Abstract
Background
Neoadjuvant chemoradiation with fluoropyrimidine followed by surgery and adjuvant chemotherapy has been the standard treatment of locally advanced stages II and III rectal cancer for many years. There is a high risk for disease recurrence; therefore, optimizing chemoradiation strategies remains an unmet need. Based on a few studies, there is evidence of the synergistic effect of VEGF/PDGFR blockade with radiation.
Methods
In this phase I, dose-escalation and dose-expansion study, we studied 3 different dose levels of lenvatinib in combination with capecitabine-based chemoradiation for locally advanced rectal cancer.
Results
A total of 20 patients were enrolled, and 19 were eligible for assessment of efficacy. The combination was well tolerated, with an MTD of 24 mg lenvatinib. The downstaging rate for the cohort and the pCR was 84.2% and 37.8%, respectively. Blood-based protein biomarkers TSP-2, VEGF-R3, and VEGF correlated with NAR score and were also differentially expressed between response categories. The NAR, or neoadjuvant rectal score, encompasses cT clinical tumor stage, pT pathological tumor stage, and pN pathological nodal stage and provides a continuous variable for evaluating clinical trial outcomes.
Conclusion
The combination of lenvatinib with capecitabine and radiation in locally advanced rectal cancer was found to be safe and tolerable, and potential blood-based biomarkers were identified.
Clinical Trial Registration
Keywords: lenvatanib, radiation, rectal cancer
In this phase I dose escalation and dose expansion study, three different dose levels of lenvatinib, in combination with capecitabine-based chemoradiation for locally advanced rectal cancer, were studied.
Lessons Learned.
In this phase I study, the combination of lenvatinib with capecitabine and radiation were considered safe for the treatment of patients with locally advanced rectal cancer.
In the cohort of 20 enrolled patients, 19 were evaluable; the downstaging rate was 84.2% and the pCR was 37.8%.
Some potential blood-based biomarkers were identified.
Discussion
Various strategies have been studied to define the ideal treatment for locally advanced rectal cancer. Pre–operative chemoradiation is known to result in better pCR rates than pre–operative radiation alone (13.7% vs 5.3%; odds ratio 2.84; 95% CI, 1.75-4.59; P < .0001) and has been the standard for several years as a treatment for locally advanced rectal cancer.1 Our study was able to achieve a pCR rate of 37.8%. However, we had no patients enrolled with T4 disease, which is known to be a characteristic of “high-risk” disease (Table 1).
Table 1.
Patient characteristics at baseline and pathological outcomes (n = 19)
| Age, years | |
| Mean (standard deviation) | 54.8 (10.7) |
| Median (min, max) | 51 (42, 72) |
| Sex, n (%) | |
| Male | 13 (68.4) |
| Female | 6 (31.6) |
| Race/ethnicity, n (%) | |
| Hispanic or Latino | 2 (10.5) |
| White | 17 (89.4) |
| Other/unknown | 1 (5.3) |
| ECOG, n (%) | |
| 0 | 18 (94.7) |
| 1 | 1 (5.3) |
| Clinical stage at diagnosis, n (%) | |
| T2N1 | 2 (10.5) |
| T3N0 | 3 (15.8) |
| T3N1 | 12 (63.2) |
| T3N2 | 2 (10.5) |
| Interval between completion of chemoXRT and surgery | |
| Median (standard deviation), days | 59 (21.7) |
| Type of surgery, n (%) | |
| LAR | 14 (73.7) |
| APR | 5 (26.3) |
| Pathological response, n (%) | |
| pCR | 7 (36.8) |
| pPR | 9 (47.4) |
| pNR | 3 (15.8) |
| NAR (Neoadjuvant rectal) score | |
| Mean (min, max) | 10.37 (0.94; 30.1) |
| Median ± standard deviation | 8.43 ± 10.32 |
Abbreviations: LAR, low anterior resection; APR, abdominoperineal resection; pCR, pathological complete response; pPR, pathological partial response; pNR, pathological non response.
