Abstract
Background
A study was initiated at Roswell Park Comprehensive Cancer Center to capture the real-world experience related to the use of CDK4/6 inhibitors (Ciclibs) for the treatment of metastatic hormone receptor-positive and HER2-negative breast cancer (HR+/HER2-).
Patients and Methods
A total of 222 patients were evaluated who received CDK4/6 inhibitors in the period from 2015 to 2021. Detailed clinical and demographic information was obtained on each patient and used to define clinical and demographic features associated with progression-free survival on CDK4/6 inhibitor-based therapies.
Results
In this real-world analysis, the majority of patients received palbociclib as the CDK4/6 inhibitor with letrozole or fulvestrant as the predominant endocrine therapies. The median progression-free survival (PFS) in the letrozole (27.6 months) and fulvestrant (17.2 months) groups were comparable to that observed in clinical trials. As expected, age at start of the treatment and menopausal status influenced endocrine therapy utilization but were not associated with PFS. Patients with recurrent disease had shorter PFS (P = .0024) than those presenting with de novo metastasis. The presence of visceral metastasis trended toward shorter PFS (P = .051). Similarly, prior endocrine therapy (P = .003) or chemotherapy (P = .036) was associated with shorter PFS. Body mass index was not associated with PFS or with dose interruption and/or modification. While the number of minorities in this analysis is limited (n = 26), these patients as a group had statistically shorter PFS on treatment (P = .002).
Conclusions
The real-world progression-free survival with CDK4/6 inhibitors mimics that observed in the clinical trial. A number of clinical and demographic features were associated with PFS on CDK4/6 inhibitor-based therapy. Further studies are ongoing to validate these findings incorporating additional cancer centers.
This article reports a real-world experience related to the use of CDK4/6 inhibitors for the treatment of metastatic hormone receptor-positive and HER2-negative breast cancer, focusing on clinical and demographic features associated with progression-free survival.
Implications for Practice.
This study provides the real-world experience with the use of CDK4/6 inhibitors in the treatment of metastatic HR+/HER2− breast cancer. The work defines clinical and demographic features that impact the response to these agents and could offer guidance in preferred strategies for select manifestations of the disease. The study also suggests the need for more study of under-represented minority populations.
Introduction
Hormone receptor-positive and HER2-negative (HR+/HER2−) breast cancer represents one of the most common disease diagnoses among women.1,2 Amongst breast cancer subtypes, HR+/HER2− is generally associated with an improved prognosis if identified at an early stage. This form of the disease is characterized by a dependence on estrogenic signaling and endocrine therapy has been the mainstay of systemic treatment for the last 3 decades.1,3-5 Such treatment in the adjuvant setting can lead to long periods of disease-free survival; however, there is a risk of metastatic recurrence even after periods of long dormancy.6-8 To mitigate recurrence, diagnostic tools have been developed that determine the risk for recurrence or metastasis (eg, OncotypeDX and Mammaprint) and the potential for a positive impact of chemotherapy on recurrence-free survival.9,10
The treatment of metastatic HR+/HER2− breast cancer has been evolving. Endocrine therapy (eg, letrozole or fulvestrant) can delay the progression of metastatic disease; however, most patients ultimately progress on treatment.1 A large number of randomized trials combined targeted agents with endocrine therapy to extend progression-free survival (PFS). These trials resulted in the approval of exemestane + everolimus for metastatic HR+/HER2− disease,11 as well as the combination of different CDK4/6 inhibitors with a variety of endocrine therapies.12-18 The CDK4/6 inhibitors: palbociclib, ribociclib, and abemaciclib are FDA-approved for the treatment of metastatic HR+/HER2− disease. In clinical trials, these agents lead to an approximate doubling of the PFS relative to the endocrine therapy alone.1,13 While these agents have differing structures, dosing regimens, and side effect profiles, the impact on PFS in the metastatic setting is incredibly consistent.19-22 These findings support the overall concept that CDK4/6 inhibitors likely exhibit the same general mechanism of action. In the treatment of HR+/HER2− metastatic breast cancer CDK4/6 inhibitors were considered highly significant in delaying the utilization of chemotherapy and enhancing patient quality of life. While effective, the use of CDK4/6 inhibitors is not generally considered curative; tumors will ultimately progress on therapy and there is a subset of cancers that appear to be intrinsically resistant.
