Abstract
Background and Objectives:
Robotic radical cystectomy (RARC) with intracorporeal urinary diversion is a technically complicated, time‐consuming procedure. The aim of this study was to present the operative, pathological, oncological, and functional outcomes of patients who underwent endopelvic fascia sparing (EPFS) RARC with intracorporeal Studer pouch formation. To the best of our knowledge, this is first series in the literature that includes EPFS RARC.
Methods:
Between October 1, 2019 and April 30, 2022, 10 bladder cancer patients underwent EPFS RARC, bilateral extended pelvic lymph node dissection with intracorporeal Studer pouch reconstruction with Balbay’s technique. Patient demographics, operative, and post-operative parameters were recorded.
Results:
Among 10 patients, 8 were male and 2 were female. Mean operative time, median estimated blood loss, and median duration of hospital stay was 530 minutes, 316 ml, and 8 days, respectively. One month postoperatively, the mean maximum flow, average flow rate, mean voided, and post-voided urine volume were 20.2 ml/sec, 4.4 ml/sec, 273.6 ml, and 3.5 ml, respectively. All of the patients were fully continent during day‐time, three had mild night-time incontinence requiring pad use (both patients 1 pad per night). During a mean 11.5 months of follow up, zero patients died. One patient with a pathological, stage 4 tumor, had nodal recurrence at six months postoperatively. No distant metastasis were detected.
Conclusion:
Endopelvic fascia sparing RARC has very promising early functional results with safe oncological outcomes and low complication rates.
Keywords: Cystectomy, Robotics, Urinary Bladder Neoplasms, Urinary Incontinence
INTRODUCTION
Radical cystectomy is the gold standard treatment modality for localized muscle invasive bladder cancer.1 Robot-assisted radical cystectomy (RARC) with intracorporeal urinary diversion is a technically complicated and time‐consuming procedure.
The introduction of robotic technology for pelvic surgeries especially in radical prostatectomy provided a different point of view for the options of performing complicated operations in the pelvis. The first reports of RARC were published by Menon et al.2 and Beecken et al.3 For more than 15 years, this procedure has been increasingly performed. Recently, we published our preliminary outcomes of RARC and intracorporeal Studer pouch formation with Balbay’s technique as a feasible minimally invasive procedure with promising operative, postoperative, oncological, and functional outcomes.4
The increasing popularity of robotic technology in urology has resulted better understanding of the urinary continence mechanisms. Anterior structure preservation was described for open and robot-assisted radical prostatectomy (RARP).5–7 Wagaskar et al. defined the Hood technique in robotic radical prostatectomy (RARP), resulting in early returning continence after surgery without compromising positive surgical margins.8
The aim of this study was to present the operative, pathological, oncological, and functional outcomes of the patients who underwent endopelvic fascia sparing (EPFS) RARC with intracorporeal Studer pouch formation with Balbay’s technique. To the best of our knowledge, this is the first series in the literature that includes EPFS RARC cases.
MATERIALS AND METHODS
Between October 1, 2019 and April 30, 2022, a total of 10 bladder cancer patients underwent EPFS RARC, bilateral extended pelvic lymph node dissection (BEPLND) with intracorporeal Studer pouch reconstruction with Balbay’s technique. All procedures were performed by an experienced, high-volume RARC robotic surgeon (MDB). Patient demographics, operative, and postoperative parameters are presented in Table 1. Subcutaneous 40 mg enoxaparin injection was administered on the first postoperative day as an anticoagulant pharmacological prophylaxis in all patients. The duration of pharmacological prophylaxis was four weeks postsurgery. Additionally, mechanical prophylaxis was performed until ambulation.
Table 1.
Demographics, Operative, and Postoperative Data of the Patients
| Demographics | |
|---|---|
| Number of patients | 10 |
| Gender: Male/Female ratio | 8/2 |
| Mean patient age (± SD) (range) (years) | 58.2 (± 3.1) (39–64) |
| Mean Body Mass Index (± SD) (range) (kg/m2) | 30.7 (± 2.1) (23–41) |
| Patients with previous intravesical BCG therapy: n (%) | 1 (10) |
| Patients with previous intravesical chemotherapy: n (%) | 0 |
| Precystectomy pathology: | |
| pT1 | 5 |
| pT2 | 5 |
| ASA Score: n (%) | |
| I | 1 |
| II | 6 |
| III | 3 |
| Previous pelvic radiation history: n (%) | 0 |
| Smoking history: | |
| ≥10 pack/year: n (%) | 4 |
| None: n (%) | 6 |
| Neoadjuvant chemotherapy: n (%) | 1 (10) |
| Mean operative time (± SD) (range) (minutes) | 530 (± 26.2) (400–650) |
| Median estimated blood loss (± SD) (range) (ml) | 316 (± 136.3) (50–1400) |
| Mean time to liquid diet (range) (days) | 1.5 (1–2) |
| Mean time to regular diet (± SD) (range) (days) | 4.1 (± 0.2) (2–7) |
| Median length of hospital stay (range) (days) | 8 (6–20) |
Abbreviations: BCC, Bacille Calmette-Guerin; SD, standard deviation; ASA, American Society of Anesthesiologists.
