current BRAF plus MEK approved cancers.
Introduction
The MAPK pathway was first implicated in the pathogenesis of melanoma, where mutations in the BRAF gene, specifically the V600E site, lead to constitutively active kinase leading to downstream cancer cell proliferation (1, 2). After discovery of the mutant BRAF V600E, efforts to inhibit this kinase were focused on developing drugs to block the active form and induce cell death of cells with overactivation of BRAF. Although tumors harboring BRAF V600 alterations respond to BRAF inhibitors, acquired resistance develops quickly. In order to avoid and/or delay resistance, a combination strategy of MEK inhibition plus BRAF inhibition was evaluated and showed synergistic benefit (3).
Beyond melanoma, mutations in the BRAF gene have also been implicated in hairy cell leukemia, colon cancer, non–small cell lung cancer (NSCLC), anaplastic thyroid cancer, and ovarian cancer among others (4). The first of the drugs to show clinical activity inhibition BRAF was vemurafenib (5) followed by dabrafenib and later by encorafenib (6). Vemurafenib, dabrafenib, and encorafenib serve as potent inhibitors of the active mutant BRAF V600E kinase. Further, inhibition of the MEK kinase, a member of the MAP kinase pathway, has also been shown to improve outcomes for patients with advanced melanoma (7–9). The success of this MEK kinase inhibition strategy has been shown in other malignancies as well (10, 11). Although BRAF plus MEK inhibitor combinations have shown responses in multiple tumors, the reason that BRAF plus MEK inhibitors were not viewed or pursued as tissue-agnostic drugs like NTRK inhibitors for NTRK fusion positive tumors is that in colon cancer there was a lack of benefit derived from single-agent use of vemurafenib (12). This unresponsiveness in colorectal cancer stalled tissue-agnostic drug development and hence tumor specific drug development pathways were pursued and approval was sought in a tumor-specific manner. However, it is important to note that the addition of an EGFR inhibition to BRAF and MEK inhibitors for colon cancer provided a significantly longer survival for patients with BRAF V600E–mutated colon cancer (13). This example shows that perhaps in some circumstances, BRAF plus MEK inhibitors may need supplemental inhibition to block an additional driver, EGFR, to overcome innate drug resistance and failure (13, 14).
Currently, the FDA approvals for BRAF plus MEK inhibitors include vemurafenib plus cobimetinib and encorafenib plus binimetinib for melanoma, dabrafenib plus trametinib for melanoma, non–small cell lung cancer, and anaplastic thyroid cancer, and vemurafenib for Erdheim-Chester disease. In addition, encorafenib plus cetuximab is FDA-approved for metastatic colorectal cancer with a BRAF V600E mutation alteration. Because mutations in the BRAF V600E are found across a breadth of tumor histologies that include a wide variety of rare and orphan cancers, perhaps the drugs that inhibit BRAF V600E and MEK should be considered as tissue-agnostic targeted drugs and pursued further for drug development (with the exception of colorectal cancer where additional EGFR inhibition is needed). Results from the vemurafenib-basket study (15) and the NCI-match trial (16) reveal that BRAF pathway inhibition is active in more than 20 unique cancer types. In this article, we review the evidence from available literature, real-world data from published case studies and clinical trials on the role of BRAF plus MEK inhibition in multiple BRAF V600–positive adult and pediatric malignancies beyond melanoma.