The mechanism of CDK4/6 inhibitor action has been extensively studied in preclinical models of HR+/HER2− breast cancer.19,23 It was initially found that these tumor models were sensitive to CDK4/6 inhibitors and that this activity cooperated with endocrine therapies.20,23 Additionally, CDK4/6 inhibitors could still function in models that were resistant to endocrine therapy.24,25 The action of CDK4/6 inhibitors is critically dependent on the RB tumor suppressor that serves to link CDK4/6 activity to downstream progression through the cell cycle.23,26,27 Thus, it has been speculated that RB-deficiency is associated with intrinsic resistance. However, recent data from multiple groups have suggested that adaptive resistance can emerge through a variety of other pathways that keep RB intact but uncouple the classical dependence on CDK4/6 for cell cycle progression.28,29 A prime example of this type of mechanism would be the overexpression of Cyclin E.29 In spite of a substantial understanding of the mechanism of action, no biomarkers have emerged for patient selection with CDK4/6 inhibitors in HR+/HER2− breast cancer. Here we report on the real-world single-institution experience with CDK4/6 inhibitors (Ciclibs). It is likely that consideration of clinical determinants of PFS will be important in shaping biomarker/precision strategies for patients.
Methods
Data Source and Patient Selection
A chart review was conducted for 222 patients who were diagnosed with HR+/HER2− breast cancer and received a CDK4/6 inhibitor from 2015 to 2021 at Roswell Park Comprehensive Cancer Center (RPCCC). Patient data from 2 studies approved by the Roswell Park Comprehensive Cancer Center Institutional Review Board was used. A retrospective chart review protocol was used to collect information on 72 patients. Subsequently a combination retrospective and prospective (NCT04526587) protocol was developed. Patients for this protocol were identified using a systematic review. Preparatory research data mining was conducted on all past breast cancer patients from RPCCC. In conjunction, all upcoming patients of the RPCCC Breast Clinic were reviewed weekly. This combination identified any study-eligible patient, which was then verified with each patient’s physician before consenting. A total of over 2500 electronic health records have been reviewed, with the majority of patients being either subtype-ineligible, not having metastatic disease, or currently on a different systemic treatment regimen. A total of 150 patients have been consented as of July 2021. Eligible patients were ≥18 years of age, had ER+/HER2− advanced breast cancer, and were treated with a CDK4/6 inhibitor. Electronic medical records were used to extract demographic information, smoking history, menopausal status, BMI, Eastern Cooperative Oncology Group (ECOG) performance status, surgery and pathology reports, genomic data, dates of diagnosis and recurrence(s), site(s) of metastases, and cancer treatment information such as any side effects/toxicities from treatment as mentioned by providers in each chart.
Statistical Analysis
The primary endpoint, PFS, was defined as the time from the first dose of ciclib therapy to either scan- or marker-proven progression, or death while on therapy. Patients who stopped ciclib therapy due to toxicities (n = 16) were not considered in PFS calculations. Patients who were prescribed a CDK4/6 inhibitor in the neoadjuvant setting (n = 3) were excluded from all analyses.
For the primary purposes of this study, patients were divided into 2 groups: patients taking an aromatase inhibitor (letrozole, anastrozole, or exemestane) along with a CDK 4/6 inhibitor, and patients taking fulvestrant along with a CDK4/6 inhibitor. The few patients prescribed Tamoxifen with a CDK4/6 inhibitor or taking CDK4/6 monotherapy (n = 2) were excluded from the analysis. Exploratory analyses were also conducted according to the 2 groups separately. Kaplan-Meier (KM) survival analysis compared with log-rank tests and univariate Cox proportional-hazards regression were used to compare PFS by demographic and endocrine therapy. Two-sided t-tests were used for comparisons between continuous demographic variables; for categorical variables, χ2 or Fisher’s exact tests were used. R (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
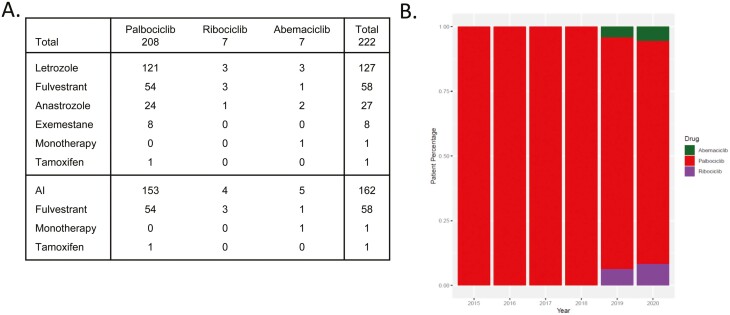
Patients were treated with CDK4/6 inhibitors at Roswell Park Comprehensive Cancer Center between 2015 and 2021 with a median follow-up of 7.69 years. Demographics and clinical features of the patients enrolled are presented in Table 1. The predominant endocrine therapies were letrozole (n = 127), fulvestrant (n = 58) with rarer utilization of exemestane, anastrozole, and tamoxifen. The use of aromatase inhibitors with CDK4/6 inhibitors (n = 162) represented the dominant overall treatment within this cohort (Fig. 1A). Of these patients 208 were treated with palbociclib starting in 2015, 7 with abemaciclib starting in 2018, and 7 with ribociclib starting in 2019 (Fig. 1B). Only one patient was treated with abemaciclib monotherapy and one patient was treated with palbociclib in combination with tamoxifen.