There is no special exclusion criteria. We performed this technique on every patient. However, previous radiotherapy or prostate surgery may prevent this dissection to be done properly. Such patients can be excluded.
Complications identified during the 0–30 day and the 31–90 postoperative period were recorded and classified according to modified Clavien‐Dindo system.
SURGICAL TECHNIQUE
With our new technique, periprostatic and periurethral endopelvic fascia (EPF) below bladder base was untouched, thus preserving puboprostatic ligaments and minimizing perisphincteric dissection.
Step By Step Technique of Endopelvic Fascia Sparing Robotic Radical Cystectomy in the Male (Figures 1–13).
Figure 1.
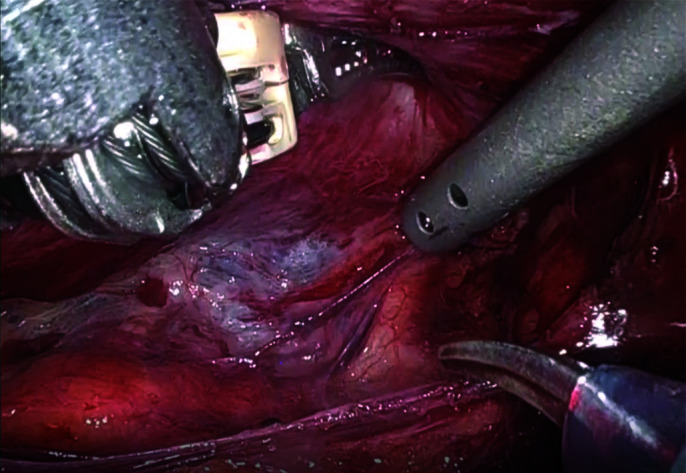
Neurovascular bundles are dissected bluntly posterolaterally.
Figure 13.
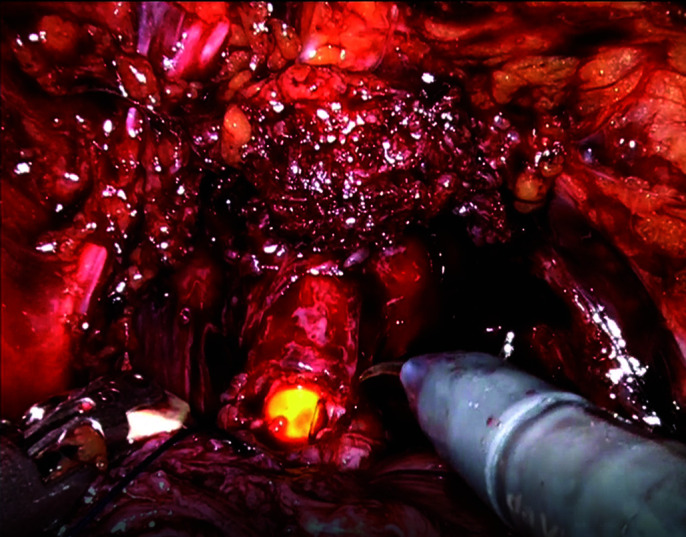
Urethra is suture-tied and cut.
Figure 2.
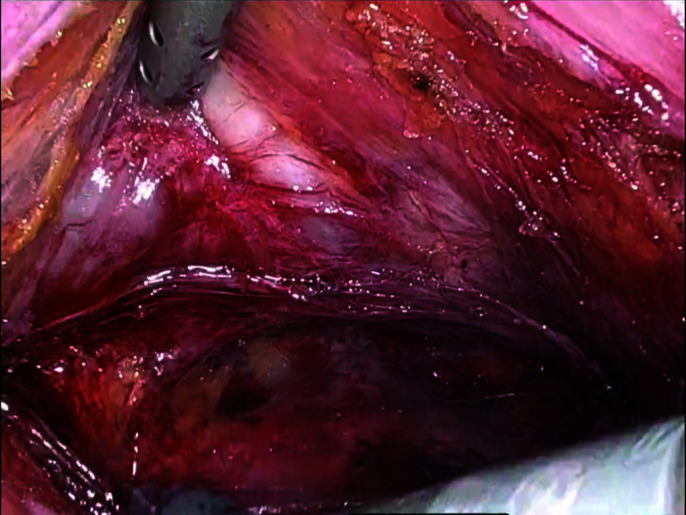
Posterior dissection completed.
Figure 3.