Prevalence of BRAF V600 alterations
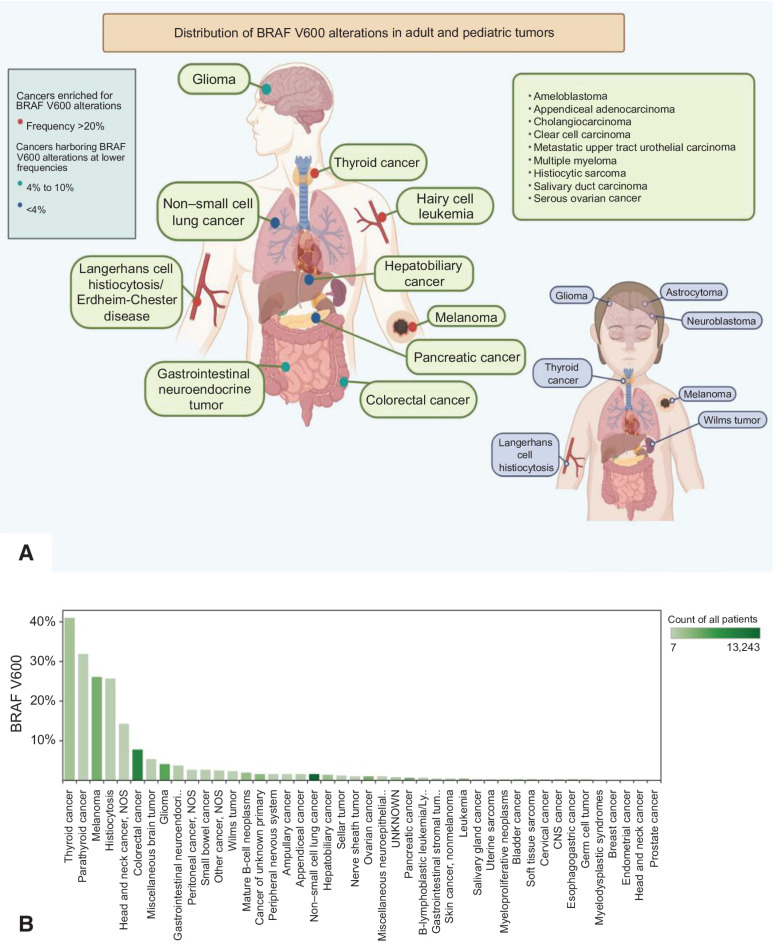
We queried the AACR GENIE database to assess the prevalence of BRAF V600E mutations across various tumor types. Among the 96,324 samples queried from the AACR GENIE database, the BRAF V600E mutation was reported in 43 different tumor types across 2,963 samples (3.07%; Fig. 1A and B). BRAF V600E was most commonly present in thyroid cancer (40.9%), parathyroid cancer (31.8%), melanoma (26.1%), Langerhans cell histiocytosis (25.7%), and head and neck cancer (14.3%). These results highlight the prevalence of BRAF V600E across various tumor types and unveil the possible opportunities for targeted therapy.
Figure 1.
A, Distribution of BRAF V600 mutations in adult and pediatric tumors. B, Frequency of BRAF V600 mutations by tumor histology. Figure panel A is a cartoon schematic with examples of various BRAF-mutated nonmelanoma cancers and the distribution in adult and pediatric tumors. Figure panel B shows the frequency of BRAF V600 mutations in 43 different tumor types across 2,963 samples in the AACR GENIE database.
Real world evidence of tissue-agnostic efficacy of BRAF inhibitors
We conducted a literature search using NCBI PubMed from 2012–2019 for case reports, series, and clinical trials using the search terms: “dabrafenib,” “trametinib,” “vemurafenib,” “encorafenib,” “binimetinib,” and “cobimetinib.” Patients with BRAF V600 mutation—positive nonmelanoma cancers were included in the analysis. The information of interest [e.g., age, previous treatments, progression-free survival (PFS), and overall survival (OS)] was manually extracted from the text and supplementary material in the manuscripts. This data was used to calculate median PFS and OS across a diverse set of nonmelanoma cancers.
The review of literature revealed 178 cases across 69 tumor types that were identified and categorized in accordance with the NIH Cancer Classification (Table 1A). The most common cases identified with the BRAF V600E mutation included Erdheim-Chester disease (n = 30, 16.9%), papillary thyroid carcinoma (n = 16, 8.9%), anaplastic thyroid carcinoma (n = 13, 7.3%), and hairy cell leukemia (n = 13, 7.3%). The mean of the patients’ ages was 43.9 years old, with a range from 5 weeks to 90 years old. Most patients were between 54 to 72 years of age (n = 35 patients or 20.4%) closely followed by patients between 1 to 18 years of age (n = 32 patients or 18.6%) being the next most common. Regarding therapy, dabrafenib (BRAF), dabrafenib plus trametinib (BRAF plus MEK), trametinib (MEK), vemurafenib (BRAF), vemurafenib plus trametinib (BRAF plus MEK), vemurafenib plus cobimetinib (BRAF plus MEK) were used in 34% (n = 56), 16% (n = 27), 4% (n = 6), 44% (n = 72), 1% (n = 1), 2% (n = 3) of cases, respectively (Table 1B).
Table 1A.