Table 1.
Patient characteristics.
| Demographic | All patients N = 222 |
Aromatase Inhibitor (%) N = 162 (72.97) |
Fulvestrant (%) N = 58 (26.13) |
|---|---|---|---|
| Age at CDK Start, y | |||
| Median | 63 | 62 | 66.5 |
| Range | [27, 89] | [27, 84] | [43, 89] |
| <65 | 125 | 99 | 26 |
| ≥65 | 97 | 63 | 32 |
| BMI at CDK start | |||
| Median | 27.85 | 27.8 | 28.1 |
| Range | [15.4, 56.2] | [15.4, 56.2] | [16.3, 47.1] |
| Underweight (<18.5) | 6 | 5 | 1 |
| Normal (18.5-24.9) | 56 | 42 | 13 |
| Overweight (25.0-29.9) | 70 | 49 | 20 |
| Obese (≥30) | 88 | 64 | 24 |
| ECOG at CDK start | |||
| 0 | 126 | 93 | 32 |
| 1 | 75 | 52 | 22 |
| 2 | 13 | 10 | 3 |
| 3 | 1 | 1 | 0 |
| Sex | |||
| Female | 219 | 159 | 58 |
| Male | 3 | 3 | 0 |
| Race/ethnicity | |||
| European | 192 | 145 | 45 |
| Asian | 3 | 1 | 2 |
| African American | 17 | 8 | 9 |
| Hispanic & Latino | 4 | 3 | 1 |
| Other | 2 | 2 | 0 |
| Menopause status at CDK start | |||
| Pre | 34 | 31 | 3 |
| Peri and post | 181 | 128 | 51 |
| Male | 3 | 3 | 0 |
| Smoking status | |||
| Never | 114 | 76 | 36 |
| Former & past | 75 | 60 | 15 |
| Current | 17 | 12 | 5 |
| Visceral status | |||
| Visceral | 103 | 69 | 32 |
| Non-visceral | 119 | 93 | 26 |
| Metastatic status at presentation | |||
| De Novo | 66 | 58 | 8 |
| Recurrent | 156 | 104 | 50 |
| Number of metastatic sites | |||
| 1 | 92 | 69 | 23 |
| 2 | 67 | 44 | 22 |
| ≥3 | 48 | 37 | 10 |
| Prior endocrine therapy | |||
| Yes | 156 | 99 | 55 |
| No | 66 | 63 | 3 |
| Prior chemotherapy | |||
| Yes | 122 | 84 | 37 |
| No | 100 | 78 | 21 |
Figure 1.
(A) Tablular representation of each patient’s hormonal therapy in addition to CDK4/6. (B) Barplot of percentages for each type of CDK4/6 prescribed at RPCCC across time, from 2015-2020.