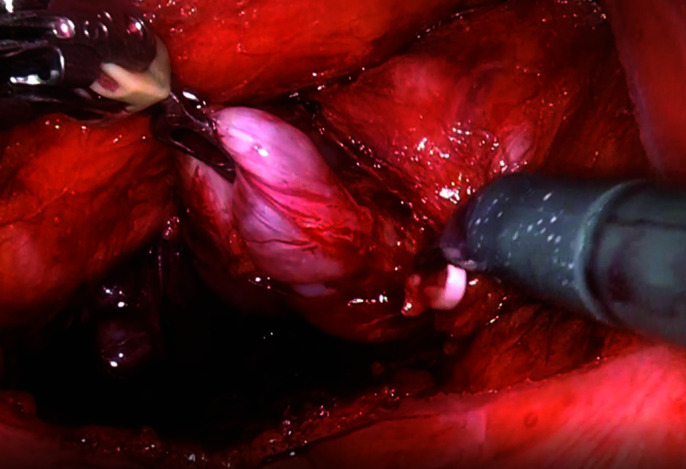
Vascular supply to the seminal vesical at its tip are clipped and marked with a Hem-o-lock® clip. This point roughly corresponds to the third sacral vertebral foramen.
Figure 4.
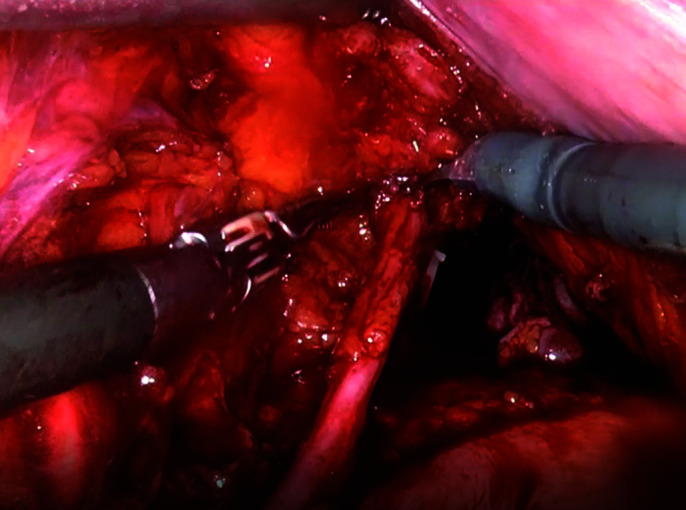
Identification and dissection of ureters. At this stage of the operation ureters are not blocked, they continue to drain the kidneys.
Figure 5.
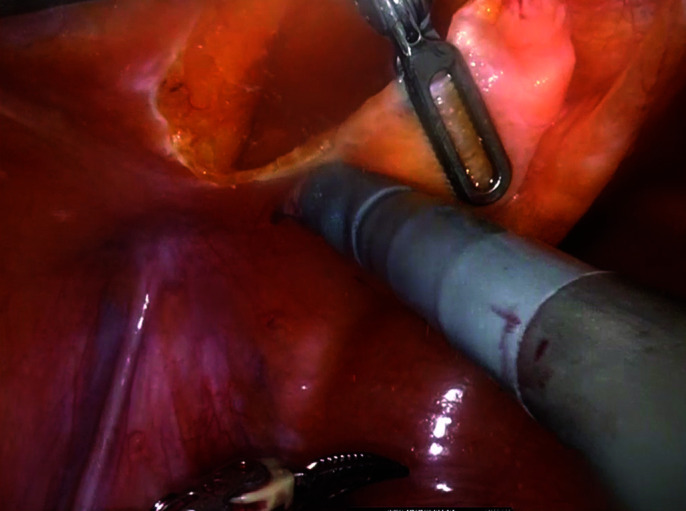
Anterior dissection (keeping urachus intact).
Figure 6.
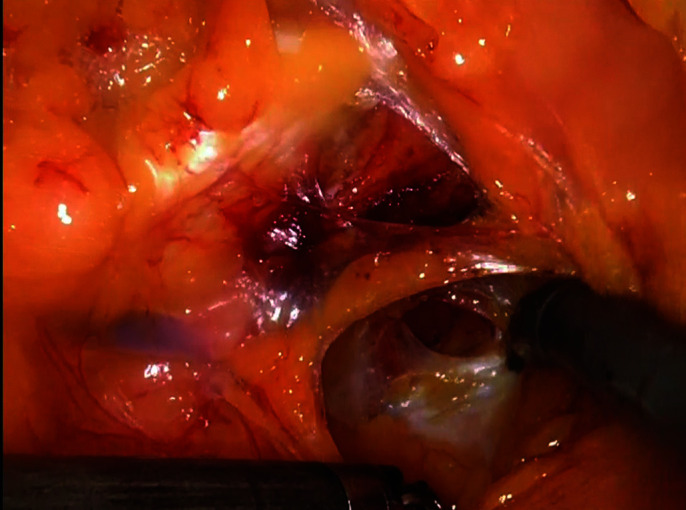
Bladder was dissected from the lateral pelvic sidewalls until exposing the endopelvic fascia distally.
Figure 7.