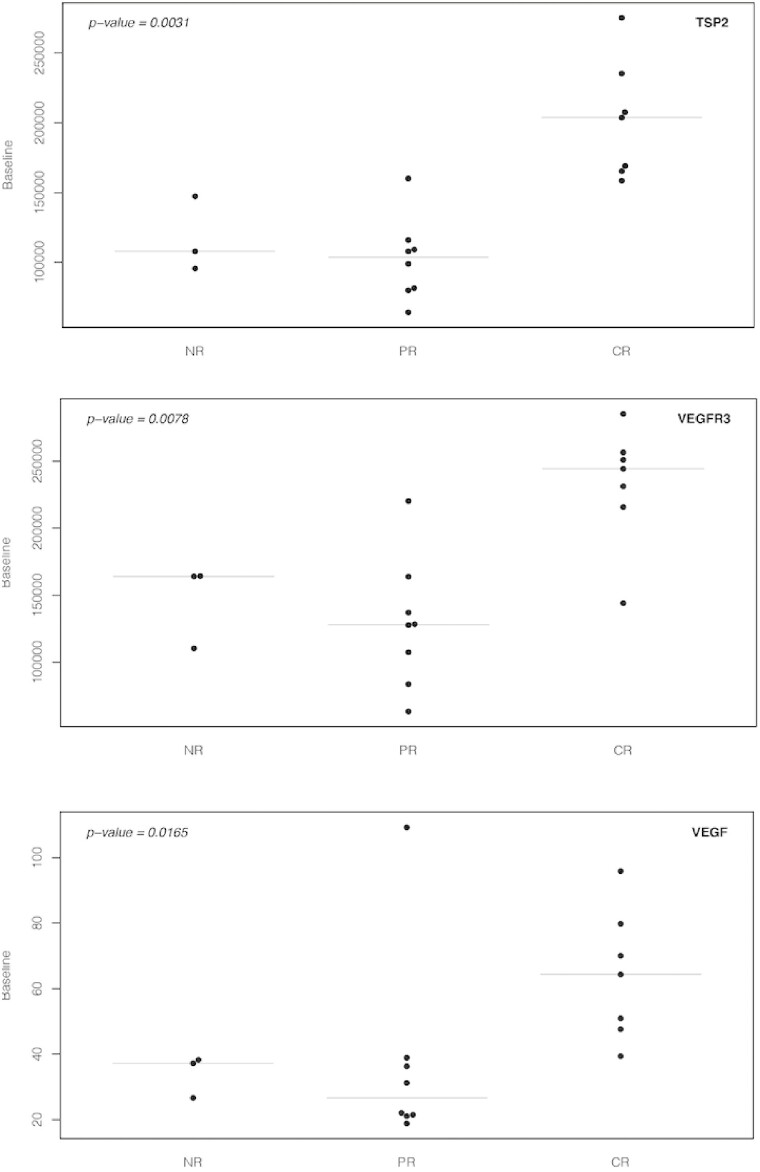
In a study of KRAS-mutated rectal cancer, the combination of capecitabine and sorafenib with radiation yielded a pCR rate of 60%.2 The downstaging rate on this study was 81.6%, comparable to the 84.2% seen in our study. The study with sorafenib did report 15% grade 3 adverse events with diarrhea and 12.5% grade 3 adverse events with hand-foot-syndrome.2 Our study did not have excess of 10% of grade 3 adverse events, and this was mostly related to hypertension more commonly seen with lenvatinib than sorafenib. Patients with high NAR scores (>16) are associated with poor overall survival, those with low scores (<8) are associated with superior overall survival, and those in the middle have intermediate survival.3 In our study, the median NAR was 8.43 and mean was 10.37, and these scores are in the intermediate range. Overall, this was a well-tolerated regimen with few adverse events and no dose-limiting toxicities. There were no treatment interruptions due to treatment. No excess post–operative complications were reported due to the study treatment except for 1 patient who had wound dehiscence that was not attributed to the study treatment. Most adverse events were low grade and in line with some side effects expected of lenvatinib. In our study, baseline levels of 3 biomarkers, TSP-2, VEGF-R3, and VEGF, correlated with NAR score, and these levels were significantly different across different response group categories (Figure 1). While previous studies with the combination of bevacizumab did not lead to success in unselected patient population, our blood-based biomarkers may be extremely beneficial to enable discernment as to which patients will benefit the most from the addition of anti-angiogenic or a mixed protein kinase inhibitor that targets other receptors in the tumor stroma to chemoradiation.
Figure 1.
Baseline levels of three markers significantly differ across outcome groups.
Trial Information
| Disease | Colorectal cancer |
| Stage of disease/treatment | Neo-adjuvant |
| Prior therapy | None |
| Type of study | Phase I, 3 + 3 |
| Primary endpoint | Maximum tolerated dose |
| Investigator’s Analysis | Active but results overtaken by other developments |
Additional Details of Endpoints or Study Design
Blood Biomarker Analyses
EDTA plasma was isolated from each patient by venipuncture at baseline (within 42 days preceding Day 1) and after completion of chemoradiation prior to surgery. The plasma levels of 25 biomarkers, including Ang-2, GP130, HGF, ICAM-1, IL-6, IL-6R, OPN, PDGF-AA, PDGF-BB, PlGF, SDF-1, TGF-b1, TGF-b2, TIMP-1, TSP-2, VCAM-1, VEGF, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2, and VEGF-R3 were measured with the CircaScan multiplex platform (Quanterix, Billerica, Massachusetts), whereas BMP-917, CD7318, and TGFb-R319 were tested as described previously.
Statistical Plan and Analyses
A standard “3+3” design was used to determine the MTD. In this study design, 3 patients were planned to be treated with a pre–determined dose of lenvatinib. The dose escalation was planned to stop with more than one DLT occurrence at any dose. Three additional patients were planned to be added if one out of 3 patients had DLT at any dose. With no DLT occurrence, three new patients were planned to be recruited to the study for the next dose of lenvatinib. The MTD of lenvatinib was defined as the highest dose level at which no more than 1 out of 6 subjects experienced DLT. At the MTD of lenvatinib, an additional expansion cohort of 10 patients was planned to be enrolled in the study to further assess the safety and efficacy of this agent in combination with capecitabine and radiation.