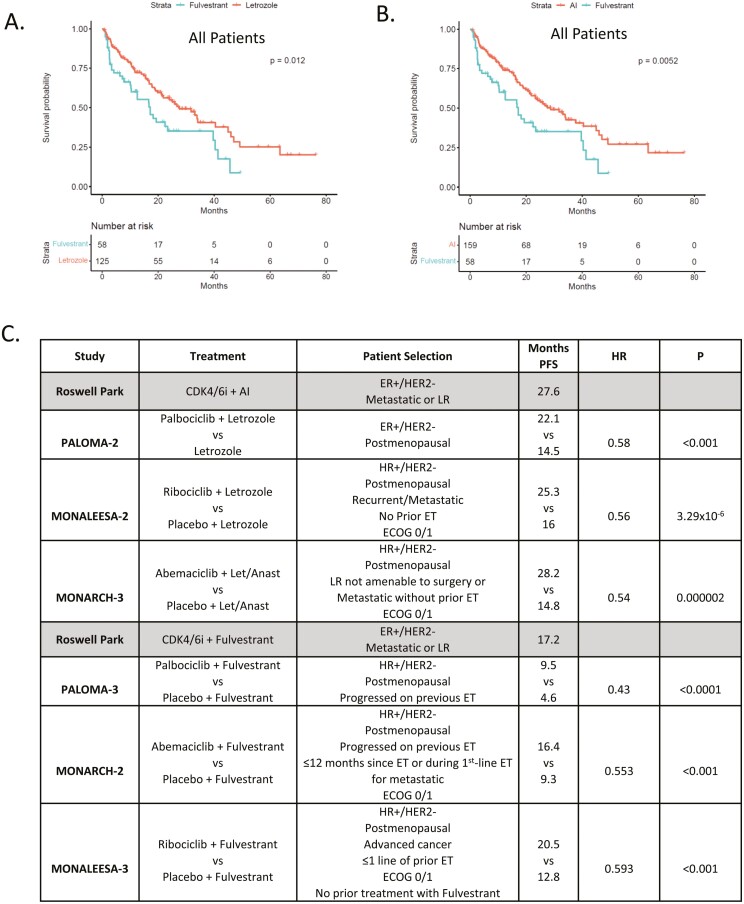
Due to the distinct utilization of endocrine therapy, patients were stratified by predominant endocrine therapy used in combination with CDK4/6 inhibitors (Fig. 2A). As expected, the PFS was shorter for patients with endocrine-resistant disease and treated with fulvestrant combinations vs. letrozole (P = .012) or all AI (P = .0052) (Fig. 2B). The median PFS in this cohort was largely comparable to those reported in the randomized clinical trials of CDK4/6 inhibitors with letrozole/aromatase inhibitors or fulvestrant (Fig. 2C).
Figure 2.
(A) Kaplan-Meier analysis of progression-free survival comparing CDK4/6i combinations with fulvestrant vs. letrozole. P = .012 by log-rank. (B) Kaplan-Meier analysis of progression-free survival comparing CDK4/6i combinations with fulvestrant vs. AI. P = .0052 by log-rank. (C) Comparison of the progression-free survival from the real-world setting vs. the indicated randomized trials.
To delineate demographic/clinical features associated with endocrine therapy, a univariate analysis was performed. As expected, prior endocrine therapy strongly favored treatment with fulvestrant (Table 2). Age and menopausal status were similarly associated with the choice of endocrine therapy, where older post-menopausal patients were more likely to be treated with fulvestrant (Table 2). Additionally, the metastatic status (recurrent vs. de novo) was associated with fulvestrant treatment. Interestingly, albeit a relatively small number of race/ethnicity other than European was associated with fulvestrant treatment in this cohort (Table 2).
Table 2.
Significant patient characteristics.