List of unique malignancies harboring BRAF V600E alteration with activity on BRAF plus or minus MEK inhibitors reported in case studies.
| Adult Wilms tumor | Lung adenocarcinoma |
| Ameloblastoma | Malignant peripheral nerve sheath tumor |
| Anaplastic astrocytoma | Malignant pleural mesothelioma |
| Anaplastic ganglioma | Metastatic ameloblastoma |
| Anaplastic pleomorphic xanthoastrocytoma | Metastatic colorectal carcinoma |
| Anaplastic thyroid carcinoma | Metastatic papillary thyroid carcinoma |
| Appendiceal adenocarcinoma | Metastatic upper tract urothelial carcinoma |
| Brainstem ganglioglioma | Multiple myeloma |
| Cholangiocarcinoma | Mutated ganglioma |
| Clear cell carcinoma | Mutated high-grade glioma |
| Colorectal adenocarcinoma | Non–small cell lung adenocarcinoma |
| Dendritic cell sarcoma | Neurofibromatosis type 1-associated glioblastoma |
| Desmoplastic infantile astrocytoma | Ovarian carcinoma |
| Encephalocraniocutaneous lipomatosis | Papillary craniopharyngioma |
| Epithelioid glioblastoma | Papillary thyroid carcinoma |
| Erdheim-Chester disease | Pediatric invasive gliofibroma |
| Ganglioma | Peduncular anaplastic ganglioma |
| Ganglioneurocytoma | Pilocytic astrocytoma |
| Gastrointestinal stromal tumor | Pilomyxoid astrocytoma |
| Glioblastoma | Pleomorphic xanthoastrocytoma |
| Glioblastoma without epithelioid cells | Pulmonary Langerhans cell histiocytosis |
| Gnathic ameloblastoma | Pilocytic astrocytoma |
| Hairy cell leukemia | Renal cell carcinoma |
| High-grade glioma | Right colon adenocarcinoma |
| Histiocytic sarcoma | Salivary duct carcinoma |
| Infiltrative pleomorphic glioma | Serous ovarian cancer |
| Langerhans cell histiocytosis | Spinal ganglioma |
| Low-grade serous ovarian adenocarcinoma | Urothelial carcinoma |
Table 1B.
Review of literature of individual case reports of BRAF V600 tumors treated with BRAF plus or minus MEK inhibitor. Table shows percentage of cases using each drug(s).
| Dabrafenib (BRAF) | 34% (n = 56) |
| Dabrafenib plus trametinib (BRAF plus MEK) | 16% (n = 27) |
| Trametinib (MEK) | 4% (n = 6) |
| Vemurafenib (BRAF) | 44% (n = 72) |
| Vemurafenib plus trametinib (BRAF plus MEK) | 1% (n = 1) |
| Vemurafenib plus cobimetinib (BRAF plus MEK) | 2% (n = 3) |
For the patients with BRAF V600E–mutated Erdheim-Chester disease the median PFS was 3.0 months, median OS was 33.0 months, and median duration of response (DOR) was 9.0 months, which included treatment with dabrafenib or vemurafenib (17–26). For the patients with BRAF V600E–mutated thyroid cancer, the median PFS was 11.3 months, median OS was 14.0 months, and median DOR was 9.3 months, which included patients treated with dabrafenib, dabrafenib plus trametinib, and vemurafenib (27–34). Overall, across a cohort of tumor histologies, median PFS was 6.5 months, and median OS was 28.5 months with a median DOR of 8.0 months (Supplementary Fig. S1).
Activity of BRAF inhibition in BRAF-positive nonmelanoma malignancies from published studies
We reviewed 16 adult studies and 6 pediatric studies conducted in nonmelanoma malignancies. Among the adult studies, responses were reported in multiple malignancies harboring a BRAF V600 alteration like hairy cell leukemia, anaplastic and papillary thyroid cancer, non–small cell lung cancer, multiple myeloma, biliary tract cancer, pancreatic cancer, and Langerhans cell histiocytosis (Table 2; refs. 10, 11, 15, 16, 35–46). Dabrafenib in combination with trametinib or vemurafenib was most commonly studied. In all studies, which compared single-agent drugs to combination therapy, the objective response rate (ORR) in combination treatment was superior. The most dramatic responses of these studied treatments were seen in hairy cell leukemia with ORR of 87% in vemurafenib plus rituximab and 78% in dabrafenib plus trametinib (44, 46). The ORR was 100% in vemurafenib alone in the U.S. study and 96% to 100% in dabrafenib alone (42, 43). Notable responses were also observed in thyroid cancer. In one of the most aggressive forms of thyroid cancer, anaplastic thyroid cancer the ORR was 69% with dabrafenib plus trametinib in an interim analysis of the ROAR study (10). Recently, definitely benefit of this combination was confirmed in an updated analysis that included the full enrollment of 36 patients and more than 4 years of additional study follow-up (47). ORR was 56%, with 50% of responders still in response at 12 months (47). In papillary thyroid cancers, ORRs were 35% in dabrafenib plus trametinib and 38.5% in vemurafenib alone (39, 40). Given these positive results, BRAF-targeted therapies may prove to be effective in various cancer types. In the vemurafenib basket study of 172 patients with 26 unique cancer types, an overall response rate of 33% was reported, and responses were observed in 13 unique cancer types (15). Interestingly, the NCI-Match study studying the combination of dabrafenib and trametinib across diverse tumor types also showed an ORR of 38% and responses in 7 distinct tumor types (16).