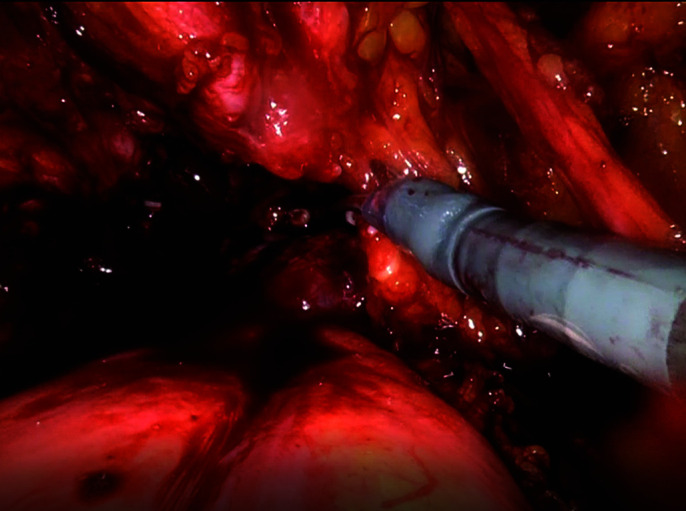
Lateral pedicles are taken down.
Figure 8.
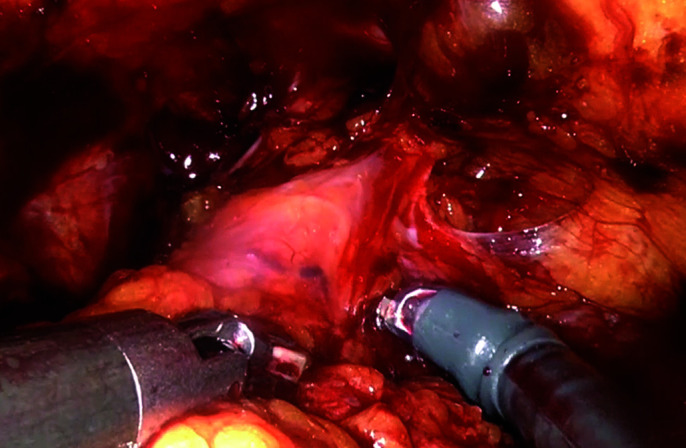
Detrussor apron is superficially incised at the bladder-prostate junction anteriorly.
Figure 9.
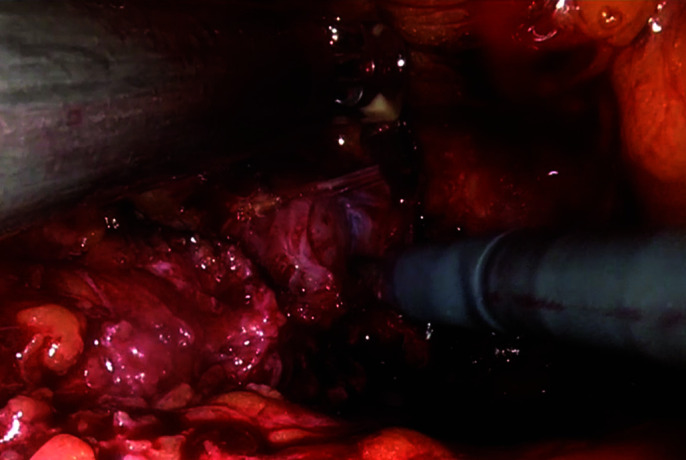
The right lateral surface of the prostate is dissected circumferentially to its apex.
Figure 10.
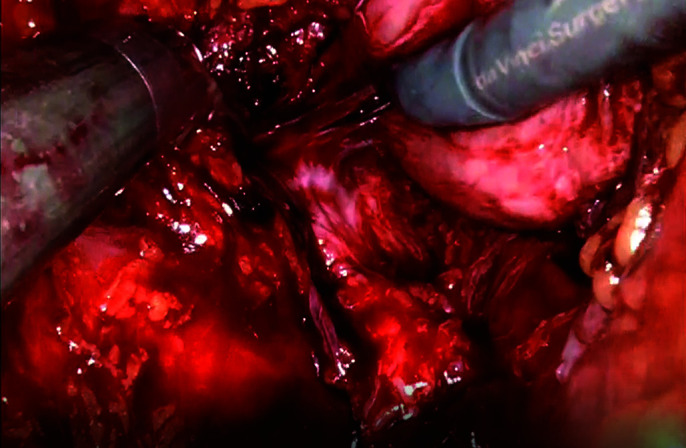
Attachments between the neurovascular bundle and the posterolateral surface of the prostate are dissected sharply and cut distally to its apex.
Figure 11.
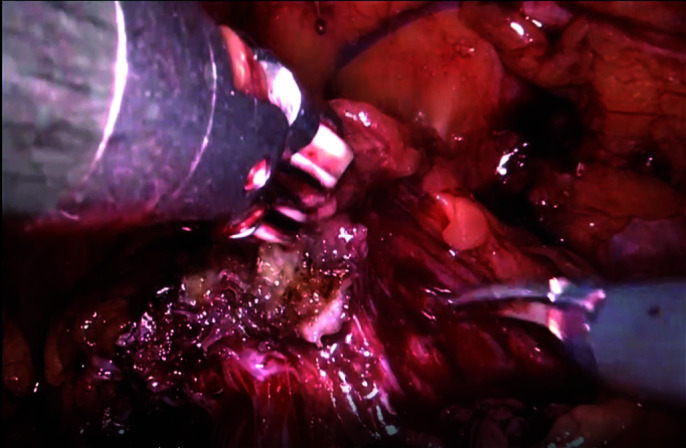
Anterior dissection of the prostate from overlying dorsal venous complex without exposing puboprostatic ligaments and deep dorsal vein.