The pathological response rate was used to assess efficacy. The response was categorized as complete response (CR), partial response (PR), and no response (NR). To test biomarker changes in response to treatment, log transformed ratios (Lratios) were calculated using the formula: log2 (post–treatment level/baseline level). Fold changes were calculated post–treatment defined as post–treatment/baseline. Waterfall plots are shown to graphically illustrate changes. The Kruskal–Wallis test was used to test the association of the different biomarkers with treatment response and bees warm plots were used to depict this graphically. Spearman’s correlation coefficient was used to test the association of the biomarkers with NAR score and scatterplots were used to depict these graphically. NAR score was calculated as [5 pN − 3(cT − pT) + 12]2/9.61. A two-sided P-value of <.05 was considered statistically significant.
Drug Information
| Generic/working name | Lenvatinib |
| Drug type | Small molecule |
| Drug class | Angiogenesis—VEGF |
| Route | Oral (po) |
| Schedule of administration | In this 3+3 dose-escalation study, patients received lenvatinib with capecitabine (850 mg/m2/BID daily) and radiation on days 1–5 each week (Monday–Friday) for a total of 5½–6 weeks (28 fractions with a total intended dose of 5040 cGy). The doses of lenvatinib tested were 14 mg daily for cohort 1, 20 mg for cohort 2, and 24 mg for cohort 3. |
| Generic/working name | Capecitabine |
| Drug type | Chemotherapy |
| Route | Oral (po) |
Dose Escalation Table
| Dose level | Dose of drug: lenvatinib (mg) | Dose of drug: capecitabine (mg/m2) | Number enrolled (cGy) |
| 1 | 14 | 850 | 5040 |
| 2 | 20 | 850 | 5040 |
| 3 | 24 | 850 | 5040 |
Patient Characteristics
| Number of patients, male | 13 |
| Number of patients, female | 6 |
| Age | Median (range): 51 (42-72) years |
| Performance status: ECOG | 0-18, 1-1, 2-0, 3-0, Unknown-0 |
| Detailed patient characteristics are shown in Table 1. |
Primary Assessment Method
| Title | Maximum tolerated dose |
|---|---|
| Number of patients screened | 24 |
| Number of patients enrolled | 20 |
| Number of patients evaluable for toxicity | 20 |
| Number of patients evaluated for efficacy | 19 |
| Evaluation method | Safety assessment was made using CTCAE v4.0. Efficacy assessment was based on pathological response evaluated by post–operative pathological staging and Neoadjuvant Rectal (NAR) score to compare the initial clinical and final pathological staging. The TNM AJCC 7th edition was used to determine the pathological staging. |
| Response assessment CR | n = 7 (37.8%) |
| Response assessment other | n = 9 (47.4%) |
Outcome Notes
Safety assessment was made using CTCAE v4.0. Assessment of efficacy was determined based on pathological response evaluated by post–operative pathological staging as well as Neoadjuvant Rectal (NAR) score to compare the initial clinical and final pathological staging. The TNM AJCC 7th edition was used to determine the pathological staging.
Seven (37.8%) patients achieved pathological complete response and additional nine patients (47.4%) had pathological downstaging. The total downstaging for the overall cohort was 84.2%. Three patients (15.8%) had no treatment response to lenvatinib and capecitabine-based neoadjuvant chemoradiation. The mean and median neoadjuvant rectal (NAR) score was 10.37 and 8.43, respectively. The median interval between completion of chemoradiation and surgery was 59 days.
Toxicities
No dose-limiting toxicities were noted. There were 5 patients treated on dose level 1 of 14 mg of lenvatinib, 3 patients on dose level 2 of 20 mg lenvatinib, and 12 patients on dose level 3 of 24 mg of lenvatinib. The most common any grade adverse events due to any cause were fatigue (n = 15), hypertension (n = 13), nausea (n = 13), radiation dermatitis (n = 10), diarrhea (n = 9) and urinary tract infection pain (n = 9). The only grade 3 adverse events due to any cause were hypertension (n = 3), decreased lymphocyte count (n = 3), increase in ALT (n = 1) and rectal pain (n = 1). The most common any grade adverse events attributed to study treatment were fatigue (n = 15), nausea (n = 13), hypertension (n = 12) and radiation dermatitis (n = 10). The only grade 3 adverse events attributed to the study drug were hypertension and a decrease in lymphocyte count (each n = 3). No treatment-related mortality occurred. The most common adverse events in cohort 3 (expansion cohort) were fatigue (n = 9), nausea (n = 8), diarrhea (n = 5) and hypertension (n = 5). The dose level 3 of 24 mg lenvatinib was established as the MTD.
Surgical Outcomes and Pathological Response
All patients enrolled in the study completed preplanned chemoradiation with concurrent capecitabine and lenvatinib and underwent surgical resection of primary rectal cancer. The median interval between completion of chemoradiation and surgery was 59 days. Fourteen patients (73.7%) underwent low anterior resection (LAR). Among patients who underwent abdominal perineal resection (APR), 1 patient died due to infectious complications from a perineal wound dehiscence. The event occurred more than 30 days but less than 90 days after surgery. Post–operative specimens were reviewed for pathological tumor regression. Seven (37.8%) patients achieved pathological complete response and additional 9 patients (47.4%) had pathological downstaging. The total downstaging for the overall cohort was 84.2%. Three patients (15.8%) had no treatment response to lenvatinib and capecitabine-based neoadjuvant chemoradiation. The mean and median neoadjuvant rectal (NAR) score were 10.37 and 8.43, respectively.