| Demographic | Letrozole vs fulvestrant | Letrozole vs other | Fulvestrant vs other | AI vs fulvestrant |
|---|---|---|---|---|
| Age at CDK start | 0.001 | 0.472 | 0.072 | 0.001 |
| BMI at CDK start | 0.792 | 0.942 | 0.796 | 0.819 |
| ECOG at CDK start | 0.751 | 0.836 | 1 | 0.832 |
| Sex | 0.553 | 1 | 1 | 0.568 |
| Race/ethnicity (EUR vs non-EUR) | 0.031 | 0.684 | 0.417 | 0.041 |
| Menopause atatus at CDK start | 0.036 | 0.643 | 0.045 | 0.017 |
| Smoking status | 0.085 | 1 | 0.337 | 0.093 |
| Visceral status | 0.145 | 0.695 | 0.508 | 0.156 |
| Metastatic status at presentation | 0.011 | 0.172 | 0.001 | 0.001 |
| Number of metastatic sites | 0.29 | 0.599 | 0.555 | 0.314 |
| Prior endocrine therapy | 2.017e-6 | 0.338 | 3.04e-6 | 2.46e‐7 |
| Prior chemotherapy | 0.115 | 0.852 | 0.394 | 0.127 |
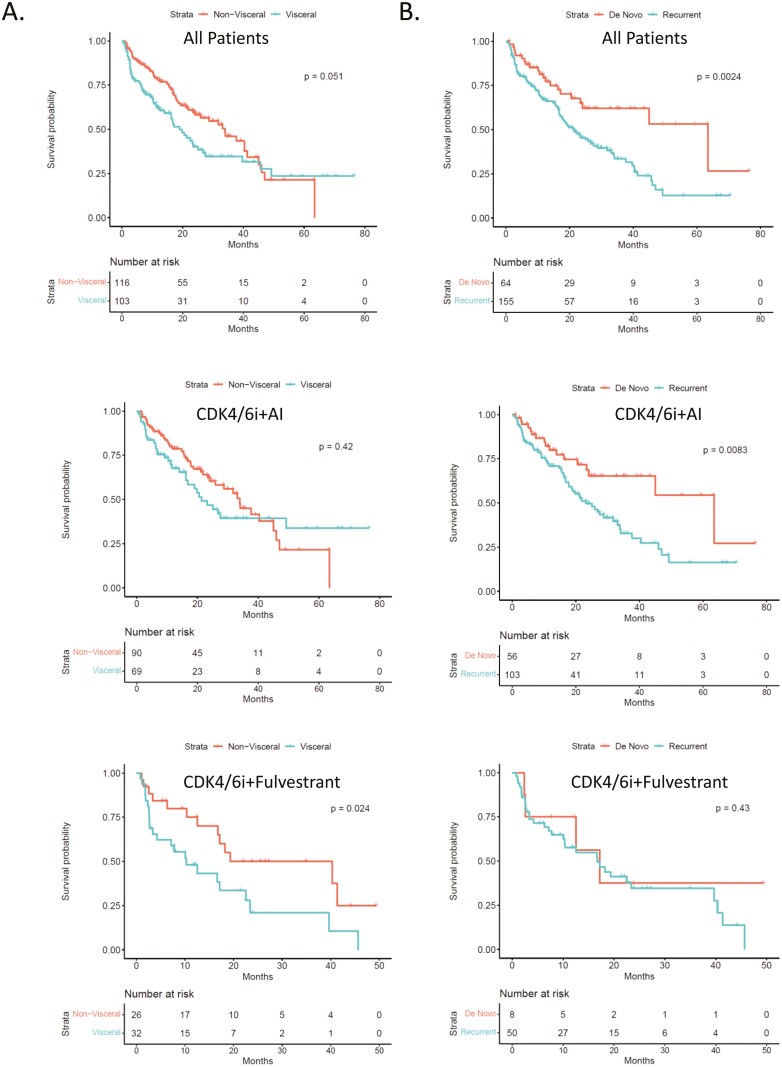
Amongst clinical features of the disease, visceral status and the manifestation of metastasis were associated with PFS in pooled treatment analysis (Fig. 3A,3B). Interestingly, these relationships maintained the most significance in distinct treatment subgroups. Namely, the visceral disease was particularly associated with short PFS in patients treated with fulvestrant and a CDK4/6 inhibitor. However, the recurrent disease was particularly associated with short PFS in patients treated with an AI+CDK4/6 inhibitor.
Figure 3.
(A) Kaplan-Meier analysis of progression-free survival comparing CDK4/6i combinations with visceral vs. non-visceral disease. (B) Kaplan-Meier analysis of progression-free survival comparing CDK 4/6i combinations with de novo and recurrent metastatic presentation. Top panel: pooled analysis; middle panel: for aromatase inhibitor combinations; Bottom panel for fulvestrant combinations.