Figure 12.
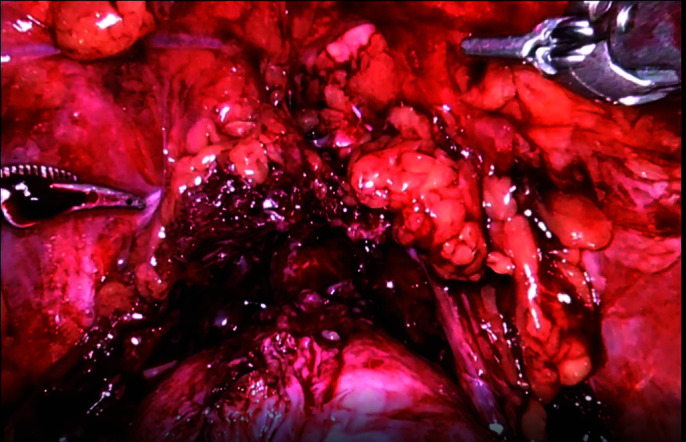
Endopelvic sparing is completed.
We prefer to use 30 degree up and down lenses interchangeably.
1-Peritoneum on the anterior wall of the pouch of Douglas is opened transversely 1 cm above its deepest reflection from the rectum.
2-The Denonvilliers' fascia at the prostate base where seminal vesicles merge with prostate is opened transversely.
3-Leaving the Denonvilliers' fascia on the posterior surface of the prostate, posterior dissection of the rectum was done from base to apex, exposing the lateral margins of the prostate.
4-The Denonvilliers' fascia over the seminal vesicles are opened along its length. Vascular supply to the seminal vesical at its tip are clipped and marked with a Hem-o-lock® clip. This point roughly corresponds to the third sacral vertebral foramen where S3 nerves, which play an important role in erectile function, exit. Distal to this point, all dissections are done athermally, including lateral borders of the seminal vesicles and prostate later in the procedure.
5-The vas deferens may also be cut to expose the lateral borders of the seminal vesicles up to the prostate base if needed.
6-The ureter is dissected along its length where it enters the bladder leaving a good amount of adventitial tissue around it. At this stage of the operation ureters are not blocked and continue to drain the kidneys.
7-The lateral to medial umbilical ligaments (urachus) on the anterior abdominal wall was freed to its most proximal level, keeping its attachment with the umbilicus intact. This maintains the traction of the specimen cranially and anteriorly which facilitates work on lateral bladder pedicles.
8-The bladder was dissected from the lateral pelvic sidewalls, exposing the endopelvic fascia distally.
9-Lateral bladder pedicles are taken down with ligature to the level of the exposed endopelvic fascia, with the Hem-o-Lock® clip marking the tip of the seminal vesical at the S3 level.
10-The bladder base and prostatic junction is estimated by gently lifting up the bladder anteriorly with the Cadiere forceps at the fourth arm and also by moving the Foley balloon back and forth. All along this junctional anterior line, tissue including the detrusor apron and dorsal venous complex is contoured with electrocautery superficially without opening the bladder. Thermal energy is applied laterally.
11-The seminal vesicle is held and pulled up medially with the fourth robotic arm at the prostatic base.
12-Monopolar scissors are used to spread the tissue and identify the prostatic base lateral to the seminal vesicles. Surrounding tissue at the junctional line of the bladder and prostate is separated with the dissector, clipped, and cut.
13-Attachments between the neurovascular bundle and the posterolateral surface of the prostate are dissected sharply, clipped, and cut distally to its apex. Unnecessary clips are avoided during this dissection.
14-The lateral and anterolateral parts of the prostate are dissected sharply starting distally from the prostatic apex cranially, underneath the detrusor apron and dorsal venous complex.
15-Lastly, the anterior dissection of the prostate from overlying dorsal venous complex is completed without exposing puboprostatic ligaments and deep dorsal vein. Most of the time, there is no need to control the dorsal venous complex. However, pinpoint cauterizations on the dorsal venous complex may be done if needed.
16-Bipolar or monopolar energy is used to control bleeding from the dorsal venous complex during anterior dissection of the prostate.
17-After complete dissection of the prostate circumferentially and the membranous urethra is exposed, both ureters are clipped and cut.
18-The urethral catheter is withdrawn, the urethra is suture-tied and cut with sharp scissors flush with the prostatic apex.
19-The whole surgical specimen, including bladder and prostate within a bag, is extracted through the Alexis port and sent for frozen section analyses of both distal ureteric ends and distal urethra.
Endopelvic Fascia and Internal Genitalia Sparing Robotic Radical Cystectomy for Female Patients
1-The peritoneum on the anterior wall of the pouch of Douglas is opened transversely one cm above its deepest reflection from the uterus on the posterior bladder wall.
2-The bladder is separated posteriorly and distally from the uterus and uterine cervix.