Table 2.
Previously published studies in adult patients with nonmelanoma BRAF-altered cancers.
| Drug | Tumor type | Number of patients | ORR or overall response rate | Other comments | Reference |
|---|---|---|---|---|---|
| Dabrafenib plus trametinib | BRAF V600E–mutant biliary tract cancer | 43 | ORR = 51% | 626 patients with biliary tract cancer were locally prescreened for the BRAFV600E mutation. On the basis of local BRAF testing, 57 patients with BRAFV600E–mutated biliary tract cancer were identified, of whom 43 were enrolled Included in NCCN guidelines | (35) |
| Vemurafenib | BRAF V600–mutant nonmelanoma malignancies | 172 | 33% | In total, 172 patients with 26 unique cancer types were treated, achieving an overall response rate of 33% and median DOR of 13 months. Responses were observed in 13 unique cancer types, including historically treatment-refractory tumor types such as cholangiocarcinoma, sarcoma, glioma, neuroendocrine carcinoma, and salivary gland carcinomas. | (15) |
| Dabrafenib plus trametinib | BRAF V600E–mutant solid tumors, lymphomas, or multiple myeloma | 29 | 38% | The median overall survival was 28.6 months. | (16) |
| Dabrafenib plus trametinib | BRAF V600E–mutant metastatic NSCLC | 93 | 68.4% | (36) | |
| Dabrafenib plus trametinib | BRAF V600E–mutant metastatic NSCLC | 36 | 64% | (11) | |
| Dabrafenib plus trametinib | BRAF V600E–mutant metastatic NSCLC | 59 | 63.2% | (37) | |
| Dabrafenib | BRAF V600E–mutant advanced non–small cell lung cancer | 84 | 33% | (38) | |
| Vemurafenib | BRAF V600E–mutant metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine | 51 | 38.5% | (39) | |
| Dabrafenib plus trametinib | Locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer | 16 (Interim data) 36 (Final data) | 69% (Interim data) 56% (Final data) | First FDA-approved therapy of anaplastic thyroid cancer. This updated analysis confirms the definitive benefit of dabrafenib plus trametinib in anaplastic thyroid cancer with long-term follow-up | (10, 47) |
| Dabrafenib plus trametinib | BRAF V600–mutant papillary thyroid carcinoma | 53 | 35% | (40) | |
| BRAF mutations in hairy cell leukemia | This study was only identifying BRAF mutations in HCL | (41) | |||
| Vemurafenib | Hairy cell leukemia that had relapsed after treatment with a purine analogue or who had disease that was refractory to purine analogues | 26 Italian study 24 U.S. study | 96% Italian study 100% U.S. study | (42) | |
| Dabrafenib | Relapsed or refractory hairy cell leukemia | 10 | 96%–100% | (43) | |
| Vemurafenib plus rituximab | Refractory or relapsed hairy cell leukemia | 30 | 87% | (44) | |
| Dabrafenib plus trametinib | BRAF V600E–mutant HGG and LGG | 45 | 33% in HGG and 69% in LGG | Included in NCCN guidelines | (45, 61) |
| Dabrafenib plus trametinib | Recurrent/refractory BRAF V600E–mutated hairy cell leukemia | 43 | 78% | (46) |
Abbreviations: HGG, high-grade glioma; LGG, low-grade glioma.
There is also evidence that BRAF V600 mutations can be targeted successfully in pediatric patients (Table 3; refs. 48–53). Among the pediatric studies, the breadth of current literature is in pediatric neuro-oncology. Pediatric gliomas are the primary tumor type studied. In three studies, favorable response were seen with ORRs of 80%, 44%, and 100% (49, 50, 52).
Table 3.