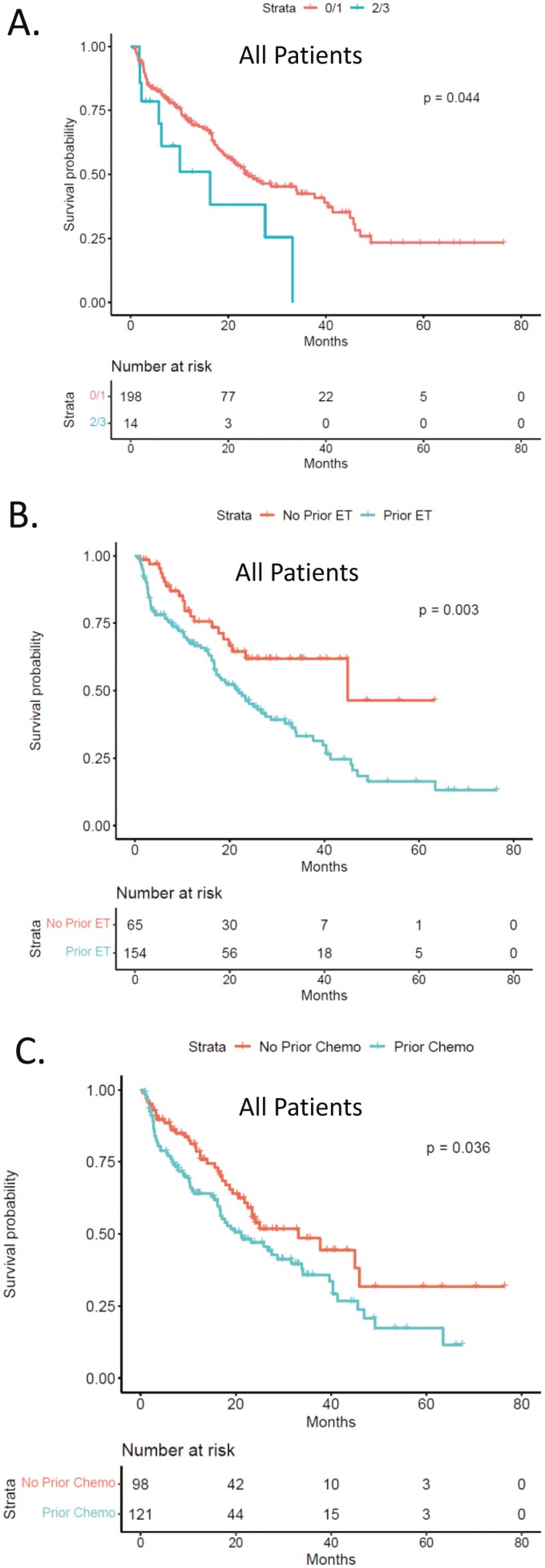
In addition to the metastatic presentation, ECOG status 2/3, while rare in this patient population, was associated with shorter PFS (Fig. 4A). Prior lines of therapy, either endocrine therapy or chemotherapy, were associated with shorter PFS in this patient population (Fig. 4B, 4C). These prior therapies had the most significance for the patient treated with CDK4/6 inhibitor with an aromatase inhibitor (Supplementary Fig. S1)
Figure 4.
(A) Kaplan-Meier analysis of progression-free survival comparing ECOG0/1 vs. ECOG2/3 status across all patients. (B) Kaplan-Meier analysis of progression-free survival comparing patients with prior endocrine therapy. P = .003 by log-rank. (C) Kaplan-Meier analysis of progression-free survival comparing patients with prior chemotherapy. P = .036 by log-rank.
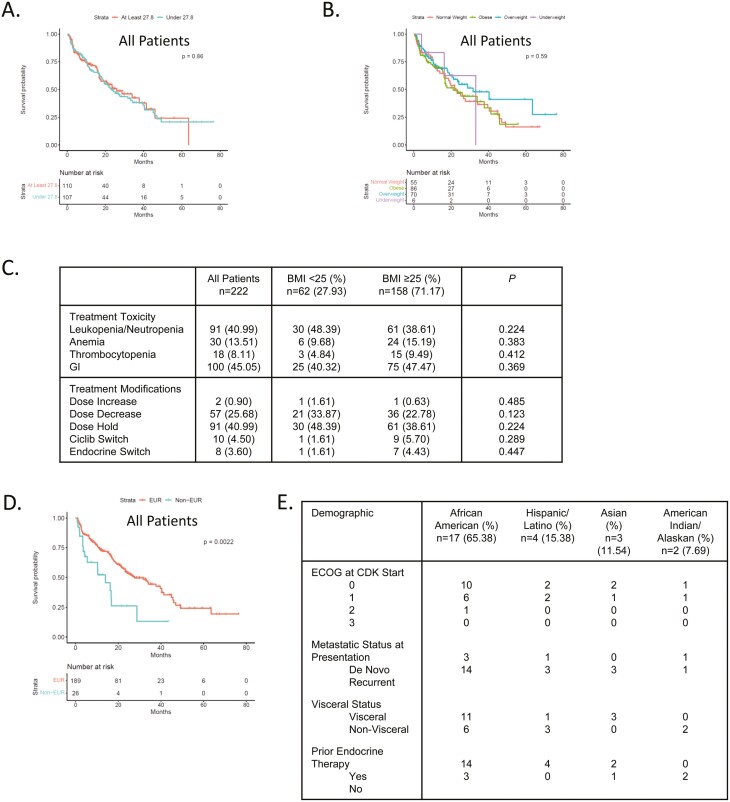
A number of demographic and clinical features were not significantly associated with PFS, including smoking, age, menopausal status, and body mass index (BMI) (Supplementary Fig. S2; Fig. 5A, B). Because body mass index could in principle influence dosing and toxicity,30 we evaluated the impact of BMI vs. dose/time modifications. There was no significant difference based on BMI (Fig. 5C). The hematological toxicities were determined from laboratory tests, the GI outcomes are self-reported not clinically validated. In the analyses of racial/ethnic outcomes, the combined minority population exhibited shorter PFS (Fig. 5Dand E; Supplementary Fig. S3). Due to the relatively small number of minority patients, subgroup analysis based on endocrine therapy was not significant but supports further study.