3-The ureters are dissected with good adventitial tissue within the broad ligaments until they enter bladder. Round ligaments are also left untouched.
4-The bladder base-urethra junction is identified with the help of Foley balloon.
5-Superficial lateral incisions below the bladder base are made towards to the urethra without opening the bladder.
6-The urethra is dissected sharply at the bladder neck circumferentially without injuring its surrounding tissue, and a vascular tape is passed underneath it. The urethra is lifted up with the help of this tape anteriorly.
7-The posterior pedicles developed between the bladder base and vaginal wall are clipped and cut close to the bladder up to the urethra, where it is lifted without thermal energy. With this maneuver, nerve bundles to the urinary sphincter traveling on the lateral and anterior vaginal wall are not injured.
8-Both ureters are clipped and cut where they enter bladder.
9-The urethral catheter is withdrawn, urethra is sutured tied, and cut with sharp scissors flush with the bladder base.
10-The surgical specimen within a bag is extracted through the Alexis port and sent for frozen section analyses of both distal ureteric ends and urethra.
EARLY FUNCTIONAL EVALUATION
At the 30-day postoperative visit, all of the study patients had uroflowmetry and post-voided residual (PVR) urine measurements. Their day and night urinary incontinence levels were classified according to their daily pad uses. Erectile function was assessed by using the first five questions of International Index of Erectile Function (IIEF-5) scores described by Rosen et al.9
STATISTICAL ANALYSIS
Descriptive techniques were performed for the overall study population. Continuous variables were reported as mean or median with standard deviation and range. Data analysis was performed using Statistical Analysis Software version 90.4 (SAS v.90.4). Institutional Review Board (IRB) approval and patient consents were obtained.
RESULTS
Ten patients were included in this study, 8 males and 2 females. Mean operative time, median estimated blood loss, and median duration of hospital stay were 530 minutes, 316 ml, and 8 days, respectively. Pathological parameters are shown in Table 2.
Table 2.
Pathological Parameters
| Parameter | |
|---|---|
| Pathological Stage (pT) | |
| 0 | 3 |
| Is | 3 |
| 1 | 1 |
| 2 | 1 |
| 3 | 1 |
| 4 | 1 |
| Lymph Node Stage (pN) | |
| 0 | 8 |
| 1 | 1 |
| 2 | 1 |
| Mean lymph node yield (± SD) (range) | 33.2 (± 3.5) (20 – 54) |
| Positive surgical margins (n) | None |
| Incidental prostatic adenocarcinoma (n) (%) | 2 (20) |
| Gleason Score 3 + 3 | 2 |
Abbreviations: SD, standard deviation; Is, in situ.
Patients developed two minor (acidosis-induced hypercalcemia and urinary tract infection) and one major complications (ileus) during the perioperative (0–30 days) period; zero complications during postoperative (31–90 days) period. One patient developed acidosis and hypercalemia after discharge and was successfully treated without need of the intensive care unit. One patient developed a urinary tract infection requiring hospitalization and parenteral antibiotics treatment. One patient had ileus during the initial hospital stay and laparoscopic adhesion removal was performed.
At the 30-day postoperative follow-up, the mean maximum flow rate, average flow rate, mean voided volume and PVR volume were 150.1 ml/sec, 30.2 ml/sec, 2640.7 ml, and 30.5 ml, respectively. All of the patients were fully continent during the day, three patients had mild night incontinence requiring pad use (1 pad/night).
On presurgical appointment, 7 patients had IIEF-5 scores of 19 and above. Among these patients, only one had moderate erectile dysfunction (16.6%), requiring oral phosphodiesterase type 5 (PDE5) inhibitors, on 30 days postoperative visit. Patient-based functional outcomes are shown in Table 3.
Table 3.
Functional Outcomes of the Patients at the One Month Visit After Endopelvic Fascia Sparing Robotic Radical Cystectomy
| P | G | pT | pN | Qmax | Qave | Volume | PVR | DPU | NPU | Pre-op ED | Postop ED | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 4 | 0 | 36 | 12 | 260 | 10 | 0 | SP | NA | ||
| 2 | M | 1 | 0 | 7 | 0.8 | 100 | 0 | 0 | SP | No ED | No ED | |
| 3 | M | 0 | 0 | 19 | 4 | 250 | 0 | SP | SP | No ED | No ED | |
| 4 | M | IS | 0 | 7 | 0.7 | 350 | 0 | 0 | SP | Severe ED | Severe ED | |
| 5 | F | IS | 2 | 40 | 5.6 | 350 | 0 | 0 | SP | NA | ||
| 6 | M | 0 | 0 | 19 | 6.1 | 588 | 0 | 0 | 1 | No ED | Moderate ED | PDE5i |
| 7 | M | IS | 1 | 20 | 2.6 | 178 | 0 | 0 | SP | No ED | No ED | |
| 8 | M | 2 | 0 | 17 | 3.6 | 162 | 0 | SP | 1 | No ED | No ED | |
| 9 | M | 3 | 0 | 17 | 5 | 225 | 20 | SP | SP | No ED | No ED | |
| 10 | M | 0 | 0 | 18 | 7 | 350 | 0 | 0 | 1 | No ED | No ED | |
Abbreviations: P, patient; G; gender; pT, pathological tumor stage; pN, pathological nodal stage; Qmax, maximum urine flow rate; Qave, average urine flow rate; DPU, day pad use; NPU, night pad use; PVR, post-voided residual urine volume; SP, safety pad; ED, erectile dysfunction; T, treatment; F, female; M, male, IS, in situ; PDE5i, phosphodiesterase type 5 inhibitor.