Previously published studies in pediatric patients with nonmelanoma BRAF-altered cancers.
| Drug | Tumor type | Number of patients | ORR or overall response rate | Other comments | Reference |
|---|---|---|---|---|---|
| Dabrafenib plus trametinib | BRAF V600E high-grade gliomas | 3 | N/A | Patient 1 remained disease free for 20 months at which time he presented with disseminated disease recurrence and died 2 months later. Patient 2 has remained on therapy with a small amount of stable disease for 32 months. Patient 3 remained on therapy with stable disease for 23 months. | (48) |
| Dabrafenib or vemurafenib | BRAF V600E pediatric gliomas | 67 | 80% | Poor prognostic factors in conventional therapies, such as concomitant homozygous deletion of CDKN2A, were not associated with lack of response to BRAF inhibition. | (49) |
| Dabrafenib | BRAF V600E pediatric low-grade glioma | 32 | 44% | (50) | |
| Dabrafenib plus trametinib | BRAF V600E Wilms tumor | 1 | N/A | The patient remains in a complete radiographic response 12 months after starting therapy and continues to receive dabrafenib and trametinib with minimal treatment-emergent toxicities. | (51) |
| Vemurafenib | Recurrent or progressive BRAF V600E mutant brain tumors | 19 | 32% | (52) | |
| Dabrafenib | BRAF V600 mutation—positive tumors | 27 | Not reported | In this first clinical trial in pediatric patients with pretreated BRAF V600–mutant tumors, dabrafenib was well tolerated while achieving target exposure levels; the average treatment duration was >1 year with many patients still on treatment. | (53) |
Totality of evidence of BRAF inhibition in adult and pediatric pan-cancers
BRAF V600 mutations are prevalent across a breadth of tumor histologies, and there are multiple nonmelanoma FDA approvals of drugs that inhibit the BRAF/MEK pathway (Table 4). A basket study of nonmelanoma BRAF V600 alterations included cohorts which derived benefit from vemurafenib (54). Similarly, in the collected case studies, thyroid cancers were one of the most abundant types of cancer, and these patients derived meaningful benefits from BRAF inhibition. Patients with hairy cell leukemia have BRAF V600 alterations in 100% of cases with a 96% response rate (41, 42). These examples suggest that BRAF V600 may be a tumor-agnostic biomarker. Critics are quick to cite findings of a study which used single-agent vemurafenib to treat BRAF V600–mutated colorectal cancer with ORR of ∼5% and PFS of 2.1 months (12). However, it may be that some cancer types, such as colorectal cancer may require additional inhibition of co-occurring alterations/pathways. By targeting additional pathways, such as EGFR, ORRs increase to 26% and PFS increases to 4.3 months (13). It is important to note that beyond colorectal cancer there is no other cancer that has a tissue-specific innate mechanism of resistance to BRAF inhibition that is prevalent widely.
Table 4.
FDA approvals of BRAF plus MEK inhibitors in nonmelanoma cancers.
| Drug/Combination | Nonmelanoma indications (Date of FDA approval) |
|---|---|
| Dabrafenib plus trametinib | Metastatic NSCLC with BRAF V600E mutation (6/22/2017) |
| Locally advanced or metastatic anaplastic thyroid cancer with BRAF V600E mutation and with no satisfactory locoregional treatment options (5/4/2018) | |
| Vemurafenib alone | Treatment of patients with Erdheim-Chester disease with BRAF V600 mutation (11/6/2017) |
| Vemurafenib plus cobimetinib | No nonmelanoma indication |
| Encorafenib | In combination with cetuximab for the treatment of adult patients with metastatic colorectal cancer with a BRAF V600E mutation (4/9/2020) |
| Encorafenib plus binimetinib | No nonmelanoma indication |
NSCLC is yet another example of BRAF V600 inhibition leading to improved outcomes with 42% ORR and PFS of 7.3 months (54). Like the findings with a median DOR of 8.0 months for all patients, 43% of patients had a response and the median treatment duration was 5.9 months with no patients progressing on vemurafenib (54). Another basket study also presented a median PFS of 5.8 months and OS of 17.6 months across an array of histologies which appears to be congruent with our findings of median PFS of 6.5 months and median OS of 28.5 months across pan-cancers (15). Brain tumors appear to be the 7th most abundant BRAF V600–mutated cancers, and in those patients vemurafenib has been shown to have an ORR of 25% and median PFS of 5.5 months (55).
To date, there are nine FDA-approved indications for BRAF plus MEK inhibitors in various malignancies including melanoma (Table 4). Single-agent vemurafenib was first approved in 2011 for unresectable or metastatic melanoma with BRAF V600E mutation followed by the combination of dabrafenib plus trametinib for the same indication in 2013, and encorafenib plus binimetinib in 2018. The combination of dabrafenib plus trametinib has also been approved for use in metastatic NSCLC with BRAF V600E mutation, as adjuvant therapy for BRAF V600E- or V600K-melanoma, and locally advanced or metastatic BRAF V600E–mutated anaplastic thyroid cancer. Vemurafenib combined with cobimetinib is approved for both BRAF V600E– or V600K–mutated metastatic melanoma. Vemurafenib as a single agent is approved for patients with BRAF V600–mutated Erdheim-Chester disease. Encorafenib is approved when used in combination with cetuximab for patients with metastatic BRAF V600E–mutated colorectal cancer.