Biomarker Analyses
Specimens for biomarker analyses were available from 18 patients. Of all the biomarkers evaluated, the highest median fold change from baseline to post-treatment was seen with PDGF-BB and PDGF-AA, with median values of 2.83 and 2.55, respectively. Expression levels of all biomarkers at baseline and post-treatment can be provided upon request. Baseline biomarker levels were also correlated with NAR score. The most significant markers were TSP-2, VEGF-R3, and VEGF with correlation coefficients being −0.672 (P = .0023), −0.529 (P = .00241) and −0.502 (P = .0337), respectively. The baseline expression of these same three markers, TSP-2, VEGF-R3, and VEGF, significantly differed across the response categories: pCR, pPR, and pNR with the highest values noted in pCR cases (P = .0031, .0078, and .0165, respectively) (Figure 1). The biomarkers that showed significant changes from baseline to post-treatment were TIMP-1 (P = .0024), BMP-9 (P = .0049), PlGF (P = .0068), VEGF-R3 (P = .0068), ICAM-1 (P = .0342), and TGF-b1 (P = .0425) (Table 2).
Table 2.
Biomarker levels at baseline and post-treatment. Fold change (post-chemo/baseline) was calculated for each patient and averaged.
| Biomarker | Unit | Baseline (n = 18) | Post-chemo (n = 12) | Fold change: median (range) |
|---|---|---|---|---|
| Ang2 | pg/mL | 396.65 (170.06-959.7) | 375.9 (184.69-827.5) | 1.01 (0.48-1.89) |
| BMP9 | pg/mL | 87.64 (37.39-382.25) | 163.49 (41.34-318.91) | 1.58 (0.89-5.53) |
| CD73 | pg/mL | 0.25 (0.01-23.29) | 0.39 (0.02-9.91) | 1.12 (0.03-143.52) |
| GP130 | ng/mL | 279.05 (187.10-432.95) | 315.30 (208.00-468.15) | 1.1 (0.5-2) |
| HGF | pg/mL | 139.48 (47.19-292.9) | 160.83 (34.26-357.6) | 1.1 (0.28-2.05) |
| ICAM1 | ng/mL | 449.65 (284.10-889.05) | 436.50 (310.55-649.90) | 1.07 (0.89-1.3) |
| IL6 | pg/mL | 2.63 (0.49-6.47) | 1.98 (0.4-6.9) | 1.31 (0.13-3.37) |
| IL6R | ng/mL | 35.13 (23.08-48.36) | 39.88 (23.06-56.01) | 1.03 (0.93-1.51) |
| OPN | ng/mL | 87.35 (43.96-235.39) | 93.41 (67.89-205.43) | 1.08 (0.65-1.75) |
| PDGFAA | pg/mL | 126.06 (7.84-1714.88) | 291.19 (69.55-3182.5) | 2.55 (0.36-22.16) |
| PDGFBB | pg/mL | 515.92 (54.34-10325) | 1893.75 (191.43-10015) | 2.83 (0.23-13.07) |
| PlGF | pg/mL | 11.8 (4.72-24.7) | 16.03 (9.43-32.54) | 1.5 (0.9-2.41) |
| SDF1 | ng/mL | 1.62 (0.58-3.17) | 1.79 (0.25-4.38) | 1.16 (0.31-3.07) |
| TGFb1 | ng/mL | 17.17 (9.72-107.88) | 26.41 (11.93-114.68) | 1.37 (0.33-6.22) |
| TGFb2 | pg/mL | 55.71 (36.04-93.22) | 79.32 (32.75-194.47) | 1.28 (0.35-3.18) |
| TGFbR3 | ng/mL | 125.93 (79.32-171.63) | 120.68 (84.08-194.68) | 1.18 (0.63-1.45) |
| TIMP1 | ng/mL | 61.58 (40.56-112.85) | 83.79 (56.82-139.55) | 1.33 (0.93-2.22) |
| TSP2 | ng/mL | 131.70 (64.05-275.30) | 189.15 (115.88-242.48) | 1.13 (0.72-2.15) |
| VCAM1 | ug/mL | 1.97 (1.49-3.40) | 2.37 (1.50-3.02) | 0.92 (0.84-1.68) |
| VEGF | pg/mL | 38.55 (18.84-109.3) | 46.59 (23.11-77.54) | 1.31 (0.24-1.88) |
| VEGFC | pg/mL | 574.06 (321.22-2488.03) | 773.27 (378.33-2669.72) | 1.25 (0.39-3.58) |
| VEGFD | ng/mL | 1.12 (0.81-2.79) | 1.27 (1.05-2.96) | 1.15 (0.75-2.67) |
| VEGFR1 | pg/mL | 63.14 (13.55-98.42) | 67.4 (29.2-146.96) | 1.15 (0.45-1.83) |
| VEGFR2 | ng/mL | 4.84 (1.13-7.28) | 4.82 (3.08-7.30) | 0.97 (0.7-1.25) |
| VEGFR3 | ng/mL | 164.04 (63.40-285.03) | 245.03 (150.23-342.40) | 1.18 (0.94-1.97) |
Adverse Events
Table 3.