Patient follow-ups occurred at a mean of 11.5 months post-surgery. At this time, two patients with pathological lymph node involvement, received adjuvant chemotherapy. No patients died during the follow-up time period. Only one female patient with pathological stage 4 tumor had retroperitoneal lymph node recurrence six months after RARC. Loop colostomy was performed by general surgery. No distant metastasis in any patients were detected.
DISCUSSION
To our knowledge, this is the first case series to assess the very early functional outcomes of RARC with an EPFS fashion. Previously, authors published their techniques and outcomes on neurovascular bundle sparing RARC, which do not include an endopelvic fascia sparing approach like the one performed in our series.2,10,11 The advantages of our technique, compared to previously published series, include better neurovascular bundle sparing with inclusion of complete sparing of endopelvic fascia and the dorsal venous complex. We observed that our technique better preserves the neurovascular bundle; in particular, functional outcomes seems very promising compared to the previously articles, including day and night urinary continence and erectile function.
Galfano et al. reported the functional and oncological results of the first 200 patients who underwent Retzius sparing RARP. Retzius sparing RARP was found to be oncologically safe and have high early continence and potency rates.12 Tewari et al. described this technique in RARP including preservation of the entire puboprostatic musculoligamentous complex, reconstructing the arcus tendinous and puboprostatic complex. By leaving lateral periurethral support tissue untouched, early continence rates improved owing to preserved structural integrity. Tewari et al. reported 88% continence rate at 12 weeks after RARP among 50 patients, which is higher than the published literature.7
Hood technique, which is similar to our technique of fascia sparing in RARC, was described for RARP in 2020. Hood technique has many advantages including early urinary continence, low positive surgical margin rate, and the ability to visualize anatomic landmarks.8 Early (four weeks) continence rate was 83% among 300 patients. In our series, day continence was 100% and night continence was 77.7% at the one-month postoperative visit. These are very promising results particularly when compared to RARP procedures.
Recently, we reported our preliminary results for RARC with intracorporeal Studer pouch formation with Balbay’s technique in 22 cases. Balbay’s technique replicates the open approach including double folding and results in sufficient pouch volume with minimal pressure for urine storage. In 17 patients with a follow-up greater than 1 year, day full continence rate was 58.8%, night full continence rate was 47.1%. With the EPFS RARC technique, our very early day and night continence rates were increased to 100% and 77.7%, respectively.4
Rocco et al. described a posterior reconstruction technique in which prior to the anastomosis, a modified posterior Rocco’s repair, the rhabdosphincter and the posterior side of the ileal neobladder neck was performed. Among 11 patients, day and night continence rates were 100% and 44% at one year post-surgery.13
In the literature, postoperative urodynamic findings were generally lacking in patients who underwent RARC with intracorporeal neobladder. In our series, the mean maximum flow rate was 15.1 ml/sec, the mean voided volume was 260 ml, and the mean PVR was below 5 ml. These results showed that Balbay’s technique using 55 cm measured ileal segments with double-folding had very promising postoperative early micturition outcomes. PVR in our series was detected to be lower than the other series in the literature. The University of South Carolina group, using Studer technique, reported a mean PVR of 268 ml.14 Checcucci et al. performed RARC with Y-shaped intracorporeal neobladder in 45 patients. At three months postoperation, the mean PVR was 154 ml.15 A Japanese group published the data of 22 patients who underwent RARC followed by intracorporeal U-shaped ileal neobladder reconstruction. The mean PVR at six and 12 months were 40 ml and 29 ml, respectively. In our series, the median PVR at one month was 3.5 ml, which may be the lowest value measured in the published literature.
We evaluated 8 male patients’ erectile functions postoperatively. Seven patients had normal erectile functions pre-operatively. At the 30-day postoperative visit, 6 patients had IIEF-5 scores 19 and above. One patient had moderate erectile dysfunction. He gained spontaneous erections with the support of PDE5 inhibitor medication.
Rocco et al. had a positive surgical margin rate of 9% (n = 1/11) in patients who underwent RARC with posterior reconstruction followed by intracorporeal neobladder. In our series none of the patients had positive surgical margin. During a mean 7.7 months of follow-up, no local recurrence was detected. Only one male patient had Clavien-Dindo grade 3 or higher complication (9%). On the postoperative sixth day, he had vomiting. A nasogastric tube was inserted and after two days of follow-up, ileus persisted. After insertion of 3 trochars into the previous locations, diagnostic laparoscopy was performed. Proximal jejunal loops were adherent to symphysis pubis. These adhesions were released by sharp dissections. On the second day of adhesion removal, he passed flatus. He tolerated regular diet well. He was discharged on the 20th day after RARC.