Conclusion
The current and growing number of indications for BRAF plus MEK inhibitors in various malignancies along with the presented case studies serve as compelling evidence that BRAF may be a tumor-agnostic target. Our literature review of cases and multiple studies are congruent with the reported nonmelanoma basket studies and show that there may be a wide variety of malignancies that could benefit from access to these drugs. Because BRAF plus MEK inhibition is standard of care in multiple tumor types, a combination approach should be used for tissue-agnostic studies as well (56–58). Furthermore, an agnostic drug indication will increase patient access to medications and potentially offer an additional line of treatment option to these rare cancer patients. The issue here is access to a potentially lifesaving therapy or a therapy conferring clinical benefit in a rare disease patient harboring a BRAF V600 alteration. If it is not approved for a particular indication, it is quite challenging to access the drug. Approval provides more efficient access to patients. The increased use of next-generation sequencing in the treatment arsenal of cancer enables potential target identification and easier approval of clinically meaningful drugs (58–60). Comprehensive review of the BRAF V600 landscape reveals the prevalence in multiple rare nonmelanoma malignancies and identifies BRAF V600 as a tissue-agnostic target.
Supplementary Material
Acknowledgments
V. Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. V. Subbiah acknowledges support of The Jacquelyn A. Brady Fund. V. Subbiah is supported by NIH grant R01CA242845. MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (1U01 CA180964), NCATS Grant UL1 TR000371 (Center for Clinical and Translational Sciences), and the MD Anderson Cancer Center Support Grant (P30 CA016672). Figure 1A was created with BioRender.com
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Authors' Disclosures
V. Subbiah reports grants from Novartis, Helsinn Pharmaceuticals during the conduct of the study; grants from Eli Lilly/Loxo Oncology, Blueprint Medicines, Turning Point Therapeutics, Boston Pharmaceuticals. In addition, V. Subbiah reports a grant and advisory board/consultant position with Eli Lilly/Loxo Oncology during the conduct of the study; research grants from Roche/Genentech, Bayer, GlaxoSmithKline, NanoCarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharmaceuticals, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, PharmaMar, Medimmune; an advisory board/consultant position with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay Therapeutics, Roche, Medimmune; travel funds from PharmaMar, Incyte, ASCO, ESMO; other support from Medscape; all outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- 2. da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res 2005;65:10686–91. [DOI] [PubMed] [Google Scholar]
- 3. Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer 2020;6:797–810. [DOI] [PubMed] [Google Scholar]
- 4. Davies MA, Samuels Y. Analysis of the genome to personalize therapy for melanoma. Oncogene 2010;29:5545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010;467:596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 2008;105:3041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–9. [DOI] [PubMed] [Google Scholar]
- 8. Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14. [DOI] [PubMed] [Google Scholar]
- 9. Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88. [DOI] [PubMed] [Google Scholar]
- 10. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600–mutant anaplastic thyroid cancer. J Clin Oncol 2018;36:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non—small cell lung cancer: an open-label, phase II trial. Lancet Oncol 2017;18:1307–16. [DOI] [PubMed] [Google Scholar]
- 12. Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 2015;33:4032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med 2019;381:1632–43. [DOI] [PubMed] [Google Scholar]
- 14. Adashek JJ, Arroyo-Martinez Y, Menta AK, Kurzrock R, Kato S. Therapeutic implications of epidermal growth factor receptor (EGFR) in the treatment of metastatic gastric/GEJ cancer. Front Oncol 2020;10:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Subbiah V, Puzanov I, Blay JY, Chau I, Lockhart AC, Raje NS, et al. Pan-cancer efficacy of vemurafenib in BRAF (V600)-mutant nonmelanoma cancers. Cancer Discov 2020;10:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salama AKS, Li S, Macrae ER, Park JI, Mitchell EP, Zwiebel JA, et al. Dabrafenib and trametinib in patients with tumors with BRAF(V600E) mutations: results of the NCI-MATCH trial subprotocol H. J Clin Oncol 2020:JCO2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhatia A, Ulaner G, Rampal R, Hyman DM, Abdel-Wahab O, Durham BH, et al. Single-agent dabrafenib for BRAF(V600E)-mutated histiocytosis. Haematologica 2018;103:e177-e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verschelden G, Van Laethem J, Velkeniers B, Ilsen B, Noeparast A, De Greve J. Significant response to dabrafenib in a patient with Erdheim-Chester disease with BRAFV600E mutation. Pol Arch Intern Med 2018;128:386–8. [DOI] [PubMed] [Google Scholar]