Adverse events reported due to any cause with lenvatinib combined with capecitabine based chemoradiation in locally advanced rectal cancer (N = 20)
| Adverse events | Cohort 1 dose level (14 mg) (N = 5) | Cohort 2 dose level (20 mg) (N = 3) | Cohort 3 dose level (24 mg) (N = 12) | Total | Total grade 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCI-CTC grade | NCI-CTC grade | NCI-CTC grade | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Blood and lymphatic system disorders | |||||||||||
| White blood cell count decreased | 2 | 2 | 0 | ||||||||
| Lymphocyte count decrease | 1 | 1 | 2 | 2 | 1 | 7 | 3 | ||||
| Neutrophil count decrease | 1 | 1 | 0 | ||||||||
| Platelet count decreased | 2 | 2 | 0 | ||||||||
| Gastrointestinal disorders | |||||||||||
| Anorexia | 2 | 1 | 3 | 6 | 0 | ||||||
| Buttock pain | 1 | 1 | 0 | ||||||||
| Alanine aminotransferase increased | 1 | 1 | 1 | ||||||||
| Aspartate aminotransferase increased | 1 | 1 | 0 | ||||||||
| Constipation | 2 | 1 | 1 | 1 | 5 | 0 | |||||
| Diarrhea | 1 | 3 | 5 | 9 | 0 | ||||||
| Dry mouth | 1 | 1 | 0 | ||||||||
| Dyspepsia | 1 | 1 | 0 | ||||||||
| Fecal Incontinence | 1 | 1 | 0 | ||||||||
| Flatulence | 1 | 1 | 1 | 3 | 0 | ||||||
| Hemorrhoids | 1 | 1 | 0 | ||||||||
| Hiccups | 1 | 1 | 0 | ||||||||
| Nausea | 2 | 3 | 7 | 1 | 13 | 0 | |||||
| Oral dysesthesia | 1 | 1 | 0 | ||||||||
| Oral pain | 1 | 1 | 0 | ||||||||
| Rectal Pain | 2 | 1 | 1 | 2 | 4 | 1 | |||||
| Proctitis | 2 | 2 | 2 | 2 | 8 | 0 | |||||
| Vomiting | 2 | 2 | 0 | ||||||||
| GI disorders—others | 1 | 3 | 2 | 6 | 0 | ||||||
| GU/GYN disorders | |||||||||||
| Creatinine elevation | 1 | 1 | 0 | ||||||||
| Erectile dysfunction | 2 | 2 | 0 | ||||||||
| Hematuria | 1 | 1 | 0 | ||||||||
| Proteinuria | 1 | 1 | 0 | ||||||||
| Urinary frequency | 1 | 1 | 0 | ||||||||
| Urinary incontinence | 1 | 1 | 0 | ||||||||
| Urinary urgency | 2 | 2 | 0 | ||||||||
| Urinary tract infection | 1 | 1 | 0 | ||||||||
| Other renal and urinary disorders | 1 | 1 | 0 | ||||||||
| Urinary tract pain | 2 | 1 | 6 | 9 | 0 | ||||||
| Breast pain | 1 | 1 | 0 | ||||||||
| Skin/cutaneous | |||||||||||
| Dermatitis radiation | 2 | 1 | 2 | 1 | 3 | 1 | 10 | 0 | |||
| Limb edema | 1 | 1 | 0 | ||||||||
| Palmar-plantar erythrodysesthesia syndrome | 1 | 1 | 0 | ||||||||
| Pruritis | 3 | 1 | 4 | 8 | 0 | ||||||
| Papulopustular rash | 1 | 1 | 0 | ||||||||
| Rash-maculo-papular | 1 | 1 | 0 | ||||||||
| Thorax/cardiovascular | |||||||||||
| Chest pain | 1 | 1 | 2 | 0 | |||||||
| Dyspnea | 1 | 1 | 1 | 3 | 0 | ||||||
| Hypertension | 1 | 3 | 3 | 3 | 3 | 13 | 3 | ||||
| Hypotension | 1 | 1 | 0 | ||||||||
| Palpitations | 1 | 1 | 0 | ||||||||
| Sinus bradycardia | 1 | 1 | 0 | ||||||||
| Electrolyte abnormalities | |||||||||||
| Hyperglycemia | 1 | 1 | 2 | 0 | |||||||
| Hypokalemia | 1 | 1 | 0 | ||||||||
| Others | |||||||||||
| Anxiety | 2 | 1 | 3 | 0 | |||||||
| Arthralgia | 1 | 1 | 0 | ||||||||
| Back pain | 1 | 1 | 0 | ||||||||
| Blurred vision | 1 | 1 | 0 | ||||||||
| Chills | 2 | 2 | 0 | ||||||||
| Depression | 1 | 1 | 2 | 0 | |||||||
| Dizziness | 1 | 1 | 0 | ||||||||
| Fatigue | 3 | 1 | 2 | 9 | 15 | 0 | |||||
| Fever | 1 | 1 | 0 | ||||||||
| Non-cardiac chest pain | 1 | 1 | 2 | 0 | |||||||
| Headache | 1 | 3 | 1 | 5 | 0 | ||||||
| Hoarseness | 3 | 3 | 0 | ||||||||
| Infections | 1 | 1 | 2 | 0 | |||||||
| Insomnia | 2 | 2 | 1 | 5 | 0 | ||||||
| Myalgia | 2 | 2 | 0 | ||||||||
| Pain | 1 | 2 | 3 | 0 | |||||||
| Pain in extremity | 1 | 1 | 0 | ||||||||
| Photophobia | 1 | 1 | 0 | ||||||||
| Somnolence | 1 | 1 | 0 | ||||||||
| Weight loss | 1 | 1 | 0 | ||||||||
| Other general disorders, non-specified | 1 | 1 | 0 | ||||||||
Table 4.