The main limitations of our study were its retrospective design, including limited number of patients and absence of comparative outcomes.
CONCLUSION
Better neurovascular bundle sparing with inclusion of complete sparing of endopelvic fascia and the dorsal venous complex show promising functional outcomes compared to previously published series. These results include day and night urinary continence and erectile function. This technique has promising safe oncological outcomes and low complication rates.
Footnotes
Disclosure: none.
Acknowledgements: none.
Conflict of interests: none.
Funding sources: none.
Informed consent: Dr. Ersin Köseoğlu declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Mevlana Derya Balbay, Department of Urology, Koç University School of Medicine, Istanbul, Turkey.; VKF American Hospital, Urology Clinic, Istanbul, Turkey.
Ersin Köseoğlu, Department of Urology, Koç University School of Medicine, Istanbul, Turkey..
Abdullah Erdem Canda, Department of Urology, Koç University School of Medicine, Istanbul, Turkey..
Arif Özkan, Urology Clinic, Koç University Hospital, Istanbul, Turkey..
Mert Kılıç, VKF American Hospital, Urology Clinic, Istanbul, Turkey..
Murat Can Kiremit, Department of Urology, Koç University School of Medicine, Istanbul, Turkey..
Ahmet Musaoğlu, VKF American Hospital, Urology Clinic, Istanbul, Turkey..
Kayhan Tarım, Department of Urology, Koç University School of Medicine, Istanbul, Turkey..
Ahmet Furkan Sarıkaya, Department of Urology, Koç University School of Medicine, Istanbul, Turkey..
References:
- 1.Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. [DOI] [PubMed] [Google Scholar]
- 2.Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003;92(3):232–236. [DOI] [PubMed] [Google Scholar]
- 3.Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol. 2003;44(3):337–339. [DOI] [PubMed] [Google Scholar]
- 4.Balbay MD, Canda AE, Kiremit MC, Koseoglu E. Intracorporeal studer pouch formation with Balbay's technique following robotic radical cystectomy for bladder cancer: experience with 22 cases with oncologic and functional outcomes. J Endourol. 2020;34(3):273–280. [DOI] [PubMed] [Google Scholar]
- 5.Cochetti G, Boni A, Barillaro F, Pohja S, Cirocchi R, Mearini E. Full neurovascular sparing extraperitoneal robotic radical prostatectomy: our experience with PERUSIA technique. J Endourol. 2017;31(1):32–37. [DOI] [PubMed] [Google Scholar]
- 6.Poore RE, McCullough DL, Jarow JP. Puboprostatic ligament sparing improves urinary continence after radical retropubic prostatectomy. Urology. 1998;51(1):67–72. [DOI] [PubMed] [Google Scholar]
- 7.Tewari AK, Bigelow K, Rao S, et al. Anatomic restoration technique of continence mechanism and preservation of puboprostatic collar: a novel modification to achieve early urinary continence in men undergoing robotic prostatectomy. Urology. 2007;69(4):726–731. [DOI] [PubMed] [Google Scholar]
- 8.Wagaskar VG, Mittal A, Sobotka S, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of Retzius and sparing the pouch of Douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol. 2021;80(2):213–221. [DOI] [PubMed] [Google Scholar]
- 9.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. [DOI] [PubMed] [Google Scholar]
- 10.Schoenberg MP, Walsh PC, Breazeale DR, Marshall FF, Mostwin JL, Brendler CB. Local recurrence and survival following nerve sparing radical cystoprostatectomy for bladder cancer: 10-year followup. J Urol. 1996;155(2):490–494. [PubMed] [Google Scholar]
- 11.Haberman K, Wittig K, Yuh B, et al. The effect of nerve-sparing robot-assisted radical cystoprostatectomy on erectile function in a preoperatively potent population. J Endourol. 2014;28(11):1352–1356. [DOI] [PubMed] [Google Scholar]
- 12.Galfano A, Di Trapani D, Sozzi F, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with >/= 1 year of follow-up. Eur Urol. 2013;64(6):974–980. [DOI] [PubMed] [Google Scholar]
- 13.Rocco B, Luciani LG, Collins J, et al. Posterior reconstruction during robotic-assisted radical cystectomy with intracorporeal orthotopic ileal neobladder: description and outcomes of a simple step. J Robot Surg. 2021;15(3):355–361. [DOI] [PubMed] [Google Scholar]
- 14.Satkunasivam R, Santomauro M, Chopra S, et al. Robotic intracorporeal orthotopic neobladder: urodynamic outcomes, urinary function, and health-related quality of life. Eur Urol. 2016;69(2):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Checcucci E, Manfredi M, Sica M, et al. Robot-assisted-radical-cystectomy with total intracorporeal Y neobladder: analysis of postoperative complications and functional outcomes with urodynamics findings. Eur J Surg Oncol. 2021. 48(3):694–702. [DOI] [PubMed] [Google Scholar]