- 19. Nordmann TM, Juengling FD, Recher M, Berger CT, Kalbermatten D, Wicki A, et al. Trametinib after disease reactivation under dabrafenib in Erdheim-Chester disease with both BRAF and KRAS mutations. Blood 2017;129:879–82. [DOI] [PubMed] [Google Scholar]
- 20. Al Bayati A, Plate T, Al Bayati M, Yan Y, Lavi ES, Rosenblatt JD. Dabrafenib and trametinib treatment for Erdheim-Chester disease with brain stem involvement. Mayo Clin Proc Innov Qual Outcomes 2018;2:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang LC, Topping KL, Gratzinger D, Brown RA, Martin BA, Silva RA, et al. Orbital and chorioretinal manifestations of Erdheim-Chester disease treated with vemurafenib. Am J Ophthalmol Case Rep 2018;11:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varadi Z, Banusz R, Csomor J, Kallay K, Varga E, Kertesz G, et al. Effective BRAF inhibitor vemurafenib therapy in a 2-year-old patient with sequentially diagnosed Langerhans cell histiocytosis and Erdheim-Chester disease. Onco Targets Ther 2017;10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stempel JM, Bustamante Alvarez JG, Carpio AM, Mittal V, Dourado C. Erdheim-Chester disease, moving away from the orphan diseases: a case report. Respir Med Case Rep 2017;20:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tzoulis C, Schwarzlmuller T, Gjerde IO, Softeland E, Neckelmann G, Biermann M, et al. Excellent response of intramedullary Erdheim-Chester disease to vemurafenib: a case report. BMC Res Notes 2015;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazor RD, Manevich-Mazor M, Kesler A, Aizenstein O, Eshed I, Jaffe R, et al. Clinical considerations and key issues in the management of patients with Erdheim-Chester disease: a seven case series. BMC Med 2014;12:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Toledano D, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol 2015;33:411–8. [DOI] [PubMed] [Google Scholar]
- 27. Lim AM, Taylor GR, Fellowes A, Cameron L, Lee B, Hicks RJ, et al. BRAF inhibition in BRAFV600E-positive anaplastic thyroid carcinoma. J Natl Compr Canc Netw 2016;14:249–54. [DOI] [PubMed] [Google Scholar]
- 28. Falchook GS, Millward M, Hong D, Naing A, Piha-Paul S, Waguespack SG, et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid 2015;25:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF(V600E)-mutated anaplastic thyroid carcinoma. Thyroid. 2019;29:1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fazeli S, Paal E, Maxwell JH, Burman KD, Nylen ES, Khosla SG. Salutary response to targeted therapy in anaplastic thyroid cancer. J Investig Med High Impact Case Rep 2019;7:2324709619890942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prager GW, Koperek O, Mayerhoefer ME, Muellauer L, Wrba F, Niederle B, et al. Sustained response to vemurafenib in a BRAF(V600E)-mutated anaplastic thyroid carcinoma patient. Thyroid. 2016;26:1515–6. [DOI] [PubMed] [Google Scholar]
- 32. Marten KA, Gudena VK. Use of vemurafenib in anaplastic thyroid carcinoma: a case report. Cancer Biol Ther 2015;16:1430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ali SM, He J, Carson W, Stephens PJ, Fiorillo J, Lipson D, et al. Extended antitumor response of a BRAF V600E papillary thyroid carcinoma to vemurafenib. Case Rep Oncol 2014;7:343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med 2013;368:684–5. [DOI] [PubMed] [Google Scholar]
- 35. Subbiah V, Lassen U, Elez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase II, open-label, single-arm, multicenter basket trial. Lancet Oncol 2020;21:1234–43. [DOI] [PubMed] [Google Scholar]
- 36. Planchard D, Besse B, Groen HJM, Hashemi SMS, Mazieres J, Kim TM, et al. Phase II study of dabrafenib plus trametinib in patients With BRAF V600E–mutant metastatic NSCLC: updated 5-year survival rates and genomic analysis. J Thorac Oncol 2022;17:103–15. [DOI] [PubMed] [Google Scholar]