Adverse events related to treatment with lenvatinib combined with capecitabine based chemoradiation in locally advanced rectal cancer (N = 20)
| Adverse events | Cohort 1 dose level (N = 5) | Cohort 2 dose level (N = 3) | Cohort 3 dose level (N = 12) | Total | Total grade 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCI-CTC grade | NCI-CTC grade | NCI-CTC grade | |||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Blood and lymphatic system disorder | |||||||||||
| Neutrophil count decreased | 1 | 1 | 0 | ||||||||
| Lymphocyte count decreased | 1 | 1 | 2 | 2 | 1 | 7 | 3 | ||||
| Platelet count decreased | 2 | 2 | 0 | ||||||||
| White blood cell count decreased | 2 | 2 | 0 | ||||||||
| Gastrointestinal disorders | |||||||||||
| Anorexia | 2 | 1 | 3 | 6 | 0 | ||||||
| Buttock pain | 1 | 1 | 0 | ||||||||
| Constipation | 1 | 1 | 2 | 0 | |||||||
| Diarrhea | 1 | 3 | 5 | 9 | 0 | ||||||
| Fecal incontinence | 1 | 1 | 0 | ||||||||
| Nausea | 2 | 3 | 7 | 1 | 13 | 0 | |||||
| Oral pain | 1 | 1 | 0 | ||||||||
| Proctitis | 2 | 2 | 2 | 1 | 7 | 0 | |||||
| Rectal pain | 1 | 1 | 2 | 0 | |||||||
| Vomiting | 2 | 2 | 0 | ||||||||
| Oral dysesthesia | 1 | 1 | 0 | ||||||||
| Other gastrointestinal disorders | 1 | 2 | 2 | 5 | 0 | ||||||
| General disorders and administration site conditions | |||||||||||
| Dry mouth | 1 | 1 | 0 | ||||||||
| Fatigue | 3 | 1 | 2 | 9 | 15 | 0 | |||||
| Injury, poisoning and procedural complications | |||||||||||
| Aspartate aminotransferase increased | 1 | 1 | 0 | ||||||||
| Skin and subcutaneous tissue disorders | |||||||||||
| Palmar plantar erythrodysesthesia syndrome | 1 | 1 | 0 | ||||||||
| Papulopustular rash | 1 | 1 | 0 | ||||||||
| Dermatitis radiation | 2 | 1 | 2 | 1 | 3 | 1 | 10 | 0 | |||
| Pruritis | 3 | 1 | 1 | 5 | 0 | ||||||
| Rash maculo-papular | 1 | 1 | 0 | ||||||||
| Cardiovascular disorders | |||||||||||
| Sinus bradycardia | 1 | 1 | 0 | ||||||||
| Hypertension | 1 | 3 | 3 | 2 | 3 | 12 | 3 | ||||
| Infections and cutaneous | |||||||||||
| Urinary tract infection | 1 | 1 | 0 | ||||||||
| Other infections and infestations | 1 | 1 | 0 | ||||||||
| Miscellaneous | |||||||||||
| Arthralgia | 1 | 1 | 0 | ||||||||
| Creatinine increased | 1 | 1 | 0 | ||||||||
| Headache | 2 | 1 | 3 | ||||||||
| Myalgia | 1 | 1 | 0 | ||||||||
| Urinary frequency | 1 | 1 | 0 | ||||||||
| Urinary incontinence | 1 | 1 | 0 | ||||||||
| Urinary urgency | 1 | 1 | 0 | ||||||||
| Urinary tract pain | 1 | 1 | 2 | 0 | |||||||
| Erectile dysfunction | 1 | 1 | 0 | ||||||||
| Hiccups | 1 | 1 | 0 | ||||||||
| Hoarseness | 3 | 3 | 0 | ||||||||
| Other renal and urinary disorders | 1 | 1 | 0 | ||||||||
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator’s assessment | Active but results overtaken by other developments |
The current standard for the treatment of locally advanced stage II/III rectal cancer is pre–operative chemoradiation with fluoropyrimidine. When compared to post–operative radiation, pre–operative radiation has some advantages: decreasing tumor volume, radiating surgery naïve tissue to potentially increase the radiation sensitivity, reducing the risk of exposing post–surgical bowel tissue and anastomosis from radiation, and increasing the likelihood of R0 resection. Various strategies have been studied to define the ideal treatment for locally advanced rectal cancer. Pre–operative chemoradiation is known to result in better pCR rates than pre–operative radiation alone (13.7% vs 5.3%; odds ratio 2.84; 95% CI, 1.75-4.59; P < .0001) and has been the standard for several years as a treatment for locally advanced rectal cancer.1 Several studies have been conducted to improve radiation sensitivity. The most common strategy studied has been to add oxaliplatin. Some studies have shown significant improvement in pCR rates4,5 with one study showing improvement in disease-free survival.4 However, the body of evidence indicates an overall higher risk for toxicities with the addition of oxaliplatin without clear overall survival benefit.5-9 In a study comparing pre–operative chemoradiation with 5FU or capecitabine with or without the addition of oxaliplatin, the pCR rates were 17.8% and 19.5%, respectively. However, the addition of oxaliplatin resulted in a significantly greater percentage of grades 3–5 diarrhea (16.5% vs 6.9%; P < .001).8 The three-year locoregional recurrence rate was similar with 5FU or capecitabine and with or without oxaliplatin.10 Similarly, the preliminary data from the ARISTOTLE trial assessing the benefit of the addition of irinotecan to capecitabine-based chemoradiation, did not reveal a statistically improved pCR rate, and showed less compliance to radiation and capecitabine along with more adverse events.11 Various studies have been conducted testing the efficacy of adding EGFR inhibitors such as cetuximab or panitumumab and anti-angiogenesis drugs such as bevacizumab to chemoradiation. However, these studies have not demonstrated significant improvement in pCR rates or have caused too much toxicity.12-14 Bevacizumab has been studied in some phase I–II trials in combination with chemoradiation. On an average, the pCR rate is 19%, but some studies have shown delay or failure to receive adjuvant therapy.15 The addition of EGFR inhibitors to chemoradiation has also not resulted in significant improvement in pCR rates and KRAS status has not been shown to be a predictor of response.12,13 The pCR rates with chemoradiation have not exceeded 20% in most studies.