Figure 5.
(A) Kaplan-Meier analysis of progression-free survival comparing median high vs. median low body mass index within the cohort. (B) Kaplan-Meier analysis of progression-free survival comparing under-weight, normal, overweight, and obese body mass index within the cohort. (C) Table summarizes the association between body mass index and different variable related to toxicity of CDK4/6 inhibitors. (D) Kaplan-Meier analysis of progression-free survival comparing European vs. all other racial/ethnic categories. P = .002 by log-rank. (E) summarizing the racial and ethnic groups in the cohort.
Discussion
CDK4/6 inhibitors have become a mainstay in the clinical treatment of HR+/HER2− metastatic breast cancer, with the possibility that they will be used for high-risk patients in the adjuvant settings.1,31 Here we explored the real-world use of these drugs at a single NCI-designated cancer center (Roswell Park Comprehensive Cancer Center). Since there are multiple CDK4/6 inhibitors that are FDA-approved, one of the key questions was simply what are the predominant ciclibs being used. In our study, palbociclib, which was the first of these agents to gain FDA-approval, remains the predominant CDK4/6 being prescribed, even with the more recent FDA approvals of abemaciclib and ribociclib. The endocrine therapy combinations are based on a variety of considerations, with an aromatase inhibitor (letrozole in particular) or fulvestrant being the most common combinations. While abemaciclib is approved as monotherapy out of the 222 patients in the study, only 1 received monotherapy. This study continues to accrue so it will be possible to determine if patterns shift with the passage of time and/or results emerging from adjuvant and other clinical studies, such as with the recent approval of abemaciclib adjuvant use for high-risk breast cancer.31
In general, the PFS observed in this real-world setting mirrored what has been observed in multiple randomized clinical trials that lead to FDA-approval.22 Notably, patients treated with aromatase inhibitors at Roswell Park had a median PFS of 27.6 months, this is highly comparable to the range of PFS that are observed in PALOMA-1 (22.1 months),12 MONARCH-3 (28.2 months),16 and MONALESSA-2 (25.3 months).17 For fulvestrant treatment the Roswell Park had a median PFS of 17.2 months; while this is superior to PALOMA-3 (9.9 months),14 it is comparable to MONARCH-2 (16. 4 months),15 and MONALEESA-3 (20.5 months).18 It should be noted that in these latter 2 studies there was significant patient selection relative to prior lines of therapy and performance status. In the real-world setting, patients with worse performance status are provided CDK4/6 inhibitor therapy. This group of patients with ECOG2/3 performance status, while small, had a shorter PFS relative to ECOG 0/1 patients. Similarly, prior lines of therapy were associated with shorter PFS; this was particularly true with prior endocrine therapy.
To date, few parameters have emerged as a guide for treatment and/or to predict disease course. Here we find that patients with recurrent disease and visceral metastasis generally had a shorter PFS relative to de novo metastasis and bone/local metastasis. These clinical features were only relevant in a certain treatment settings. The reason for this remains unclear; however, small numbers in some of the sub-groups could confound these analyses. In a recently published meta-analysis of randomized clinical studies, visceral metastasis and recurrent metastatic disease are associated with a shorter PFS across CDK4/6 treatment groups, consistent with the findings in the Roswell Park cohort.22
A high BMI could be associated with lesser drug exposure due to the FDA-approved standard dose of palbociclib (125 mg) that was largely used at Roswell Park. However, BMI had no impact on PFS in our study. This is consistent with recently published studies analyzing BMI relative to abemaciclib-based therapy.30 Conversely, we expected that a higher BMI could limit toxicity, as was recently reported with abemaciclib;30 however, BMI was not associated with toxicity measures in our cohort. This could potentially reflect differential metabolism and toxicity profiles of abemaciclib vs. palbociclib.
The understanding of the role of race/ethnicity in the response to CDK4/6 inhibitors is relatively limited.22 Out of ~4000 patients enrolled in the Phase III clinical studies, less than 100 were of African descent and the predominant racial group beyond European ancestry was Asian. Therefore, investigating treatment outcomes and trends in diverse patient populations is of importance. In our cohort, the predominant non-European patient group was of African descent accounting for ~8% of patients. As a whole, the non-European grouping experienced shorter PFS relative to patients of European descent. Due to the overall small size of these populations, sub-group analysis is challenging and additional analyses and accrual will be required. We found that African Americans entered treatment with CDK4/6 inhibitors disproportionately with recurrent disease and treatment with fulvestrant, both of which associate with shorter PFS. These findings support additional study and the prospect of tailored interventions for select patient groups with metastatic HR+/HER2- breast cancer.