- 37. Planchard D, Besse B, Groen HJM, Souquet PJ, Quoix E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non–small cell lung cancer: an open-label, multicenter phase II trial. Lancet Oncol 2016;17:984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non–small cell lung cancer: a single-arm, multicenter, open-label, phase II trial. Lancet Oncol 2016;17:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomized, multicenter, open-label, phase II trial. Lancet Oncol 2016;17:1272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah MH, Wei L, Wirth LJ, Daniels GA, De Souza JA, Timmers CD, et al. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J Clin Oncol 2017;35:6022-. [Google Scholar]
- 41. Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy cell leukemia. N Engl J Med 2011;364:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tiacci E, Park JH, De Carolis L, Chung SS, Broccoli A, Scott S, et al. Targeting mutant BRAF in relapsed or refractory hairy cell leukemia. N Engl J Med 2015;373:1733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tiacci E, De Carolis L, Simonetti E, Merluzzi M, Bennati A, Perriello VM, et al. Safety and efficacy of the BRAF inhibitor dabrafenib in relapsed or refractory hairy cell leukemia: a pilot phase II clinical trial. Leukemia 2021;35:3314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tiacci E, De Carolis L, Simonetti E, Capponi M, Ambrosetti A, Lucia E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy cell leukemia. N Engl J Med 2021;384:1810–23. [DOI] [PubMed] [Google Scholar]
- 45. Subbiah V, Stein A, van den Bent M, Wick A, de Vos FY, von Bubnoff N, et al. Abstract CT025: Dabrafenib plus trametinib in BRAF V600E-mutant high-grade (HGG) and low-grade glioma (LGG). Cancer Res 2021;81:CT025. [Google Scholar]
- 46. Kreitman RJ, Moreau P, Hutchings M, Gazzah A, Blay J-Y, Wainberg ZA, et al. Treatment with combination of dabrafenib and trametinib in patients with recurrent/refractory BRAF V600E-- mutated hairy cell leukemia (HCL). Blood 2018;132:391-. [Google Scholar]
- 47. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: updated analysis from the phase II ROAR basket study. Ann Oncol 2022;33:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toll SA, Tran HN, Cotter J, Judkins AR, Tamrazi B, Biegel JA, et al. Sustained response of three pediatric BRAF(V600E) mutated high-grade gliomas to combined BRAF and MEK inhibitor therapy. Oncotarget 2019;10:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nobre L, Zapotocky M, Ramaswamy V, Ryall S, Bennett J, Alderete D, et al. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol 2020;4:PO.19.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation– -positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res 2019;25:7303–11. [DOI] [PubMed] [Google Scholar]
- 51. Obasaju P, Shahab S, Dunn E, Rhee DS, Jiang L, Dome JS, et al. BRAF V600E-- mutated metastatic pediatric Wilms tumor with complete response to targeted RAF/MEK inhibition. Cold Spring Harb Mol Case Stud 2020;6:a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nicolaides T, Nazemi KJ, Crawford J, Kilburn L, Minturn J, Gajjar A, et al. Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: pacific pediatric neuro-oncology consortium study (PNOC-002). Oncotarget 2020;11:1942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kieran MW, Geoerger B, Dunkel IJ, Broniscer A, Hargrave D, Hingorani P, et al. A phase I and pharmacokinetic study of oral dabrafenib in children and adolescent patients with recurrent or refractory BRAF V600 mutation-– positive solid tumors. Clin Cancer Res 2019;25:7294–302. [DOI] [PubMed] [Google Scholar]
- 54. Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol 2018;36:3477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kato S, Adashek JJ, Shaya J, Okamura R, Jimenez RE, Lee S, et al. Concomitant MEK and cyclin gene alterations: implications for response to targeted therapeutics. Clin Cancer Res 2021;27:2792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kato S, Okamura R, Adashek JJ, Khalid N, Lee S, Nguyen V, et al. Targeting G1/S phase cell-cycle genomic alterations and accompanying co-alterations with individualized CDK4/6 inhibitor-based regimens. JCI Insight 2021;6:e142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kato S, Kim KH, Lim HJ, Boichard A, Nikanjam M, Weihe E, et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun 2020;11:4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Adashek JJ, Subbiah V, Kurzrock R. From tissue-agnostic to N-of-one therapies: (R)evolution of the precision paradigm. Trends Cancer 2021;7:15–28. [DOI] [PubMed] [Google Scholar]
- 60. Adashek JJ, Kato S, Parulkar R, Szeto CW, Sanborn JZ, Vaske CJ, et al. Transcriptomic silencing as a potential mechanism of treatment resistance. JCI Insight 2020;5:e134824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicenter, open-label, single-arm, phase II, basket trial. Lancet Oncol 2022;23:53–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.