Our study was able to achieve a pCR rate of 37.8%. However, we had no T4 cases in the study, which is known to be a characteristic of “high-risk” disease. In a study of patients with KRAS-mutated rectal cancer, the combination of capecitabine and sorafenib with radiation yielded a pCR rate of 60%.2 Sorafenib is a protein kinase inhibitor with activity against VEGF, PDGFR and RAS, similar to lenvatinib. The downstaging rate on this study was 81.6% very comparable to 84.2% seen in our study. We did not collect information on KRAS mutation status in our study and therefore, it might be possible that the combination has better efficacy in patients with tumors bearing KRAS mutations. The study with sorafenib did report 15% grade 3 adverse events with diarrhea and 12.5% grade 3 adverse events with hand-foot-syndrome. Our study did not have an excess of 10% of grade 3 adverse events and this was mostly related to hypertension more commonly seen with lenvatinib than sorafenib. Our group has also previously evaluated the combination of 5FU and sorafenib with radiation. We showed that the pCR rate was 33% and downstaging occurred in 85.7%.16 These results were demonstrated in patients unselected based on KRAS mutation status.
The NAR score has been validated in many datasets, but prospective validation of its association with overall survival is lacking. There can be potentially 3 different NAR categories depending on the value. Patients with high NAR scores (>16) are associated with poor overall survival, those with low scores (<8) are associated with superior overall survival and those in the middle have intermediate survival.3 In our study, the median NAR was 8.43 and the mean was 10.37, and these scores are in the intermediate range. It would be useful to validate the association of NAR score with survival in a larger study of this combination.
Overall, this was a well-tolerated regimen with few adverse events and no dose-limiting toxicities. There were no treatment interruptions due to treatment. No excess post–operative complications were reported due to the study treatment except for 1 patient who had wound dehiscence but was not attributed to the study treatment. Most adverse events were low grade and in line with some side effects expected of lenvatinib.
To this date, there are no reliable predictive biomarkers for TKIs. In our study, the baseline level of 3 biomarkers TSP-2, VEGF-R3, and VEGF correlated with NAR score and these levels were significantly different across different response group categories. TSP-2 encodes of thrombospondin-2 which has anti-angiogenesis properties.17 Patients with lower levels of TSP-2 at baseline did not show significant pathological response, possibly due to TSP-2 induced hypoxia. Hypoxia overall can lead to radioresistance. However, hypoxia can lead to the secretion of angiogenic factors such as VEGF and when combined with anti-angiogenesis agents can increase sensitivity to radiation.18 Thus, we postulate that tumors in a hypoxic environment have activation of angiogenic signaling that may increase the sensitivity to radiation combined with antiangiogenic agents. While previous studies with the combination of bevacizumab did not lead to success in unselected patient population, our blood-based biomarkers may be beneficial to enable discernment as to which patients will benefit the most from the addition of anti-angiogenic or a mixed protein kinase inhibitor that targets other receptors in the tumor stroma to chemoradiation.
There are some limitations to this study. This is a single-arm, single-institution study. Therefore, the results of the trial will need to be confirmed in a larger randomized trial. In the NRG-GI002 study, the TNT approach was tested with independent arms of combination chemoradiation with pembrolizumab or veliparib. The experimental arms with combination pembrolizumab or veliparib did not significantly improve pCR or NAR score; however, the combination of pembrolizumab or veliparib was considered safe when administered with chemoradiation for locally advanced rectal cancer.19,20 In the era of total neoadjuvant treatment (TNT), this approach may seem outdated. However, we believe that this approach can be integrated as a treatment arm for the chemoradiation portion to help ensure more superior pCR rates and increase the chances for non–operative management. There is also increasing use of circulating tumor DNA (ctDNA) in stages II and III colorectal cancers. There are currently prospective trials ongoing to assess the utilization of ctDNA for escalation/de-escalation of systemic therapy in locally advanced colorectal cancer.21 This study is the first of its kind that has reported safety, efficacy, and correlative biomarker analyses of the novel combination of lenvatinib with capecitabine and radiation in locally advanced rectal cancer. We believe that the integration of ctDNA with the blood-based biomarkers will add significant value in designing a large trial with the combination of lenvatinib and capecitabine with radiation and identify the patients that will most likely benefit from the combination.