Supplementary Material
Contributor Information
Erik S Knudsen, Department of Molecular and Cellular Biology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Emily Schultz, Center for Personalized Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Deanna Hamilton, Center for Personalized Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Kris Attwood, Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Stephen Edge, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Tracey O’Connor, Department of Medical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Ellis Levine, Department of Medical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Agnieszka K Witkiewicz, Center for Personalized Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA; Department of Pathology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: A.W., E.K., E.S. Provision of study material/patients: D.H., E.S., S.E., T.O’C., E.L. Collection and/or assembly of data: D.H., E.S., A.W., E.K. Data analysis and interpretation: K.A., E.S., E.K., A.W. Manuscript writing: E.K., A.W., E.S. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Waks AG, Winer EP.. Breast cancer treatment: a review. JAMA. 2019;321(3):288-300. [DOI] [PubMed] [Google Scholar]
- 2. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. [DOI] [PubMed] [Google Scholar]
- 3. Briest S, Davidson NE.. Aromatase inhibitors for breast cancer. Rev Endocr Metab Disord. 2007;8(3):215-228. [DOI] [PubMed] [Google Scholar]
- 4. Brodie AM, Dowsett M, Coombes RC.. Aromatase inhibitors as new endocrine therapy for breast cancer. Cancer Treat Res. 1988;39:51-65. [DOI] [PubMed] [Google Scholar]
- 5. McGuire WL, Horwitz KB, Pearson OH, Segaloff A.. Current status of estrogen and progesterone receptors in breast cancer. Cancer. 1977;39(6 Suppl):2934-2947. [DOI] [PubMed] [Google Scholar]
- 6. Cuzick J. Predicting late recurrence in ER-positive breast cancer. Nat Rev Clin Oncol. 2019;16(7):406-408. [DOI] [PubMed] [Google Scholar]
- 7. Rueda OM, Sammut SJ, Seoane JA, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567(7748):399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruhstaller T, Giobbie-Hurder A, Colleoni M, et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: long-term follow-up of the BIG 1-98 trial. J Clin Oncol. 2019;37:105-114, https://doi.org/10.1200/JCO.18.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dowsett M, Dunbier AK.. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008;14(24):8019-8026. [DOI] [PubMed] [Google Scholar]
- 10. Albain KS, Paik S, van’t Veer L.. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast. 2009;18 Suppl 3:S141-S145. [DOI] [PubMed] [Google Scholar]
- 11. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35. [DOI] [PubMed] [Google Scholar]
- 13. O’Leary B, Finn RS, Turner NC.. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417-430. [DOI] [PubMed] [Google Scholar]
- 14. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. [DOI] [PubMed] [Google Scholar]
- 15. Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. [DOI] [PubMed] [Google Scholar]
- 16. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638-3646. [DOI] [PubMed] [Google Scholar]
- 17. O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat. 2018;168(1):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465-2472. [DOI] [PubMed] [Google Scholar]
- 19. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES.. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knudsen ES, Shapiro GI, Keyomarsi K.. Selective CDK4/6 inhibitors: biologic outcomes, determinants of sensitivity, mechanisms of resistance, combinatorial approaches, and pharmacodynamic biomarkers. Am Soc Clin Oncol Educ Book. 2020;40:115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360-1369. [DOI] [PubMed] [Google Scholar]
- 22. Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250-260. [DOI] [PubMed] [Google Scholar]
- 23. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thangavel C, Dean JL, Ertel A, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18(3):333-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wardell SE, Ellis MJ, Alley HM, et al. Efficacy of SERD/SERM Hybrid-CDK4/6 inhibitor combinations in models of endocrine therapy-resistant breast cancer. Clin Cancer Res. 2015;21(22):5121-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knudsen ES, Witkiewicz AK.. the strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer. 2017;3(1):39-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumarasamy V, Vail P, Nambiar R, Witkiewicz AK, Knudsen ES.. Functional determinants of cell-cycle plasticity and sensitivity to CDK4/6 inhibition. Cancer Res. 2020. https://doi.org/:10.1158/0008-5472.CAN-20-2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019. https://doi.org/10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franzoi MA, Eiger D, Ameye L, et al. Clinical Implications of body mass index in metastatic breast cancer patients treated with abemaciclib and endocrine therapy. J Natl Cancer Inst. 2021;113(4):462-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston SRD, Harbeck N, Hegg R, et al. ; monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.