Contributor Information
Rutika Mehta, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Jessica Frakes, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Jongphil Kim, Department of Biostatistics and Bioinformatics H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Andrew Nixon, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Yingmiao Liu, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Lauren Howard, Department of Biostatistics and Bioinformatics, Duke University, Durham, NC, USA.
Maria E Martinez Jimenez, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Estrella Carballido, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Iman Imanirad, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Julian Sanchez, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Sophie Dessureault, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Hao Xie, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Seth Felder, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Ibrahim Sahin, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Sarah Hoffe, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Mokenge Malafa, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Richard Kim, Department of Gastrointestinal Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Funding
This study was supported by an unrestricted grant from Eisai.
Conflict of Interest
Rutika Mehta: Eli Lilly (C/A), BMS, Astellas (SAB), Daiichi Sankyo, Natera (H); Andrew Nixon: Eli Lilly, GSK, Promega Corporation, Leap Therapeutics, AdjuVolt Therapeutics (C/A), Genentech, HTG Molecular Diagnostics, MedImmune/AstraZeneca, Medpacto, Promega Corporation, Seattle Genetics (RF), Core Correlatives Sciences Committee (NCTN-CCSC, Chair); Richard Kim: QED, Lilly, BMS, Bayer (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Kim R, Prithviraj GK, Shridhar R, et al. Phase I study of pre-operative continuous 5-FU and sorafenib with external radiation therapy in locally advanced rectal adenocarcinoma. Radiother Oncol. 2016;118(2):382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Moos R, Koeberle D, Schacher S, et al. ; Swiss Group for Clinical Cancer Research (SAKK). Neoadjuvant radiotherapy combined with capecitabine and sorafenib in patients with advanced KRAS-mutated rectal cancer: a phase I/II trial (SAKK 41/08). Eur J Cancer. 2018;89:82-89. [DOI] [PubMed] [Google Scholar]
- 3. Glynne-Jones R, Glynne-Jones S.. The concept and use of the neoadjuvant rectal score as a composite endpoint in rectal cancer. Lancet Oncol. 2021;22(7):e314-e326. [DOI] [PubMed] [Google Scholar]
- 4. Bosset JF, Collette L, Calais G, et al. ; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114-1123. [DOI] [PubMed] [Google Scholar]
- 5. Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34(27):3300-3307. [DOI] [PubMed] [Google Scholar]
- 6. Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. [DOI] [PubMed] [Google Scholar]
- 7. Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773-2780. [DOI] [PubMed] [Google Scholar]
- 8. Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638-1644. [DOI] [PubMed] [Google Scholar]
- 9. O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32(18):1927-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/- oxaliplatin in locally advanced rectal cancer: Final results of PETACC-6. J Clin Oncol. 2018;36(15_suppl):3500-3500. [Google Scholar]
- 11. Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11):djv 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sebag-Montefiore D, Adams R, Gollins S, et al. ARISTOTLE: a phase III trial comparing concurrent capecitabine with capecitabine and irinotecan (Ir) chemoradiation as preoperative treatment for MRI-defined locally advanced rectal cancer (LARC). J Clin Oncol. 2020;38(15_suppl):4101-4101. [Google Scholar]
- 13. Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30(14):1620-1627. [DOI] [PubMed] [Google Scholar]
- 14. Helbling D, Bodoky G, Gautschi O, et al. Neoadjuvant chemoradiotherapy with or without panitumumab in patients with wild-type KRAS, locally advanced rectal cancer (LARC): a randomized, multicenter, phase II trial SAKK 41/07. Ann Oncol. 2013;24(3):718-725. [DOI] [PubMed] [Google Scholar]
- 15. Landry JC, Feng Y, Prabhu RS, et al. Phase II trial of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: 5-year clinical outcomes ECOG-ACRIN cancer research group E3204. Oncologist. 2015;20(6):615-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salazar R, Capdevila J, Laquente B, et al. A randomized phase II study of capecitabine-based chemoradiation with or without bevacizumab in resectable locally advanced rectal cancer: clinical and biological features. BMC Cancer. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z.. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000;19(7):557-568. [DOI] [PubMed] [Google Scholar]
- 18. Wachsberger P, Burd R, Dicker AP.. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9(6):1957-1971. [PubMed] [Google Scholar]
- 19. George TJ, Yothers G, Hong TS, et al. NRG-GI002: a phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—First experimental arm (EA) initial results. J Clin Oncol. 2019;37(15_suppl):3505-3505. [Google Scholar]
- 20. Rahma OE, Yothers G, Hong TS, et al. NRG-GI002: a phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—Pembrolizumab experimental arm (EA) primary results. J Clin Oncol. 2021;39(3_suppl):8. [Google Scholar]
- 21. Kasi PM, Sawyer S, Guilford J, et al. A multicenter study to evaluate the impact of circulating tumor DNA guided therapy (BESPOKE) in patients with stage II and III colorectal cancer. Ann Oncol. 2020;31(4):S459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.